Abstract
This review examines the production of the biopolymer curdlan, synthesized by Agrobacterium species (sp.), on processing coproducts and plant lignocellulosic hydrolysates. Curdlan is a β-(1→3)-D-glucan that has various food, non-food and biomedical applications. A number of carbon sources support bacterial curdlan production upon depletion of nitrogen in the culture medium. The influence of culture medium pH is critical to the synthesis of curdlan. The biosynthesis of the β-(1→3)-D-glucan is likely controlled by a regulatory protein that controls the genes involved in the bacterial production of curdlan. Curdlan overproducer mutant strains have been isolated from Agrobacterium sp. ATCC 31749 and ATCC 31750 by chemical mutagenesis and different selection procedures. Several processing coproducts of crops have been utilized to support the production of curdlan. Of the processing coproducts investigated, cassava starch waste hydrolysate as a carbon source or wheat bran as a nitrogen source supported the highest curdlan production by ATCC 31749 grown at 30 °C. To a lesser extent, plant biomass hydrolysates have been explored as possible substrates for curdlan production by ATCC 31749. Prairie cordgrass hydrolysates have been shown to support curdlan production by ATCC 31749 although a curdlan overproducer mutant strain, derived from ATCC 31749, was shown to support nearly double the level of ATCC 31749 curdlan production under the same growth conditions.
1. Introduction
Curdlan is an extracellular polysaccharide synthesized by Agrobacterium species (previously classified taxonomically as Alcaligenes faecalis var. myxogenes) that has a complex tertiary structure [1,2,3]. The unbranched, high-molecular-weight polysaccharide (average molecular weight ranges between 5.3 × 104 and 2 × 106) is composed of approximately 135 glucosyl residues linked by β-D-(1→3) bonds [2,4]. Curdlan dissolves in dilute alkali solutions but is insoluble in water or acidic solutions [2]. When curdlan forms a gel, a transition from being flexible and disordered to forming a rigid and structured state has been noted [4]. Curdlan can exist as a thermoreversible, low-set gel or a thermo-irreversible, high-set gel depending upon heating temperature [4]. It was noted that curdlan is stable against freezing and thawing [5]. It has the ability to absorb up to 100 times its weight in water [4,6]. Using electron microscopy, the formation of long microfibrils in curdlan was directly related to gel strength [7]. The biopolymer can be stained with dyes such as Aniline Blue [8].
It has been reported that curdlan shows no toxicity, carcinogenicity or any effect on animal reproduction [9]. Further, curdlan was found to not affect the caloric intake of rodents [9]. Having demonstrated the polysaccharide gum to be safe in animals, this carbohydrate polymer was approved for use by the U.S. Food and Drug Administration in December 1996 [9]. Curdlan has applications in the food and pharmaceutical industries [5,6,9,10,11]. Curdlan can serve as a thickening agent in the food industry such as in jellies, noodles and ice cream [4,5]. It may be able to serve as a fat replacement in foods [6]. The polysaccharide gum has also been used in animal feeds [12]. Concerning non-food applications, biomedical applications include being used for its immunostimulatory effects such as being a biological response modifier of both innate and adaptive immune responses [4,11]. It has been reported that curdlan can inhibit tumor growth and that curdlan can activate leukocytes and induce cytokine production in humans [11,13]. Curdlan has been used to encapsulate several drugs [11]. This biopolymer can be hydrolyzed using acid hydrolysis as well as by enzyme degradation [4]. The chemical derivatization of curdlan by carboxymethylation, phosphorylation and sulfation has been used to broaden its range of potential commercial applications [4]. A sulfated curdlan derivative can be effective against HIV [11]. Hydroxyethylated curdlan has also been shown to be effective in the time-controlled release of protein [14]. Curdlan is sold commercially by InvivoGen (San Diego, CA) for immunological use. Other non-food uses of curdlan are as an additive to cement to improve its fluidity due to its ability to absorb water or as an enzyme immobilization support [15].
2. Effect of Culture Conditions on Curdlan Production by Agrobacterium Strains
2.1. Effect of Carbon Source
It has been suggested that curdlan may serve as a biofilm to protect the bacterium from harsh environmental conditions [16]. During the post-stationary period of an excess carbon source-containing nitrogen-limited batch or continuous bacterial culture, curdlan synthesis initiates [3,17,18,19]. Carbon sources, including glucose, sucrose, fructose, galactose and maltose, support curdlan production by Agrobacterium species (sp.) ATCC 31749 [3,19,20,21,22,23,24,25]. It was observed that curdlan production by ATCC 31749 grown at 30 °C for 120 h was nearly identical when 3% glucose or maltose served as the carbon source [23]. In another study, it was reported that 2% glucose, sucrose or maltose supported higher curdlan production by ATCC 31749 after 96 h at 30 °C than did 2% fructose or galactose [24]. When analogues of glucose-related sugars were supplemented as carbon sources, ATCC 31749 supported the synthesis of modified carbohydrate polymers [26]. Using 13C-glucose as a carbon source for curdlan synthesis, it was determined that 60% of the curdlan formed was derived from the labelled glucose [27]. The increased expression of ABS transporters is thought to promote glucose channeling into bacterial curdlan synthesis [11]. Using 3% sucrose as the carbon source, ATCC 31749 produced slightly lower levels of curdlan after 120 h at 30 °C [22]. When curdlan production by ATCC 31750 was studied, glucose, fructose, galactose, maltose, sucrose, lactose and raffinose (10%) were tested as possible carbon sources for curdlan production after 72 h at 30 °C [25]. Maltose was found to support greater curdlan production in ATCC 31750 than the other carbon sources examined with galactose supporting the least curdlan [25]. For ATCC 31750, the specific growth rate on a sucrose-containing medium at 30 °C was greatest at pH 7.0 while the specific curdlan production rate was greatest at pH 5.0 after nitrogen depletion in a fermentor [15,28]. Subsequently, a two-step fed-batch process involving ATCC 31750 was developed where the initial pH of the sucrose-containing medium was adjusted to pH 7.0 to allow biomass production and lowered to pH 6.5 to stimulate curdlan production [25].
2.2. Effect of Phosphate
In ATCC 31750, an analysis of the optimal phosphate concentration in shake flask medium cultures to support curdlan production was between 0.1 and 0.3 g/L [29]. The phosphate level in the culture medium had to be sufficient to support cell growth [29]. Curdlan production by ATCC 31749 was shown to increase when low-polyphosphates were added to a glucose-containing culture medium [30]. The genes encoding the polyphosphate metabolizing enzymes were confirmed to be present in ATCC 31749 [30]. The inclusion of both (NaPO3)6 and CaCO3 in the culture medium was necessary to stimulate curdlan production by ATCC 31749 [30]. It was observed that optimal curdlan production (42.8 g/L) by ATCC 31749 at 30 °C occurred after 120 h when the dissolved oxygen concentration in the stirred tank fermentor was 60% [31]. The curdlan gel strength did not appear to be affected by the level of dissolved oxygen in the fermentor [31]. It was also noted that the intracellular levels of NAD+, UMP, UDP-glucose, NADH, AMP, ADP and ATP in ATCC 31749 cells were affected by the concentration of dissolved oxygen in the medium [31]. With curdlan biosynthesis being dependent on ATP production by the tricarboxylic acid cycle and the electron transfer chain, the dissolved oxygen level would be expected to be a factor in allowing polysaccharide synthesis to continue (Figure 1).
2.3. Effect of Pyrimidine Base Supplementation
The supplementation of pyrimidine bases has been examined to determine what effect their addition has on curdlan synthesis considering that UTP is critical to the formation of UDP-glucose. The addition of the pyrimidine bases uracil, cytosine, thymine, dihydrouracil or orotic acid to a sucrose-containing culture medium of Agrobacterium sp. ATCC 31749 was found to affect curdlan production to different degrees [21]. The inclusion of uracil or cytosine to the culture medium of ATCC 31749 following 48 h of growth at 30 °C caused an increase of curdlan concentration by 1.7-fold or 1.5-fold, respectively, after 120 h of growth compared to curdlan production by ATCC 31749 if a pyrimidine base was not supplemented into the medium [21]. In contrast, the supplementation of the pyrimidine bases thymine, dihydrouracil or orotic acid had no effect upon curdlan production by ATCC 31749 following 120 h of growth [21]. Uracil supplementation to the culture medium elevated biomass production by 3.0-fold after 120 h of growth of ATCC 31749 compared to the control cultures which was thought to be causing the observed increase in curdlan production [21]. It should be noted that the increase in curdlan production by ATCC 31749 following cytosine addition into the culture medium is likely due to the conversion of cytosine into uracil by Agrobacterium species. It has been found that cells of Agrobacterium species contain the enzyme cytosine deaminase, which catalyzes the deamination of cytosine to uracil [21]. Uracil addition to the sucrose-containing culture medium has also been investigated in Agrobacterium sp. ATCC 31750. The addition of uracil to the culture medium increased the curdlan yield from sucrose-grown cells of ATCC 31750 [32]. This effect of uracil supplementation on curdlan yield appeared to confirm the importance of UDP-glucose (Figure 1) as a substrate for polysaccharide formation [33]. The cellular synthesis of UDP-glucose is costly in the sense that it requires two ATP molecules to synthesize the nucleotide sugar [3]. When curdlan synthesis is initiated in ATCC 31750, the intracellular concentrations of UMP and AMP were highly increased [33]. As nitrogen was depleted, the level of UMP rose approximately 5-fold [33]. It was previously noted for ATCC 31750 that uracil supplementation following 46 h to a sucrose-containing culture medium elevated curdlan production relative to an unsupplemented culture after 160 h of growth [32]. Biomass production by ATCC 31750 after 80 h was observed to increase slightly after uracil addition at 46 h [32]. With little increase in biomass after 80 h, it was suggested that uracil addition affected curdlan production by increasing its pool of uridine nucleotides. It is known that UMP levels in ATCC 31750 increased by more than 4-fold after 12 h of nitrogen depletion [33]. Proteomic analysis was also examined in ATCC 31750 following uracil addition to a sucrose-containing culture medium where the cells were grown in a fermentor at 37 °C for 90 h [34]. Although the concentration of UTP-glucose-1-phosphate uridylyltransferase was only elevated by 3%, the concentrations of β-1,3-glucan synthase catalytic unit and phosphoglucomutase were enhanced by 35 or 30%, respectively [34]. The concentration of the pyrimidine salvage enzyme uracil phosphoribosyltransferase was also increased following uracil addition as might be expected [34]. An elevation in the levels of UTP and UDP-glucose was detected following uracil addition to the culture medium [34].
2.4. Effect of Tween-80 Supplementation
It has been shown that the non-ionic surfactant Tween-80 (polyethylene glycol sorbitan monooleate), when added to a glucose-supplemented culture medium at concentration less than 0.3%, stimulated curdlan synthesis by ATCC 31749 at 30 °C [35]. The presence of Tween-80 in the culture medium did not influence cell growth but was found to increase the level of glycosyltransferase activity [35]. Another study also explored the effect of growing ATCC 31749 at 30 °C following the addition of Tween-80 to a glucose-based culture medium [36]. Tween-80 (16 g/L) addition to the bacterial culture resulted in maximum curdlan production but synthesized a curdlan with a looser ultrastructure that was not compact but more dispersive [36]. It was concluded that the presence of Tween-80 in the medium caused an increase in the membrane permeability of the ATCC 31749 cells plus increased ATP and UTP regeneration allowing a higher level of UDP-glucose to be synthesized [36].
3. Genetics and Regulation of Curdlan Biosynthesis
The genome sequence of ATCC 31749 has been determined to better understand how curdlan biosynthesis is regulated [37]. The genes involved in curdlan biosynthesis have been mapped to two loci in ATCC 31749 [38]. One locus contains three genes including crdS (that is homologous to glycosyltransferases such as cellulose synthase), crdA and crdC (the role of the latter two genes is unknown) while the second locus contains the gene crdR which encodes a positive transcriptional regulatory gene [16,38]. The expression of the genes crdS, crdA and crdC was increased when crdR was overexpressed [16]. The expression of crdR was noted to be elevated in the stationary phase relative to the exponential phase [16]. In the overexpressed crdR mutant strain of ATCC 31749, curdlan production by the mutant strain was 1.4-fold higher than curdlan production by strain ATCC 31749 grown on a glucose-based culture medium for 120 h at 30 °C [22]. When a crdR knockout strain was constructed, it was deficient for curdlan production, but its biomass production was unaffected [16]. It was found that the gene for phosphatidylserine synthase in ATCC 31749 was necessary for the strain to produce high-molecular-weight curdlan indicating the phospholipid composition affected curdlan synthesis [39]. The genes responsible for curdlan biosynthesis in ATCC 31749 were examined at 30 °C for transcriptional regulation in glucose-grown cells [40]. It was concluded that enhanced translation of genes involved in the tricarboxylic acid cycle, the electron transfer chain and UDP-glucose biosynthesis was occurring [40]. The effect of pH on the expression of enzymes involved in curdlan production has been documented for ATCC 31750 sucrose-grown cells in a fermentor incubated at 37 °C. It has been found that when the culture medium pH is reduced from 7.0 to 5.5, the levels of proteins involved directly or indirectly in curdlan synthesis are elevated. The β-1,3-glucan synthase catalytic unit was 10-fold higher in the pH 5.5-grown cells compared to the pH 7.0-grown cells [41]. Similarly, a 3-fold higher concentration of UTP-glucose-1-phosphate uridylyltransferase was detected when the cells were grown at pH 5.5 compared to 7.0 [41]. The pH downshift also increased the concentrations of phosphoglucomutase and orotidine 5’-monophosphate decarboxylase [41]. The reduction in pH from 7.0 to 5.5 also increased the level of UDP-glucose in the cells [41]. With nitrogen depletion being critical in the synthesis of curdlan, curdlan production in a ntrC mutant strain of ATCC 31749 was investigated after growth in a glucose-containing medium [42]. The ntrC gene is involved in activating genes that respond to nitrogen starvation [42,43]. It was observed that curdlan production was very low in the ntrC mutant strain after 72 h compared to the parent strain [42]. Further the biopolymer synthesized by the mutant strain was not a β-glucan [42].
Curdlan overproducer mutant strains have been previously isolated in Agrobacterium sp. ATCC 31749 and 31750 [12,13,23]. A mutant strain of ATCC 31749 was isolated by chemical mutagenesis and ampicillin-resistance exhibiting curdlan overproduction [23]. The mutant strain produced the highest levels of curdlan after growth for 120 h at 30 °C using 3% glucose or maltose as a carbon source [23]. Biomass production by the mutant strain was slightly higher than what was observed for its parent strain on glucose or maltose after 120 h at 30 °C and the elevated biomass production could be related to the increase in curdlan production [23]. Two prior studies have reported the isolation of curdlan overproducer mutants of ATCC 31750 [12,13]. In the initial study, ATCC 31750 cells were subjected to chemical mutagenesis and screened on aniline blue-containing solid medium to identify mutant R259 [13]. The mutant strain grown on a sucrose-containing medium at 30 °C for 120 produced 76 g/L curdlan while its parent strain ATCC 31750 produced 64 g/L under the same conditions [13]. The yield for the mutant strain was determined to be 0.55 g curdlan/g sucrose compared to 0.45 g curdlan/g sucrose for ATCC 31750 [13]. It was confirmed that curdlan was the biopolymer being synthesized based on its structural analysis [13]. In the second study, a mutant strain, identified as M5, was also isolated from ATCC 31750 by chemical mutagenesis and screening on an aniline blue-containing solid medium [12]. Strain M5 synthesized a 1.6-fold higher curdlan concentration and 1.4-fold higher yield than its parent strain following growth at 30 °C for 120 h on a glucose-containing medium in a stirred bioreactor [12]. Interestingly, the pH optimum in the mutant strain for curdlan production and cell growth was 7.0, unlike what was observed in its parent strain. The polysaccharide gum being synthesized by strain M5 was identified as curdlan following analysis of its structural properties [12].
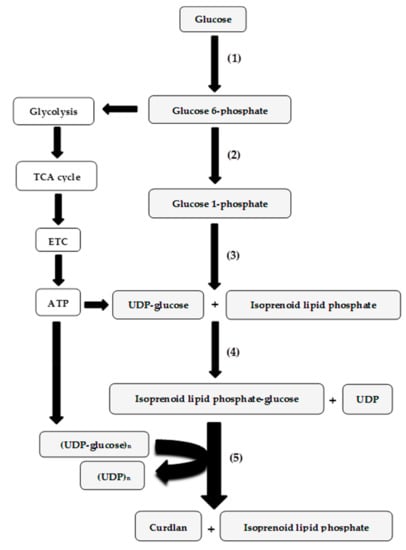
Figure 1.
The metabolic pathways in Agrobacterium thought to be involved in curdlan synthesis [33,40,42]. The pathway enzymes involved include (1) hexokinase, (2) phosphoglucomutase, (3) UDP-glucose pyrophosphorylase, (4) glycosyltransferase, and (5) curdlan synthase. Abbreviations: TCA cycle, tricarboxylic acid cycle; ETC, electron transport chain. The pathway for curdlan biosynthesis is shaded in grey, while the other metabolic pathways contributing to ATP formation are not shaded.
4. Curdlan Production by Agrobacterium Strains Grown on Processing Coproducts
A variety of processing coproducts of crops have been tested as alternative carbon or nitrogen sources to support bacterial curdlan synthesis (Table 1). The majority of studies have utilized Agrobacterium sp. ATCC 31749 to test the coproducts for their ability to sustain curdlan production by substituting for glucose as a carbon source. The ability of ATCC 31749 to use the crop-derived coproducts corn glucose syrup, corn maltose syrup and cassava glucose syrup was examined [20]. The highest curdlan yield by ATCC 31749 was obtained at 85% when corn maltose syrup served as the carbon source [20]. The curdlan yield by ATCC 31749 dropped substantially to 55% or 44%, respectively, when corn glucose syrup or cassava glucose syrup served as the carbon source in the culture medium [20]. In another study, the ability of the ethanol-processing coproduct condensed corn distillers’ solubles was investigated as both a carbon and nitrogen source to support curdlan production by ATCC 31749 [22]. It was found that 400 g/L condensed corn distillers’ solubles supported curdlan production by ATCC 31749 at a 1.4-fold higher level than if the strain was grown on 3% glucose for 120 h at 30 °C [22]. It was suggested that the 3-fold increased biomass production by ATCC 31749 after growth on condensed corn distillers’ solubles compared to glucose for 120 h at 30 °C could be the reason for the enhanced curdlan production [22]. In a study reporting the isolation of a strain exhibiting enhanced curdlan production compared to its parent strain ATCC 31749, a high maltose corn syrup was utilized as a carbon source in the culture medium [23]. The findings indicated that 3% high maltose corn syrup supported less curdlan production by ATCC 31749 or the mutant strain than if 3% glucose or maltose served as the carbon source in the medium after 120 h of growth at 30 °C [23]. Biomass production by ATCC 31749 or the mutant strain on the high maltose corn syrup was similar after 120 h of growth at 30 °C compared to glucose or maltose as the carbon source [23]. The strain exhibiting enhanced curdlan production also was investigated for its ability to utilize 400 g/L condensed corn distillers’ solubles to support curdlan and biomass production after 120 h at 30 °C [44]. The results indicated that the mutant strain synthesized about the same curdlan and biomass levels as its parent ATCC 31749 on this ethanol-processing coproduct [44]. In a recent study, the ability of a cassava starch waste hydrolysate (50 g/L), prepared by treatment with amylase, to sustain curdlan production by ATCC 31749 was compared to the use of 50 g/L glucose as a carbon source [24]. It was observed that the starch waste hydrolysate caused a slight increase in curdlan production by ATCC 31749 after 96 h at 30 °C compared to ATCC 31749 grown in the glucose-containing medium. It was concluded that cassava starch waste hydrolysate could serve as a substitute for glucose as a carbon source which could lower the production cost of curdlan [24]. The juice extracted from the discarded bottom part of asparagus spears was investigated for its possible use as a supplement to support curdlan production by ATCC 31749 in a sucrose-containing medium [45]. When the 10% juice was added to the sucrose-containing culture medium, ATCC 31749 produced 40.2 g/L curdlan after 168 h of growth at 30 °C [45]. The juice supplement was found to support a higher level of curdlan production by ATCC 31749 compared to when the strain was grown in the sucrose-containing medium alone [45]. It was thought that the enhanced curdlan production was due to the juice extracted from the discarded asparagus spears being rich in minerals and vitamins [45]. The utilization of a mixed species fermentation system involving Agrobacterium sp. ATCC 31749 and the fungus Trichoderma harzianum GIM 3.442 to synthesize curdlan has been reported where wheat bran was used as a nitrogen source [46]. It was determined that this fermentation system could produce more curdlan than ATCC 31749 alone could synthesize on a glucose-containing medium [46]. With respect to the use of a processing coproduct to support curdlan synthesis by Agrobacterium sp. ATCC 31750, sugar cane molasses was explored as a possible alternative to sucrose as a carbon source [25]. Using fed-batch cultivation, the addition of sugar cane molasses to the ATCC 31749 culture medium resulted in 42 g/L curdlan being synthesized after 120 h, with its yield being comparable to using sucrose as a carbon source [25].

Table 1.
Curdlan production and yield by strains grown on processing coproduct or hydrolyzed lignocellulosic biomass.
5. Curdlan Production by Agrobacterium Strains Grown on Plant Lignocellulosic Hydrolysates
Rather than produce curdlan from glucose, a viable alternative could be the hydrolysis of the cellulose within plant biomass providing glucose as a carbon source for the biobased synthesis of curdlan [49]. An excellent source of plant biomass are prairie grasses, considering their high yields with little fertilizer input plus their high cellulose content [50]. One such prairie grass is the North American prairie cordgrass (Spartina pectinata), which contains 33% cellulose [47]. If the plant biomass is treated at high temperature and pressure, it is possible to release a high concentration of cellulose from the biomass [50]. Subsequent enzymatic treatment by cellulase and cellobiase can be used to degrade cellulose to glucose which is a substrate for curdlan production [47,48]. Using a prairie cordgrass hydrolysate to support curdlan production by ATCC 31749, exogenous nitrogen source addition to a hydrolysate-containing medium was shown to be important. It was found that the optimal level of ammonium phosphate in the culture medium for curdlan production by ATCC 31749 was dependent on how the hydrolysate was prepared [47]. In a prior study, the prairie cordgrass hydrolysate was treated to produce a solids-only or complete hydrolysate [47]. Using the solids-only hydrolysate, the highest curdlan level produced by ATCC 31749 was noted when it was grown for 144 h at 30 °C on a medium containing 2.2 mM ammonium phosphate [47]. Utilizing the complete hydrolysate, the highest curdlan concentration was synthesized by ATCC 31749 on a medium containing 3.3 mM ammonium phosphate [47]. Curdlan production and yield by ATCC 31749 after 144 h at 30 °C on the medium containing the complete hydrolysate and 3.3 mM ammonium phosphate was greater than what was observed for ATCC 31749 after 144 h of growth at 30 °C on the solids-only medium containing 2.2 mM ammonium phosphate [47]. Cellular biomass production by ATCC 31749 after 144 h of growth on the complete hydrolysate medium was greater than its biomass production on the solids-only hydrolysate medium [47]. The ability of a curdlan overproducer mutant strain to utilize a prairie cordgrass hydrolysate to support curdlan production by ATCC 31749 was also examined in relation to the ammonium phosphate concentration present in the medium [48]. The highest curdlan concentration and yield was produced by the mutant strain grown at 30 °C and 144 h on a medium containing the complete hydrolysate and 3.3 mM ammonium phosphate [48]. Curdlan production by the mutant strain grown under the same culture conditions on the complete prairie cordgrass hydrolysate as its parent strain ATCC 31749 was 1.8-fold higher (Table 1). It should be noted that the curdlan yields from the prairie cordgrass hydrolysates were low compared to yields obtained using crop processing coproducts (Table 1). The metabolic engineering of ATCC 31749 to utilize cellobiose to produce curdlan could be a major advancement in being able to utilize plant lignocellulosic biomass to synthesize the biopolymer [51]. It has been reported that a recombinant ATCC 31749 strain containing cellulose phosphorylase can effectively utilize cellobiose. This strain was shown to produce a higher curdlan concentration after 144 h at 30 °C on cellobiose than the curdlan level synthesized by ATCC 31749 on sucrose [51]. It is likely that such recombinant strains of Agrobacterium sp. will need to be engineered if plant lignocellulosic biomass is to be cost-effectively used for commercial curdlan production.
6. Conclusions
Overall, it is clear from the literature that curdlan is a commercially important biopolymer that has a wide array of food, non-food and medical applications. The primary obstacle to further commercialization of curdlan production is its being competitive with the cost of other commercially utilized polysaccharide gums. The majority of the research exploring curdlan production by Agrobacterium sp. has focused on culture conditions where various sugars have been tested as carbon sources. There have been far fewer studies investigating bacterial curdlan production using low value crop processing coproducts instead of sugars to ferment the biopolymer. Curdlan synthesis by Agrobacterium sp. on low value of crop processing coproducts could be an answer to make the commercial production of curdlan more cost-effective. Similarly, there have been few reports of plant lignocellulosic hydrolysates being used to sustain Agrobacterium sp. curdlan production. Low value prairie grasses that contain high cellulose content can be enzymatically hydrolyzed to release glucose. The glucose-containing hydrolysate could subsequently be supplemented into culture medium to support bacterial curdlan production. Again, the use of lignocellulosic hydrolysates of prairie grasses for curdlan synthesis by Agrobacterium sp. could make the production of this biopolymer more economical if the curdlan yields could be increased. It is clear from the literature that studies exploring the utilization of plant lignocellulosic hydrolysates are few in number and should be an area that is investigated in future studies. The genetic engineering of Agrobacterium sp. strains to hydrolyze cellobiose may be an important advancement in using plant biomass for curdlan production [51]. This advancement is important because it would eliminate the need to use a fermentation system involving a fungal strain to hydrolyze cellobiose [46]. New environmentally friendly, biobased approaches that allow curdlan to be produced efficiently and cost effectively by recombinant Agrobacterium sp. strains from crop processing coproducts and plant biomass need to be developed to expand the future commercial applications of this biopolymer.
Author Contributions
The author wrote and edited this article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Welch Foundation Grant T-0014, NIFA Grant No. 2011-67010-20051 and South Dakota Agricultural Experiment Station Grant SD00H434-12.
Conflicts of Interest
The author declares no conflict of interest.
References
- Harada, T.; Fujimori, F.; Hirose, S.; Masada, M. Growth and β-glucan 10C3K production by a mutant of Alcaligenes faecalis var myxogenes in defined medium. Agric. Biol. Chem. 1966, 30, 764–769. [Google Scholar] [CrossRef]
- Harada, T.; Misaki, A.; Saito, H. Curdlan: A bacterial gel-forming β-1,3-glucan. Arch. Biochem. Biophys. 1968, 124, 292–298. [Google Scholar] [CrossRef]
- Phillips, K.R.; Lawford, H.G. Theoretical maximum and observed product yields associated with curdlan production by Alcaligenes faecalis. Can. J. Microbiol. 1983, 29, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Edgar, K.J. Properties, chemistry and applications of the bioactive polysaccharide curdlan. Biomacromolecules 2014, 15, 1079–1096. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Konno, A.; Taguchi, T.; Tawada, T.; Kasai, H.; Toda, J.; Terasaki, T. Curdlan: Properties and applications to foods. J. Food Sci. 1991, 56, 769–772,776. [Google Scholar] [CrossRef]
- Jezequel, V. Curdlan: A new functional β-glucan. Cereal Foods World 1998, 43, 361–364. [Google Scholar]
- Koreeda, A.; Harada, T.; Ogawa, K.; Sato, S.; Kasai, N. Study of the ultrastructure of gel-forming (1→3)-β-D-glucan (curdlan-type polysaccharide) by electron microscopy. Carbohydr. Res. 1974, 33, 396–399. [Google Scholar] [CrossRef]
- Nakanishi, I.; Kimura, K.; Kusui, S.; Yamazaki, E. Complex formation of gel-forming bacterial (1→3)-β-D-glucan (curdlan-type polysaccharides) with dyes in aqueous solution. Carbohydr. Res. 1974, 32, 47–52. [Google Scholar] [CrossRef]
- Spicer, E.F.J.; Goldenthal, E.I.; Ikeda, T. A toxicological assessment of curdlan. Food Chem. Toxicol. 1999, 37, 455–479. [Google Scholar] [CrossRef]
- Kanke, M.; Koda, K.; Koda, Y.; Katayama, H. Application of curdlan to controlled drug delivery. I. The preparation and evaluation of theophylline-containing curdlan tablets. Pharm. Res. 1992, 9, 414–418. [Google Scholar] [CrossRef]
- Zhan, X.B.; Lin, C.C.; Zhang, H.T. Recent advances in curdlan biosynthesis, biotechnological production, and applications. Appl. Microbiol. Biotechnol. 2012, 93, 525–553. [Google Scholar] [CrossRef] [PubMed]
- Kalyanasundaram, G.T.; Doble, M.; Gummadi, S.N. Production and downstream processing of (1→3)-β-D-glucan from mutant strain of Agrobacterium sp. ATCC 31750. AMB Express 2012, 2, 31. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-K.; Ryu, K.-E.; Choi, W.-A.; Rhee, Y.-H.; Lee, I.-Y. Enhanced production of (1→3)-β-D-glucan by a mutant strain of Agrobacterium species. Biochem. Bioeng. J. 2003, 16, 163–168. [Google Scholar] [CrossRef]
- Kim, B.S.; Jung, I.D.; Kim, J.S.; Lee, J.-H.; Lee, I.Y.; Lee, K.B. Curdlan gels as protein delivery vehicles. Biotechnol. Lett. 2000, 22, 1127–1130. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, I.-Y.; Kim, M.-K.; Park, Y.H. Optimal pH control of batch processes for production of curdlan by Agrobacterium species. J. Ind. Microbiol. Biotechnol. 1999, 23, 143–148. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, C.; Yang, L.; Zhao, L.; Lin, C.; Liu, Z.; Mao, Z. CdR function in a curdlan-producing Agrobacterium sp. ATCC31749 strain. BMC Microbiol. 2015, 15, 25. [Google Scholar] [CrossRef]
- Lawford, H.G.; Rousseau, J.D. Production of β-1,3-glucan exopolysaccharide in low shear systems: The requirement for high oxygen tension. Appl. Biochem. Biotechnol. 1992, 34/35, 597–612. [Google Scholar] [CrossRef]
- Wu, J.R.; Yu, L.J.; Zhan, X.B.; Zheng, Z.Y.; Lu, J.; Lin, C.C. NtrC-dependent regulatory network for curdlan biosynthesis in response to nitrogen limitation in Agrobacterium sp. ATCC 31749. Process Biochem. 2012, 47, 1552–1558. [Google Scholar] [CrossRef]
- Jiang, L. Effect of nitrogen source on curdlan production by Alcaligenes faecalis ATCC 31749. Int. J. Biol. Macromol. 2013, 52, 218–220. [Google Scholar] [CrossRef]
- Portilho, M.; Matioli, G.; Zanin, G.; de Moraes, F.F.; Scamparini, A.R. Production of insoluble exopolysaccharide of Agrobacterium sp. (ATCC 31749 and IFO 13140). Appl. Biochem. Biotechnol. 2006, 129–132, 864–869. [Google Scholar] [CrossRef]
- West, T.P. Pyrimidine base supplementation affects curdlan production by Agrobacterium sp. ATCC 31749. J. Basic Microbiol. 2006, 46, 153–157. [Google Scholar] [CrossRef] [PubMed]
- West, T.P.; Nemmers, B. Curdlan production by Agrobacterium sp. ATCC 31749 on an ethanol fermentation coproduct. J. Basic Microbiol. 2008, 48, 65–68. [Google Scholar] [CrossRef] [PubMed]
- West, T.P. Elevated curdlan production by a mutant of Agrobacterium sp. ATCC 31749. J. Basic Microbiol. 2009, 49, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lu, M.; Fang, Y.; Wu, L.; Xu, Y.; Wang, S. Production of curdlan grown on cassava starch waste hydrolysates. J. Polym. Environ. 2018, 26, 33–38. [Google Scholar] [CrossRef]
- Lee, I.-Y.; Seo, W.T.; Kim, M.K.; Park, C.S.; Park, Y.H. Production of curdlan using sucrose or sugar cane molasses by two-step fed-batch cultivation of Agrobacterium species. J. Ind. Microbiol. Biotechnol. 1997, 18, 255–259. [Google Scholar] [CrossRef]
- Lee, J.W.; Yeomans, W.G.; Allen, A.L.; Kaplan, D.L.; Deng, F.; Gross, R.A. Exopolymers from curdlan production: Incorporation of glucose-related sugars by Agrobacterium sp. strain ATCC 31749. Can. J. Microbiol. 1997, 43, 149–156. [Google Scholar] [CrossRef]
- Kai, A.; Ishino, T.; Arashida, T.; Hatanaka, K.; Akaike, T.; Matsuzaki, K.; Kaneko, Y.; Mimura, T. Biosynthesis of curdlan from culture media containing 13C-labeled glucose as the carbon source. Carbohydr. Res. 1993, 240, 153–159. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, Y.H. Optimal production of curdlan by Agrobacterium sp. with feedback inferential control of optimal pH profile. Biotechnol. Lett. 2001, 23, 525–530. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, I.Y.; Lee, J.H.; Kim, K.T.; Rhee, Y.H.; Park, Y.H. Residual phosphate concentration under nitrogen-limiting conditions regulates curdlan production in Agrobacterium species. J. Ind. Microbiol. Biotechnol. 2000, 25, 180–183. [Google Scholar] [CrossRef]
- Yu, L.; Wu, J.; Liu, J.; Zhan, X.; Zheng, Z.; Lin, C.C. Enhanced curdlan production in Agrobacterium sp. ATCC 31749 by addition of low-polyphosphates. Biotechnol. Bioprocess Eng. 2011, 16, 34–41. [Google Scholar] [CrossRef]
- Zhang, H.T.; Zhan, X.B.; Zheng, Y.Z.; Wu, J.R.; English, N.; Yu, X.B.; Lin, C.C. Improved curdlan fermentation process based on optimization of dissolved oxygen combined with pH control and metabolic characterization of Agrobacterium sp. ATCC 31749. Appl. Microbiol. Biotechnol. 2012, 93, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, I.Y. Optimization of uracil addition for curdlan (β-1→3-glucan) production by Agrobacterium sp. Biotechnol. Lett. 2001, 23, 1131–1134. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, I.Y.; Ko, J.H.; Rhee, Y.H.; Park, Y.H. Higher intracellular levels of uridine monophosphate under nitrogen-limited conditions enhance metabolic flux of curdlan synthesis in Agrobacterium species. Biotechnol. Bioeng. 1999, 62, 317–323. [Google Scholar] [CrossRef]
- Jin, L.-H.; Lee, J.-H. Effect of uracil addition on proteomic profiles and 1,3-β-glucan production in Agrobacterium sp. Biotechnol. Appl. Biochem. 2014, 61, 280–288. [Google Scholar] [PubMed]
- Xia, Z. Effect of Tween 80 on the production of curdlan by Alcaligenes faecalis ATCC 31749. Carbohydr. Polym. 2013, 98, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhu, L.; Gao, M.; Zheng, Z.; Wu, J.; Zhan, X. Influence of Tween-80 on the production of water-insoluble curdlan from Agrobacterium sp. Int. J. Biol. Macromol. 2018, 106, 611–619. [Google Scholar] [CrossRef]
- Ruffing, A.M.; Castro-Melchor, M.; Hu, W.-S.; Chen, R.R. Genome sequence of the curdlan-producing Agrobacterium sp. strain ATCC 31749. J. Bacteriol. 2011, 193, 4294–4295. [Google Scholar] [CrossRef]
- Stasinopoulos, S.J.; Fisher, P.R.; Stone, B.A.; Stanisich, V.A. Detection of two loci involved in (1→3)-β-glucan (curdlan) biosynthesis by Agrobacterium sp. ATCC31749, and comparative sequence analysis of the putative curdlan synthase gene. Glycobiology 1999, 9, 31–41. [Google Scholar] [CrossRef]
- Karnezis, T.; Fisher, H.C.; Neumann, G.M.; Stone, B.A.; Stanisich, V.A. Cloning and characterization of the phosphatidylserine synthase gene of Agrobacterium sp. ATCC 31749 and effect of its inactivation on production of high-molecular-mass (1→3)-β-D-glucan (curdlan). J. Bacteriol. 2002, 184, 4114–4123. [Google Scholar] [CrossRef]
- Zhang, H.; Setubal, J.C.; Zhan, X.; Zheng, Z.; Yu, L.; Wu, J.; Chen, D. Component identification of electron transport chains in curdlan-producing Agrobacterium sp. ATCC 31749 and its genome-specific prediction using comparative genome and phylogenetic tree analysis. J. Ind. Microbiol. Biotechnol. 2011, 38, 667–677. [Google Scholar] [CrossRef]
- Jin, L.-H.; Um, H.-J.; Yin, C.-J.; Kim, Y.-H.; Lee, J.-H. Proteomic analysis of curdlan-producing Agrobacterium sp. in response to pH downshift. J. Biotechnol. 2008, 138, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.-J.; Wu, J.-R.; Zheng, Z.-Y.; Zhan, X.-B.; Lin, C.C. Changes in curdlan biosynthesis and nitrogenous compounds utilization characterized in ntrC mutant of Agrobacterium sp. ATCC 31749. Curr. Microbiol. 2011, 63, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, A.; Ma, F.; Yang, J.; Xie, Y. Genetic control and regulatory mechanisms of succinoglycan and curdlan biosynthesis in genus Agrobacterium. Appl. Microbiol. Biotechnol. 2016, 100, 6183–6192. [Google Scholar] [CrossRef] [PubMed]
- West, T.P. Polysaccharide production by an Agrobacterium sp. curdlan overproducer mutant on a grain fermentation coproduct. Res. J. Microbiol. 2012, 7, 273–279. [Google Scholar] [CrossRef]
- Anane, R.F.; Sun, H.; Zhao, L.; Wang, L.; Lin, C.; Mao, Z. Improved curdlan production with discarded bottom parts of asparagus spear. Microb. Cell Factories 2017, 16, 59. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, L.; Ding, H.; Gao, M.; Zheng, Z.; Wu, J.; Zhan, X. Enhanced production of curdlan by coupled fermentation system of Agrobacterium sp. ATCC 31749 and Trichoderma harzianum GIM 3.442. Carbohydr. Polym. 2017, 157, 1687–1694. [Google Scholar] [CrossRef]
- West, T.P. Effect of nitrogen source concentration on curdlan production by Agrobacterium sp. ATCC 31749 grown on prairie cordgrass hydrolysates. Prep. Biochem. Biotechnol. 2016, 46, 85–90. [Google Scholar] [CrossRef]
- West, T.P.; Peterson, J.L. Production of the polysaccharide curdlan by an Agrobacterium strain grown on a plant biomass hydrolysate. Can. J. Microbiol. 2014, 60, 53–56. [Google Scholar] [CrossRef]
- Zhang, Y.-H.P. Reviving the carbohydrate economy via multi-product lignocellulose biorefineries. J. Ind. Microbiol. Biotechnol. 2008, 35, 367–375. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, Z.; Labavitch, J.M.; Wang, D.; Teter, S.A.; Jenkins, B.M. Evaluation of different biomass materials as feedstock for fermentable sugar production. In Applied Biochemistry and Biotechnology; Humana Press: Totowa, NJ, USA, 2007; Volume 136–140, pp. 423–435. [Google Scholar]
- Shin, H.-D.; Liu, L.; Kim, M.-K.; Park, Y.-I.; Chen, R. Metabolic engineering of Agrobacterium sp. ATCC31749 for curdlan production from cellobiose. J. Ind. Microbiol. Biotechnol. 2016, 43, 1323–1331. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).