Production of the Polysaccharide Curdlan by Agrobacterium species on Processing Coproducts and Plant Lignocellulosic Hydrolysates
Abstract
1. Introduction
2. Effect of Culture Conditions on Curdlan Production by Agrobacterium Strains
2.1. Effect of Carbon Source
2.2. Effect of Phosphate
2.3. Effect of Pyrimidine Base Supplementation
2.4. Effect of Tween-80 Supplementation
3. Genetics and Regulation of Curdlan Biosynthesis
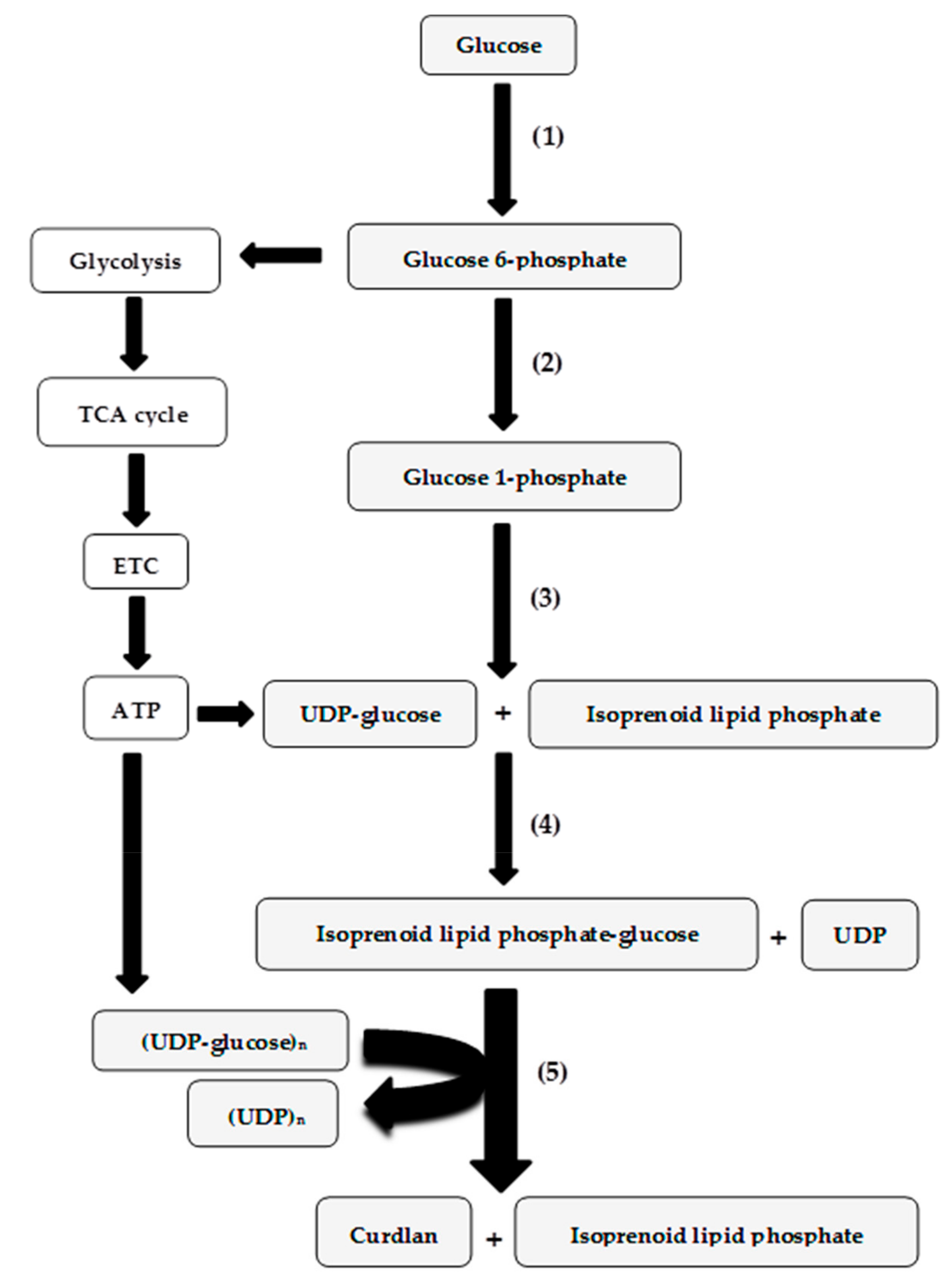
4. Curdlan Production by Agrobacterium Strains Grown on Processing Coproducts
5. Curdlan Production by Agrobacterium Strains Grown on Plant Lignocellulosic Hydrolysates
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harada, T.; Fujimori, F.; Hirose, S.; Masada, M. Growth and β-glucan 10C3K production by a mutant of Alcaligenes faecalis var myxogenes in defined medium. Agric. Biol. Chem. 1966, 30, 764–769. [Google Scholar] [CrossRef]
- Harada, T.; Misaki, A.; Saito, H. Curdlan: A bacterial gel-forming β-1,3-glucan. Arch. Biochem. Biophys. 1968, 124, 292–298. [Google Scholar] [CrossRef]
- Phillips, K.R.; Lawford, H.G. Theoretical maximum and observed product yields associated with curdlan production by Alcaligenes faecalis. Can. J. Microbiol. 1983, 29, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Edgar, K.J. Properties, chemistry and applications of the bioactive polysaccharide curdlan. Biomacromolecules 2014, 15, 1079–1096. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Konno, A.; Taguchi, T.; Tawada, T.; Kasai, H.; Toda, J.; Terasaki, T. Curdlan: Properties and applications to foods. J. Food Sci. 1991, 56, 769–772,776. [Google Scholar] [CrossRef]
- Jezequel, V. Curdlan: A new functional β-glucan. Cereal Foods World 1998, 43, 361–364. [Google Scholar]
- Koreeda, A.; Harada, T.; Ogawa, K.; Sato, S.; Kasai, N. Study of the ultrastructure of gel-forming (1→3)-β-D-glucan (curdlan-type polysaccharide) by electron microscopy. Carbohydr. Res. 1974, 33, 396–399. [Google Scholar] [CrossRef]
- Nakanishi, I.; Kimura, K.; Kusui, S.; Yamazaki, E. Complex formation of gel-forming bacterial (1→3)-β-D-glucan (curdlan-type polysaccharides) with dyes in aqueous solution. Carbohydr. Res. 1974, 32, 47–52. [Google Scholar] [CrossRef]
- Spicer, E.F.J.; Goldenthal, E.I.; Ikeda, T. A toxicological assessment of curdlan. Food Chem. Toxicol. 1999, 37, 455–479. [Google Scholar] [CrossRef]
- Kanke, M.; Koda, K.; Koda, Y.; Katayama, H. Application of curdlan to controlled drug delivery. I. The preparation and evaluation of theophylline-containing curdlan tablets. Pharm. Res. 1992, 9, 414–418. [Google Scholar] [CrossRef]
- Zhan, X.B.; Lin, C.C.; Zhang, H.T. Recent advances in curdlan biosynthesis, biotechnological production, and applications. Appl. Microbiol. Biotechnol. 2012, 93, 525–553. [Google Scholar] [CrossRef] [PubMed]
- Kalyanasundaram, G.T.; Doble, M.; Gummadi, S.N. Production and downstream processing of (1→3)-β-D-glucan from mutant strain of Agrobacterium sp. ATCC 31750. AMB Express 2012, 2, 31. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-K.; Ryu, K.-E.; Choi, W.-A.; Rhee, Y.-H.; Lee, I.-Y. Enhanced production of (1→3)-β-D-glucan by a mutant strain of Agrobacterium species. Biochem. Bioeng. J. 2003, 16, 163–168. [Google Scholar] [CrossRef]
- Kim, B.S.; Jung, I.D.; Kim, J.S.; Lee, J.-H.; Lee, I.Y.; Lee, K.B. Curdlan gels as protein delivery vehicles. Biotechnol. Lett. 2000, 22, 1127–1130. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, I.-Y.; Kim, M.-K.; Park, Y.H. Optimal pH control of batch processes for production of curdlan by Agrobacterium species. J. Ind. Microbiol. Biotechnol. 1999, 23, 143–148. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, C.; Yang, L.; Zhao, L.; Lin, C.; Liu, Z.; Mao, Z. CdR function in a curdlan-producing Agrobacterium sp. ATCC31749 strain. BMC Microbiol. 2015, 15, 25. [Google Scholar] [CrossRef]
- Lawford, H.G.; Rousseau, J.D. Production of β-1,3-glucan exopolysaccharide in low shear systems: The requirement for high oxygen tension. Appl. Biochem. Biotechnol. 1992, 34/35, 597–612. [Google Scholar] [CrossRef]
- Wu, J.R.; Yu, L.J.; Zhan, X.B.; Zheng, Z.Y.; Lu, J.; Lin, C.C. NtrC-dependent regulatory network for curdlan biosynthesis in response to nitrogen limitation in Agrobacterium sp. ATCC 31749. Process Biochem. 2012, 47, 1552–1558. [Google Scholar] [CrossRef]
- Jiang, L. Effect of nitrogen source on curdlan production by Alcaligenes faecalis ATCC 31749. Int. J. Biol. Macromol. 2013, 52, 218–220. [Google Scholar] [CrossRef]
- Portilho, M.; Matioli, G.; Zanin, G.; de Moraes, F.F.; Scamparini, A.R. Production of insoluble exopolysaccharide of Agrobacterium sp. (ATCC 31749 and IFO 13140). Appl. Biochem. Biotechnol. 2006, 129–132, 864–869. [Google Scholar] [CrossRef]
- West, T.P. Pyrimidine base supplementation affects curdlan production by Agrobacterium sp. ATCC 31749. J. Basic Microbiol. 2006, 46, 153–157. [Google Scholar] [CrossRef] [PubMed]
- West, T.P.; Nemmers, B. Curdlan production by Agrobacterium sp. ATCC 31749 on an ethanol fermentation coproduct. J. Basic Microbiol. 2008, 48, 65–68. [Google Scholar] [CrossRef] [PubMed]
- West, T.P. Elevated curdlan production by a mutant of Agrobacterium sp. ATCC 31749. J. Basic Microbiol. 2009, 49, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lu, M.; Fang, Y.; Wu, L.; Xu, Y.; Wang, S. Production of curdlan grown on cassava starch waste hydrolysates. J. Polym. Environ. 2018, 26, 33–38. [Google Scholar] [CrossRef]
- Lee, I.-Y.; Seo, W.T.; Kim, M.K.; Park, C.S.; Park, Y.H. Production of curdlan using sucrose or sugar cane molasses by two-step fed-batch cultivation of Agrobacterium species. J. Ind. Microbiol. Biotechnol. 1997, 18, 255–259. [Google Scholar] [CrossRef]
- Lee, J.W.; Yeomans, W.G.; Allen, A.L.; Kaplan, D.L.; Deng, F.; Gross, R.A. Exopolymers from curdlan production: Incorporation of glucose-related sugars by Agrobacterium sp. strain ATCC 31749. Can. J. Microbiol. 1997, 43, 149–156. [Google Scholar] [CrossRef]
- Kai, A.; Ishino, T.; Arashida, T.; Hatanaka, K.; Akaike, T.; Matsuzaki, K.; Kaneko, Y.; Mimura, T. Biosynthesis of curdlan from culture media containing 13C-labeled glucose as the carbon source. Carbohydr. Res. 1993, 240, 153–159. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, Y.H. Optimal production of curdlan by Agrobacterium sp. with feedback inferential control of optimal pH profile. Biotechnol. Lett. 2001, 23, 525–530. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, I.Y.; Lee, J.H.; Kim, K.T.; Rhee, Y.H.; Park, Y.H. Residual phosphate concentration under nitrogen-limiting conditions regulates curdlan production in Agrobacterium species. J. Ind. Microbiol. Biotechnol. 2000, 25, 180–183. [Google Scholar] [CrossRef]
- Yu, L.; Wu, J.; Liu, J.; Zhan, X.; Zheng, Z.; Lin, C.C. Enhanced curdlan production in Agrobacterium sp. ATCC 31749 by addition of low-polyphosphates. Biotechnol. Bioprocess Eng. 2011, 16, 34–41. [Google Scholar] [CrossRef]
- Zhang, H.T.; Zhan, X.B.; Zheng, Y.Z.; Wu, J.R.; English, N.; Yu, X.B.; Lin, C.C. Improved curdlan fermentation process based on optimization of dissolved oxygen combined with pH control and metabolic characterization of Agrobacterium sp. ATCC 31749. Appl. Microbiol. Biotechnol. 2012, 93, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, I.Y. Optimization of uracil addition for curdlan (β-1→3-glucan) production by Agrobacterium sp. Biotechnol. Lett. 2001, 23, 1131–1134. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, I.Y.; Ko, J.H.; Rhee, Y.H.; Park, Y.H. Higher intracellular levels of uridine monophosphate under nitrogen-limited conditions enhance metabolic flux of curdlan synthesis in Agrobacterium species. Biotechnol. Bioeng. 1999, 62, 317–323. [Google Scholar] [CrossRef]
- Jin, L.-H.; Lee, J.-H. Effect of uracil addition on proteomic profiles and 1,3-β-glucan production in Agrobacterium sp. Biotechnol. Appl. Biochem. 2014, 61, 280–288. [Google Scholar] [PubMed]
- Xia, Z. Effect of Tween 80 on the production of curdlan by Alcaligenes faecalis ATCC 31749. Carbohydr. Polym. 2013, 98, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhu, L.; Gao, M.; Zheng, Z.; Wu, J.; Zhan, X. Influence of Tween-80 on the production of water-insoluble curdlan from Agrobacterium sp. Int. J. Biol. Macromol. 2018, 106, 611–619. [Google Scholar] [CrossRef]
- Ruffing, A.M.; Castro-Melchor, M.; Hu, W.-S.; Chen, R.R. Genome sequence of the curdlan-producing Agrobacterium sp. strain ATCC 31749. J. Bacteriol. 2011, 193, 4294–4295. [Google Scholar] [CrossRef]
- Stasinopoulos, S.J.; Fisher, P.R.; Stone, B.A.; Stanisich, V.A. Detection of two loci involved in (1→3)-β-glucan (curdlan) biosynthesis by Agrobacterium sp. ATCC31749, and comparative sequence analysis of the putative curdlan synthase gene. Glycobiology 1999, 9, 31–41. [Google Scholar] [CrossRef]
- Karnezis, T.; Fisher, H.C.; Neumann, G.M.; Stone, B.A.; Stanisich, V.A. Cloning and characterization of the phosphatidylserine synthase gene of Agrobacterium sp. ATCC 31749 and effect of its inactivation on production of high-molecular-mass (1→3)-β-D-glucan (curdlan). J. Bacteriol. 2002, 184, 4114–4123. [Google Scholar] [CrossRef]
- Zhang, H.; Setubal, J.C.; Zhan, X.; Zheng, Z.; Yu, L.; Wu, J.; Chen, D. Component identification of electron transport chains in curdlan-producing Agrobacterium sp. ATCC 31749 and its genome-specific prediction using comparative genome and phylogenetic tree analysis. J. Ind. Microbiol. Biotechnol. 2011, 38, 667–677. [Google Scholar] [CrossRef]
- Jin, L.-H.; Um, H.-J.; Yin, C.-J.; Kim, Y.-H.; Lee, J.-H. Proteomic analysis of curdlan-producing Agrobacterium sp. in response to pH downshift. J. Biotechnol. 2008, 138, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.-J.; Wu, J.-R.; Zheng, Z.-Y.; Zhan, X.-B.; Lin, C.C. Changes in curdlan biosynthesis and nitrogenous compounds utilization characterized in ntrC mutant of Agrobacterium sp. ATCC 31749. Curr. Microbiol. 2011, 63, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, A.; Ma, F.; Yang, J.; Xie, Y. Genetic control and regulatory mechanisms of succinoglycan and curdlan biosynthesis in genus Agrobacterium. Appl. Microbiol. Biotechnol. 2016, 100, 6183–6192. [Google Scholar] [CrossRef] [PubMed]
- West, T.P. Polysaccharide production by an Agrobacterium sp. curdlan overproducer mutant on a grain fermentation coproduct. Res. J. Microbiol. 2012, 7, 273–279. [Google Scholar] [CrossRef]
- Anane, R.F.; Sun, H.; Zhao, L.; Wang, L.; Lin, C.; Mao, Z. Improved curdlan production with discarded bottom parts of asparagus spear. Microb. Cell Factories 2017, 16, 59. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, L.; Ding, H.; Gao, M.; Zheng, Z.; Wu, J.; Zhan, X. Enhanced production of curdlan by coupled fermentation system of Agrobacterium sp. ATCC 31749 and Trichoderma harzianum GIM 3.442. Carbohydr. Polym. 2017, 157, 1687–1694. [Google Scholar] [CrossRef]
- West, T.P. Effect of nitrogen source concentration on curdlan production by Agrobacterium sp. ATCC 31749 grown on prairie cordgrass hydrolysates. Prep. Biochem. Biotechnol. 2016, 46, 85–90. [Google Scholar] [CrossRef]
- West, T.P.; Peterson, J.L. Production of the polysaccharide curdlan by an Agrobacterium strain grown on a plant biomass hydrolysate. Can. J. Microbiol. 2014, 60, 53–56. [Google Scholar] [CrossRef]
- Zhang, Y.-H.P. Reviving the carbohydrate economy via multi-product lignocellulose biorefineries. J. Ind. Microbiol. Biotechnol. 2008, 35, 367–375. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, Z.; Labavitch, J.M.; Wang, D.; Teter, S.A.; Jenkins, B.M. Evaluation of different biomass materials as feedstock for fermentable sugar production. In Applied Biochemistry and Biotechnology; Humana Press: Totowa, NJ, USA, 2007; Volume 136–140, pp. 423–435. [Google Scholar]
- Shin, H.-D.; Liu, L.; Kim, M.-K.; Park, Y.-I.; Chen, R. Metabolic engineering of Agrobacterium sp. ATCC31749 for curdlan production from cellobiose. J. Ind. Microbiol. Biotechnol. 2016, 43, 1323–1331. [Google Scholar] [CrossRef]
| Coproduct/Hydrolyzed Plant Biomass | Agrobacterium sp. Strain | Growth Conditions | Curdlan (g/L) | Yield (g/g) | Reference |
|---|---|---|---|---|---|
| Cassava glucose syrup | ATCC 31749 | 360 h, 30 °C | ND | 0.44 | [20] |
| Corn glucose syrup | ATCC 31749 | 360 h, 30 °C | ND | 0.55 | [20] |
| Corn maltose corn syrup | ATCC 31749 | 360 h, 30 °C | ND | 0.85 | [20] |
| Condensed corn distillers’ solubles | ATCC 31749 | 120 h, 30 °C | 7.7 | ND | [22] |
| Corn maltose syrup | ATCC 31749 | 120 h, 30 °C | 4.9 | ND | [23] |
| Corn maltose syrup | ECP-1 | 120 h, 30 °C | 7.4 | ND | [23] |
| Cassava starch waste | ATCC 31749 | 96 h, 30 °C | 21.2 | ND | [24] |
| Sugar cane molasses | ATCC 31750 | 120 h, 30 °C | 42.0 | 0.35 | [25] |
| Condensed corn distillers’ solubles | ECP-1 | 120 h, 30 °C | 5.4 | ND | [44] |
| Asparagus spear juice | ATCC 31749 | 168 h, 30 °C | 40.2 | 0.56 | [45] |
| Wheat bran | ATCC 31749 | 144 h, 30 °C | 47.9 | 0.60 | [46] |
| Prairie cordgrass | ATCC 31749 | 144 h, 30 °C | 9.9 | 0.01 | [47] |
| Prairie cordgrass | ECP-1 | 144 h, 30 °C | 18.0 | 0.01 | [48] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
West, T.P. Production of the Polysaccharide Curdlan by Agrobacterium species on Processing Coproducts and Plant Lignocellulosic Hydrolysates. Fermentation 2020, 6, 16. https://doi.org/10.3390/fermentation6010016
West TP. Production of the Polysaccharide Curdlan by Agrobacterium species on Processing Coproducts and Plant Lignocellulosic Hydrolysates. Fermentation. 2020; 6(1):16. https://doi.org/10.3390/fermentation6010016
Chicago/Turabian StyleWest, Thomas P. 2020. "Production of the Polysaccharide Curdlan by Agrobacterium species on Processing Coproducts and Plant Lignocellulosic Hydrolysates" Fermentation 6, no. 1: 16. https://doi.org/10.3390/fermentation6010016
APA StyleWest, T. P. (2020). Production of the Polysaccharide Curdlan by Agrobacterium species on Processing Coproducts and Plant Lignocellulosic Hydrolysates. Fermentation, 6(1), 16. https://doi.org/10.3390/fermentation6010016




