Abstract
Wine sensory experience includes flavor, aroma, color, and (for some) even acoustic traits, which impact consumer acceptance. The quality of the wine can be negatively impacted by the presence of off-flavors and aromas, or dubious colors, or sediments present in the bottle or glass, after pouring (coloring matter that precipitates or calcium bitartrate crystals). Flavor profiles of wines are the result of a vast number of variations in vineyard and winery production, including grape selection, winemaker’s knowledge and technique, and tools used to produce wines with a specific flavor. Wine color, besides being provided by the grape varieties, can also be manipulated during the winemaking. One of the most important “tools” for modulating flavor and color in wines is the choice of the yeasts. During alcoholic fermentation, the wine yeasts extract and metabolize compounds from the grape must by modifying grape-derived molecules, producing flavor-active compounds, and promoting the formation of stable pigments by the production and release of fermentative metabolites that affect the formation of vitisin A and B type pyranoanthocyanins. This review covers the role of Saccharomyces and non-Saccharomyces yeasts, as well as lactic acid bacteria, on the perceived flavor and color of wines and the choice that winemakers can make by choosing to perform co-inoculation or sequential inoculation, a choice that will help them to achieve the best performance in enhancing these wine sensory qualities, avoiding spoilage and the production of defective flavor or color compounds.
1. Introduction
1.1. The Human Senses in Wine Evaluation
Five senses are involved in perceiving wine sensory quality: sight, taste, hearing, touch, and smell. Color perception results from the stimulus of the retina by light (wavelengths 380 to 760 nm). In wine, color and appearance are the first attributes by which quality is assessed. According to Spence [], color is the most important product-intrinsic indicator used by consumers when searching, purchasing, and subsequently consuming food or a libation. Color, clarity, and hue affect the perception of other attributes such as flavor due to the association with color. For example, a yellow/green beverage is expected to have a lemon flavor and an acidic taste.
Taste is a chemical sense and happens when taste stimuli contact with the taste receptors located on the tongue, called taste buds. Humans can distinguish six basic tastes: sweet, sour, salty, bitter, umami, and fatty [,]. Between 20 and 30 levels of intensity can be distinguished for each taste, and each taste quality represents different nutritional or physiological requirements, or a potential dietary risk [].
Sound (waves which strike the eardrum, causing it to vibrate []) is also important when judging a wine. For instances, when we hear a champagne cork popping, it is a sign that the wine has an enjoyable gas.
Texture in wine can be defined as the total sum of kinesthetic sensations derived from oral manipulation. It encompasses mouthfeel, masticatory properties, residual properties, and even visual and auditory properties [].
Aroma and flavor are chemical senses stimulated by the chemical properties of odor molecules which must reach the olfactory bulb to interact with olfactory cells in the olfactory mucosa []; therefore, to smell, molecules must be airborne (i.e., volatile). The sensory term which we call “flavor” is a mingled experience based on human judgment, built on personal differences in perception thresholds.
In conclusion, and as reported by Swiegers et al. [], all of the senses play a key role in wine/flavor development—color, aroma, mouthfeel, sound, and, ultimately, taste. Altogether, these sensory perceptions are very complex. Wine contains many flavor and aroma-active compounds. Terpenes, methoxypyrazines, esters, ethanol and other alcohols and aldehydes impart distinct flavors and aromas (floral, pepper, fruit, woody and vinylic flavors, among others) to wine [,,]. The taste of wine can be described as sweet, sour, salty, umami, bitter, and, to a lesser extent, fat []. These properties are the result of the presence of sugars, polyols, salts, polyphenols, flavonoid compounds, amino acids, and fatty acids. Compounds such as glycerol, polysaccharides, and mannoproteins contribute to the viscosity and mouthfeel of wines []; grape anthocyanins contribute to the color [], and ethanol, by sheer mass, also carries other alcohols along, promoting a mouth-warming effect [].
1.2. Main Wine Aroma and Flavor Compounds from the Fermentative Origin
Yeast and bacteria are vital to the development of wine flavor. Many biosynthetic pathways, in wine yeast and malolactic bacteria, are responsible for the formation of wine aroma and flavor. However, we cannot discard the other factors that can also influence the wine chemical composition, such as viticultural practices, grape-must composition, pH, fermentation temperature, and technological aspects associated with the vinification process []. So, depending on their origin, wine aroma and flavor compounds can be named varietal aromas (originating from the grapes), fermentative aromas (originating during alcoholic and malolactic fermentations), and aging aromas (developed during the reductive or oxidative wine-aging that depends on storage conditions) [].
Most of the wine aroma and flavor compounds are produced or released during wine fermentation due to microbial activities of Saccharomyces and non-Saccharomyces yeast genera (Brettanomyces, Candida, Debaryomyces, Hanseniaspora, Hansenula, Kloeckera, Kluyveromyces, Lachancea, Metschnikowia, Pichia, Saccharomycodes, Schizosaccharomyces, Torulaspora, and Zygosaccharomyces). Both in spontaneous and inoculated wine fermentations, non-Saccharomyces are important in early stages of the fermentation, before Saccharomyces becomes dominant in the culture, and contribute meaningfully to the global aroma profile of wines by producing flavor-active compounds [,].
A group of aroma compounds has been directly linked to specific varietal flavors and aromas in wines [,]. Most of these compounds are present at low concentrations in both grapes and fermented wine. These aroma compounds are found in grapes in the form of non-odorant precursors that, due to the metabolic activity of Saccharomyces and non-Saccharomyces yeast during fermentation, are transformed to aromas and flavor that are of great relevance in the sensory perception of wines [] (Table 1).

Table 1.
Main odorants contributing to varietal aromas of some monovarietal wines.
During alcoholic fermentation, some yeast, mainly non-Saccharomyces yeasts, can release β-glucosidases that hydrolyze the glycosidic bonds of the odorless non-volatile glycoside linked forms of monoterpenes (geraniol, linalool, nerol, among others), releasing the odor compounds to the wine []. Volatile thiols that give Sauvignon blanc wines their characteristic aroma (bell pepper, black currant, grapefruit, and citrus peel) are not present in grape juice but occur in grape must as odorless, non-volatile, cysteine-bound conjugates. During fermentation, the wine yeasts are responsible for the cleaving of the thiol from the precursor [].
However, the major groups of aromas and flavor compounds from the fermentative origin are ethanol, higher alcohols or fusel alcohols, and esters. The biosynthetic pathways responsible for the formation of higher alcohols, the Ehrlich pathway, or the enzymes responsible for the formation of esters, have been studied in wine yeasts [].
Higher alcohols are derived from amino acid catabolism via a pathway that was first described by Ehrlich [] and later revised by Neubauer and Fromherz in 1911 []. Amino acids that are assimilated by the Ehrlich pathway (valine, leucine, isoleucine, methionine, and phenylalanine), present in grape must are metabolized by yeasts, sequentially, throughout the fermentation. Figure 1 shows the metabolism of phenylalanine with the production of 2-phenylethanol and, after oxidation of phenylacetaldehyde, the formation of phenylacetate. Both compounds possess a pleasant rose-like aroma/flavor.
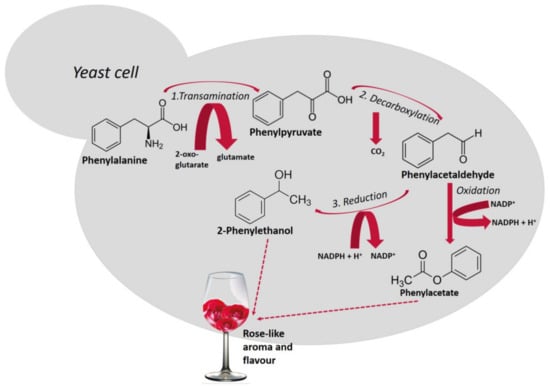
Figure 1.
Schematic representation of the Ehrlich pathway for the catabolism of the aromatic amino acid, phenylalanine leading to the formation of 2-phenylethanol []. This biosynthetic pathway consists of three steps (reactions 1, 2 and 3): first, amino acids are deaminated to the corresponding α-ketoacids, in reactions catalyzed by transaminases. In a second step, α-ketoacids are decarboxylated and converted to their corresponding aldehydes (five decarboxylases are involved in this process), in a third step, alcohol dehydrogenases (Adh1p to Adh6p and Sfa1p) catalyze the reduction of aldehydes to their corresponding higher alcohols [].
Studies have shown that profiles and concentrations of higher alcohols produced vary by yeast species, even when the fermentation conditions are similar, which indicates that the mechanisms that regulate the Ehrlich pathway are diverse in non-Saccharomyces yeasts compared to Saccharomyces [,]. So, Ehrlich pathway mechanisms should be explored in detail in non-Saccharomyces yeasts as it contributes to the formation of important and flavorful wine aromas [].
The most important esters are synthetized by yeasts during alcoholic fermentation as a detoxification mechanism since they are less toxic than their correspondent alcohol or acidic precursors. Moreover, their synthesis serves as a mechanism for the regeneration of free CoA from its conjugates [,].
Esters (Figure 2) that contribute to wine aroma, derived from fermentation, belong to two categories: the acetate esters of higher alcohols and the ethyl esters of medium-chain fatty acids (MCFA). Acetate esters are formed inside the yeast cell, and in S. cerevisiae the reaction is metabolized by two alcohol acetyltransferases, AATase I and AATase II (encoded by genes ATF1 and ATF2 [,]). Eat1p is responsible for the production of acetate and propanoate esters [,]. Most medium-chain fatty acid ethyl ester biosynthesis during fermentation is catalyzed by two enzymes, Eht1p and Eeb1p [,].
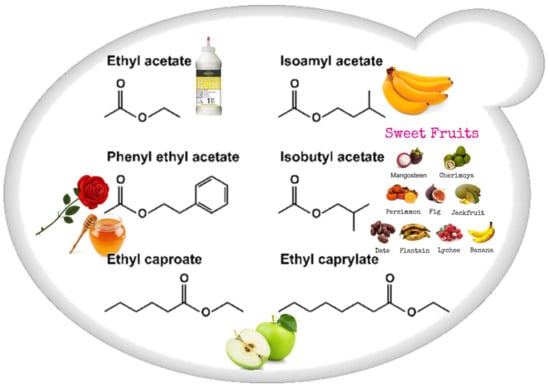
Figure 2.
Schematic representation of the most important wine esters: ethyl acetate (glue-like aroma), isoamyl acetate (banana aroma), 2-phenylethyl acetate (roses and honey aromas), isobutyl acetate (sweet-fruits aromas), and ethyl caproate and ethyl caprylate with a sour-apple aroma [].
Volatile fatty acids also contribute to the flavor and aroma of the wine. During yeast fermentation, long-chain fatty acids (LCFAs) are also formed via the fatty-acid synthesis pathway from acetyl-CoA in concentrations varying from ng/L to g/L []. Medium-chain fatty acids (MCFAs (C6 to C12)) are produced primarily by yeasts as intermediates in the biosynthesis of LCFAs that are prematurely released from the fatty acid synthase complex. These acids (Table 2) directly contribute to the flavor of wine or serve as substrates that participate in the formation of ethyl acetates []. As most have unpleasant aromas (see Table 2), their formation should be minimized.

Table 2.
Main medium-chain fatty acids (MCFAs (C6 to C12)), produced by yeasts during alcoholic fermentation.
Sulfur-containing compounds can also be formed by yeasts during alcoholic fermentation. They are usually perceived as off-flavors. The sulfur-containing compounds can be derived from the grape and the metabolic activities of yeast and bacteria. They can also occur due to the chemical reactions during the wine aging and storage and also due to environmental contamination []. They can be formed by enzymatic mechanism as the products of metabolic and fermentative pathways whose substrates are both amino acids and some sulfur-containing pesticides. When wine microorganisms metabolize these thiols, the sulfur compounds formed are considered off-flavors [] which convey negative notes such as cabbage, garlic, onion, rotten eggs, rubber, and sulfur to wines []. However, there are some volatile thiols that may confer enjoyable aromatic notes at trace levels, such as 4-mercapto-4-methylpentan-2-one (4MMP), 3-mercaptohexan-1-ol (3MH), already mentioned in Table 1, and 3-mercaptohexyl acetate (3MHA), important for the characterization of the typical Sauvignon Blanc wine aroma [,,].
Finally, another important family of aromatic compounds present in wines are the carbonyl compounds. In this group we may include acetaldehyde, acrolein, ethyl carbamate, formaldehyde, and furfural []. Several factors may contribute to the presence of carbonyl compounds in wines, including the fermentation of over-ripe grapes and increasing the maceration time, probably due to increased concentration of the precursors like amino acids and glucose in the must []. Due to their carbonyl group, carbonyl compounds present a high reactivity with the nucleophile’s cellular constituents [] and may cause cell damage. So, these compounds are toxic, and their formation should be avoided.
2. Yeast Modulation of Wine Aroma and Flavor Compounds
2.1. Non-Saccharomyces and Saccharomyces Co-inoculation vs. Sequential Inoculation
The wine industry attempts to diversify producing wines with distinctive characteristics and creating high-quality new products. A true test for winemakers is to blend several grapes, grown on different soil and climate conditions (terroir), with a developing science of yeast and bacterial metabolism, to produce the most enjoyable wine []. Many winemakers today use commercial yeast and bacteria starter cultures for alcoholic and malolactic fermentation, respectively. The selection of a “fit-for-purpose” starter strain has a pivotal role in optimizing flavor and aroma.
There is no consensus on the impact of indigenous yeasts on wine sensory properties; while some researchers show a positive effect, others show negative effects on the wine chemical composition and sensory properties [,]. For example, Varela et al. [] showed an increase in the concentration of some higher alcohols and esters in wines produced with autochthonous yeasts compared to the wines produced with commercial yeasts. Moreover, some non-Saccharomyces yeast may increase the concentration of biogenic amines in wines [].
In red winemaking, significant increases in the concentrations of desirable compounds such as ethyl lactate (sweet, fruity, acidic, ethereal with a brown nuance aroma), 2,3-butanediol (buttery aroma), 2-phenylethanol, and 2-phenylethyl acetate (both with a rose-like scent) can be obtained when non-Saccharomyces yeasts are introduced into the fermentation process [,].
Another selection criterion for non-Saccharomyces yeasts, when aiming to improve the wine aroma, is the presence of β-glucosidase activity that favors the hydrolysis of the non-volatile aromatic precursors from the grape [,]. Non-Saccharomyces species display superior β-glucosidase activity to that of Saccharomyces species, which has been defined as intracellular and strain-dependent [].
Budic-Leto et al. [] found that Prosek, a traditional Croatian dessert wine, produced with native and inoculated yeasts differed in its volatile compounds. Using descriptive sensory analysis, it was shown that the sensory properties of the wines were significantly different depending on the type of fermentation, namely determined for the attributes strawberry jam aroma, and fullness. So, recently, non-Saccharomyces yeast species have been suggested for winemaking as they could contribute to the improvement of wine quality mostly in terms of aromatic characteristics [,]. Thus, starter cultures composed of non-Saccharomyces yeasts together with S. cerevisiae have been used for co-, or sequential wine fermentations [].
Co-inoculation involving S. cerevisiae and non-Saccharomyces yeasts species typically results in the disappearance (or the presence in relative low amounts) and loss of viability of non-Saccharomyces [,]. Though S. cerevisiae dominance can be explained by the depletion of sugar and nutrients from the grape must followed by ethanol production and lack of oxygen, some direct mechanisms for yeast species antagonism have also been described: (i) killer factors (so-called killer toxins or killer proteins), which are secreted peptides, encoded by extrachromosomal elements of S. cerevisiae that affect other yeast species []; (ii) similar compounds have also been described for Torulaspora delbrueckii species [] and for the genera Pichia, Kluyveromyces, Lachancea, Candida, Cryptococcus, Debaryomyces, Hanseniaspora, Hansenula, Kluyveromyces, Metschnikowia, Torulopsis, Ustilago, Williopsis, and Zygosaccharomyces, indicating that the killer phenomenon is indeed widespread among yeasts. []; (iii) S. cerevisiae are also able to secret antimicrobial peptides (AMPs) during alcoholic fermentation that are active against wine-related yeasts (e.g., Dekkera bruxellensis) and bacteria (e.g., Oenococcus oeni). These AMPs correspond to fragments of the S. cerevisiae glyceraldehyde 3-phosphate dehydrogenase (GAPDH) protein [].
Several authors agree that the sequential culture is better than the mixed culture, especially because it allows for a greater expression of the metabolism of non-Saccharomyces yeasts at the beginning of fermentation [,]. However, as recently reported by Loira et al. [], the winemaker selection criteria for performing co-inoculation or sequential inoculation with the appropriate non-Saccharomyces is dependent on the characteristics of the wine to be produced, including the desired sensory properties. The ratio of inoculation (non-Saccharomyces vs. Saccharomyces) is also a subject that must be considered. Moreover, the contribution of the inoculation of non-Saccharomyces strains to wine fermentation can be direct or indirect, through biological interactions with S. cerevisiae. Recently, Renault et al. [,] described a synergic interaction between S. cerevisiae and T. delbrueckii resulting in increased levels of 3-sulfanylhexan-1-ol a compound that presents a sulphurous aroma and an initially fruity flavor. However, with over-aging, the aroma/flavor evolves to savory and chicken meaty with roasted coffee shades and a hint of fruitiness [].
Not long ago, García et al. [] performed small-scale fermentations where they studied the oenological characterization of five non-Saccharomyces native yeast species under several co-culture conditions in combination with a selected strain of S. cerevisiae, aiming to improve the sensory characteristics of the Malvar wines. Sequential inoculations were elaborated with S. cerevisiae CLI 889 in combination with several non-Saccharomyces: (i) T. delbrueckii CLI 918, which produced wines with a lower ethanol content and higher fruity and floral aroma; (ii) C. stellata CLI 920, which augmented the aroma complexity and glycerol content; (iii) L. thermotolerans 9-6C, which produced an increase in acidity and floral and ripe-fruit aroma and a lower volatile acidity; (iv) Schizosaccharomyces pombe, which produced wines with fruity aromas; and (v) M. pulcherrima, which produced wines with lower volatile acidity and an increase of glycerol and ripe-fruit aroma.
Continuing their work on Malvar wines, García et al. [] performed fermentations at the pilot scale, using sequential-inoculation strategies which resulted in wines that tasters were able to distinguish from the controls. Moreover, the wines were most appreciated, namely, those produced in sequential cultures with T. delbrueckii CLI 918/S. cerevisiae CLI 889 and C. stellata CLI 920/S. cerevisiae CLI 889 and, also, with mixed and sequential cultures of L. thermotolerans 9-6C/S. cerevisiae CLI 889 strains. Studies have shown that sequential cultures can produce more different wines, when compared with the controls, providing sensory properties associated with the non-Saccharomyces strains. Some strains of T. delbrueckii in sequential fermentation with S. cerevisiae can produce significant amounts of 3-ethoxy-1-propanol [], with a fruity-like aroma with a low perception threshold, 0.1 mg/L [].
However, sequential inoculation is not only favorable for positive aromas sequential. It is a fermentation technique that can be used to prevent or diminish the production of some undesirable compounds, augmenting the production of others. Viana et al. [] reported a decrease in the higher alcohol’s concentration (considered as possessing a fuel-like aroma) from 452.5 mg/L (control) to 306.2 mg/L when carrying out mixed fermentations with H. osmophila and S. cerevisiae. Moreover, higher concentrations of 2-phenylethyl acetate (rose-like aroma) were obtained. A higher intensity of fruitiness was also detected in these wines when compared to the control wine, obtained throughout the fermentation of a pure S. cerevisiae culture.
2.2. Saccharomyces and Lactic Acid Bacteria co inoculation vs. Sequential Inoculation
The vinification involves different microbiological processes, mainly alcoholic fermentation (AF) conducted by Saccharomyces cerevisiae and malolactic fermentation (MLF) conducted by lactic acid bacteria (Oenococcus oeni). These two distinctive fermentation processes represent an essential step in the improvement of the quality of red wines [].
MLF naturally occurs after AF, however, the timing of the start of MLF depends on several parameters like temperature, pH, alcoholic degree and the concentration of sulfur dioxide (SO2), as well as on certain yeast metabolites available, such as medium-chain fatty acids and peptidic fractions [,]. The success of spontaneous MLF is not always guaranteed, and the addition of starter culture can improve its viability. To overcome this issue, the winemakers may carry out traditional LAB inoculation after alcoholic fermentation (sequential inoculation), or simultaneous inoculation in the must with yeast (co-inoculation). The co-inoculation has, in the last several years, been adopted by some winemakers, particularly in warm climates with higher temperatures, where high concentrations of ethanol can inhibit LAB growth [].
There are not many studies focusing on the impact of the co-inoculation technique on the aromatic and biochemical profile of wines. Abrahamse and Bartowsky [] and Knoll et al. [] have shown that the timing of inoculation with LAB in both white and red wines could influence the profile of aromatic yeast-derived compounds such as higher alcohols, terpene, esters, and fatty acids. However, these works were performed at the lab scale, and no sensory evaluation was performed on the wines.
Antalick and collaborators [] demonstrated the impact of the timing of inoculation with LAB on the metabolic profile of wines manufactured at production scale. They have clearly shown that this technique has an impact on the aromatic profile of the wines, mainly in the presence of lactic and fruity notes. Co-inoculation can modulate the intensity of these descriptors, due to the production/degradation of metabolites or by the development of an aromatic mask over the short and long term. Moreover, they discuss that the metabolic and aromatic changes that occur with co-inoculation depend strongly on the yeast and LAB strains, as well as on the composition of the must. Co-inoculation of musts at the beginning of vinification can also lead to a faster vinification process without an excessive increase in volatile acidity [].
Due to the importance of the interaction between yeasts (Saccharomyces and non-Saccharomyces) and bacteria during wine processing, Berbegal et al. [] applied a next-generation sequencing (NGS) analysis to several fermentation modalities: uninoculated must, pied-de-cuve, S. cerevisiae, S. cerevisiae, and Torulaspora delbrueckii co-inoculated and sequentially inoculated, along with S. cerevisiae and Metschnikowia pulcherrima co-inoculated and sequentially inoculated, continued by spontaneous malolactic consortium to perform MLF. Each experimental trial led to the different taxonomic composition of the bacterial communities of the malolactic consortia, in terms of prokaryotic phyla and genera. Among other interesting findings, they found that MLF was delayed when M. pulcherrima was inoculated and was even inhibited when the inoculated yeast strain was T. delbrueckii []. Thus, an antagonistic effect of M. pulcherrima and especially T. delbrueckii on lactic bacteria population has been proven, which may be due to the ability of T. delbrueckii to produce “toxins” or killer factors that prevent bacteria growth [] and to the ability of M. pulcherrima to produce high amounts of pulcherrimin (an iron chelator) that inhibits the growth of bacteria [].
3. Yeast Modulation of Wine Color and Pigment Formation
Anthocyanins and their derivatives, originating in the grapes, are the main pigments responsible for the red wine color, and their structural modifications result in a characteristic variation of color in red wines, from pale ruby (young red wine) to deep purple-red color (aged red wine) [,]. Such variations can also result in changes in wine mouthfeel and flavor.
Saccharomyces yeasts can directly or indirectly contribute to wine color, altering color parameters such as intensity and tonality by: (i) increasing the formation of stable pigment precursors (vinyl phenols, acetaldehyde, and pyruvic acid); and (ii) modifying the pH due to organic acid metabolism (production or consumption) [].
Pyruvic acid and acetaldehyde promote the formation of vitisins of types A and B, respectively [,], during must fermentation, (Figure 3A). Vitisins contribute more to wine color parameters than unmodified anthocyanins and exhibit a hypsochromic shift, i.e., a change of spectral band position (in the absorption, reflectance, transmittance, or emission spectrum) to a shorter wavelength corresponding to a higher frequency. Vitisins can change towards an orange-red hue due to the long conjugation afforded by pyran ring []. Moreover, their color expression remains stable against discoloration due to the presence of sulfur dioxide (bleaching capacity) or changes in pH [] (Figure 4).
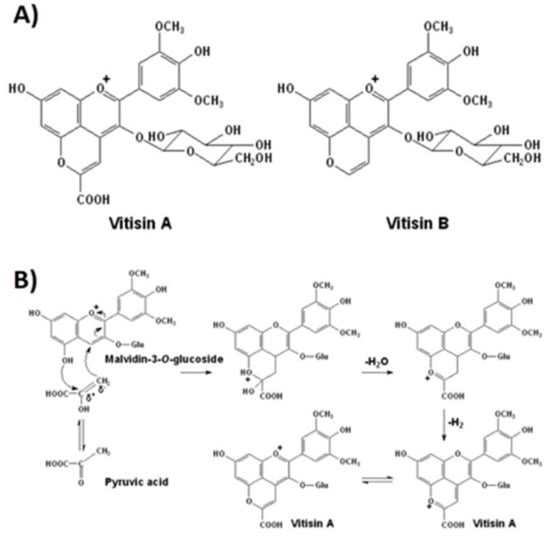
Figure 3.
(A) Structures of vitisin A and vitisin B generated from malvidin-3-O-glucoside. (B) The formation mechanism of vitisin A produced by condensation of an anthocyanin (malvidin-3-O-glucoside) with pyruvic acid [,,].
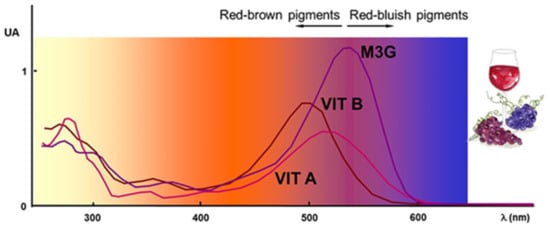
Figure 4.
UV-visible spectra of malvidin-3-O-glucoside and vitisins A and B. Adapted from [].
During the fermentation process, practices like pellicular maceration that increase the extraction of anthocyanins from the skins of grape berries will promote the formation of vitisins []; also, acetaldehyde and pyruvic acid, as mentioned before, are important intermediate compounds in yeast metabolism and influence wine vitisin content [,,].
During sugars’ fermentation by yeasts, pyruvate is metabolized into acetaldehyde, with the latter being the terminal electron acceptor in the formation of ethanol. Acetaldehyde and pyruvate, formed in yeast cytoplasm, are rapidly metabolized (the first is reduced to ethanol, and the second is either decarboxylated to acetaldehyde or used in the formation of acetyl-CoA). However, some of the acetaldehyde and pyruvate molecules, through cell lysis, pass to the wine medium and are sufficiently reactive to attack other molecules, enabling the transformation of anthocyanins into compounds such as pyranoanthocyanins and their secondary generated pigments (anthocyanin oligomers and polymeric anthocyanin) [,]. Pyroanthocyanins are the most important group of anthocyanin derivatives present in fermented beverages, including wine, and the A-type vitisins or carboxy-pyroanthocyanins are produced, as mentioned before, by condensation of anthocyanin with pyruvic acid [] (Figure 3B).
In terms of vitisin kinetic formation, during S. cerevisiae fermentation, type-A vitisins are produced in the first six days of fermentation (when pyruvic acid is available). At the end of fermentation, when nutrients are limited, the amount of acetaldehyde is high enough to lead to the formation of type-B vitisins [,]. So, to generate a more pleasant red wine color, before fermentation the winemaker must select the wine yeast strains that will be able to increase anthocyanin extraction and/or can produce more pyruvic acid and acetaldehyde. Postponing or starting MLF early can prevent the consumption of pyruvic acid and acetaldehyde by lactic acid bacteria [], increasing the possibility for vitisin synthesis.
Oxidative fermentation (fermentation in barrels or with micro-oxygenation) and wine aging in wood give rise to pyruvic acid and acetaldehyde, increasing the vitisin levels and, consequently, color intensity and stability []. Yellowish α-pyranone-anthocyanins called oxovitisins were also described by He et al. [] in aged red wines derived from the direct oxidation of A-type vitisins. Moreover, A-type vitisins are the pyranoanthocyanins detected in higher concentrations in port wines. Port wine is made by stopping the fermentation process with the addition of wine spirit “aguardente”, leaving the wine with a high concentration of sugars. So, when fermentation is stopped, the pyruvic acid concentration is relatively high and increases after wine fortification [].
Vinylphenolic pyroanthocyanin adducts result from the condensation between vinyl phenols and anthocyanins. These color compounds also show high color stability []. Yeast strains are also able to affect the concentration and the composition of wine tannins as well as the degree of tannin polymerization [], thus, indirectly throughout yeast actions affecting stabilization of anthocyanins, and consequently, stabilization of color can occur due to reaction between anthocyanins and tannins forming pigmented tannins and through copigmentation of anthocyanins [].
Additionally, what about non-Saccharomyces wine yeasts? Well, we have already mentioned that an improvement in fermentation quality, efficiency, and wine pleasantness is obtained when sequential or co-inoculation of non-Saccharomyces and Saccharomyces yeast is performed.
Torulaspora delbrueckii is one non-Saccharomyces species available commercially (Viniflora® Harmony.nsac and Viniflora® Melody.nsac, Zymaflore® Alpha, BIODIVA®, and Viniferm NS-TD® are some commercial examples), therefore, this yeast could be a good candidate for wine color improvement as it is reported to have a positive influence on the taste and aroma of wines. For instance, Pinotage grape must inoculate with T. delbrueckii originated red wines improved in color intensity (anthocyanins) and mouthfeel (flavanols) when compared to the control (musts inoculated with S. cerevisiae) []. However, T. delbrueckii has poor production of acetaldehyde [], thus being a poor candidate for wine color improvement in the context of B-type vitisins.
Other non-Saccharomyces yeasts, not yet available as commercial products, but studied in the academic community, could be good candidates for wine color improvement. Medina et al. [] reported that in the case of co-fermentation of Metschnikowia and Hanseniaspora with S. cerevisiae, only an increase in the content of B-type vitisins occurred, probably due to the enhanced acetaldehyde formation.
Further experiments performed by sequential inoculation of Schizosaccharomyces pombe and Lachancea thermotolerans exposed an increase of type-A vitisins when compared with the control S. cerevisiae []. Also, several authors detected interesting features in non-Saccharomyces yeasts. P. guilliermondii strains presenting a high hydroxycinnamate decarboxylase activity may improve the formation of vinylphenolic pyranoanthocyanins; non-Saccharomyces yeasts, such as Candida valida, Metschnikowia pulcherrima, Kloeckera apiculata and Starmerella bombicola, which synthesize and release pectolytic enzymes, can improve wine color due to the extraction of a greater amount of polyphenolic compounds during fermentation and by facilitating clarification and filtration processes [,,,].
4. The Role of Saccharomyces and non-Saccharomyces Mannoproteins in Aroma and Color of Wines
Mannoproteins are highly glycosylated glycoproteins located on the external layer of the yeast cell wall, representing 35% to 40% of the S. cerevisiae cell wall (Figure 5) []. Mannoproteins are composed of 10% to 20% protein and 80% to 90% d-mannose associated with residues of d-glucose and N-acetylglucosamine [].
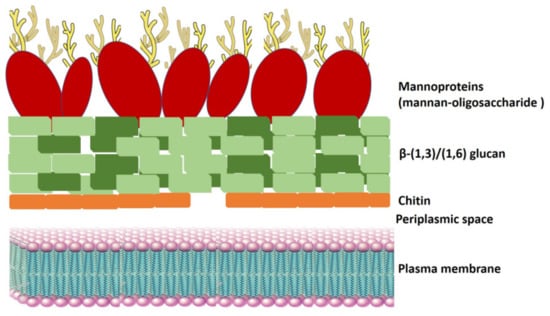
Figure 5.
Schematic representation of the yeast cell wall. The yeast cell wall is composed of mannan–oligosaccharide (mannoproteins), complex polymers of β-(1,3)/(1,6) glucan, and chitin. As shown in Figure 5, mannoproteins are located on the surface of the cell wall.
In wine, we can find two groups of mannoproteins: one made up of those secreted into wine by yeast during alcoholic fermentation (100–150 mg/L) with molecular weights from 5000 to more than 800,000 Da [], the other one composed by those that are released into the wine due to the autolysis of yeasts during aging on lees, probably through the cleavage of linkages between mannoproteins, glucans, and chitin [,].
The presence of these mannoproteins in wines has many positive consequences, from the reduction of the protein haze in white wines [] to decreasing astringency of red wines, by increased inhibition of tannin aggregation [,]. Among other positive factors, mannoproteins also interact with wine volatile compounds []. So, these yeast-derived glycoprotein complexes can have positive effects on the technological and sensorial properties of wines [].
In terms of improving wine palatability and mouth feel, yeast mannoproteins promote the increase of wine sweetness [] and improve the aroma persistence and complexity [,]. However, the number of mannoproteins released by yeast into wine can vary concerning the strain and the chemical–physical and compositional conditions of the wine system [,].
Their presence can also affect the release of volatile compounds, affecting the final perception of the wine []. The physicochemical interactions between aroma compounds and mannoproteins depends on the nature of the volatile, since a greater degree of interaction is often observed with hydrophobic compounds [], as well as the conformational structure of the mannoproteins []; moreover, Chalier et al. [] demonstrated that both the glycosidic and peptidic parts of the mannoproteins may interact well with the aroma compounds.
It has been shown that the use of mannoproteins (in low amounts) or the contact of the wine with fine lees increases the levels of esters (ethyl hexanoate, methyl, and ethyl hexadecanoate, which present fruity aromas), due to the esterification of fatty acids released during yeast fermentation or yeast autolysis [,], while higher amounts increase, in excess, fatty acid content, producing yeasty, herbaceous, and cheese-like smells []. However, it has been suggested that mannoproteins can be used to remove or reduce the incidence of wine off-flavors—4-ethylguaiacol and 4-ethylphenol. The sorption of these compounds to the yeast walls could be due to their interactions with the functional groups of the mannoproteins and the free amino acids on the surface of the cell walls [].
The release of mannoproteins into the wine is not a physiological characteristic of just S. cerevisiae yeast strains. In 2014, Domizio and collaborators [], studied eight non-Saccharomyces wine strains (H. osmophila, L. thermotolerans, M. pulcherrima, P. fermentans, S. ludwigii, S. bacillaris, T. delbrueckii, and Z. florentinus) in mixed inocula fermentations of a synthetic polysaccharide-free grape juice for their ability to release mannoproteins. The eight non-Saccharomyces yeasts confirmed a higher capacity to release polysaccharides when compared to S. cerevisiae. Moreover, Pérez-Través et al. [] also studied the ability of natural hybrids of S. cerevisiae × S. krudriavzevii to release mannoproteins. Interestingly, they found that this strain, in the fermentation conditions studied, was able to produce a higher quantity of mannoproteins when compared with the sample in which only S. cerevisiae was used. Furthermore, the authors also found that the genome interaction in hybrids creates a biological ecosystem that boosts the release of mannoproteins.
As mentioned before, the presence of mannoproteins in wines, namely red wines, promotes tannin stability and reduction of astringency [,]. The interaction between mannoproteins and wine phenolic compounds is a matter of interest, as studies show that they may have an impact on color stability [,]. However, results are contradictory, as some authors state that there was no positive interaction between mannoproteins and color compounds and that the interaction between mannoproteins and tannins results in a decrease of wine tannin content due to the precipitation of tannin and mannoprotein [,,,], thus being responsible for a decrease in wine color intensity or lower filterability [], whereas, others state that mannoproteins appear to stabilize anthocyanin-derived pigments, from a colloidal point of view, avoiding their aggregation and further precipitation []. The study of the exhaustive pigment composition of wines has shown that the addition of mannoproteins can stabilize type-A vitisins and other derivative pigments [].
5. Final Remarks
Wines are complex and evolve physiochemically and sensorially through time. Most of the wine aroma and flavor compounds are produced or released during wine fermentation due to microbial activity of Saccharomyces, non-Saccharomyces yeast genera, and lactic acid bacteria. A true challenge for winemakers is the selection of a “fit-for-purpose” microbial starter culture or culture strains that can have a crucial role in optimizing flavor, aroma, and color of wines, among other sensory properties.
Co-inoculation involving S. cerevisiae and non-Saccharomyces yeasts species may result in the death or loss of variability of non-Saccharomyces, once S. cerevisiae dominates the fermentation and is stress-resistant to the inhibitory ethanol effect. So, several authors suggest that the sequential inoculation (non-Saccharomyces followed by S. cerevisiae) is a better technique than the mixed culture, allowing a higher expression of the metabolism of non-Saccharomyces. Nevertheless, the ratio of inoculation (non-Saccharomyces vs. Saccharomyces) must be taken into account, especially if the wine should present a special and desirable characteristic such as the expression of a peculiar aroma-flavor, or even the inhibition of the production of a specific family of compounds like, for instance, higher alcohols.
Concerning the MLF, the co-inoculation process has been adopted by some winemakers, in warm climates, where high concentrations of ethanol can inhibit lactic acid bacteria (LAB) growth. Co-inoculation of musts at the beginning of vinification can also accelerate the process without an excessive increase in volatile acidity. However, winemakers must be aware of the possible interactions between yeasts (Saccharomyces and non-Saccharomyces) and LAB during wine processing. LAB feed on dead and lysed yeast cells but some non-Saccharomyces may delay (M. pulcherrima) or inhibit (T. delbrueckii) bacterial growth, thus inhibiting the occurrence of MLF.
Regarding wine color characteristics, especially red wine, Saccharomyces and non-Saccharomyces yeasts can directly or indirectly contribute to wine intensity and tonality, by increasing the formation of stable pigment precursors (vinyl phenols, acetaldehyde, and pyruvic acid) and by modifying the pH due to organic acid metabolism. Pyruvic acid is necessary for vitisin synthesis; these important pigments contribute more to wine color parameters than unmodified anthocyanins. Concerning acetaldehyde, this compound has several negative impacts (one on health, the other as a potent binder of SO2), so its formation, although beneficial to wine color, should be avoided. Also, it is important to choose the right time for promoting MLF, either spontaneously or by inoculation of a commercial LAB strain, to prevent consumption of pyruvic acid by lactic acid bacteria and to promote vitisin synthesis.
Finally, mannoproteins may have a positive effect on sensory perception of red wine, reducing astringency and bitterness and encouraging aroma revelation and odor complexity, but further studies are necessary in order to unravel the possible stabilization mechanism and the relationship between Saccharomyces and non-Saccharomyces mannoprotein characteristics and their ability to stabilize wine color.
So, in conclusion, knowledge and control of yeast and bacteria can help winemakers enhance the sensory quality of their wines for flavor and color.
Funding
We appreciate the financial support provided to the Research Unit in Vila Real [grant number UID/QUI/00616/2019] by FCT-Portugal and COMPETE
Acknowledgments
The author wants to acknowledge Interreg Program for the financial support of the Project IBERPHENOL, Project Number 0377_IBERPHENOL_6_E, co-financed by European Regional Development Fund (ERDF) through POCTEP 2014-2020.
Conflicts of Interest
The author declares no conflict of interest
References
- Spence, C. On the psychological impact of food colour. Flavour 2015, 4, 2044–7248. [Google Scholar] [CrossRef]
- Mattes, R.D. Fat Taste in Humans: Is It a Primary. In Fat Detection: Taste, Texture, and Post Ingestive Effects; Montmayeur, J.P., le Coutre, J., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 167–193. [Google Scholar]
- Melis, M.; Tomassini Barbarossa, I. Taste Perception of Sweet, Sour, Salty, Bitter, and Umami and Changes Due to l-Arginine Supplementation, as a Function of Genetic Ability to Taste 6-n-Propylthiouracil. Nutrients 2017, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, N.; Roper, S.D. The cell biology of taste. J. Cell Biol. 2010, 190, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Raghu, M.A. Study to Explore the Effects of Sound Vibrations on Consciousness. Int. J. Soc. Work Hum. Serv. Pract. 2018, 6, 75–88. Available online: http://www.hrpub.org/download/20180730/IJRH2-19290514.pdf (accessed on 15 September 2019).
- Barham, P.; Skibsted, L.H.; Bredie, W.L.P.; Frøst, M.B.; Møller, P.; Risbo, J.; Snitkjær, P.; Mortensen, L.M. Molecular Gastronomy: A New Emerging Scientific Discipline. Chem. Rev. 2010, 110, 2313–2365. [Google Scholar] [CrossRef]
- Feher, J. The Chemical senses. In Quantitative Human Physiology, an Introduction, 2nd ed.; Feher, J., Ed.; Academic Press: London, UK, 2017; pp. 427–439. [Google Scholar]
- Swiegers, J.; Bartowsky, E.; Henschke, P.; Pretorius, I. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Bloem, A.; Bertrand, A.; Lonvaud-Funel, A.; de Revel, G. Vanillin production from simple phenols by wine-associated lactic acid bacteria. Lett. Appl. Microbiol. 2007, 44, 62–67. [Google Scholar] [CrossRef]
- Bloem, A.; Lonvaud-Funel, A.; de Revel, G. Hydrolysis of glycosidically bound flavour compounds from oak wood by Oenococcus Oeni. Food Microbiol. 2008, 25, 99–104. [Google Scholar] [CrossRef]
- Regodón-Mateos, J.; Pérez-Nevado, F.; Ramírez-Fernández, M. Influence of Saccharomyces cerevisiae yeast strain on the major volatile compounds of wine. Enzyme Microb. Technol. 2006, 40, 151–157. [Google Scholar] [CrossRef]
- Vilela, A.; Inês, A.; Cosme, F. Is wine savory? Umami taste in wine. SDRP J. Food Sci. Technol. 2016, 1, 100–105. [Google Scholar] [CrossRef][Green Version]
- Moreno-Arribas, M.; Polo, M. Winemaking biochemistry and microbiology: Current knowledge and future trends. Crit. Rev. Food Sci. Nutr. 2005, 45, 265–286. [Google Scholar] [CrossRef] [PubMed]
- Victor-Freitas, A.P.; Fernandes, A.; Oliveira, J.; Teixeira, N.; Mateus, N. A review of the current knowledge of red wine colour. OENO One 2017, 51. [Google Scholar] [CrossRef]
- Jordão, A.M.; Vilela, A.; Cosme, F. From Sugar of Grape to Alcohol of Wine: Sensorial Impact of Alcohol in Wine. Beverages 2015, 1, 292–310. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M. Microbial Contribution to Wine Aroma and Its Intended Use for Wine Quality Improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef]
- García, V.; Vásquez, H.; Fonseca, F.; Manzanares, P.; Viana, F.; Martínez, C.; Ganga, M. Effects of using mixed wine yeast cultures in the production of chardonnay wines. Rev. Argent. Microbiol. 2010, 42, 226–229. [Google Scholar]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef]
- Polásková, P.; Herszage, J.; Ebeler, S. Wine flavor: Chemistry in a glass. Chem. Soc. Rev. 2008, 37, 2478–2489. [Google Scholar] [CrossRef]
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascués, E.; Calderón, F.; Benito, A.; Rauhut, D.; Santos, A.; et al. Effects on varietal aromas during wine making: A review of the impact of varietal aromas on the flavor of wine. Appl. Microbiol. Biotechnol. 2019, 103, 7425–7450. [Google Scholar] [CrossRef]
- Lanaridis, P.; Salaha, M.J.; Tzourou, I.; Tsoutsouras, E.; Karagiannis, S. Volatile Compounds in Grapes and Wines From Two Muscat Varieties Cultivated In Greek Islands. J. Int. Sci. Vigne Vin 2002, 36, 39–47. [Google Scholar] [CrossRef]
- Ong, P.K.C.; Acree, T.E. Similarities in the Aroma Chemistry of Gewürztraminer Variety Wines and Lychee (Litchi chinesis Sonn.) Fruit. J. Agric. Food Chem. 1999, 47, 665–670. [Google Scholar] [CrossRef]
- Arévalo Villena, M.; Úbeda Iranzo, J.; Cordero Otero, R.; Briones Pérez, A. Optimization of a rapid method for studying the cellular location of β-glucosidase activity in wine yeasts. J. Appl. Microbiol. 2005, 99, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Marais, J. Sauvignon blanc Cultivar Aroma - A Review. S. Afr. J. Enol. Vitic. 1994, 15, 41–45. [Google Scholar] [CrossRef][Green Version]
- Carien, C.; Wessel, J. A comprehensive review on Sauvignon Blanc aroma with a focus on certain positive volatile thiols. Food Res. Int. 2012, 45, 287–298. [Google Scholar] [CrossRef]
- Sacks, G.L.; Gates, M.J.; Ferry, F.X.; Lavin, E.H.; Kurtz, A.J.; Acree, T.E. Sensory Threshold of 1,1,6-Trimethyl-1,2-dihydronaphthalene (TDN) and Concentrations in Young Riesling and Non-Riesling Wines. J. Agric. Food Chem. 2012, 60, 2998–3004. [Google Scholar] [CrossRef] [PubMed]
- Darriet, P.; Thibon, C.; Dubourdieu, D. Aroma and Aroma Precursors in Grape Berry. In Aroma and Aroma Precursors in Grape Berry; Hernâni Gerós, M., Manuela, C., Serge, D., Eds.; Bentham Science Publishers: Sharjah, UAE, 2012; pp. 111–136. [Google Scholar] [CrossRef]
- Herderich, M.J.; Siebert, T.E.; Parker, M.; Capone, D.L.; Mayr, C.; Zhang, P.; Geffroy, O.; Williamson, P.; Francis, I.L. Synthesis of The Ongoing Works on Rotundone, an Aromatic Compound Responsible of the Peppery Notes in Wines. Internet J. Enol. Vitic. 2013, 6, 1–6. [Google Scholar]
- Claus, H.; Mojsov, K. Enzymes for Wine Fermentation: Current and Perspective Applications. Fermentation 2018, 4, 52. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Capone, D.L.; Pardon, K.H.; Elsey, G.M.; Sefton, M.A.; Francis, I.L.; Pretorius, I.S. Engineering volatile thiol release in Saccharomyces cerevisiae for improved wine aroma. Yeast 2007, 24, 561–574. [Google Scholar] [CrossRef]
- Gamero, A.; Belloch, C.; Querol, A. Genomic and transcriptomic analysis of aroma synthesis in two hybrids between Saccharomyces cerevisiae and S. kudriavzevii in winemaking conditions. Microb. Cell Fact. 2015, 14, 128. [Google Scholar] [CrossRef]
- Ehrlich, F. Über die bedingungen der fuselölbildung und über ihren zusammenhang mit dem eiweissaufbau der hefe. Ber. Dtsch. Chem. Ges. 1907, 40, 1027–1047. [Google Scholar] [CrossRef]
- Neubauer, O.; Fromherz, K. Über den Abbau der Aminosäuren bei der Hefegärung. Hoppe-Seyler’s Z Physiol. Chem. 1911, 70, 326–350. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.M.; van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich Pathway for Fusel Alcohol Production: A Century of Research on Saccharomyces cerevisiae Metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Parapouli, M.; Sfakianaki, A.; Monokrousos, N.; Perisynakis, A.; Hatziloukas, E. Comparative transcriptional analysis of flavour-biosynthetic genes of a native Saccharomyces cerevisiae strain fermenting in its natural must environment, vs. a commercial strain and correlation of the genes’ activities with the produced flavour compounds. J. Biol. Res. Thessalon. 2019, 26. [Google Scholar] [CrossRef] [PubMed]
- Gamero, A.; Quintilla, R.; Groenewald, M.; Alkema, W.; Boekhout, T.; Hazelwood, L. High-throughput screening of a large collection of non-conventional yeasts reveals their potential for aroma formation in food fermentation. Food Microbiol. 2016, 60, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Rathbone, D.; Asimont, S.; Adden, R.; Ebeler, S.E. Dynamic changes in ester formation during chardonnay juice fermentations with different yeast inoculation and initial brix conditions. Am. J. Enol. Vitic. 2004, 55, 346–354. [Google Scholar]
- Swiegers, J.H.; Saerens, S.M.G.; Pretorius, I.S. Novel yeast strains as tools for adjusting the flavour of fermented beverages to market specifications. In Biotechnology in Flavour Production, 2nd ed.; Havkin-Frenkel, D., Dudai, N., Eds.; Wiley Online Library: Oxford, UK, 2016; pp. 62–132. [Google Scholar] [CrossRef]
- Kruis, A.J.; Levisson, M.; Mars, A.E.; van der Ploeg, M.; Garcés Daza, F.; Ellena, V.; Kengen, S.W.M.; van der Oost, J.; Weusthuis, R.A. Ethyl acetate production by the elusive alcohol acetyltransferase from yeast. Metab. Eng. 2017, 41, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Kruis, A.J.; Brigida, G.; Jonker, T.; Mars, A.E.; van Rijswijck, I.M.H.; Wolkers-Rooijackers Judith, C.M.; Smid, E.J.; Jan, S.; Verstrepen, K.J.; Kengen, S.W.M.; et al. Contribution of Eat1 and other alcohol acyltransferases to ester production in Saccharomyces cerevisiae. Front Microbiol. 2018, 9, 3202. [Google Scholar] [CrossRef] [PubMed]
- Querol, A.; Perez-Torrado, R.; Alonso-del-Real, J.; Minebois, R.; Stribny, J.; Oliveira, B.M.; Barrio, E. New trends in the uses of yeasts in oenology. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Elsevier: Cambridge, UK, 2018; pp. 177–210. [Google Scholar]
- Mato, I.; Suarez-Luque, S.; Huidobro, J.F. Simple determination of main organic acids in grape juice and wine by using capillary zone electrophoresis with direct UV detection. Food Chem. 2007, 102, 104–112. [Google Scholar] [CrossRef]
- Duan, L.L.; Shi, Y.; Jiang, R.; Yang, Q.; Wang, Y.Q.; Liu, P.T.; Duan, C.Q.; Yan, G.L. Effects of adding unsaturated fatty acids on fatty acid composition of Saccharomyces cerevisiae and major volatile compounds in wine. S. Afr. J. Enol. Vitic. 2015, 36, 285–295. Available online: http://www.scielo.org.za/scielo.php?script=sci_arttext&pid=S2224-79042015000200001&lng=en&tlng=en (accessed on 15 September 2019). [CrossRef]
- Zhao, P.; Gao, J.; Qian, M.; Li, H. Characterization of the Key Aroma Compounds in Chinese Syrah Wine by Gas Chromatography-Olfactometry-Mass Spectrometry and Aroma Reconstitution Studies. Molecules 2017, 22, 1045. [Google Scholar] [CrossRef]
- Landaud, S.; Helinck, S.; Bonnarme, P. Formation of volatile sulfur compounds and metabolism of methionine and other sulfur compounds in fermented food. Appl. Microbiol. Biotechnol. 2008, 7, 1191–1205. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Pretorius, I.S. Microbial formation and modification of flavour and off-flavour compounds in wine. In Biology of Microorganisms on Grapes, in Must and Wine; König, H., Unden, G., Fröhlich, J., Eds.; Springer: Heidelberg, Germany, 2008; pp. 211–233. [Google Scholar] [CrossRef]
- Vermeulen, C.; Gijs, L.; Collin, S. Sensorial contribution and formation pathways of thiols in foods: A review. Food Rev. Int. 2005, 21, 69–137. [Google Scholar] [CrossRef]
- Tominaga, T.; Murat, M.L.; Dubourdieu, D. Development of a method analyzing the volatile thiols involved in the characteristic aroma of wines made from Vitis vinifera L. cv. Sauvignon blanc. J. Agric. Food Chem. 1998, 46, 1044–1048. [Google Scholar] [CrossRef]
- Ferreira, D.C.; Hernandes, K.C.; Nicolli, K.P.; Souza-Silva, E.A.; Manfroi, V.; Alcaraz Zini, C.; Elisa Welke, J. Development of a method for determination of target toxic carbonyl compounds in must and wine using HS-SPME-GC/MS-SIM after preliminary GC×GC/TOFMS analyses. Food Anal. Methods 2019, 12, 108–120. [Google Scholar] [CrossRef]
- Lago, L.O.; Nicolli, K.P.; Marques, A.B.; Zini, C.A.; Welke, J.E. Influence of ripeness and maceration of the grapes on levels of furan and carbonyl compounds in wine – Simultaneous quantitative determination and assessment of the exposure risk to these compounds. Food Chem. 2017, 230, 594–603. [Google Scholar] [CrossRef]
- Semchyshyn, H.M. Reactive Carbonyl Species In Vivo: Generation and Dual Biological Effects. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Bozoudi, D.; Tsaltas, D. Grape Microbiome: Potential and Opportunities as a Source of Starter Cultures. In Grape and Wine Biotechnology; InTech: Rijeka, Croatia, 2016. [Google Scholar]
- Jemec, P.K.; Cadez, N.; Zagorc, T.; Bubic, V.; Zupec, A.; Raspor, P. Yeast population dynamics in five spontaneous fermentations of Malvasia must. Food Microbiol. 2001, 18, 247–259. [Google Scholar] [CrossRef]
- Combina, M.; Elía Mercado, L.; Catania, C.; Ganga, A.; Martinez, C. Dynamics of indigenous yeast populations during spontaneous fermentation of wines from Mendoza, Argentina. Int. J. Food Microbiol. 2005, 99, 237–243. [Google Scholar] [CrossRef]
- Varela, C.; Siebert, T.; Cozzolino, D.; Rose, L.; McLean, H.; Henschke, P.A. Discovering a chemical basis for differentiating wines made by fermentation with ‘wild’ indigenous and inoculated yeasts: Role of yeast volatile compounds. Aust. J. Grape Wine Res. 2009, 15, 238–248. [Google Scholar] [CrossRef]
- Restuccia, D.; Loizzo, M.R.; Spizzirri, U.G. Accumulation of Biogenic Amines in Wine: Role of Alcoholic and Malolactic Fermentation. Fermentation 2018, 4, 6. [Google Scholar] [CrossRef]
- Clemente-Jimenez, J.; Mingorance-Cazorla, L.; Martínez-Rodríguez, S.; Las HerasVázquez, F.; Rodríguez-Vico, F. Influence of sequential yeast mixtures on wine fermentation. Int. J. Food Microbiol. 2005, 98, 301–308. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.J.; di Stefano, R. Description of the β-glucosidase activity of wine yeasts. Food Microbiol. 1997, 14, 583–591. [Google Scholar] [CrossRef]
- Van Rensburg, P.; Pretorius, I. Enzymes in winemaking: Harnessing natural catalysts for efficient bio-transformations-a review. S. Afr. J. Enol. Vitic. Spec. Issue 2000, 21, 52–73. [Google Scholar]
- Budić-Leto, I.; Zdunic, G.; Banovic, M.; Kovacevic-Ganic, K.; Tomic-Potrebujes, I.; Lovric, T. Fermentative aroma compounds and sensory descriptors of traditional Croatian dessert wine Prošek from Plavac mali cv. Food Technol. Biotechnol. 2010, 48, 530–537. [Google Scholar] [CrossRef]
- Puertas, B.; Jiménez, M.J.; Cantos-Villar, E.; Cantoral, J.M.; Rodriguez, M. Use of Torulaspora delbrueckii and Saccharomyces cerevisiae in semi-industrial sequential inoculation to improve quality of Palomino and Chardonnay wines in warm climates. J. Appl. Microbiol. 2016, 122, 733–746. [Google Scholar] [CrossRef]
- García, M.; Arroyo, T.; Crespo, J.; Cabellos, J.M.; Esteve-Zarzoso, B. Use of native non-Saccharomyces strain: A new strategy in D.O. “Vinos de Madrid” (Spain) wines elaboration. Eur. J. Food Sci. Technol. 2017, 5, 1–31. Available online: https://www.eajournals.org/journals/european-journal-of-food-science-and-technology-ejfst/vol-5-issue-2-april-2017/use-native-non-saccharomyces-strain-new-strategy-d-o-vinos-de-madrid-spain-wines-elaboration/ (accessed on 30 September 2019).
- Rossouw, D.; Bauer, F.F. Exploring the phenotypic space of non-Saccharomyces wine yeast biodiversity. Food Microbiol. 2016, 55, 32–46. [Google Scholar] [CrossRef]
- Wang, C.; Mas, A.; Esteve-Zarzoso, B. Interaction between Hanseniaspora uvarum and Saccharomyces cerevisiae during alcoholic fermentation. Int. J. Food Microbiol. 2015, 206, 67–74. [Google Scholar] [CrossRef]
- Wang, C.; Mas, A.; Esteve-Zarzoso, B. The interaction between Saccharomyces cerevisiae and non-Saccharomyces yeast during alcoholic fermentation is species and strain specific. Front. Microbiol. 2016, 7, 502. [Google Scholar] [CrossRef]
- Schaffrath, R.; Meinhard, F.; Klassen, R. Yeast Killer Toxins: Fundamentals and Applications. In Physiology and Genetics, 2nd ed.; Anke Schüffler, A., Ed.; Springer International Publishing AG: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Velázquez, R.; Zamora, E.; Álvarez, M.L.; Hernández, L.M.; Ramírez, M. Effects of new Torulaspora delbrueckii killer yeasts on the must fermentation kinetics and aroma compounds of white table wine. Front. Microbiol. 2015, 6, 1222. [Google Scholar] [CrossRef]
- El-Banna, A.A.; El-Sahn, M.A.; Shehata, M.G. Yeasts Producing Killer Toxins: An Overview. Alex. J. Food Sci. Technol. 2011, 8, 41–53. [Google Scholar] [CrossRef]
- Branco, P.; Francisco, D.; Chambon, C.; Hébraud, M.; Arneborg, N.; Almeida, M.G.; Caldeira, J.; Albergaria, H. Identification of novel GAPDH-derived antimicrobial peptides secreted by Saccharomyces cerevisiae and involved in wine microbial interactions. Appl. Microbiol. Biotechnol. 2014, 98, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Loira, I.; Morata, A.; Comuzzo, P.; Callejo, M.J.; González, C.; Calderón, F.; Suárez-Lepe, J.A. Use of Schizosaccharomyces pombe and Torulaspora delbrueckii strains in mixed and sequential fermentations to improve red wine sensory quality. Food Res. Int. 2015, 76, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Curiel, J.A.; Morales, P.; Gonzalez, R.; Tronchoni, J. Different Non-Saccharomyces Yeast Species Stimulate Nutrient Consumption in S. cerevisiae Mixed Cultures. Front Microbiol. 2017, 31, 2121. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Bañuelos, M.A.; Suárez-Lepe, J.A. Isolation, selection, and identification techniques for non-Saccharomyces yeasts of oenological interest. In Biotechnological Progress and Beverage Consumption; Grumezescu Alexandru, M., Holban Alina, M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 467–521. [Google Scholar] [CrossRef]
- Renault, P.; Coulon, J.; de Revel, G.; Barbe, J.C.; Bely, M. Increase of fruity aroma during mixed T. delbrueckii/S. cerevisiae wine fermentation is linked to specific esters enhancement. Int. J. Food Microbiol. 2015, 207, 40–48. [Google Scholar] [CrossRef]
- Renault, P.; Coulon, J.; Moine, V.; Thibon, C.; Bely, M. Enhanced 3-sulfanylhexan-1-ol production in sequential mixed fermentation with Torulaspora delbrueckii/Saccharomyces cerevisiae reveals a situation of synergistic interaction between two industrial strains. Front. Microbiol. 2016, 7, 293. [Google Scholar] [CrossRef]
- Mosciano, G. Successful flavors: From formulation to QC to applications and beyond. In Successful Flavors; Mosciano, G., Ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2006; p. 240. ISBN 10 1932633197. [Google Scholar]
- García, M.; Esteve-Zarzoso, B.; Crespo, J.; Cabellos, J.M.; Arroyo, T. Yeast Monitoring of Wine Mixed or Sequential Fermentations Made by Native Strains from D.O. “Vinos de Madrid” Using Real-Time Quantitative PCR. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Loira, I.; Vejarano, R.; Bañuelos, M.A.; Morata, A.; Tesfaye, W.; Uthurry, C.; Villa, A.; Cintora, I.; Suárez-Lepe, J.A. Influence of sequential fermentation with Torulaspora delbrueckii and Saccharomyces cerevisiae on wine quality. LWT Food Sci. Technol. 2014, 59, 915–922. [Google Scholar] [CrossRef]
- Peinado, R.; Moreno, J.; Medina, M.; Mauricio, J. Changes in volatile compounds and aromatic series in sherry wine with high gluconic acid levels subjected to aging by submerged flor yeast cultures. Biotechnol. Lett. 2004, 26, 757–762. [Google Scholar] [CrossRef]
- Viana, F.; Gil, J.V.; Vallés, S.; Manzanares, P. Increasing the levels of 2-phenylethyl acetate in wine through the use of a mixed culture of Hanseniaspora osmophila and Saccharomyces cerevisiae. Int. J. Food Microbiol. 2009, 135, 68–74. [Google Scholar] [CrossRef]
- Berbegal, C.; Spano, G.; Tristezza, M.; Grieco, F.; Capozzi, V. Microbial Resources and Innovation in the Wine Production Sector. S. Afr. J. Enol. Vitic. 2017, 38, 156–166. [Google Scholar] [CrossRef]
- Alexandre, H.; Costello, P.J.; Remize, F.; Guzzo, J.; Guilloux-Benatier, M. Saccharomyces cerevisiae-Oenococcus oeni interactions in wine: Current knowledge and perspectives. Int. J. Food Microbiol. 2004, 93, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Nehme, N.; Mathieu, F.; Taillandier, P. Impact of the co-culture of Saccharomyces cerevisiae-Oenococcus oeni on malolactic fermentation performance and partial characterization of a yeast-derived inhibitory peptidic fraction. Food Microbiol. 2010, 27, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Zapparoli, G.; Tosi, E.; Azzolini, M.; Vagnoli, P.; Krieger, S. Bacterial inoculation strategies for the achievement of malolactic fermentation in high-alcohol wines. S. Afr. J. Enol. Vitic. 2009, 30, 49–55. [Google Scholar] [CrossRef]
- Abrahamse, C.E.; Bartowsky, E.J. Timing of malolactic fermentation inoculation in Shiraz grape must and wine: Influence on chemical composition. World J. Microbiol. Biotechnol. 2012, 28, 255–265. [Google Scholar] [CrossRef]
- Knoll, C.; Fritsch, S.; Schnell, S.; Grossmann, M.; Krieger-Weber, S.; Du Toit, M.; Rauhut, D. Impact of different malolactic fermentation inoculation scenarios on Riesling wine aroma. World J. Microbiol. Biotechnol. 2012, 28, 1143–1153. [Google Scholar] [CrossRef]
- Antalick, G.; Perello, M.; de Revel, G. Co-inoculation with Yeast and LAB Under Winery Conditions: Modification of the Aromatic Profile of Merlot Wines. S. Afr. J. Enol. Vitic. 2013, 34, 223–232. [Google Scholar] [CrossRef]
- Cañas, P.M.; Romero, E.G.; Pérez-Martín, F.; Seseña, S.; Palop, M.L. Sequential inoculation versus co-inoculation in Cabernet Franc wine fermentation. Food Sci Technol Int. 2015, 21, 203–212. [Google Scholar] [CrossRef]
- Berbegal, C.; Borruso, L.; Fragasso, M.; Tufariello, M.; Russo, P.; Brusetti, L.; Spano, G.; Capozzi, V. A Metagenomic-Based Approach for the Characterization of Bacterial Diversity Associated with Spontaneous Malolactic Fermentations in Wine. Int. J. Mol. Sci. 2019, 120, 3980. [Google Scholar] [CrossRef]
- Kántor, A.; Hutková, J.; Petrová, J.; Hleba, L.; Kačániová, M. Antimicrobial activity of pulcherrimin pigment produced by Metschnikowia pulcherrima against various yeast species. J. Microbiol. Biotech. Food Sci. 2015, 5, 282–285. [Google Scholar] [CrossRef]
- Delgado-Vargas, F.; Jiménez-Aparicio, A.R.; Paredes-Lopez, O. Natural Pigments: Carotenoids, Anthocyanins, and Betalains — Characteristics, Biosynthesis, Processing, and Stability. Crit. Rev. Food Sci. Nut. 2000, 40, 173–289. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Na-Na, L.; Lin, M.; Qiu-Hong, P.; Jun, W.; Malcolm, J.R.; Chang-Qing, D. Anthocyanins and their variation in red wines. II. Anthocyanin derived pigments and their color evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suáez, J.A. Pyruvic Ácid and acetaldehyde production by different strains of Saccharomyces cerevisiae: Relationship with vitisin A and B formation in red wines. J. Agric. Food Chem. 2003, 51, 7402–7409. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Calderón, F.; Gonzalez, C.; Gómez-Cordovés, M.C.; Suarez, C.J.A. Formation of the highly stable pyranoanthocyanins (vitisins A and B) in red wines by the addition of pyruvic acid and acetaldehyde. Food Chem. 2007, 100, 1144–1152. [Google Scholar] [CrossRef]
- Schwarz, M.; Wabnitz, T.C.; Winterhalter, P. Pathway Leading to the Formation of Anthocyanin−Vinylphenol Adducts and Related Pigments in Red Wines. J. Agric. Food Chem. 2003, 51, 3682–3687. [Google Scholar] [CrossRef]
- Fulcrand, H.; Benabdeljalil, C.; Rigaud, J.; Chenyier, V.; Moutounet, M. A new class of wine pigments generated by reaction between pyruvic acid and grape anthocyanins. Phytochemistry 1998, 47, 1401–1407. [Google Scholar] [CrossRef]
- Victor-Freitas, A.P.; Mateus, N. Formation of pyranoanthocyanins in red wines: A new and diverse class of anthocyanin derivatives. Anal. Bioanal. Chem. 2011, 401, 1463–1473. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Suárez-Lepe, J.A. Influence of Yeasts in Wine Colour. In Grape and Wine Biotechnology; Morata, A., Loira, I., Eds.; IntechOpen: London, UK, 2016; pp. 285–305. [Google Scholar] [CrossRef]
- Ruta, L.L.; Farcasanu, I.C. Anthocyanins and Anthocyanin-Derived Products in Yeast-Fermented Beverages. Antioxidants (Basel) 2019, 8, 182. [Google Scholar] [CrossRef]
- Asenstorfer, R.E.; Markides, A.J.; Iland, P.G.; Jones, G.P. Formation of vitisin A during red wine fermentation and maturation. Aust. J. Grape Wine Res. 2003, 9, 40–46. [Google Scholar] [CrossRef]
- Osborne, J.P.; Orduña, R.M.; Pilone, G.J.; Liu, S.Q. Acetaldehyde metabolism by wine lactic acid bacteria. FEMS Microbiol. Lett. 2000, 191, 51–55. [Google Scholar] [CrossRef]
- He, J.R.; Oliveira, J.; Silva, A.M.-S.; Mateus, N.; Victor-Freitas, A.P. Oxovitisins: A New Class of Neutral Pyranone-anthocyanin Derivatives in Red Wines. J. Agric. Food Chem. 2010, 58, 8814–8819. [Google Scholar] [CrossRef] [PubMed]
- Carew, A.L.; Smith, P.; Close, D.C.; Curtin, C.; Dambergs, R.G. Yeast Effects on Pinot noir Wine Phenolics, Color, and Tannin Composition. J. Agric. Food Chem. 2013, 61, 9892–9898. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A. Wine colour. In Woodhead Publishing Series in Food Science, Technology and Nutrition; Reynolds, A.G., Ed.; Woodhead Publishing: Sawston, UK, 2010; pp. 73–104. [Google Scholar] [CrossRef]
- Minnaar, P.P.; Ntushelo, N.; Ngqumba, Z.; van Breda, V.; Jolly, N.P. Effect of Torulaspora delbrueckii yeast on the anthocyanin and flavanol concentrations of Cabernet franc and Pinotage wines. S. Afr. J. Enol. Vitic. 2015, 36, 50–58. Available online: http://www.scielo.org.za/scielo.php?script=sci_arttext&pid=S2224-79042015000100013&lng=en&tlng=en (accessed on 25 September 2019). [CrossRef]
- Pacheco, A.; Santos, J.; Chaves, S.; Almeida, J.; Leão, C.; Sousa, M.J. The Emerging Role of the Yeast Torulaspora delbrueckii. In Bread and Wine Production: Using Genetic Manipulation to Study Molecular Basis of Physiological Responses, Structure and Function of Food Engineering; Eissa, A.A., Ed.; IntechOpen: London, UK, 2012; pp. 339–370. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Fariña, L.; Dellacassa, E.; Carrau, F. Non-Saccharomyces and Saccharomyces strains co-fermentation increases acetaldehyde accumulation: Effect on anthocyanin-derived pigments in Tannat red wines. Yeast 2016, 33, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Calderón, F.; Benito, S. The Combined Use of Schizosaccharomyces pombe and Lachancea thermotolerans-Effect on the Anthocyanin Wine Composition. Molecules 2017, 22. [Google Scholar] [CrossRef]
- Benito, S.; Morata, A.; Palomero, F.; Gonzalez, M.; Suárez-Lepe, J. Formation of vinylphenolic pyranoanthocyanins by Saccharomyces cerevisiae and Pichia guillermondii in red wines produced following different fermentation strategies. Food Chem. 2011, 124, 15–23. [Google Scholar] [CrossRef]
- Strauss, M.; Jolly, N.; Lambrechts, M.; Van Rensburg, P. Screening for the production of extracellular hydrolytic enzymes by non-Saccharomyces wine yeasts. J. Appl. Microbiol. 2001, 91, 182–190. [Google Scholar] [CrossRef]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef]
- Rodrigues, A.; Ricardo-Da-Silva, J.M.; Lucas, C.; Laureano, O. Effect of commercial mannoproteins on wine colour and tannins stability. Food Chem. 2012, 131, 907–914. [Google Scholar] [CrossRef]
- Doco, T.; Quellec, N.; Moutounet, M. Polysaccharide patterns during the ageing of Carignan noir red wines. Am. J. Enol. Vitic. 1999, 50, 25–32. [Google Scholar]
- Chalier, P.; Angot, B.; Delteil, D.; Doco, T.; Gunata, Z. Interactions between aroma compounds and whole mannoprotein isolated from Saccharomyces cerevisiae strains. Food Chem. 2007, 100, 22–30. [Google Scholar] [CrossRef]
- Dupin, I.; Mc Kinnon, B.M.; Ryan, C.; Boulay, M.; Markides, A.J.; Jones, G.P.; Williams, P.J.; Waters, E.J. Saccharomyces cerevisiae, mannoproteins that protect wine from protein haze: Their release during fermentation and lees contact and a proposal mechanism of action. J. Agric. Food Chem. 2000, 48, 3098–3105. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.; Francis, L.; Williams, P.; Kwitkowski, M.; Gawel, R.; Cheynier, V.; Waters, E. The mouth-feel properties of polysaccharides and anthocyanins in a wine like medium. Food Chem. 2004, 85, 519–525. [Google Scholar] [CrossRef]
- Riou, V.; Vernhet, A.; Doco, T.; Moutonnet, M. Aggregation of grape seed tannins in model wine – effect of wine polysaccharides. Food Hydrocol. 2002, 16, 17–23. [Google Scholar] [CrossRef]
- Juega, M.; Nunez, Y.P.; Carrascosa, A.V.; Martinez-Rodriguez, A.J. Influence of yeast mannoproteins in the aroma improvement of white wines. J Food Sci. 2012, 77, M499–M504. [Google Scholar] [CrossRef] [PubMed]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology, Volume 1: The Microbiology of Wine and Vinifications; John Wiley and Sons: Hoboken, NJ, USA, 2006; Volume 1. [Google Scholar]
- Guadalupe, Z.; Palacios, A.; Ayestarán, B. Maceration enzymes and mannoproteins: A possible strategy to increase colloidal stability and colour extraction in red wines. J. Agric. Food Chem. 2007, 55, 4854–4862. [Google Scholar] [CrossRef]
- Costa, G.P.; Nicolli, K.P.; Welke, J.E.; Manfroi, V.; Zini, C.A. Volatile Profile of Sparkling Wines Produced with the Addition of Mannoproteins or Lees before Second Fermentation Performed with Free and Immobilized Yeasts. J. Braz. Chem. Soc. 2018, 29, 1866–1875. [Google Scholar] [CrossRef]
- Doco, T.; Vuchot, P.; Cheynier, V.; Moutounet, M. Structural modification of wine arabinogalactans during aging on lees. Am. J. Enol. Vitic. 2003, 54, 150–157. [Google Scholar]
- Lubbers, S.; Voilley, A.; Feuillat, M.; Charpentier, C. Influence of mannoproteins from yeast on the aroma intensity of a model wine. LWT-Food Sci. Technol. 1994, 27, 108–114. [Google Scholar] [CrossRef]
- Braschi, G.; Ricci, A.; Grazia, L.; Versari, A.; Patrignani, F.; Lanciotti, R. Mannoprotein Content and Volatile Molecule Profiles of Trebbiano Wines Obtained by Saccharomyces cerevisiae and Saccharomyces bayanus Strains. Fermentation 2019, 5, 66. [Google Scholar] [CrossRef]
- Comuzzo, P.; Tat, L.; Tonizzo, A.; Battistutta, F. Yeast derivatives (extracts and autolysates) in winemaking: Release of volatile compounds and effects on wine aroma volatility. Food Chem. 2006, 99, 217–230. [Google Scholar] [CrossRef]
- Nieto-Rojo, R.; Ancín-Azpilicueta, C.; Garrido, J.J. Sorption of 4-ethylguaiacol and 4-ethylphenol on yeast cell walls, using a synthetic wine. Food Chem. 2014, 152, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Domizio, P.; Liu, Y.; Bisson, L.F.; Barile, D. Use of non-Saccharomyces wine yeasts as novel sources of mannoproteins in wine. Food Microbiol. 2014, 43, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Través, L.; Querol, A.; Pérez-Torrado, R. Increased mannoprotein content in wines produced by Saccharomyces kudriavzevii × Saccharomyces cerevisiae hybrids. Int. J. Food Microbiol. 2016, 237, 35–38. [Google Scholar] [CrossRef]
- Escot, S.; Feulliat, M.; Dulau, L.; Charpentier, C. Release of polysaccharides by yeasts and the influence of released polysaccharides on colour stability and wine astringency. Aust. J. Grape Wine Res. 2001, 7, 153–159. [Google Scholar] [CrossRef]
- Poncet-Legrand, C.; Doco, T.; Williams, P.; Vernhet, A. Inhibition of grape seed tannin aggregation by wine mannoproteins: Effect of polysaccharide molecular weight. Am. J. Enol. Vitic. 2007, 58, 87–91. [Google Scholar]
- Guadalupe, Z.; Ayestarán, B. Effect of commercial mannoprotein addition on polysaccharide, polyphenolic, and colour composition in red wines. J. Agric. Food Chem. 2008, 56, 9022–9029. [Google Scholar] [CrossRef]
- Guadalupe, Z.; Martínez, L.; Ayestarán, B. Yeast mannoproteins in red winemaking. Effect on polysaccharide, polyphenolic and colour composition. Am. J. Enol. Vitic. 2010, 61, 191–200. [Google Scholar]
- Morata, A.; Gomez-Cordoves, M.C.; Suberviola, J.; Bartolome, B.; Colomo, B.; Suarez, J.A. Adsorption of anthocyanins by yeast cell walls during the fermentation of red wines. J. Agric. Food Chem. 2003, 51, 4084–4088. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; García-Estévez, I.; Puente, V.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Color Stabilization of Red Wines. A Chemical and Colloidal Approach. J. Agric. Food Chem. 2014, 62, 6984–6994. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).