Effect of Sequential Inoculation with Non-Saccharomyces and Saccharomyces Yeasts on Riesling Wine Chemical Composition
Abstract
1. Introduction
2. Materials and Method
2.1. Yeast Strains
2.2. Vinification
2.3. Fermentation Kinetics
2.4. Chemical Compounds Analysis
2.4.1. HPLC
2.4.2. FTIR
2.5. Volatile Compounds Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Fermentation Kinetics
3.1.1. Yeast Population Kinetics
3.1.2. Alcoholic Fermentation Kinetics
3.2. Chemical Monitoring
3.3. Volatile Compounds
3.3.1. Esters
3.3.2. Higher Alcohols
3.3.3. Fatty Acids
3.3.4. Terpenes
3.3.5. Low Volatile Sulfur Compounds
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rapp, A.; Mandery, H. ChemInform Abstract: Wine Aroma. Chem. Informationsd. 1986, 42, 873. [Google Scholar] [CrossRef]
- Fischer, U. Wine aroma. In Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 9783540493389. [Google Scholar]
- German, J.B.; Yeritzian, C.; Tolstoguzov, V.B. Olfaction, where nutrition, memory and immunity intersect. In Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 9783540493389. [Google Scholar]
- Martin, D.M.; Chiang, A.; Lund, S.T.; Bohlmann, J. Biosynthesis of wine aroma: Transcript profiles of hydroxymethylbutenyl diphosphate reductase, geranyl diphosphate synthase, and linalool/nerolidol synthase parallel monoterpenol glycoside accumulation in Gewürztraminer grapes. Planta 2012, 236, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Schüttler, A.; Friedel, M.; Jung, R.; Rauhut, D.; Darriet, P. Characterizing aromatic typicality of riesling wines: Merging volatile compositional and sensory aspects. Food Res. Int. 2015, 69, 26–37. [Google Scholar] [CrossRef]
- Tominaga, T.; Masneuf, I.; Dubourdieu, D. Powerful Aromatic Volatile Thiols in Wines Made from Several Vitis vinifera Grape Varieties and Their Releasing Mechanism. In Nutraceutical Beverages: Chemistry, Nutrition, and Health Effects; American Chemical Society: Washington, DC, USA, 2009. [Google Scholar]
- Peña-Gallego, A.; Hernández-Orte, P.; Cacho, J.; Ferreira, V. S-Cysteinylated and S-glutathionylated thiol precursors in grapes. A review. Food Chem. 2012, 131, 1–13. [Google Scholar] [CrossRef]
- Tominaga, T.; Murat, M.-L.; Dubourdieu, D. Development of a Method for Analyzing the Volatile Thiols Involved in the Characteristic Aroma of Wines Made from Vitis vinifera L. Cv. Sauvignon Blanc. J. Agric. Food Chem. 1998, 46, 1044–1048. [Google Scholar] [CrossRef]
- Roujou de Boubee, D.; Van Leeuwen, C.; Dubourdieu, D. Organoleptic impact of 2-methoxy-3-isobutylpyrazine on red Bordeaux and Loire wines. Effect of environmental conditions on concentrations in grapes during ripening. J. Agric. Food Chem. 2000, 48, 4830–4834. [Google Scholar] [CrossRef] [PubMed]
- Rusjan, D.; Strlič, M.; Košmerl, T.; Prosen, H. The response of monoterpenes to different enzyme preparations in Gewürztraminer (Vitis vinifera L.) wines. S. Afr. J. Enol. Vitic. 2009, 30, 56–64. [Google Scholar] [CrossRef][Green Version]
- Gamero, A.; Quintilla, R.; Groenewald, M.; Alkema, W.; Boekhout, T.; Hazelwood, L. High-throughput screening of a large collection of non-conventional yeasts reveals their potential for aroma formation in food fermentation. Food Microbiol. 2016, 60, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Ruiz, J.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Benito, S.; Santos, A. Influence of Torulaspora delbrueckii in varietal thiol (3-SH and 4-MSP) release in wine sequential fermentations. Int. J. Food Microbiol. 2017, 257, 183–191. [Google Scholar] [CrossRef]
- Ruiz, J.; Belda, I.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Santos, A.; Benito, S. Analytical impact of Metschnikowia pulcherrima in the volatile profile of Verdejo white wines. Appl. Microbiol. Biotechnol. 2018, 102, 8501–8509. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.; Bartowsky, E.; Jiranek, V. Screening of Lactobacillus spp. and Pediococcus spp. for glycosidase activities that are important in oenology. J. Appl. Microbiol. 2005, 95, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Saerens, S.M.G.; Verstrepen, K.J.; Van Laere, S.D.M.; Voet, A.R.D.; Van Dijck, P.; Delvaux, F.R.; Thevelein, J.M. The Saccharomyces cerevisiae EHT1 and EEB1 genes encode novel enzymes with medium-chain fatty acid ethyl ester synthesis and hydrolysis capacity. J. Biol. Chem. 2006, 281, 4446–4456. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.M.; Swiegers, J.H.; Varela, C.; Pretorius, I.S.; Agosin, E. Influence of wine fermentation temperature on the synthesis of yeast-derived volatile aroma compounds. Appl. Microbiol. Biotechnol. 2007, 77, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Ugliano, M.; Henschke, P.A. Yeasts and wine flavour. In Wine Chemistry and Biochemistry; Springer: New York, NY, USA, 2009; ISBN 9780387741161. [Google Scholar]
- Benito, S. The impacts of Schizosaccharomyces on winemaking. Appl. Microbiol. Biotechnol. 2019, 103, 4291–4312. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.; Palomero, F.; Morata, A.; Calderón, F.; Suárez-Lepe, J.A. A method for estimating Dekkera/Brettanomyces populations in wines. J. Appl. Microbiol. 2009, 106, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.; Farías, M.E. Improvement of wine organoleptic characteristics by non-Saccharomyces yeasts. Appl. Microbiol. 2010, 2, 908–919. [Google Scholar]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef]
- Contreras, A.; Curtin, C.; Varela, C. Yeast population dynamics reveal a potential ‘collaboration’ between Metschnikowia pulcherrima and Saccharomyces uvarum for the production of reduced alcohol wines during Shiraz fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 1885–1895. [Google Scholar] [CrossRef]
- Maturano, Y.P.; Mestre, M.V.; Kuchen, B.; Toro, M.E.; Mercado, L.A.; Vazquez, F.; Combina, M. Optimization of fermentation-relevant factors: A strategy to reduce ethanol in red wine by sequential culture of native yeasts. Int. J. Food Microbiol. 2019, 289, 40–48. [Google Scholar] [CrossRef]
- Andorra, I.; Monteiro, M.; Esteve-Zarzoso, B.; Albergaria, H.; Mas, A. Analysis and direct quantification of Saccharomyces cerevisiae and Hanseniaspora guilliermondii populations during alcoholic fermentation by fluorescence in situ hybridization, flow cytometry and quantitative PCR. Food Microbiol. 2011, 28, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.L.; Fierro-Risco, J.; Ríos-Reina, R.; Ubeda, C.; Paneque, P. Influence of Saccharomyces cerevisiae and Lachancea thermotolerans co-inoculation on volatile profile in fermentations of a must with a high sugar content. Food Chem. 2019, 276, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Benito, S. The impact of Torulaspora delbrueckii yeast in winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 3081–3094. [Google Scholar] [CrossRef] [PubMed]
- Benito, S. The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 6775–6790. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Schneider, A.; Gerbi, V.; Redoglia, M. A Rapid HPLC Method for Separation and Determination of Major Organic Acids in Grape Musts and Wines. Am. J. Enol. Vitic. 1987, 38, 151–155. [Google Scholar]
- Semmler, H.; Sponholz, W.-R.; Rauhut, D. Standard Operating Procedure (SOP) for the Analysis of Organic Acids in Wines by HPLC; Hochschule Geisenheim University: Geisenheim, Germany, 2017. [Google Scholar]
- Baumgartner, D.; Bill, R.; Roth, I. Traubenmostanalyse mit Hilfe der. FTIR-Spektroskopie. Schweizerische Zeitschrift Obs. Weinbau 2001, 2, 46–48. [Google Scholar]
- Patz, C.D.; David, A.; Thente, K.; Kuerbel, P.; Dietrich, H. Wine analysis with FTIR spectrometry. Wein-Wiss. Vitic. Enol. Sci. 1999, 54, 80–87. [Google Scholar]
- Rapp, A.; Yavas, I.; Hastrich, H. Einfache und schnelle Anreicherung (“Kaltronmethode”) von Aromastoffen des Weines und deren quantitative Bestimmung mittels Kapillargaschromatographie. Dtsch. Leb. 1994, 90, 171–174. [Google Scholar]
- Fritsch, S.; Brezina, S.; Ebert, K.; Rauhut, D. Standard Operating Procedure (SOP) for the Analysis of the Fermentation Bouquet in Wines; Department of Microbiology Biochemistry Hochschule Geisenheim University: Geisenheim, Germany, 2017. [Google Scholar]
- Fritsch, S.; Ebert, K.; Brand, M.; Rauhut, D. Standard Operating Procedure (SOP) for the Analysis of Terpens and Norisoprenoids in Wines; Department of Microbiology Biochemistry Hochschule Geisenheim University: Geisenheim, Germany, 2017. [Google Scholar]
- Rauhut, D.; Beisert, B.; Berres, M.; Gawron-Scibek, M.; Kürbel, H. Pulse flame photometric detection: An innovative technique to analyse volatile sulfur compounds in wine and other beverages. In State-of-the-Art in Flavour Chemistry and Biology; Hofmann, T., Rothe, M., Schieberle, P., Eds.; Deutsche Forschungsanstalt für Lebensmittelchemie: Garching, Germany, 2005; pp. 363–367. ISBN 3-00-015809-X. [Google Scholar]
- Rauhut, D.; Beisert, B. Standard Operating Procedure (SOP) for the Analysis of Low Volatile Sulfur Compounds Using Headspace and GC-PFPD; Department of Microbiology Biochemistry Hochschule Geisenheim University: Geisenheim, Germany, 2017. [Google Scholar]
- Taillandier, P.; Lai, Q.P.; Julien-Ortiz, A.; Brandam, C. Interactions between Torulaspora delbrueckii and Saccharomyces cerevisiae in wine fermentation: Influence of inoculation and nitrogen content. World J. Microbiol. Biotechnol. 2014, 30, 1959–1967. [Google Scholar] [CrossRef]
- Gutiérrez, A.R.; Santamaría, P.; Epifanio, S.; Garijo, P.; López, R. Ecology of spontaneous fermentation in one winery during 5 consecutive years. Lett. Appl. Microbiol. 1999, 29, 411–415. [Google Scholar] [CrossRef]
- Nissen, P.; Neilsen, D.; Arneborg, N. The relative glucose uptake abilities of non-Saccharomyces yeasts play a role in their coexistence with Saccharomyces cerevisiae in mixed cultures. Appl. Microbiol. Biotechnol. 2004, 64, 543–550. [Google Scholar] [CrossRef]
- Stratford, M.; Anslow, P.A. Comparison of the inhibitory action on Saccharomyces cerevisiae of weak- acid preservatives, uncouplers, and medium-chain fatty acids. FEMS Microbiol. Lett. 1996, 142, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Stanley, G.A.; Douglas, N.G.; Every, E.J.; Tzanatos, T.; Pamment, N.B. Inhibition and stimulation of yeast growth by acetaldehyde. Biotechnol. Lett. 1993, 15, 1199–1204. [Google Scholar] [CrossRef]
- Palfree, R.G.E.; Bussey, H. Yeast Killer Toxin: Purification and Characterisation of the Protein Toxin from Saccharomyces cerevisiae. Eur. J. Biochem. 1979, 93, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; San Mauro, M.; Bravo, E.; Marquina, D. PMKT2, a new killer toxin from Pichia membranifaciens, and its promising biotechnological properties for control of the spoilage yeast Brettanomyces bruxellensis. Microbiology 2009, 155, 624–634. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Alonso, A.; Marquina, D.; Santos, A. The biology of Pichia membranifaciens killer toxins. Toxins 2017, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Albergaria, H.; Francisco, D.; Gori, K.; Arneborg, N.; Gírio, F. Saccharomyces cerevisiae CCMI 885 secretes peptides that inhibit the growth of some non-Saccharomyces wine-related strains. Appl. Microbiol. Biotechnol. 2010, 86, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Holzberg, I.; Finn, R.K.; Steinkraus, K.H. A kinetic study of the alcoholic fermentation of grape juice. Biotechnol. Bioeng. 1967, 9, 413–427. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Azzolini, M.; Tosi, E.; Lorenzini, M.; Finato, F.; Zapparoli, G. Contribution to the aroma of white wines by controlled Torulaspora delbrueckii cultures in association with Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2015, 31, 277–293. [Google Scholar] [CrossRef]
- Sun, S.Y.; Gong, H.S.; Jiang, X.M.; Zhao, Y.P. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae on alcoholic fermentation behaviour and wine aroma of cherry wines. Food Microbiol. 2014, 44, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Mallet, S.; Arellano, M.; Boulet, J.C.; Couderc, F. Determination of tartaric acid in solid wine residues by capillary electrophoresis and indirect UV detection. J. Chromatogr. A 1999, 853, 181–184. [Google Scholar] [CrossRef]
- Gao, C.; Fleet, G.H. Degradation of malic and tartaric acids by high density cell suspensions of wine yeasts. Food Microbiol. 1995, 12, 65–71. [Google Scholar] [CrossRef]
- Seo, S.-H.; Rhee, C.-H.; Park, H.-D. Degradation of malic acid by Issatchenkia orientalis KMBL 5774, an acidophilic yeast strain isolated from Korean grape wine pomace. J. Microbiol. 2007, 45, 521–527. [Google Scholar] [PubMed]
- Kim, D.H.; Hong, Y.A.; Park, H.D. Co-fermentation of grape must by Issatchenkia orientalis and Saccharomyces cerevisiae reduces the malic acid content in wine. Biotechnol. Lett. 2008, 30, 1633–1688. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.K.; Lee, H.J.; Park, H.J.; Hong, Y.A.; Rhee, I.K.; Lee, W.H.; Choi, S.W.; Lee, O.S.; Park, H.D. Degradation of malic acid in wine by immobilized Issatchenkia orientalis cells with oriental oak charcoal and alginate. Lett. Appl. Microbiol. 2010, 50, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.; Palomero, F.; Gálvez, L.; Morata, A.; Calderón, F.; Palmero, D.; Suárez-Lepe, J.A. Quality and composition of red wine fermented with Schizosaccharomyces pombe as sole fermentative yeast, and in mixed and sequential fermentations with Saccharomyces cerevisiae. Food Technol. Biotechnol. 2014, 52, 376. [Google Scholar]
- Kapsopoulou, K.; Kapaklis, A.; Spyropoulos, H. Growth and fermentation characteristics of a strain of the wine yeast Kluyveromyces thermotolerans isolated in Greece. World J. Microbiol. Biotechnol. 2005, 21, 1599–1602. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Benito, S. Combined use of S. pombe and L. thermotolerans in winemaking. Beneficial effects determined through the study of wines’ analytical characteristics. Molecules 2016, 21, 1744. [Google Scholar] [CrossRef] [PubMed]
- Escribano, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine aromatic compound production and fermentative behaviour within different non-Saccharomyces species and clones. J. Appl. Microbiol. 2018, 124, 1521–1531. [Google Scholar] [CrossRef]
- Escribano-Viana, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine aroma evolution throughout alcoholic fermentation sequentially inoculated with non-Saccharomyces/Saccharomyces yeasts. Food Res. Int. 2018, 112, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bartowsky, E.J.; Henschke, P.A. Acetic acid bacteria spoilage of bottled red wine—A review. Int. J. Food Microbiol. 2008, 125, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Sadoudi, M.; Tourdot-Maréchal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacón, J.J.; Ballester, J.; Vichi, S.; Guérin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast-yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Hidalgo, C.; Henschke, P.A.; Chambers, P.J.; Curtin, C.; Varela, C. Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl. Environ. Microbiol. 2014, 80, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Vilela, A. Lachancea thermotolerans, the Non-Saccharomyces Yeast that Reduces the Volatile Acidity of Wines. Fermentation 2018, 4, 56. [Google Scholar] [CrossRef]
- Porter, T.J.; Divol, B.; Setati, M.E. Lachancea yeast species: Origin, biochemical characteristics and oenological significance. Food Res. Int. 2019, 119, 378–389. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef]
- García, M.; Esteve-Zarzoso, B.; Arroyo, T. Non-Saccharomyces Yeasts: Biotechnological Role for Wine Production. In Grape and Wine Biotechnology; InTech: London, UK, 2016. [Google Scholar]
- du Plessis, H.; du Toit, M.; Nieuwoudt, H.; van der Rijst, M.; Kidd, M.; Jolly, N. Effect of Saccharomyces, Non-Saccharomyces Yeasts and Malolactic Fermentation Strategies on Fermentation Kinetics and Flavor of Shiraz Wines. Fermentation 2017, 3, 64. [Google Scholar] [CrossRef]
- García, M.; Esteve-Zarzoso, B.; Cabellos, J.; Arroyo, T. Advances in the Study of Candida stellata. Fermentation 2018, 4, 74. [Google Scholar] [CrossRef]
- Zohre, D.E.; Erten, H. The influence of Kloeckera apiculata and Candida pulcherrima yeasts on wine fermentation. Process Biochem. 2002, 38, 319–324. [Google Scholar] [CrossRef]
- Clemente-Jimenez, J.M.; Mingorance-Cazorla, L.; Martínez-Rodríguez, S.; Las Heras-Vázquez, F.J.; Rodríguez-Vico, F. Molecular characterization and oenological properties of wine yeasts isolated during spontaneous fermentation of six varieties of grape must. Food Microbiol. 2004, 21, 149–155. [Google Scholar] [CrossRef]
- Varela, C.; Sengler, F.; Solomon, M.; Curtin, C. Volatile flavour profile of reduced alcohol wines fermented with the non-conventional yeast species Metschnikowia pulcherrima and Saccharomyces uvarum. Food Chem. 2016, 29, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Navascués, E.; Marquina, D.; Santos, A.; Calderon, F.; Benito, S. Dynamic analysis of physiological properties of Torulaspora delbrueckii in wine fermentations and its incidence on wine quality. Appl. Microbiol. Biotechnol. 2015, 99, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Amaya-Delgado, L.; Herrera-López, E.J.; Arrizon, J.; Arellano-Plaza, M.; Gschaedler, A. Performance evaluation of Pichia kluyveri, Kluyveromyces marxianus and Saccharomyces cerevisiae in industrial tequila fermentation. World J. Microbiol. Biotechnol. 2013, 29, 875–881. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, D.; Lee, P.R.; Liu, S.Q. Assessment of volatile and non-volatile compounds in durian wines fermented with four commercial non-Saccharomyces yeasts. J. Sci. Food Agric. 2016, 96, 1511–1521. [Google Scholar] [CrossRef]
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascués, E.; Calderón, F.; Benito, A.; Rauhut, D.; Benito, S. Effects on varietal aromas during wine making: A review of the impact of varietal aromas on the flavor of wine. Appl. Microbiol. Biotechnol. 2019, 1–26. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes—A review. Green Chem. 2011, 13, 2658–2671. [Google Scholar] [CrossRef]
- Mestres, M.; Busto, O.; Guasch, J. Analysis of organic sulfur compounds in wine aroma. J. Chromatogr. A 2000, 881, 569–581. [Google Scholar] [CrossRef]
- Jiranek, V.; Langridge, P.; Henschke, P.A. Regulation of hydrogen sulfide liberation in wine-producing Saccharomyces cerevisiae strains by assimilable nitrogen. Appl. Environ. Microbiol. 1995, 61, 461–467. [Google Scholar]
- Mendes-Ferreira, A.; Mendes-Faia, A.; Leão, C. Survey of hydrogen sulphide production by wine yeasts. J. Food Prot. 2002, 65, 1033–1037. [Google Scholar] [CrossRef]
- Renault, P.; Miot-Sertier, C.; Marullo, P.; Hernández-Orte, P.; Lagarrigue, L.; Lonvaud-Funel, A.; Bely, M. Genetic characterization and phenotypic variability in Torulaspora delbrueckii species: Potential applications in the wine industry. Int. J. Food Microbiol. 2009, 134, 201–210. [Google Scholar] [CrossRef]
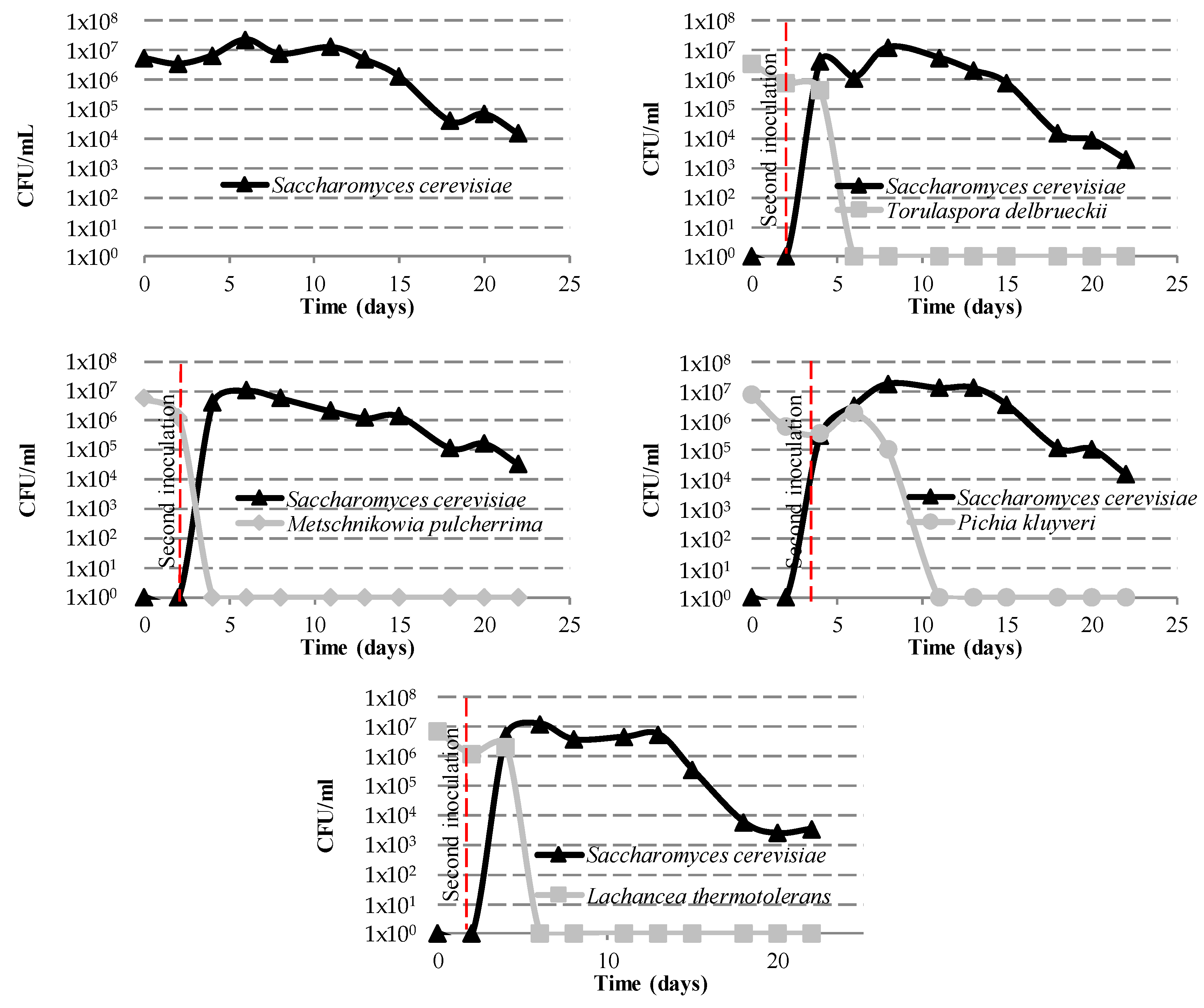
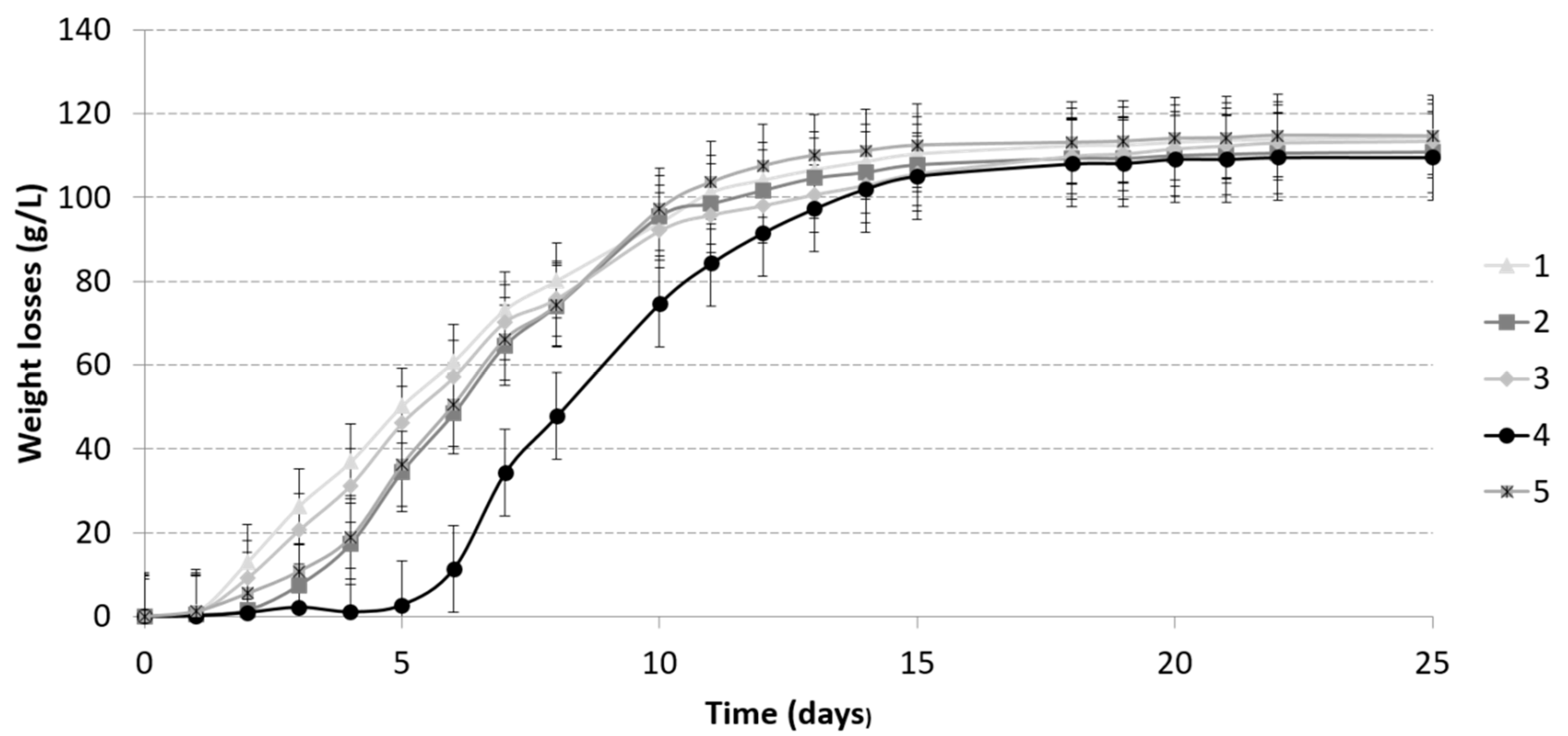
| Compounds | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Tartaric acid (g/L) | 4.72 ± 0.18a | 4.38 ± 0.02b | 4.43 ± 0.01b | 4.43 ± 0.01b | 4.63 ± 0.28ab |
| Malic acid (g/L) | 2.28 ± 0.00d | 2.21 ± 0.00c | 2.10 ± 0.01a | 2.21 ± 0.01c | 2.17 ± 0.01b |
| Shikimic acid (mg/L) | 50.19 ± 0.06b | 49.94 ± 0.36ab | 49.36 ± 0.25a | 49.86 ± 0.34ab | 49.64 ± 0.37ab |
| Lactic acid (g/L) | 0.21 ± 0.01a | 0.17 ± 0.01a | 0.21 ± 0.01a | 0.19 ± 0.00a | 1.51 ± 0.04b |
| Acetic acid (g/L) | 0.25 ± 0.03ab | 0.21 ± 0.03a | 0.30 ± 0.03b | 0.23 ± 0.02a | 0.31 ± 0.02b |
| Citric acid (g/L) | 0.14 ± 0.02a | 0.14 ± 0.01a | 0.14 ± 0.00a | 0.15 ± 0.02a | 0.13 ± 0.01a |
| Residual sugars (g/L) | 4.4 ± 0.25b | 2.8 ± 0.23a | 4.5 ± 0.30b | 2.9 ± 0.16a | 3.0 ± 0.11a |
| pH | 3.1 ± 0.00a | 3.2 ± 0.00b | 3.2 ± 0.00b | 3.2 ± 0.00b | 3.1 ± 0.00a |
| Ethanol (% v/v) | 13.20 ± 0.19b | 13.17 ± 0.44b | 12.98 ± 0.35a | 13.04 ± 0.28a | 12.96 ± 0.31a |
| Glycerol (g/L) | 5.8 ± 0.04a | 6.6 ± 0.04b | 7.0 ± 0.05c | 7.1 ± 0.05c | 7.4 ± 0.07d |
| Compounds | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Esters | |||||
| Ethyl esters | |||||
| Ethyl lactate (mg/L) | nq | nq | nq | nq | 52.26 ± 3.27 |
| i-Ethyl butanoate (µg/L) | nd | nd | nd | nd | nd |
| Ethyl butanoate (µg/L) | 432.19 ± 31.18b | 308.35 ± 16.36a | 332.37 ± 20.24a | 278.46 ± 26.92a | 304.86 ± 15.42a |
| Ethyl hexanoate (µg/L) | 1876.06 ± 69.54bc | 1798.42 ± 45.10b | 1994.32 ± 45.97c | 1551.1898.47a | 1518.12 ± 28.25a |
| Ethyl octanoate (µg/L) | 1472.32 ± 89.10ab | 1367.63 ± 33.80a | 1672.57 ± 186.55b | 1440.66 ± 122.99ab | 1364.97 ± 96.58a |
| Ethyl decanoate (µg/L) | 442.63 ± 57.49a | 437.23 ± 37.76a | 553.12 ± 87.48a | 546.90 ± 60.22a | 581.35 ± 47.92a |
| Diethyl succinate (µg/L) | nq | nq | nq | nq | nq |
| Ethyl 2-hydroxy-4-methyl valerate (µg/L) | nq | nq | nq | nq | nq |
| Total ethyl esters (µg/L) | 4223.20 ± 152.74a | 3911.63 ± 18.66a | 4552.38 ± 305.74a | 3817.20 ± 282.13a | 56031.95 ± 3282.57b |
| Acetates | |||||
| Ethyl acetate (mg/L) | 159.18 ± 7.20b | 135.02 ± 9.10a | 115.53 ± 6.80a | 184.01 ± 5.02c | 127.97 ± 10.25a |
| Isoamyl acetate and 2-methyl butyl acetate (µg/L) | 3927.41 ± 186.64a | 4446.21 ± 205.56bc | 3779.80 ± 90.54a | 4751.71 ± 257.79c | 4154.59 ± 163.59ab |
| Hexyl acetate (µg/L) | 694.15 ± 29.53b | 666.82 ± 11.07b | 626.61 ± 38.94ab | 590.44 ± 34.53a | 592.86 ± 23.73a |
| Ethyl phenylacetate (µg/L) | nq | nq | nq | nq | nq |
| 2-Phenyl-ethyl acetate (µg/L) | 429.43 ± 9.15b | 510.21 ± 16.05c | 352.56 ± 14.12a | 885.06 ± 28.39d | 375.91 ± 8.12a |
| Total acetates (µg/L) | 164229.49 ± 7358.44c | 140647.20 ± 9289.39b | 120290.10 ± 6811.04a | 190236.09 ± 5279.61d | 133095.47 ± 10391.01ab |
| Total esters (µg/L) | 168452.69 ± 7369.92b | 144558.83 ± 9282.59a | 124842.48 ± 6791.85a | 194053.29 ± 5518.21c | 189127.42 ± 9985.27c |
| Higher alcohols | |||||
| 3-Methyl-butanol and 2-methyl-butanol (mg/L) | 188.62 ± 9.08a | 230.96 ± 8.93b | 212.96 ± 11.85ab | 224.83 ± 15.01b | 212.78 ± 10.67ab |
| 2-Phenyl-ethanol (mg/L) | 13.74 ± 1.20a | 23.42 ± 0.95c | 18.00 ± 0.60b | 28.62 ± 1.39d | 23.38 ± 1.00c |
| Hexanol (µg/L) | 1131.24 ± 37.40b | 1208.40 ± 53.76bc | 1277.45 ± 71.62c | 677.55 ± 15.71a | 1415.87 ± 43.94d |
| Total higher alcohols (mg/L) | 203.48 ± 9.95a | 255.59 ± 9.61b | 232.23 ± 11.84ab | 254.14 ± 15.91b | 237.58 ± 11.63b |
| Fatty acids | |||||
| Hexanoic acid (mg/L) | 11.13 ± 0.30cd | 10.78 ± 0.17bc | 11.38 ± 0.14d | 10.41 ± 0.15ab | 9.90 ± 0.21ab |
| Octanoic acid (mg/L) | 11.99 ± 0.20c | 10.96 ± 0.25bc | 11.56 ± 0.75c | 10.31 ± 0.35b | 9.05 ± 0.41a |
| Decanoic acid (µg/L) | 4209.93 ± 141.19c | 3975.70 ± 119.78bc | 4233.22 ± 178.75c | 3434.45 ± 229.12a | 3718.80 ± 67.31ab |
| i-Valeric acid (µg/L) | 1446.64 ± 12.53a | 1493.16 ± 21.54a | 1458.47 ± 20.22a | 2093.35 ± 19.51b | 1491.55 ± 19.36a |
| Total fatty acids (mg/L) | 28.79 ± 0.38c | 27.21 ± 0.39bc | 28.64 ± 1.03c | 26.26 ± 0.67b | 24.15 ± 0.51a |
| Terpenes | |||||
| Linalool oxide 1 (µg/L) | nq | nq | nq | nq | nq |
| Linalool oxide 2 (µg/L) | nq | nq | nq | nq | nq |
| Linalool (µg/L) | 54.52 ± 1.96a | 56.06 ± 1.16a | 56.97 ± 1.49a | 56.61 ± 2.17a | 57.85 ± 2.54a |
| α-Terpineol (µg/L) | 37.27 ± 0.64a | 37.46 ± 0.89a | 36.92 ± 0.61a | 38.51 ± 0.90a | 37.81 ± 0.60a |
| Total terpenes (µg/L) | 91.80 ± 2.15a | 93.52 ± 1.05a | 93.89 ± 2.01a | 95.12 ± 2.65a | 95.65 ± 2.91a |
| Low volatile sulfur compounds | |||||
| H2S | 3.48 ± 028a | 5.85 ± 0.15b | 6.28 ± 0.29bc | 7.65 ± 0.87cd | 7.78 ± 0.82d |
| MeSH | nq | nq | nq | nq | nq |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutraive, O.; Benito, S.; Fritsch, S.; Beisert, B.; Patz, C.-D.; Rauhut, D. Effect of Sequential Inoculation with Non-Saccharomyces and Saccharomyces Yeasts on Riesling Wine Chemical Composition. Fermentation 2019, 5, 79. https://doi.org/10.3390/fermentation5030079
Dutraive O, Benito S, Fritsch S, Beisert B, Patz C-D, Rauhut D. Effect of Sequential Inoculation with Non-Saccharomyces and Saccharomyces Yeasts on Riesling Wine Chemical Composition. Fermentation. 2019; 5(3):79. https://doi.org/10.3390/fermentation5030079
Chicago/Turabian StyleDutraive, Ophélie, Santiago Benito, Stefanie Fritsch, Beata Beisert, Claus-Dieter Patz, and Doris Rauhut. 2019. "Effect of Sequential Inoculation with Non-Saccharomyces and Saccharomyces Yeasts on Riesling Wine Chemical Composition" Fermentation 5, no. 3: 79. https://doi.org/10.3390/fermentation5030079
APA StyleDutraive, O., Benito, S., Fritsch, S., Beisert, B., Patz, C.-D., & Rauhut, D. (2019). Effect of Sequential Inoculation with Non-Saccharomyces and Saccharomyces Yeasts on Riesling Wine Chemical Composition. Fermentation, 5(3), 79. https://doi.org/10.3390/fermentation5030079





