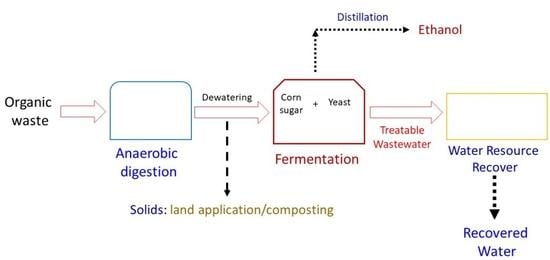
Harnessing the Residual Nutrients in Anaerobic Digestate for Ethanol Fermentation and Digestate Remediation Using Saccharomyces cerevisiae
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
2.2. Preparation of Anaerobic Digestate (ADE)
2.3. Ethanol Fermentation in ADE
2.4. Effect of ADE-Borne Nutrients on Fermentation of Glucose by S. cerevisiae
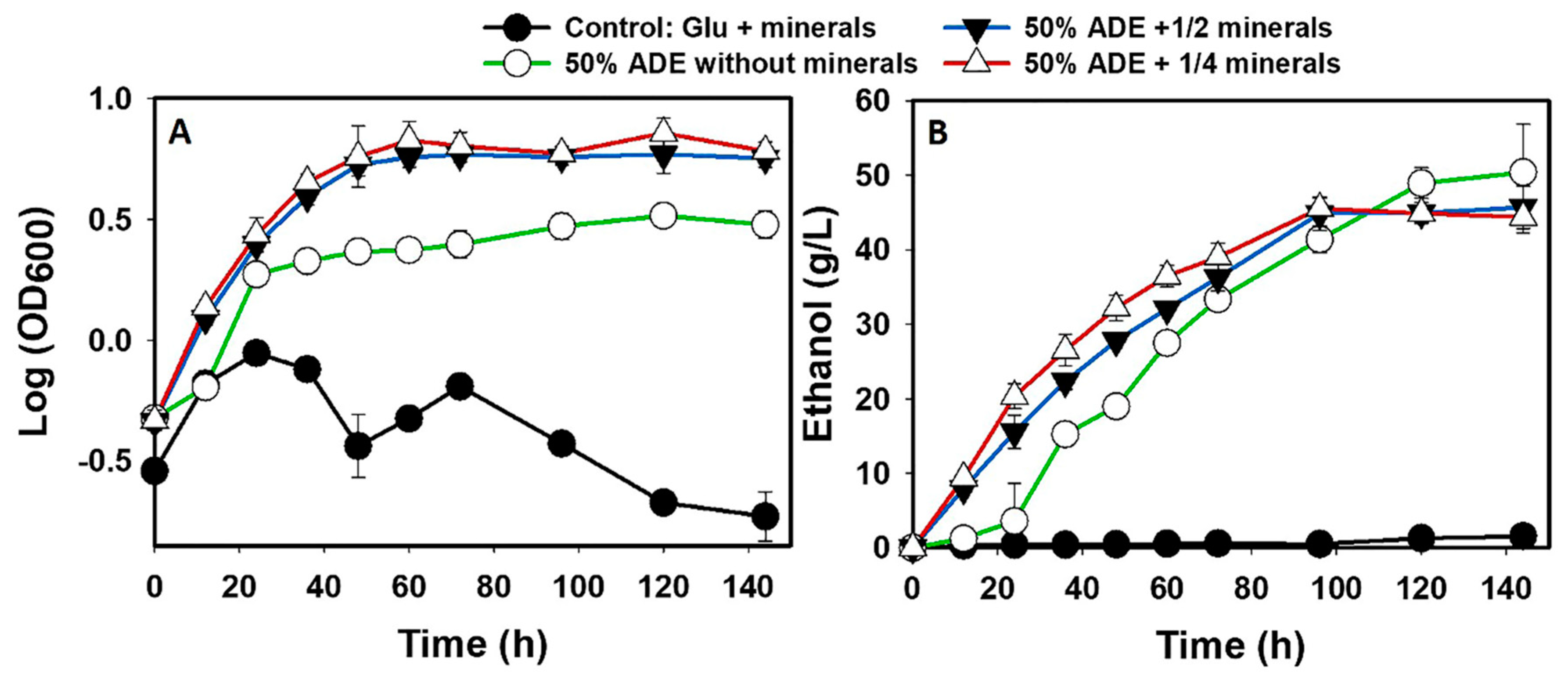
2.4.1. Effect of Metals on Glucose Fermentation in ADE
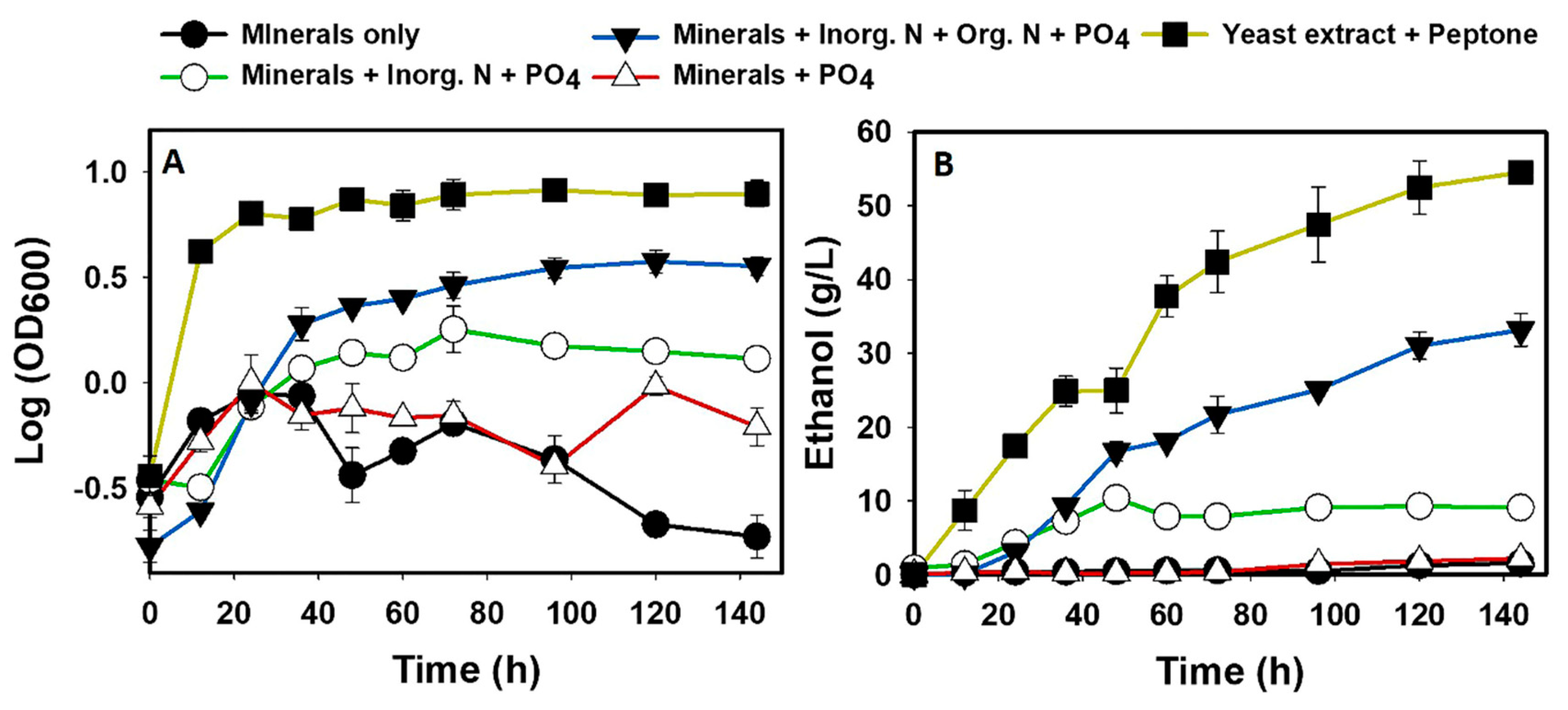
2.4.2. Combined Effects of Metals, Nitrogen, and Phosphorus on Glucose Fermentation in ADE
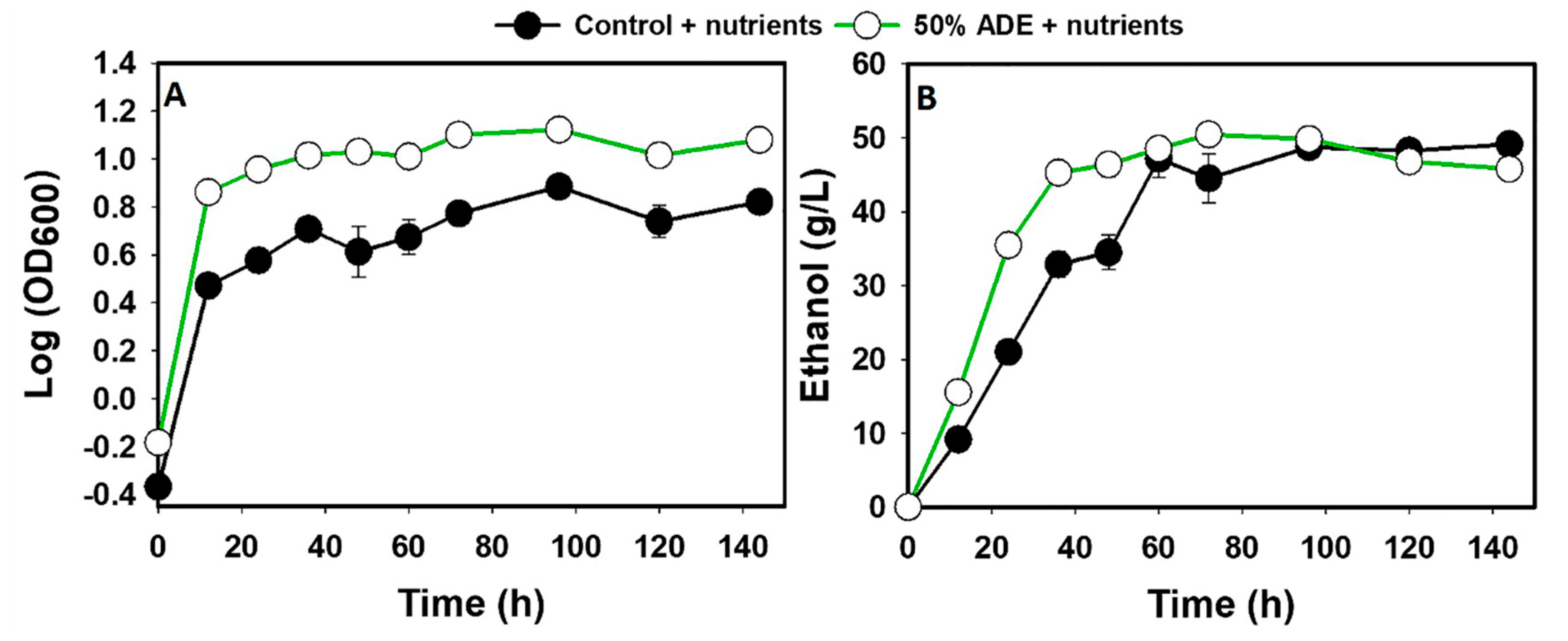
2.4.3. Effect of Excess Nitrogen on Glucose Fermentation in ADE
2.5. Analytical Methods
2.6. Statistical Analysis
3. Results
3.1. The fermentation Profiles of S. cerevisiae Grown in ADE
3.2. Removal of Nutrients from the ADE by S. cerevisiae
4. Discussion
4.1. ADE Clarification and Nutrient Removal
4.2. Effects of the ADE-Borne Nutrients on Growth and Ethanol Production by S. cerevisiae
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Satchwell, A.J.; Scown, C.D.; Smoith, S.J.; Amirebrahimi, J.; Jin, L.; Kirchstetter, T.W.; Brown, N.J.; Preble, C.V. Accelerating the deployment of anaerobi digestion to meet zero waste goals. Environ. Sci. Technol. 2018, 52, 13663–13669. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.L.; Crutchnik, D.; Franchi, O.; Pavissich, J.P.; Belmonte, M.; Pedrouso, A.; Mosquera-Correl, A.; Val del Rio, A. Nitrogen and phosphorus recovery from anaerobically pretreated agro-food wastes: A review. Front. Sustain. Food Syst. 2019, 2, 91. [Google Scholar] [CrossRef]
- Driver, J.; Lijmbach, D.; Steen, I. Why recover phosphorus for recycling, and how? Environ. Technol. 1991, 20, 651–662. [Google Scholar] [CrossRef]
- Münch, E.V.; Barr, K. Controlled struvite crystallization for removing phosphorus from anaerobic digester sidestreams. Wat. Res. 2001, 35, 151–159. [Google Scholar] [CrossRef]
- Sun, G.L.; Reynolds, E.E.; Belcher, A.M. Designing yeast as plant-like hyperaccumulators for heavy metals. Nature Commun. 2019, 10, 5080. [Google Scholar] [CrossRef]
- Soares, E.V.; Hebbelinck, K.; Soares, H.M.V.M. Toxic effects caused by heavy metals in the yeast Saccharomyces cerevisiae: A comparative study. Can J. Microbiol. 2003, 49, 336–343. [Google Scholar] [CrossRef]
- Hosiner, D.; Gerber, S.; Lichtenberg-Frate, H.; Glaser, W.; Schuller, C.; Klipp, E. Impact of acute metal stress in Saccharomyces cerevisiae. PLoS ONE 2014, 9, e83330. [Google Scholar] [CrossRef]
- Agu, C.V.; Ujor, V.; Ezeji, T.C. Allopurinol supplementation of the growth medium enhances the fermentation of lignocellulosic hydrolysates to ethanol by Saccharomyces cerevisiae. Biocat. Agric. Biotechnol. 2018, 14, 151–159. [Google Scholar] [CrossRef]
- Okonkwo, C.C.; Azam, M.M.; Ezeji, T.C.; Qureshi, N. Enhancing ethanol production from cellulosic sugars using Scheffersomyces (Pichia) stipitis. Bioprocess Biosyst. Eng. 2016, 39, 1023–1032. [Google Scholar] [CrossRef]
- Quinones, R.; Luliucci, R.J.; Behnke, G.; Brown, R.; Shoup, D.; Riedel, T.M.; Plavchak, C.; Lininger, B.E.; Spehar, J.M. Moving towards fast characterization of polymorphic drugs by solid-state NMR spectroscopy. J. Pharm. Biomed. Anal. 2018, 148, 163–169. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of peotein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bely, M.; Sablayrolles, J.; Barre, P. Automatic detection of assimilable nitrogen deficiencies during alcoholic fermentation in oenological conditions. J. Ferment. Bioeng. 1990, 70, 246–252. [Google Scholar] [CrossRef]
- Jiranek, V.; Langridge, P.; Henschke, P.A. Regulation of hydrogen sulfide liberation in wine-Producing Saccharomyces cerevisiae strains by assimilable nitrogen. Appl. Environ. Microbiol. 1995, 61, 461–467. [Google Scholar] [CrossRef] [PubMed]
- de Souza, R.B.; de Menezes, J.A.; de Souza, R.F.R.; Dutra, E.D.; de Morais, M.A.A., Jr. Mineral composition of sugarcane juice and its influence on the ethanol fermentation. Appl. Biochem. Biotechnol. 2015, 175, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.A.; Payne, R.W.; Yarrow, D. Yeasts: Characteristics and Identification; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Siverio, J.M. Assimilation of nitrate by yeasts. FEMS Microbiol. Rev. 2002, 26, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Anthonisen, A.C.; Loehr, R.C.; Prakasam, T.B.S.; Srinath, E.G. Inhibition of nitrification by ammonia and nitrous acid. J. Water Pollut. Control Fed. 1976, 48, 835–852. [Google Scholar]
- Nishimura, K.; Igarashi, K.; Kakinuma, Y. Proton gradient-driven nickel uptake by vacuolar membrane vesicles of Saccharomyces cerevisiae. J. Bacteriol. 1998, 180, 1962–1965. [Google Scholar] [CrossRef]
- Kücükgöze, G.; Alkim, C.; Yilmaz, Ü.; Kisakesen, H.I.; Gündüz, S.; Akman, S.; Çakar, P. Evolutionary engineering and transcriptomic analysis of nickel-resistant Saccharomyces cerevisiae. FEMS Yeast Res. 2013, 13, 731–746. [Google Scholar] [CrossRef]
- Lazard, M.; Blanquet, S.; Fisicaro, P.; Labbarraque, G.; Plateau, P. Uptake of selenite by Saccharomyces cerevisiae involves the high and low affinity orthophosphate transporters. J. Biol. Chem. 2010, 285, 32029–32037. [Google Scholar] [CrossRef]
- Brasser, H.J.; Krijger, G.C.; van Meerten, T.G.; Wolterbeek, H.T. Influence of silicon on cobalt, zinc, and magnesium in Baker’s yeast, Saccharomyces cerevisiae. Biol. Trace Elem. Res. 2006, 112, 175–189. [Google Scholar] [CrossRef]
- Cetin, E.; Karakas, E.; Dulegurkgen, E.; Ovez, S.; Kolukirik, M.; Yilmaz, G. Effects of high-concentration influent suspended solids on aerobic granulation in pilot-scale sequencing batch reactors treating real domestic wastewater. Water Res. 2018, 131, 74–89. [Google Scholar] [CrossRef] [PubMed]
- ter Schure, E.G.; van Riel, N.A.W.; Verrips, C.T. The role of ammonia metabolism in nitrogen catabolite repression in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2000, 24, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.L.; Pramer, D. Minor element composition of yeast extract. J. Bacteriol. 1962, 84, 869–870. [Google Scholar] [CrossRef] [PubMed]
- Andrews, A.T. The composition, structure and origin of proteose-peptone component 5 of bovine milk. Eur. J. Biochem. 1978, 90, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Albers, E.; Larsson, C.; Liden, G.; Niklasson, C.; Gustafsson, L. Influence of the nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl. Environ. Microbiol. 1996, 62, 3187–3195. [Google Scholar] [CrossRef]

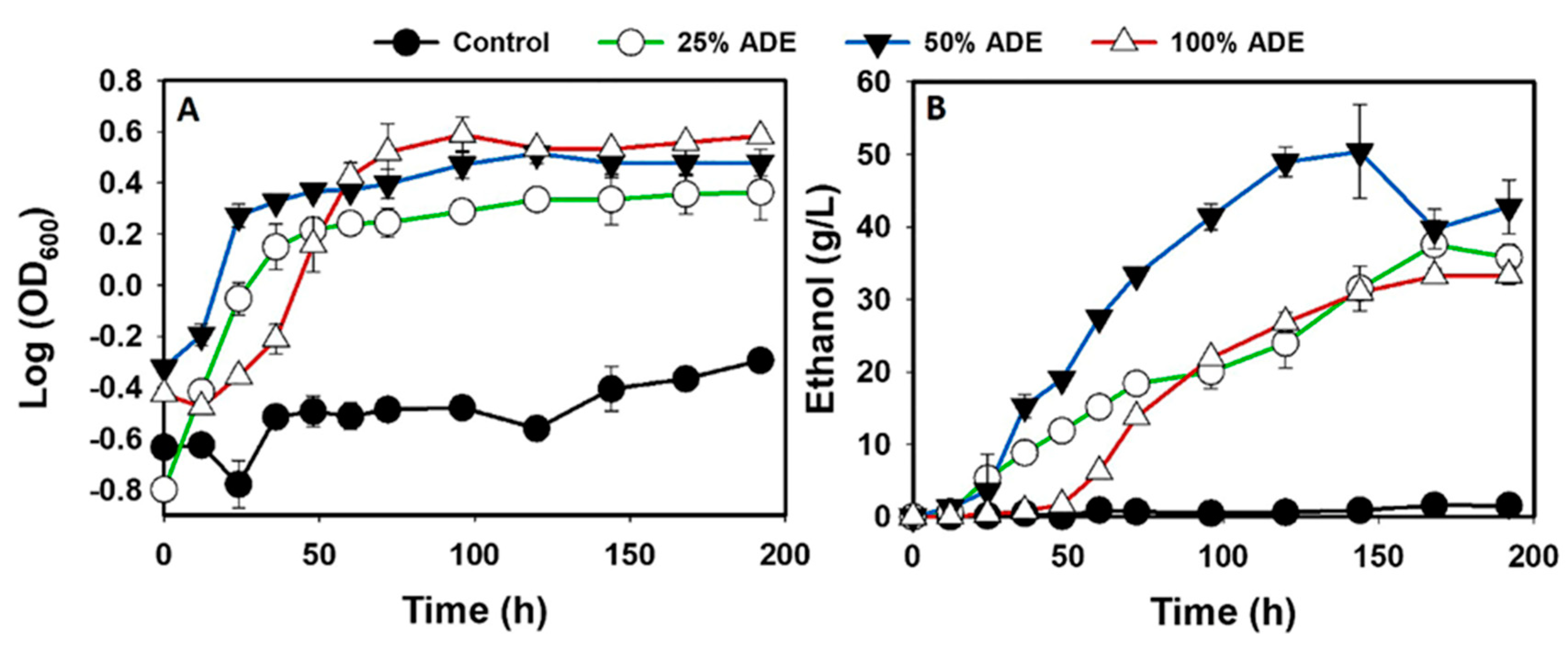
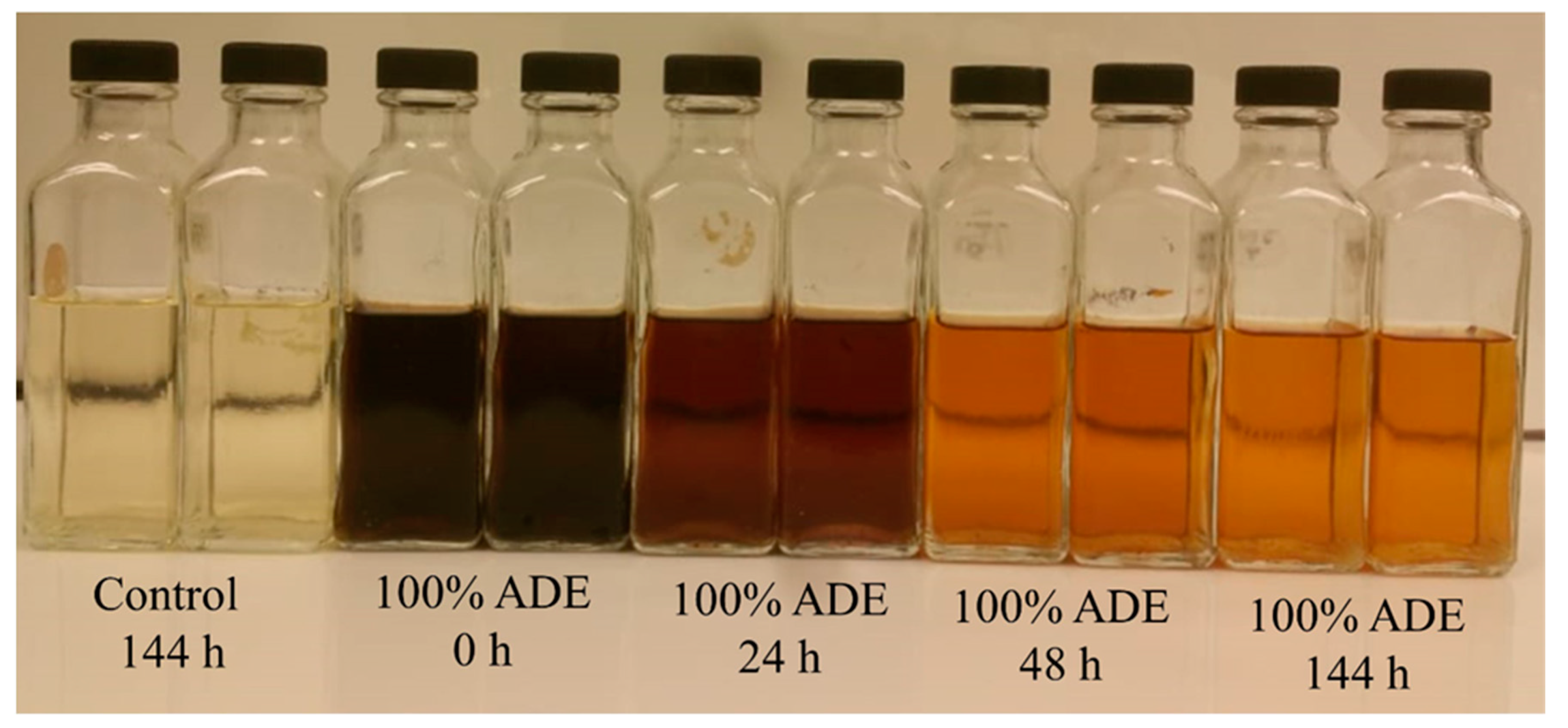
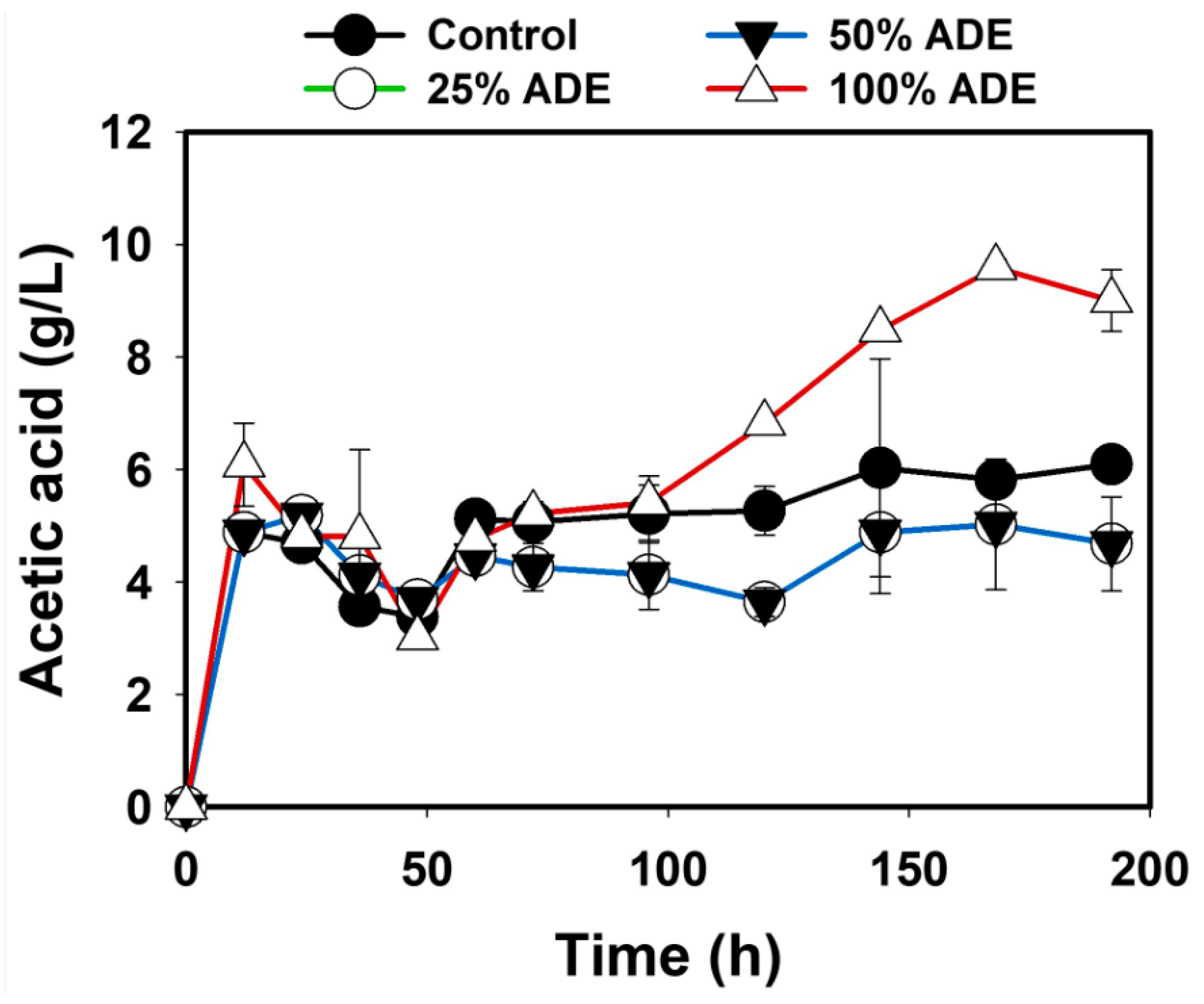
| Treatment | Maximum Ethanol Concentration (g/L) | Ethanol Yield (g/g) | Ethanol Productivity (g/L/h) | Initial glucose Concentration (g/L) | Final Glucose Concentration (g/L) | Glucose Consumed (g/L) |
|---|---|---|---|---|---|---|
| Control medium | 1.6 ± 0.9a | 0.18 ± 0.10a | 0.01 ± 0.01a | 111.7 ± 0.3a | 102.3 ± 0.0a | 9.1 ± 0.3a |
| 25% ADE | 37.5 ± 3.3b,c | 0.39 ± 0.08b | 0.22 ± 0.02b | 117.2 ± 9.5a | 22.5 ± 0.3b | 94.7 ± 9.4b |
| 50% ADE | 50.4 ± 6.4b | 0.45 ± 0.06b,c | 0.35 ± 0.04c | 111.7 ± 0.3a | 0.0 ± 0.0c | 111.7 ± 0.3b |
| 100% ADE | 33.3 ± 1.2c | 0.34 ± 0.03b | 0.17 ± 0.01b | 111.7 ± 0.3a | 13.7 ± 5.0d | 98.5 ± 5.2b |
| Nutrient | Initial Concentration (mg/L) | Final Concentration (mg/L) | Fold Decrease | Initial Concentration (mg/L) | Final Concentration (mg/L) | Fold Decrease |
|---|---|---|---|---|---|---|
| *50% ADE | *Control Medium | |||||
| Aluminum | 0.491 ± 0.06a | 0.073 ± 0.005b | 6.7 | 0.474 ± 0.080a | 0.069 ± 0.008b | 7.0 |
| Copper | 0.229 ± 0.01a | 0.038 ± 0.02b | 6.0 | 0.007 ± 0.001a | 0.0026 ± 0.019b | 2.7 |
| Magnesium | 435 ± 8.49a | 360 ± 5.41b | 1.2 | 58.657 ± 0.320a | 18.957 ± 0.525b | 3.1 |
| Manganese | 2.300 ± 0.03a | 0.514 ± 0.02b | 4.5 | 0.489 ± 0.001a | 0.106 ± 0.002b | 4.6 |
| Molybdenum | 0.013 ± 0.004a | 0.0063 ± 0.00b | 2.1 | 0.007 ± 0.000a | 0.002 ± 0.0005b | 3.5 |
| Zinc | 0.600 ± 0.02a | 0.116 ± 0.07b | 5.2 | 0.426 ± 0.021a | 0.404 ± 0.069a | 1.1 |
| NH4-N | 400 ± 7.94a | 0.400 ± 0.61b | 1000.0 | 266.000 ± 5.196a | 1.471 ± 0.494b | 181.0 |
| NO3-N | 0.200 ± 0.02a | 0.243 ± 0.02a | - | 13.494 ± 0.101a | 10.000 ± 1.080a | 1.4 |
| Total nitrogen | 471 ± 5.33a | 4.510 ± 0.52b | 104.4 | 291.333 ± 5.508a | 2.330 ± 2.222b | 131.0 |
| Iron | 1.2 ± 0.02a | 1.010 ± 0.14a | 1.2 | 1.600 ± 0.006a | 0.913 ± 0.390a | 1.8 |
| Potassium | 2.078 ± 46.48a | 1.239±29.08b | 1.7 | 1566.670 ± 8.021a | 61.173 ± 14.902b | 25.6 |
| Sodium | 243 ± 7.75a | 204 ± 3.49b | 1.2 | 43.527 ± 0.071a | 116.253 ± 2.059b | - |
| Nickel | 0.046 ± 0.003a | 0.055 ± 0.001b | - | 0.005 ± 0.000a | 0.001 ± 0.0002b | 5.0 |
| Sulfur | 21.400 ± 1.10a | 12.471 ± 0.56b | 1.7 | 12.190 ± 0.06a | 27.90 ± 0.581b | - |
| Sulfate | 16.400 ± 2.19a | 4.400 ± 0.04b | 3.7 | 13.494 ± 0.101a | 9.961 ± 1.080a | 1.4 |
| Selenium | 0.251 ± 0.047a | 0.335 ± 0.015b | - | 0.111 ± 0.025a | 0.321 ± 0.028b | - |
| Silicon | 17.000 ± 0.29a | 22.600 ± 0.37b | - | 0.441 ± 0.006a | 3.962 ± 0.170b | - |
| Chloride | 1.312 ± 11.01a | 554 ± 3.32b | 2.4 | 907.371 ± 1.320a | 110.073 ± 1.960b | 8.2 |
| Bromide | 1.300 ± 0.05a | 0.920 ± 0.08b | 1.4 | 0.26 ± 0.000a | 0.052 ± 0.000b | 5.0 |
| Phosphate (PO4-P) | 960.00 ± 1.00a | 494.67 ± 8.08b | 1.9 | 954.333 ± 3.060a | 2.51 ± 2.45b | 380.2 |
| Total phosphorus | 1261.33 ± 1.53a | 576.67 ± 17.47b | 2.2 | 1255.333 ± 22.300a | 3.34 ± 2.61b | 376.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ujor, V.C.; Okonkwo, C.C.; Rush, B.B.; McCrea, G.E.; Ezeji, T.C. Harnessing the Residual Nutrients in Anaerobic Digestate for Ethanol Fermentation and Digestate Remediation Using Saccharomyces cerevisiae. Fermentation 2020, 6, 52. https://doi.org/10.3390/fermentation6020052
Ujor VC, Okonkwo CC, Rush BB, McCrea GE, Ezeji TC. Harnessing the Residual Nutrients in Anaerobic Digestate for Ethanol Fermentation and Digestate Remediation Using Saccharomyces cerevisiae. Fermentation. 2020; 6(2):52. https://doi.org/10.3390/fermentation6020052
Chicago/Turabian StyleUjor, Victor Chinomso, Christopher Chukwudi Okonkwo, Brennen Bradley Rush, Grace Ellen McCrea, and Thaddeus Chukwuemeka Ezeji. 2020. "Harnessing the Residual Nutrients in Anaerobic Digestate for Ethanol Fermentation and Digestate Remediation Using Saccharomyces cerevisiae" Fermentation 6, no. 2: 52. https://doi.org/10.3390/fermentation6020052
APA StyleUjor, V. C., Okonkwo, C. C., Rush, B. B., McCrea, G. E., & Ezeji, T. C. (2020). Harnessing the Residual Nutrients in Anaerobic Digestate for Ethanol Fermentation and Digestate Remediation Using Saccharomyces cerevisiae. Fermentation, 6(2), 52. https://doi.org/10.3390/fermentation6020052