Modifications of Phenolic Compounds, Biogenic Amines, and Volatile Compounds in Cabernet Gernishct Wine through Malolactic Fermentation by Lactobacillus plantarum and Oenococcus oeni
Abstract
1. Introduction
2. Materials and Methods
2.1. Alcoholic Fermentation, Malolactic Fermentation, and Chemicals
2.2. Analysis of Phenolic Compounds
2.3. Analysis of Biogenic Amines
2.4. Analysis of Volatile Compounds
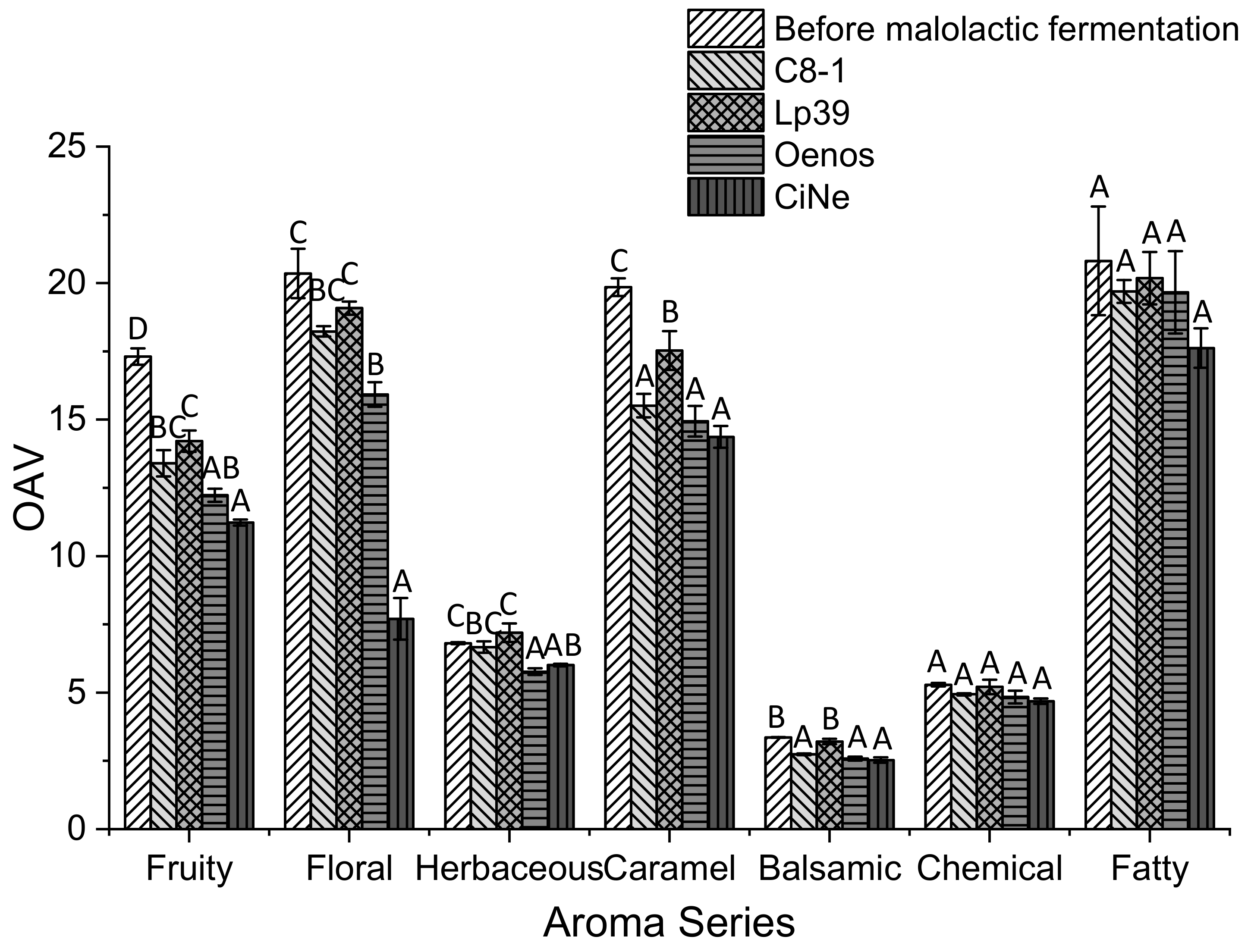
2.5. Odor Activity Value and Aroma Profile
2.6. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Compounds
3.2. Biogenic Amines
3.3. Volatile Compounds
3.3.1. Esters
3.3.2. Higher Alcohols
3.3.3. Aldehydes/Ketones
3.3.4. Fatty Acids
3.3.5. Terpene Derivatives
3.3.6. Others
3.4. Aroma Profile
3.5. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gil-Sánchez, I.; Bartolomé Suáldea, B.; Victoria Moreno-Arribas, M. Chapter 6—Malolactic Fermentation. In Red Wine Technology; Morata, A., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 85–98. [Google Scholar] [CrossRef]
- Pérez-Martín, F.; Izquierdo-Cañas, P.M.; Seseña, S.; García-Romero, E.; Palop, M.L. Aromatic compounds released from natural precursors by selected Oenococcus oeni strains during malolactic fermentation. Eur. Food Res. Technol. 2015, 240, 609–618. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.A.; G-Alegría, E.; Polo, M.C.; Tenorio, C.; Martín-Alvarez, P.J.; Calvo De La Banda, M.T.; Ruiz-Larrea, F.; Moreno-Arribas, M.V. Wine volatile and amino acid composition after malolactic fermentation: Effect of Oenococcus oeni and Lactobacillus plantarum starter cultures. J. Agric. Food Chem. 2005, 53, 8729–8735. [Google Scholar] [CrossRef] [PubMed]
- Boido, E.; Lloret, A.; Medina, K.; Carrau, F.; Dellacassa, E. Effect of β-Glycosidase Activity of Oenococcus oeni on the Glycosylated Flavor Precursors of Tannat Wine during Malolactic Fermentation. J. Agric. Food Chem. 2002, 50, 2344–2349. [Google Scholar] [CrossRef] [PubMed]
- Valdés La Hens, D.; Bravo-Ferrada, B.M.; Delfederico, L.; Caballero, A.C.; Semorile, L.C. Prevalence of Lactobacillus plantarum and Oenococcus oeni during spontaneous malolactic fermentation in Patagonian red wines revealed by polymerase chain reaction-denaturing gradient gel electrophoresis with two targeted genes. Aust. J. Grape Wine Res. 2015, 21, 49–56. [Google Scholar] [CrossRef]
- Pramateftaki, P.V.; Metafa, M.; Kallithraka, S.; Lanaridis, P. Evolution of malolactic bacteria and biogenic amines during spontaneous malolactic fermentations in a Greek winery. Lett. Appl. Microbiol. 2006, 43, 155–160. [Google Scholar] [CrossRef] [PubMed]
- López, I.; Núñez, R.L.; Lozano, C.T.; Larrea, F.R. Performance of malolactic fermentation by inoculation of selected Lactobacillus plantarum and Oenococcus oeni strains isolated from Rioja red wines. VITIS 2008, 47, 123–129. [Google Scholar]
- Restuccia, D.; Loizzo, M.R.; Spizzirri, U.G. Accumulation of Biogenic Amines in Wine: Role of Alcoholic and Malolactic Fermentation. Fermentation 2018, 4, 6. [Google Scholar] [CrossRef]
- Iorizzo, M.; Testa, B.; Lombardi, S.J.; García-Ruiz, A.; Muñoz-González, C.; Bartolomé, B.; Moreno-Arribas, M.V. Selection and technological potential of Lactobacillus plantarum bacteria suitable for wine malolactic fermentation and grape aroma release. LWT 2016, 73, 557–566. [Google Scholar] [CrossRef]
- Hernandez, T.; Estrella, I.; Perez-Gordo, M.; Alegria, E.G.; Tenorio, C.; Ruiz-Larrrea, F.; Moreno-Arribas, M.V. Contribution of malolactic fermentation by Oenococcus oeni and Lactobacillus plantarum to the changes in the nonanthocyanin polyphenolic composition of red wine. J. Agric. Food Chem. 2007, 55, 5260–5266. [Google Scholar] [CrossRef]
- Lerm, E.; Engelbrecht, L.; Toit, M.D. Selection and characterisation of Oenococcus oeni and Lactobacillus plantarum South African wine isolates for use as malolactic fermentation starter cultures. S. Afr. J. Enol. Vitic. 2011, 32, 280–295. [Google Scholar] [CrossRef]
- Capozzi, V.; Russo, P.; Ladero, V.; Fiocco, D.; Alvarez, M.A.; Grieco, F.; Spano, G. Biogenic Amines Degradation by Lactobacillus plantarum: Toward a Potential Application in Wine. Front. Microbiol. 2012, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, N.S.; Bravo-Ferrada, B.M.; Pozo-Bayón, M.Á.; Semorile, L.; Elizabeth Tymczyszyn, E. Changes in the volatile profile of Pinot noir wines caused by Patagonian Lactobacillus plantarum and Oenococcus oeni strains. Food Res. Int. 2018, 106, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, N.; Tymczyszyn, E.E.; Semorile, L.C.; La Hens, D.V.; Delfederico, L.; Hollmann, A.; Bravo-Ferrada, B. Lactobacillus plantarum as a malolactic starter culture in winemaking: A new (old) player? Electron. J. Biotechnol. 2018, 38, 10–18. [Google Scholar] [CrossRef]
- Du Toit, M.; Engelbrecht, L.; Lerm, E.; Krieger-Weber, S. Lactobacillus: The Next Generation of Malolactic Fermentation Starter Cultures—An Overview. Food Bioprocess. Technol. 2011, 4, 876–906. [Google Scholar] [CrossRef]
- Spano, G.; Beneduce, L.; Tarantino, D.; Zapparoli, G.; Massa, S. Characterization of Lactobacillus plantarum from wine must by PCR species–specific and RAPD–PCR. Lett. Appl. Microbiol. 2002, 35, 370–374. [Google Scholar] [CrossRef]
- Grimaldi, A.; Bartowsky, E.; Jiranek, V. Screening of Lactobacillus spp. and Pediococcus spp. for glycosidase activities that are important in oenology. J. Appl. Microbiol. 2005, 99, 1061–1069. [Google Scholar] [CrossRef]
- Grimaldi, A.; Bartowsky, E.; Jiranek, V. A survey of glycosidase activities of commercial wine strains of Oenococcus oeni. Int. J. Food Microbiol. 2005, 105, 233–244. [Google Scholar] [CrossRef]
- Matthews, A.; Grimaldi, A.; Walker, M.; Bartowsky, E.; Grbin, P.; Jiranek, V. Lactic Acid Bacteria as a Potential Source of Enzymes for Use in Vinification. Appl. Environ. Microbiol. 2004, 70, 5715–5731. [Google Scholar] [CrossRef]
- Liu, S.-Q. Malolactic fermentation in wine – beyond deacidification. J. Appl. Microbiol. 2002, 92, 589–601. [Google Scholar] [CrossRef]
- Li, S.; He, F.; Zhu, B.; Wang, J.; Duan, C. Comparison of phenolic and chromatic characteristics of dry red wines made from native Chinese grape species and Vitis vinifera. Int. J. Food Prop. 2016, 20, 2134–2146. [Google Scholar] [CrossRef]
- He, F.; Liang, N.; Mu, L.; Pan, Q.; Wang, J.; Reeves, M.J.; Duan, C. Anthocyanins and their variation in red wines I. Monomeric anthocyanins and their color expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef] [PubMed]
- Vivas, N.; Augustin, M.; Lonvaud-Funel, A. Influence of oak wood and grape tannins on the lactic acid bacterium Œenococcus oeni (Leuconostoc oenos, 8413). J. Sci. Food Agric. 2000, 80, 1675–1678. [Google Scholar] [CrossRef]
- Campos, F.M.; Couto, J.A.; Hogg, T.A. Influence of phenolic acids on growth and inactivation of Oenococcus oeni and Lactobacillus hilgardii. J. Appl. Microbiol. 2003, 94, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Lombardi, S.J.; Renzo, T.D.; Testa, B.; Coppola, R.; Sorrentino, E. Effect of phenolic compounds on the growth and L-malic acid metabolism of Oenococcus oeni. J. Biotechnol. 2012, 6, 1225–1231. [Google Scholar] [CrossRef]
- García-Ruiz, A.; Bartolomé, B.; Martínez-Rodríguez, A.J.; Pueyo, E.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V. Potential of phenolic compounds for controlling lactic acid bacteria growth in wine. Food Control 2008, 19, 835–841. [Google Scholar] [CrossRef]
- Devi, A.; Anu-Appaiah, K.A. Diverse physiological and metabolic adaptations by Lactobacillus plantarum and Oenococcus oeni in response to the phenolic stress during wine fermentation. Food Chem. 2018, 268, 101–109. [Google Scholar] [CrossRef]
- Siebert, T.E.; Smyth, H.E.; Capone, D.L.; Neuwöhner, C.; Pardon, K.H.; Skouroumounis, G.K.; Herderich, M.J.; Sefton, M.A.; Pollnitz, A.P. Stable isotope dilution analysis of wine fermentation products by HS-SPME-GC-MS. Anal. Bioanal. Chem. 2005, 381, 937–947. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Pretorius, I.S. Yeast Modulation of Wine Flavor. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2005; Volume 57, pp. 131–175. [Google Scholar] [CrossRef]
- López, R.; López-Alfaro, I.; Gutiérrez, A.R.; Tenorio, C.; Garijo, P.; González-Arenzana, L.; Santamaría, P. Malolactic fermentation of Tempranillo wine: Contribution of the lactic acid bacteria inoculation to sensory quality and chemical composition. Int. J. Food Sci. Technol. 2011, 46, 2373–2381. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Borneman, A.R. Genomic variations of Oenococcus oeni strains and the potential to impact on malolactic fermentation and aroma compounds in wine. Appl. Microbiol. Biotechnol. 2011, 92, 441–447. [Google Scholar] [CrossRef]
- Antalick, G.; Perello, M.-C.; De Revel, G. Characterization of Fruity Aroma Modifications in Red Wines during Malolactic Fermentation. J. Agric. Food Chem. 2012, 60, 12371–12383. [Google Scholar] [CrossRef]
- Bravo-Ferrada, B.M.; Hollmann, A.; Brizuela, N.; La Hens, D.V.; Tymczyszyn, E.; Semorile, L. Growth and consumption of l-malic acid in wine-like medium by acclimated and non-acclimated cultures of Patagonian Oenococcus oeni strains. Folia Microbiol. 2016, 61, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Knoll, C.; Fritsch, S.; Schnell, S.; Grossmann, M.; Krieger-Weber, S.; Du Toit, M.; Rauhut, D. Impact of different malolactic fermentation inoculation scenarios on Riesling wine aroma. World J. Microbiol. Biotechnol. 2012, 28, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, N.S.; Bravo-Ferrada, B.M.; Curilén, Y.; Delfederico, L.; Caballero, A.; Semorile, L.; Pozo-Bayón, M.Á.; Tymczyszyn, E.E. Advantages of Using Blend Cultures of Native L. plantarum and O. oeni Strains to Induce Malolactic Fermentation of Patagonian Malbec Wine. Front. Microbiol. 2018, 9, 2109. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Y.; Yang, Y.P.; Peng, Q.; Han, Y. Biogenic amines in wine: A review. Int. J. Food Sci. Technol. 2015, 50, 1523–1532. [Google Scholar] [CrossRef]
- Izquierdo Cañas, P.M.; Gómez Alonso, S.; Ruiz Pérez, P.; Seseña Prieto, S.; García Romero, E.; Palop Herreros, M.L. Biogenic Amine Production by Oenococcus oeni Isolates from Malolactic Fermentation of Tempranillo Wine. J. Food Prot. 2009, 72, 907–910. [Google Scholar] [CrossRef]
- Guerrini, S.; Mangani, S.; Granchi, L.; Vincenzini, M. Biogenic Amine Production by Oenococcus oeni. Curr. Microbiol. 2002, 44, 374–378. [Google Scholar] [CrossRef]
- Cinquanta, L.; De Stefano, G.; Formato, D.; Niro, S.; Panfili, G. Effect of pH on malolactic fermentation in southern Italian wines. Eur. Food Res. Technol. 2018, 244, 1261–1268. [Google Scholar] [CrossRef]
- Landete, J.M.; Ferrer, S.; Pardo, I. Biogenic amine production by lactic acid bacteria, acetic bacteria and yeast isolated from wine. Food Control 2007, 18, 1569–1574. [Google Scholar] [CrossRef]
- Landete, J.M.; Ferrer, S.; Pardo, I. Which lactic acid bacteria are responsible for histamine production in wine? J. Appl. Microbiol. 2005, 99, 580–586. [Google Scholar] [CrossRef]
- Sun, S.Y.; Chen, Z.X.; Jin, C.W. Combined influence of lactic acid bacteria starter and final pH on the induction of malolactic fermentation and quality of cherry wines. LWT 2018, 89, 449–456. [Google Scholar] [CrossRef]
- Wang, S.; Li, S.; Zhao, H.; Gu, P.; Chen, Y.; Zhang, B.; Zhu, B. Acetaldehyde released by Lactobacillus plantarum enhances accumulation of pyranoanthocyanins in wine during malolactic fermentation. Food Res. Int. 2018, 108, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Alonso, S.; Hermosín-Gutiérrez, I.; García-Romero, E. Simultaneous HPLC Analysis of Biogenic Amines, Amino Acids, and Ammonium Ion as Aminoenone Derivatives in Wine and Beer Samples. J. Agric. Food Chem. 2007, 55, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhu, B.; Wang, Y.; Lu, L.; Lan, Y.; Reeves, M.J.; Duan, C. Influence of pre-fermentation cold maceration treatment on aroma compounds of Cabernet Sauvignon wines fermented in different industrial scale fermenters. Food Chem. 2014, 154, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Nemanic, J.; Vanzo, A.; Vrhovsek, U.J.V.J.O.G.R. Effect of red wine maceration techniques on oligomeric and polymeric proanthocyanidins in wine, cv. Blaufränkisch. VITIS 2002, 41, 47–51. [Google Scholar]
- Jiménez, N.; Curiel, J.A.; Reverón, I.; De Las Rivas, B.; Muñoz, R. Uncovering the Lactobacillus plantarum WCFS1 Gallate Decarboxylase Involved in Tannin Degradation. Appl. Environ. Microbiol. 2013, 79, 4253–4263. [Google Scholar] [CrossRef]
- Reverón, I.; Jiménez, N.; Curiel, J.A.; Peñas, E.; López De Felipe, F.; De Las Rivas, B.; Muñoz, R. Differential Gene Expression by Lactobacillus plantarum WCFS1 in Response to Phenolic Compounds Reveals New Genes Involved in Tannin Degradation. Appl. Environ. Microbiol. 2017, 83, e03387-16. [Google Scholar] [CrossRef]
- Silva, I.; Campos, F.M.; Hogg, T.; Couto, J.A. Factors influencing the production of volatile phenols by wine lactic acid bacteria. Int. J. Food Microbiol. 2011, 145, 471–475. [Google Scholar] [CrossRef]
- Vaquero, I.; Marcobal, Á.; Muñoz, R. Tannase activity by lactic acid bacteria isolated from grape must and wine. Int. J. Food Microbiol. 2004, 96, 199–204. [Google Scholar] [CrossRef]
- María, E.T.; José María, L.; Inés, R.; Laura, S.; Blanca, D.L.R.; Rosario, M.O. A Lactobacillus plantarum esterase active on a broad range of phenolic esters. Appl. Environ. Microbiol. 2015, 81, 3235–3242. [Google Scholar] [CrossRef]
- Arena, M.E.; Manca De Nadra, M.C. Biogenic amine production by Lactobacillus. J. Appl. Microbiol. 2001, 90, 158–162. [Google Scholar] [CrossRef]
- Saerens, S.M.; Delvaux, F.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters affecting ethyl ester production by Saccharomyces cerevisiae during fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Li, T.; Yang, H.; Ren, J.; Zhang, B.; Zhu, B. Dibasic Ammonium Phosphate Application Enhances Aromatic Compound Concentration in Bog Bilberry Syrup Wine. Molecules 2017, 22, 52. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Duan, S.; Zhao, L.; Gao, Z.; Luo, M.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, S. Aroma characterization based on aromatic series analysis in table grapes. Sci Rep. 2016, 6, 31116. [Google Scholar] [CrossRef] [PubMed]
- Narain, N.; Nigam, N.; De sousa galvão, M. CHAPTER 20—Passion Fruit. In Handbook of Fruit and Vegetable Flavors; Hui, Y.H., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 345–389. [Google Scholar] [CrossRef]
- Noble, A.C.; Arnold, R.A.; Buechsenstein, J.; Leach, E.J.; Schmidt, J.O.; Stern, P.M. Modification of a Standardized System of Wine Aroma Terminology. Am. J. Enol. Vitic. 1991, 38, 143–146. [Google Scholar]
- Leffingwell & Associates. Odor & Flavor Detection Thresholds in Water (In Parts per Billion). Available online: http://www.leffingwell.com/odorthre.htm (accessed on 21 September 2019).
- The Good Scents Company Information System. Available online: http://www.thegoodscentscompany.com (accessed on 21 September 2019).
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Sonni, F.; Moore, E.G.; Chinnici, F.; Riponi, C.; Smyth, H.E. Characterisation of Australian Verdelho wines from the Queensland Granite Belt region. Food Chem. 2016, 196, 1163–1171. [Google Scholar] [CrossRef]
- Juan, F.S.; Cacho, J.; Ferreira, V.; Escudero, A. Aroma Chemical Composition of Red Wines from Different Price Categories and Its Relationship to Quality. J. Agric. Food Chem. 2012, 60, 5045–5056. [Google Scholar] [CrossRef]
- Christensen, L.P.; Edelenbos, M.; Kreutzmann, S. Fruits and Vegetables of Moderate Climate. In Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Berger, R.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 135–187. [Google Scholar]
- Sabon, I.; De Revel, G.; Kotseridis, Y.; Bertrand, A. Determination of Volatile Compounds in Grenache Wines in Relation with Different Terroirs in the Rhone Valley. J. Agric. Food Chem. 2002, 50, 6341–6345. [Google Scholar] [CrossRef]
- Sánchez-Palomo, E.; Gómez García-Carpintero, E.; Alonso-Villegas, R.; González-Viñas, M.A. Characterization of aroma compounds of Verdejo white wines from the La Mancha region by odour activity values. Flavour Fragr. J. 2010, 25, 456–462. [Google Scholar] [CrossRef]
- Costello, P.J.; Siebert, T.E.; Solomon, M.R.; Bartowsky, E.J. Synthesis of fruity ethyl esters by acyl coenzyme A: Alcohol acyltransferase and reverse esterase activities in Oenococcus oeni and Lactobacillus plantarum. J. Appl. Microbiol. 2013, 114, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Gammacurta, M.; Lytra, G.; Marchal, A.; Marchand, S.; Christophe Barbe, J.; Moine, V.; De Revel, G. Influence of lactic acid bacteria strains on ester concentrations in red wines: Specific impact on branched hydroxylated compounds. Food Chem. 2018, 239, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Delaquis, P.; Cliff, M.; King, M.; Girard, B.; Hall, J.; Reynolds, A. Effect of Two Commercial Malolactic Cultures on the Chemical and Sensory Properties of Chancellor Wines Vinified with Different Yeasts and Fermentation Temperatures. Am. J. Enol. Vitic. 2000, 51, 42–48. [Google Scholar]
- Maicas, S.; Gil, J.-V.; Pardo, I.; Ferrer, S. Improvement of volatile composition of wines by controlled addition of malolactic bacteria. Food Res. Int. 1999, 32, 491–496. [Google Scholar] [CrossRef]
- Gámbaro, A.; Boido, E.; Zlotejablko, A.; Medina, K.; Lloret, A.; Dellacassa, E.; Carrau, F. Effect of malolactic fermentation on the aroma properties of Tannat wine. Aust. J. Grape Wine Res. 2001, 7, 27–32. [Google Scholar] [CrossRef]
- Ugliano, M.; Henschke, P.A. Yeasts and Wine Flavour. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 313–392. [Google Scholar] [CrossRef]
- Bakker, J.; Clarke, R.J. Wine Flavour Chemistry, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Miranda-López, R.; Libbey, L.M.; Watson, B.T.; Mcdaniel, M.R. Identification of Additional Odor-Active Compounds in Pinot noir Wines. Am. J. Enol. Vitic. 1992, 43, 90–92. [Google Scholar]
- Ugliano, M.; Moio, L. Changes in the Concentration of Yeast-Derived Volatile Compounds of Red Wine during Malolactic Fermentation with Four Commercial Starter Cultures of Oenococcus oeni. J. Agric. Food Chem. 2005, 53, 10134–10139. [Google Scholar] [CrossRef]
- Knoll, C.; Fritsch, S.; Schnell, S.; Grossmann, M.; Rauhut, D.; Du Toit, M. Influence of pH and ethanol on malolactic fermentation and volatile aroma compound composition in white wines. LWT 2011, 44, 2077–2086. [Google Scholar] [CrossRef]
- Sun, S.; Gong, H.; Liu, W.; Jin, C. Application and validation of autochthonous Lactobacillus plantarum starter cultures for controlled malolactic fermentation and its influence on the aromatic profile of cherry wines. Food Microbiol. 2016, 55, 16–24. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Oliveira, P.; Baumes, R.L.; Maia, O. Changes in aromatic characteristics of Loureiro and Alvarinho wines during maturation. J Food Compos. Anal. 2008, 21, 695–707. [Google Scholar] [CrossRef]
- Silva Ferreira, A.C.; Guedes De Pinho, P. Nor-isoprenoids profile during port wine ageing—Influence of some technological parameters. Anal. Chim. Acta 2004, 513, 169–176. [Google Scholar] [CrossRef]
- Brizuela, N.S.; Bravo-Ferrada, B.M.; La Hens, D.V.; Hollmann, A.; Delfederico, L.; Caballero, A.; Tymczyszyn, E.E.; Semorile, L. Comparative vinification assays with selected Patagonian strains of Oenococcus oeni and Lactobacillus plantarum. LWT 2017, 77, 348–355. [Google Scholar] [CrossRef]
- Mansfield, A.K.; Zoecklein, B.W.; Whiton, R.S. Quantification of Glycosidase Activity in Selected Strains of Brettanomyces bruxellensis and Oenococcus oeni. Am. J. Enol. Vitic. 2002, 53, 303–307. [Google Scholar]
- Grimaldi, A.; Mclean, H.; Jiranek, V. Identification and Partial Characterization of Glycosidic Activities of Commercial Strains of the Lactic Acid Bacterium, Oenococcus oeni. Am. J. Enol. Vitic. 2000, 51, 362–369. [Google Scholar]
- Ugliano, M.; Genovese, A.; Moio, L. Hydrolysis of Wine Aroma Precursors during Malolactic Fermentation with Four Commercial Starter Cultures of Oenococcus oeni. J. Agric. Food Chem. 2003, 51, 5073–5078. [Google Scholar] [CrossRef]
- Cavin, J.F.; Andioc, V.; Etievant, P.X.; Divies, C. Ability of Wine Lactic Acid Bacteria to Metabolize Phenol Carboxylic Acids. Am. J. Enol. Vitic. 1993, 44, 76–80. [Google Scholar]
- Chatonnet, P.; Dubourdieu, D.; Boidron, J.N. The Influence of Brettanomyces/Dekkera sp. Yeasts and Lactic Acid Bacteria on the Ethylphenol Content of Red Wines. Am. J. Enol. Vitic. 1995, 46, 463–468. [Google Scholar]
- Van Beek, S.; Priest, F.G. Decarboxylation of Substituted Cinnamic Acids by Lactic Acid Bacteria Isolated during Malt Whisky Fermentation. Appl. Environ. Microbiol. 2000, 66, 5322–5328. [Google Scholar] [CrossRef]

| Phenolic Compounds 1 | Before Malolactic Fermentation | L. plantarum C8-1 | L. plantarum Lp39 | O. oeni Oenos | O. oeni CiNe |
|---|---|---|---|---|---|
| Procyanin B1 | 17.28 ± 0.33 B | 16.64 ± 0.39 AB | 15.69 ± 0.70 A | 16.53 ± 0.52 AB | 15.80 ± 0.37 A |
| Procyanin B2 | 4.72 ± 0.06 B | 5.09 ± 0.10 C | 4.49 ± 0.22 AB | 4.20 ± 0.15 A | 4.34 ± 0.08 A |
| Procyanin C1 | 2.74 ± 0.09 B | 2.77 ± 0.41 B | 2.02 ± 0.05 A | 2.67 ± 0.18 AB | 2.91 ± 0.34 B |
| Catechin | 20.71 ± 0.46 B | 18.20 ± 0.03 A | 16.71 ± 0.85 A | 18.91 ± 1.74 AB | 18.20 ± 0.18 A |
| Epicatechin | 34.92 ± 0.09 C | 27.71 ± 0.14 AB | 25.13 ± 1.03 A | 28.44 ± 1.93 B | 27.94 ± 0.03 B |
| Epigallocatechin | 0.52 ± 0.01 B | 0.47 ± 0.02 A | 0.44 ± 0.02 A | 0.56 ± 0.01 B | 0.45 ± 0.01 A |
| Gallocatechin | 8.80 ± 0.09 A | 8.87 ± 0.20 A | 7.98 ± 0.66 A | 9.13 ± 0.68 A | 8.77 ± 0.16 A |
| Total flavanols | 89.70 ± 0.21 C | 79.75 ± 0.19 AB | 72.47 ± 3.53 A | 80.43 ± 5.18 B | 78.41 ± 1.16 AB |
| Myricetin | 0.32 ± 0.01 B | 0.23 ± 0.01 A | 0.25 ± 0.02 A | 0.26 ± 0.02 A | 0.25 ± 0.00 A |
| Myricetin-glucoside | 2.78 ± 0.03 C | 2.41 ± 0.03 AB | 2.29 ± 0.11 A | 2.52 ± 0.09 B | 2.40 ± 0.03 AB |
| Quercetin-galactoside | 0.50 ± 0.00 B | 0.44 ± 0.01 A | 0.42 ± 0.02 A | 0.43 ± 0.00 A | 0.44 ± 0.02 A |
| Quercetin-glucuronide | 0.42 ± 0.03 B | 0.27 ± 0.01 AB | 0.16 ± 0.07 A | 0.23 ± 0.10 A | 0.24 ± 0.02 A |
| Dihydroquercetin | 0.12 ± 0.00 AB | 0.06 ± 0.04 A | 0.06 ± 0.02 AB | 0.12 ± 0.03 B | 0.08 ± 0.00 AB |
| Syringetin-glucoside | 0.94 ± 0.01 C | 0.86 ± 0.00 B | 0.81 ± 0.01 A | 0.80 ± 0.03 A | 0.80 ± 0.00 A |
| Total flavonols | 5.08 ± 0.06 B | 4.27 ± 0.09 A | 4.00 ± 0.23 A | 4.35 ± 0.27 A | 4.21 ± 0.08 A |
| Gallic acid | 0.68 ± 0.42 A | 3.85 ± 0.17 B | 6.65 ± 0.17 D | 6.17 ± 0.03 D | 5.31 ± 0.40 C |
| Protocatechuic acid | 0.26 ± 0.02 B | 0.08 ± 0.01 A | 0.42 ± 0.03 E | 0.38 ± 0.01 D | 0.33 ± 0.01 C |
| Caffeic acid | tr | tr | 4.08 ± 0.41 A | 8.97 ± 0.40 C | 7.56 ± 0.26 B |
| 4-Hydroxycinnamic acid | tr | tr | 0.59 ± 0.04 A | 1.13 ± 0.09 B | 1.05 ± 0.01 B |
| Total phenolic acids | 0.94 ± 0.41 A | 3.93 ± 0.17 B | 11.74 ± 0.65 C | 16.64 ± 0.46 E | 14.24 ± 0.68 D |
| Total phenolic compounds | 95.72±0.68 AB | 87.95 ± 0.11 A | 88.20 ± 4.41 A | 101.42 ± 5.92 B | 96.86 ± 0.40 AB |
| Biogenic Amines 1 | Before Malolactic Fermentation | L. plantarum C8-1 | L. plantarum Lp39 | O. oeni Oenos | O. oeni CiNe |
|---|---|---|---|---|---|
| Histamine | 5.06 ± 0.03 A | 4.87 ± 0.16 A | 6.26 ± 0.17 B | 8.20 ± 0.28 C | 8.04 ± 0.35 C |
| Spermine | 0.05 ± 0.00 A | 0.07 ± 0.00 B | 0.07 ± 0.01 B | 0.07 ± 0.00 B | 0.07 ± 0.00 B |
| Spermidine | 0.89 ± 0.10 AB | 0.80 ± 0.05 A | 0.96 ± 0.01 B | 0.85 ± 0.00 AB | 0.93 ± 0.03 AB |
| Tyramine | 0.05 ± 0.00 B | 0.02 ± 0.01 A | 0.05 ± 0.00 B | 0.02 ± 0.01 A | 0.02 ± 0.01 A |
| Putrescine | 1.10 ± 0.02 C | 1.05 ± 0.02 BC | 1.07 ± 0.02 C | 0.99 ± 0.01 B | 0.91 ± 0.05 A |
| Cadaverine | 0.02 ± 0.00 C | 0.01 ± 0.00 A | 0.01 ± 0.00 AB | 0.01 ± 0.00 BC | 0.01 ± 0.00 A |
| Phenethylamine | 0.13 ± 0.03 A | 0.12 ± 0.00 A | 0.11 ± 0.02 A | 0.12 ± 0.00 A | 0.11 ± 0.00 A |
| Total biogenic amines | 7.29 ± 0.11 A | 6.94 ± 0.21 A | 8.54 ± 0.23 B | 10.28 ± 0.28 C | 10.08 ± 0.33 C |
| Code | RI 1, 2, 3 | Before Malolactic Fermentation | L. plantarum C8-1 | L. plantarum Lp39 | O. oeni Oenos | O. oeni CiNe | |
|---|---|---|---|---|---|---|---|
| Esters | |||||||
| Acetate esters | |||||||
| E1 | Ethyl acetate a (mg/L) | 801.9 | 41.31 ± 0.15 B | 33.66 ± 0.39 A | 39.44 ± 1.24 B | 31.82 ± 0.87 A | 31.09 ± 1.23 A |
| E2 | Isoamyl acetate a | 1120.3 | 801.25 ± 3.71 C | 339.49 ± 38.62 B | 359.04 ± 14.14 B | 287.38 ± 10.61 AB | 201.10 ± 36.87 A |
| E3 | Phenethyl acetate a | 1802.9 | 2.70 ± 0.09 B | 7.14 ± 0.23 C | 2.25 ± 0.04 A | 2.24 ± 0.03 A | 2.22 ± 0.03 A |
| Total acetate esters (mg/L) | 42.12 ± 0.15 B | 34.01 ± 0.43 A | 39.80 ± 1.25 B | 32.11 ± 0.88 A | 31.30 ± 1.27 A | ||
| Ethyl esters | |||||||
| E4 | Ethyl butanoate a | 1019.5 | 646.55 ± 28.58 C | 303.41 ± 8.72 AB | 364.32 ± 25.93 B | 242.74 ± 39.73 A | 217.36 ± 22.17 A |
| E5 | Ethyl hexanoate a | 1229.2 | 543.51 ± 0.41 C | 263.05 ± 37.13 AB | 343.08 ± 16.92 B | 255.41 ± 12.59 AB | 208.76 ± 60.22 A |
| E6 | Ethyl heptanoate a | 1329 | 1.42 ± 0.01 B | 1.04 ± 0.21 AB | 1.12 ± 0.09 AB | 1.19 ± 0.07 AB | 0.64 ± 0.32 A |
| E7 | Ethyl lactate a (mg/L) | 1336.2 | 4.96 ± 0.20 A | 116.95 ± 3.30 C | 71.12 ± 3.39 B | 66.22 ± 5.32 B | 64.70 ± 2.36 B |
| E8 | 2-Hydroxyisovaleric acid ethyl ester b (mg/L) | 1420 | tr | 2.80 ± 0.05 | tr | tr | tr |
| E9 | Ethyl octanoate a | 1429.9 | 33.21 ± 0.20 C | 31.05 ± 0.99 BC | 32.94 ± 0.34 C | 27.17 ± 0.02 B | 21.37 ± 2.55 A |
| E10 | Ethyl nonanoate a | 1531.3 | 0.78 ± 0.00 A | 1.24 ± 0.06 BC | 1.18 ± 0.04 ABC | 1.44 ± 0.17 C | 0.80 ± 0.16 AB |
| E11 | Ethyl decanoate b | 1630.7 | 36.48 ± 0.46 B | 34.26 ± 0.08 B | 35.71 ± 0.30 B | 34.36 ± 0.88 B | 30.32 ± 1.41 A |
| E12 | Ethyl benzoate b | 1663.1 | 11.81 ± 1.73 C | 6.51 ± 0.30 A | 8.08 ± 0.11 AB | 8.57 ± 0.21 ABC | 11.15 ± 0.47 BC |
| E13 | Diethyl succinate a | 1667.5 | 124.19 ± 15.40 A | 263.66 ± 14.01 B | 226.67 ± 0.77 B | 137.73 ± 9.62 A | 130.25 ± 9.63 A |
| E14 | Ethyl dodecanoate a | 1826.7 | 29.45 ± 0.18 A | 29.21 ± 0.08 A | 29.52 ± 0.14 A | 29.85 ± 0.82 A | 29.19 ± 0.16 A |
| E15 | Ethyl dihydrocinnamate b | 1867 | 2.25 ± 0.09 B | 1.66 ± 0.10 A | 1.76 ± 0.12 A | 1.75 ± 0.01 A | 1.54 ± 0.06 A |
| E16 | Ethyl isopentyl succinate b | 1878 | 14.95 ± 1.89 B | 16.39 ± 1.98 B | 15.27 ± 2.00 B | 5.47 ± 1.88 A | 4.39 ± 1.73 A |
| E17 | Ethyl cinnamate b | 2079.3 | 54.32 ± 14.90 B | 29.63 ± 2.42 AB | 25.12 ± 0.70 A | 22.56 ± 1.17 A | 20.70 ± 0.31 A |
| E18 | Ethyl hexadecanoate b | 2179 | 29.33 ± 0.06 A | 29.24 ± 0.09 A | 29.29 ± 0.02 A | 29.35 ± 0.10 A | 29.16 ± 0.02 A |
| Total ethyl esters (mg/L) | 6.49 ± 0.27 A | 120.76 ± 3.29 C | 72.23 ± 3.38 B | 67.02 ± 5.30 B | 65.40 ± 2.28 B | ||
| Other esters | |||||||
| E19 | Isopentyl isopentanoate b | 1290 | 3.67 ± 0.01 | tr | tr | tr | tr |
| E20 | Methyl octanoate a | 1384.1 | 1.57 ± 0.13 C | 0.93 ± 0.08 B | 1.05 ± 0.03 B | 0.71 ± 0.01 AB | 0.43 ± 0.15 A |
| E21 | Isoamyl hexanoate a | 1454.4 | 3.98 ± 0.06 B | 3.89 ± 0.03 B | 3.91 ± 0.05 B | 3.54 ± 0.02 A | 3.53 ± 0.08 A |
| E22 | Isoamyl lactate a | 1562.8 | 194.55 ± 4.00 A | 653.64 ± 99.40 C | 422.7 ± 10.10 B | 400.48 ± 5.28 B | 378.39 ± 21.03 B |
| E23 | Isoamyl octanoate b | 1650.8 | 111.54 ± 40.15 AB | 136.50 ± 20.67 B | 127.35 ± 2.89 AB | 93.77 ± 5.37 AB | 43.26 ± 14.53 A |
| E24 | Methyl salicylate a | 1769.4 | 21.19 ± 1.21 A | 20.03 ± 0.94 A | 36.88 ± 2.18 B | 31.75 ± 1.20 B | 34.92 ± 0.72 B |
| Total other esters | 336.49 ± 45.19 A | 814.99 ± 120.97 C | 591.90 ± 15.25 BC | 530.25 ± 1.07 AB | 460.53 ± 5.71 AB | ||
| Total esters (mg/L) | 48.95 ± 0.16 A | 155.58 ± 3.59 D | 112.63 ± 4.64 C | 99.66 ± 6.18 BC | 97.16 ± 1.02 B | ||
| Higher alcohols | |||||||
| H1 | Isobutanol b (mg/L) | 1089.1 | 19.56 ± 0.49 B | 18.97 ± 0.49 AB | 19.67 ± 0.74 B | 16.87 ± 0.69 A | 18.48 ± 0.62 AB |
| H2 | Isoamyl alcohol a (mg/L) | 1200.1 | 274.86 ± 3.39 A | 265.07 ± 8.42 A | 279.37 ± 12.88 A | 254.94 ± 11.52 A | 262.15 ± 3.87 A |
| H3 | 3-Methylpentanol a | 1319 | 127.34 ± 1.68 A | 122.6 ± 10.14 A | 129.33 ± 4.90 A | 116.51 ± 4.79 A | 118.42 ± 0.30 A |
| H4 | 1-Hexanol a (mg/L) | 1345.1 | 5.29 ± 0.04 B | 5.20 ± 0.19 B | 5.31 ± 0.29 B | 4.04 ± 0.10 A | 4.22 ± 0.05 A |
| H5 | (E)-3-Hexen-1-ol a | 1356.2 | 6.00 ± 3.87 A | 18.63 ± 2.85 C | 17.25 ± 1.04 BC | 1.83 ± 1.23 A | 8.54 ± 1.72 AB |
| H6 | (Z)-3-Hexen-1-ol a | 1377.2 | 337.11 ± 21.35 A | 401.45 ± 12.98 A | 370.44 ± 14.70 A | 352.69 ± 19.78 A | 348.44 ± 11.02 A |
| H7 | 2-Ethyl-1-hexanol a | 1480.4 | 7.79 ± 0.48 A | 11.75 ± 0.39 B | 11.81 ± 0.01 B | 10.87 ± 0.23 B | 11.96 ± 1.00 B |
| H8 | 3-Ethyl-4-methylpentanol b | 1501.1 | 35.39 ± 1.69 A | 37.96 ± 0.77 A | 38.54 ± 0.99 A | 37.08 ± 0.54 A | 36.09 ± 2.06 A |
| H9 | 2-Nonanol a | 1509.9 | 1.81 ± 0.10 A | 1.73 ± 0.03 A | 3.10 ± 0.12 B | 1.80 ± 0.19 A | 1.51 ± 0.14 A |
| H10 | 2,3-Butanediol b | 1531.8 | 387.95 ± 14.47 A | 317.36 ± 38.57 A | 542.79 ± 155.35 A | 336.66 ± 54.59 A | 322.23 ± 59.76 A |
| H11 | 1-Octanol a | 1549.2 | 26.11 ± 3.24 A | 30.08 ± 0.88 AB | 34.38 ± 0.03 BC | 36.90 ± 1.61 BC | 38.89 ± 2.46 C |
| H12 | 1-Decanol b | 1746.9 | 3.12 ± 0.12 A | 2.43 ± 0.01 A | 2.48 ± 0.32 A | 2.60 ± 0.27 A | 2.99 ± 0.99 A |
| H12 | Benzyl alcohol a | 1856.6 | 169.47 ± 35.69 A | 275.53 ± 6.46 A | 244.54 ± 2.78 A | 210.57 ± 16.29 A | 314.76 ± 155.88 A |
| H13 | 2-Phenylethanol a (mg/L) | 1889.3 | 25.74 ± 1.53 AB | 26.19 ± 0.89 AB | 29.76 ± 0.20 B | 25.56 ± 2.22 AB | 21.98 ± 2.68 A |
| Total higher alcohols (mg/L) | 326.55 ± 5.52 A | 316.65 ± 9.99 A | 335.5 ± 14.29 A | 302.52 ± 13.20 A | 308.04 ± 7.33 A | ||
| Aldehydes/Ketones | |||||||
| AK1 | Acetaldehyde b (mg/L) | 655 | 34.23 ± 2.06 A | 30.94 ± 1.41 A | 31.23 ± 1.55 A | 28.33 ± 1.68 A | 29.30 ± 0.77 A |
| AK2 | 2,6-Dimethyl-4-heptanone b | 1170.5 | 196.54 ± 18.68 A | 150.26 ± 48.15 A | 186.86 ± 13.33 A | 178.15 ± 7.74 A | 147.30 ± 40.39 A |
| AK3 | Nonanal b | 1390.9 | 3.92 ± 2.55 A | 4.46 ± 2.64 A | 3.31 ± 0.88 A | 2.44 ± 0.23 A | 2.24 ± 0.41 A |
| AK4 | Decanal a | 1495 | 0.81 ± 0.05 A | 0.97 ± 0.03 A | 1.10 ± 0.11 A | 0.97 ± 0.36 A | 1.32 ± 0.68 A |
| AK5 | Benzaldehyde a | 1524.2 | 38.50 ± 17.71 A | 27.86 ± 1.33 A | 28.22 ± 3.00 A | 34.96 ± 1.20 A | 71.62 ± 53.00 A |
| AK6 | 3,4-Dimethylbenzaldehyde b | 1806.6 | 482.89 ± 5.85 A | 458.21 ± 23.11 A | 550.95 ± 28.40 AB | 748.42 ± 113.34 B | 517.37 ± 21.26 A |
| Total aldehydes/ketones (mg/L) | 34.95 ± 2.06 A | 31.59 ± 1.38 A | 32.00 ± 1.57 A | 29.29 ± 1.56 A | 30.04 ± 0.78 A | ||
| Fatty acids | |||||||
| F1 | Acetic acid b (mg/L) | 1443.3 | 1.87 ± 0.15 A | 2.32 ± 0.49 A | 2.51 ± 0.02 A | 1.97 ± 0.01 A | 2.05 ± 1.05 A |
| F2 | Isobutyric acid a (mg/L) | 1556.8 | 1.74 ± 0.05 A | 1.61 ± 0.08 A | 1.64 ± 0.04 A | 1.61 ± 0.16 A | 1.55 ± 0.11 A |
| F3 | Butyric acid a (mg/L) | 1615.9 | 1.04 ± 0.09 A | 0.99 ± 0.06 A | 1.09 ± 0.07 A | 0.97 ± 0.09 A | 0.86 ± 0.05 A |
| F4 | Isovaleric acid b (mg/L) | 1656.3 | 1.02 ± 0.02 A | 1.02 ± 0.01 A | 1.03 ± 0.01 A | 1.19 ± 0.04 B | 1.15 ± 0.02 B |
| F5 | Hexanoic acid a (mg/L) | 1821 | 2.74 ± 0.35 A | 2.40 ± 0.05 A | 2.43 ± 0.08 A | 2.70 ± 0.21 A | 2.33 ± 0.08 A |
| F6 | Octanoic acida (mg/L) | 2007.7 | 1.84 ± 0.30 B | 1.53 ± 0.03 AB | 1.50 ± 0.05 AB | 1.48 ± 0.13 AB | 1.15 ± 0.08 A |
| F7 | n-Decanoic acid a | 2178.8 | 173.81 ± 3.09 B | 168.69 ± 0.27 AB | 167.34 ± 0.35 A | 167.23 ± 0.01 A | 166.89 ± 0.10 A |
| Total fatty acids (mg/L) | 10.42 ± 0.66 A | 10.03 ± 0.72 A | 10.36 ± 0.24 A | 10.07 ± 0.62 A | 9.25 ± 1.40 A | ||
| Terpene derivatives | |||||||
| T1 | α-Ionone a | 1528.5 | 2.95 ± 0.13 A | 2.15 ± 0.53 A | 2.67 ± 0.15 A | 2.38 ± 0.12 A | 3.30 ± 0.47 A |
| T2 | Linalool a | 1537.7 | 7.05 ± 0.27 A | 6.69 ± 0.19 A | 6.54 ± 0.24 A | 13.26 ± 0.24 B | 19.47 ± 0.25 C |
| T3 | 4-Terpineol a | 1597.5 | 2.40 ± 0.66 A | 2.45 ± 0.70 A | 2.19 ± 0.12 A | 2.07 ± 0.20 A | 2.27 ± 0.26 A |
| T4 | Hotrienol b | 1600.9 | 0.80 ± 0.02 A | 0.78 ± 0.15 A | 0.79 ± 0.04 A | 0.70 ± 0.00 A | 0.88 ± 0.01 A |
| T5 | α-Terpineol a | 1689 | 1.08 ± 0.16 A | 1.76 ± 0.06 A | 1.79 ± 0.25 A | 3.32 ± 0.01 B | 3.95 ± 0.45 B |
| T6 | D-Citronellol a | 1750.7 | 13.23 ± 0.02 A | 12.83 ± 2.81 A | 10.99 ± 0.35 A | 11.07 ± 0.09 A | 10.68 ± 0.28 A |
| T7 | β-Damascenone a | 1808.4 | 20.45 ± 1.12 A | 20.25 ± 0.3 A | 21.53 ± 0.72 A | 19.63 ± 0.54 A | 19.44 ± 1.56 A |
| T8 | β-Iononea | 1916 | 1.10 ± 0.04 C | 0.94 ± 0.00 B | 0.95 ± 0.01 B | 0.74 ± 0.01 A | tr |
| T9 | Cadalene b | 2150.9 | 9.04 ± 0.78 B | 3.71 ± 0.96 A | 4.27 ± 0.36 A | 6.07 ± 0.00 A | 5.49 ± 0.43 A |
| T10 | TDN 4, b | 1737.9 | 1.70 ± 0.04 C | 1.31 ± 0.00 A | 1.39 ± 0.02 AB | 1.46 ± 0.07 AB | 1.51 ± 0.04 B |
| Total terpene derivatives | 59.80 ± 1.59 AB | 52.86 ± 2.34 A | 53.10 ± 1.95 A | 60.72 ± 0.70 AB | 66.99 ± 3.75 B | ||
| Others | |||||||
| O1 | Styrene a | 1255.5 | 10.79 ± 0.79 B | 3.72 ± 0.06 A | 3.97 ± 0.13 A | 2.86 ± 0.04 A | 2.99 ± 0.03 A |
| O2 | Dihydro-2-methyl-3(2H)-Thiophenone b | 1527.4 | 124.16 ± 1.35 D | 47.02 ± 2.21 BC | 60.81 ± 6.08 C | 32.87 ± 0.05 AB | 21.98 ± 7.52 A |
| O3 | Methionol b | 1708 | 2.67 ± 0.34 BC | 2.18 ± 0.27 ABC | 3.24 ± 0.36 C | 2.00 ± 0.20 AB | 1.58 ± 0.04 A |
| O4 | Naphthalene a | 1736.5 | 1.39 ± 0.03 A | 1.40 ± 0.00 A | 1.44 ± 0.07 A | 1.43 ± 0.05 A | 1.39 ± 0.06 A |
| O5 | 2-Methylnaphthalene b | 1872.1 | 55.41 ± 0.50 A | 45.41 ± 2.98 A | 57.39 ± 11.84 A | 43.52 ± 5.36 A | 43.07 ± 6.00 A |
| O6 | Phenol a | 1967.6 | 56.30 ± 7.84 A | 66.79 ± 4.62 A | 60.66 ± 2.41 A | 61.43 ± 5.76 A | 58.42 ± 2.04 A |
| O7 | 4-Ethylguaiacol a | 1990.4 | 37.84 ± 4.09 A | 56.90 ± 1.81 B | 36.14 ± 1.54 A | 34.27 ± 0.69 A | 30.35 ± 1.19 A |
| O8 | 4-Ethylphenol a | 2107 | 68.85 ± 28.63 A | 129.45 ± 5.35 B | 34.47 ± 0.93 A | 31.03 ± 1.70 A | 27.79 ± 0.73 A |
| O9 | 2,3-Dihydrobenzofuran b | 2274.1 | 20.07 ± 4.48 A | 17.03 ± 0.12 A | tr | tr | tr |
| O10 | 1-Methylnaphthalene b | 1841 | 1.00 ± 0.02 A | 1.00 ± 0.01 A | 1.02 ± 0.03 A | 1.00 ± 0.00 A | 1.00 ± 0.01 A |
| Total others | 378.47 ± 48.07 B | 370.90 ± 12.23 B | 259.14 ± 18.46 A | 210.40 ± 13.75 A | 188.57 ± 5.48 A |
| Volatile Compounds 1 | Odor Threshold (μg/L) | Descriptor 2 | Aroma Series | Before Malolactic Fermentation | L. plantarum C8-1 | L. plantarum Lp39 | O. oeni Oenos | O. oeni CiNe |
|---|---|---|---|---|---|---|---|---|
| Ethyl acetate | 12,300 [55] | Pineapple, fruity, solvent, balsamic [55] | 1,4,5 [55] | 3.36 ± 0.01 B | 2.74 ± 0.03 A | 3.21 ± 0.10 B | 2.59 ± 0.07 A | 2.53 ± 0.10 A |
| Isoamyl acetate | 160 [45] | Banana [45] | 1 [45] | 5.01 ± 0.02 C | 2.12 ± 0.24 B | 2.24 ± 0.09 B | 1.80 ± 0.07 AB | 1.26 ± 0.23 A |
| Ethyl butanoate | 400 [45] | Banana, pineapple, strawberry [45] | 1 [45] | 1.62 ± 0.07 C | 0.76 ± 0.02 AB | 0.91 ± 0.06 B | 0.61 ± 0.10 A | 0.54 ± 0.06 A |
| Ethyl hexanoate | 80 [55] | Strawberry, apple, banana [55] | 4 [55] | 6.79 ± 0.01 C | 3.29 ± 0.46 AB | 4.29 ± 0.21 B | 3.19 ± 0.16 AB | 2.61 ± 0.75 A |
| Ethyl heptanoate | 2 [56] | Winelike, brandy, fruity [56] | 1,6 [56] | 0.71 ± 0.00 B | 0.52 ± 0.10 AB | 0.56 ± 0.04 AB | 0.59 ± 0.03 AB | 0.32 ± 0.16 A |
| Ethyl lactate | 154,636 [45] | Fruity, buttery [45] | 1,7 [45] | 0.03 ± 0.00 A | 0.76 ± 0.02 C | 0.46 ± 0.02 B | 0.43 ± 0.03 B | 0.42 ± 0.02 B |
| Isoamyl hexanoate | 2.7 [57] | Fruity, banana, apple, pineapple, green [55] | 1,3 [58] | 1.47 ± 0.02 B | 1.44 ± 0.01 B | 1.45 ± 0.02 B | 1.31 ± 0.01 A | 1.31 ± 0.03 A |
| Methyl salicylate | 40 [59] | wintergreen, mint [60] | 3 [58] | 0.53 ± 0.03 A | 0.50 ± 0.02 A | 0.92 ± 0.05 B | 0.79 ± 0.03 B | 0.87 ± 0.02 B |
| Isoamyl alcohol | 60,000 [55] | Solvent, sweet, alcohol, nail polish [55] | 4,6,7 [55] | 4.58 ± 0.06 A | 4.42 ± 0.14 A | 4.66 ± 0.21 A | 4.25 ± 0.19 A | 4.37 ± 0.06 A |
| 1-Hexanol | 1100 [45] | Herbaceous, grass, woody [45] | 3 [45] | 4.81 ± 0.04 B | 4.73 ± 0.17 B | 4.83 ± 0.26 B | 3.67 ± 0.09 A | 3.84 ± 0.04 A |
| 2-Phenylethanol | 14,000 [45] | Roses, honey [45] | 2 [45] | 1.84 ± 0.11 AB | 1.87 ± 0.06 AB | 2.13 ± 0.01 B | 1.83 ± 0.16 AB | 1.57 ± 0.19 A |
| Butyric acid | 173 [61] | Cheese, fatty [62] | 7 [62] | 6.00 ± 0.51 A | 5.75 ± 0.34 A | 6.28 ± 0.43 A | 5.61 ± 0.53 A | 4.99 ± 0.28 A |
| Hexanoic acid | 420 [45] | Cheese, fatty [45] | 7 [45] | 6.52 ± 0.82 A | 5.70 ± 0.13 A | 5.78 ± 0.19 A | 6.42 ± 0.49 A | 5.54 ± 0.19 A |
| Octanoic acid | 500 [45] | Rancid, cheese, fatty acid [45] | 7 [45] | 3.68 ± 0.60 B | 3.06 ± 0.07 AB | 3.00 ± 0.11 AB | 2.96 ± 0.27 AB | 2.30 ± 0.16 A |
| α-Ionone | 2.6 [63] | Flowery, violet [64] | 2 [58] | 1.13 ± 0.05 A | 0.83 ± 0.20 A | 1.03 ± 0.06 A | 0.92 ± 0.05 A | 1.27 ± 0.18 A |
| β-Damascenone | 4 [65] | Sweet, exotic flowers, stewed apple [66] | 1,2,4 [66] | 5.11 ± 0.28 A | 5.06 ± 0.08 A | 5.38 ± 0.18 A | 4.91 ± 0.14 A | 4.86 ± 0.39 A |
| β-Ionone | 0.09 [45] | Violets, rose [66] | 2 [45] | 12.27 ± 0.46 C | 10.47 ± 0.00 B | 10.55 ± 0.10 B | 8.28 ± 0.10 A | tr |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-Y.; Zhu, H.-Z.; Lan, Y.-B.; Liu, R.-J.; Liu, Y.-R.; Zhang, B.-L.; Zhu, B.-Q. Modifications of Phenolic Compounds, Biogenic Amines, and Volatile Compounds in Cabernet Gernishct Wine through Malolactic Fermentation by Lactobacillus plantarum and Oenococcus oeni. Fermentation 2020, 6, 15. https://doi.org/10.3390/fermentation6010015
Wang S-Y, Zhu H-Z, Lan Y-B, Liu R-J, Liu Y-R, Zhang B-L, Zhu B-Q. Modifications of Phenolic Compounds, Biogenic Amines, and Volatile Compounds in Cabernet Gernishct Wine through Malolactic Fermentation by Lactobacillus plantarum and Oenococcus oeni. Fermentation. 2020; 6(1):15. https://doi.org/10.3390/fermentation6010015
Chicago/Turabian StyleWang, Shao-Yang, Hai-Zhen Zhu, Yi-Bin Lan, Ruo-Jin Liu, Ya-Ran Liu, Bo-Lin Zhang, and Bao-Qing Zhu. 2020. "Modifications of Phenolic Compounds, Biogenic Amines, and Volatile Compounds in Cabernet Gernishct Wine through Malolactic Fermentation by Lactobacillus plantarum and Oenococcus oeni" Fermentation 6, no. 1: 15. https://doi.org/10.3390/fermentation6010015
APA StyleWang, S.-Y., Zhu, H.-Z., Lan, Y.-B., Liu, R.-J., Liu, Y.-R., Zhang, B.-L., & Zhu, B.-Q. (2020). Modifications of Phenolic Compounds, Biogenic Amines, and Volatile Compounds in Cabernet Gernishct Wine through Malolactic Fermentation by Lactobacillus plantarum and Oenococcus oeni. Fermentation, 6(1), 15. https://doi.org/10.3390/fermentation6010015





