Determination of Nutrient Supplementation by Means of ATR-FTIR Spectroscopy during Wine Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Nutrient Additions and Enological Analysis
2.2. Infrared Spectroscopy Measurements
2.3. Statistical and Chemometric Analyses
3. Results and Discussion
3.1. Enological Parameters and Fermentation Process
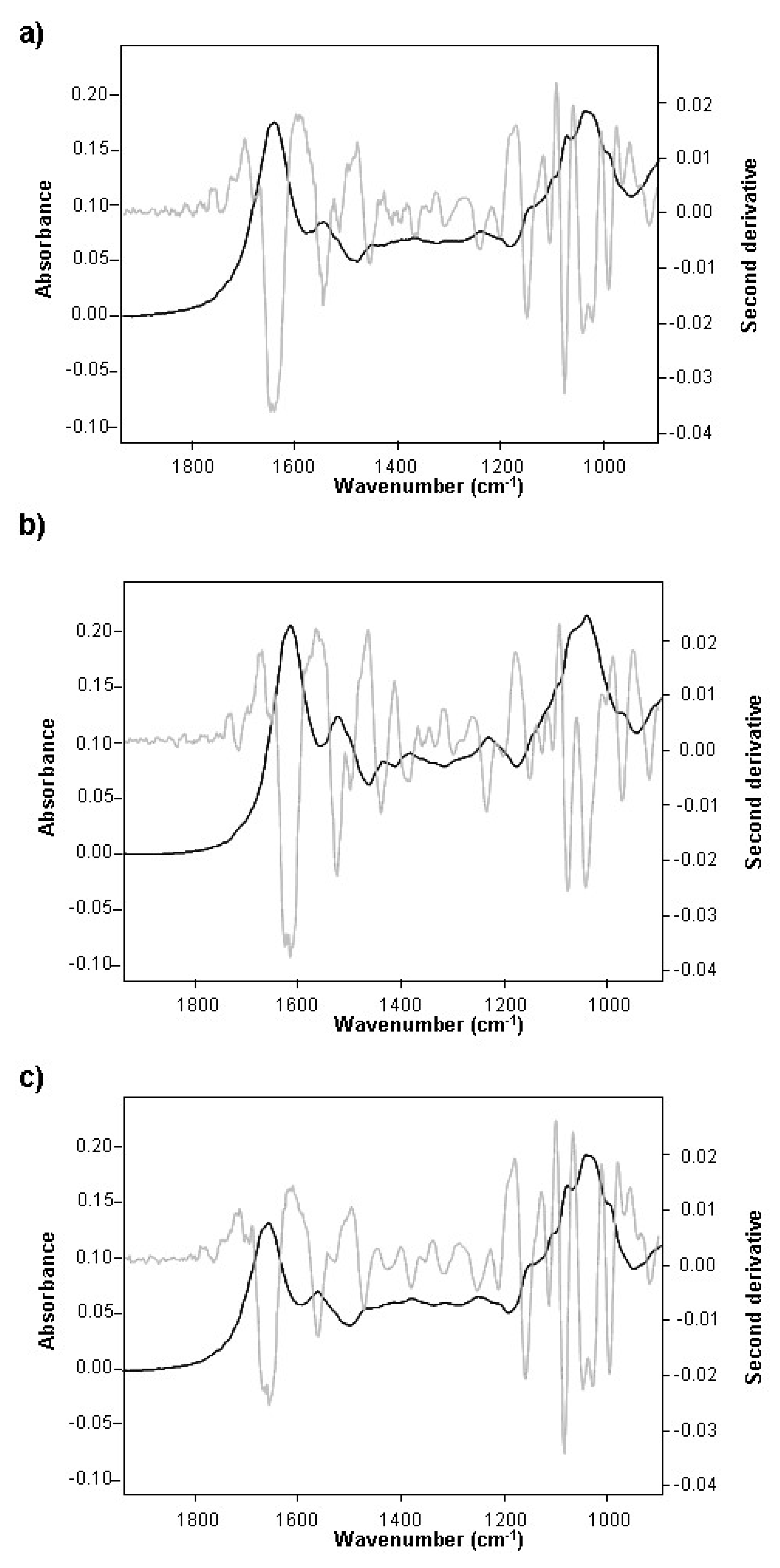
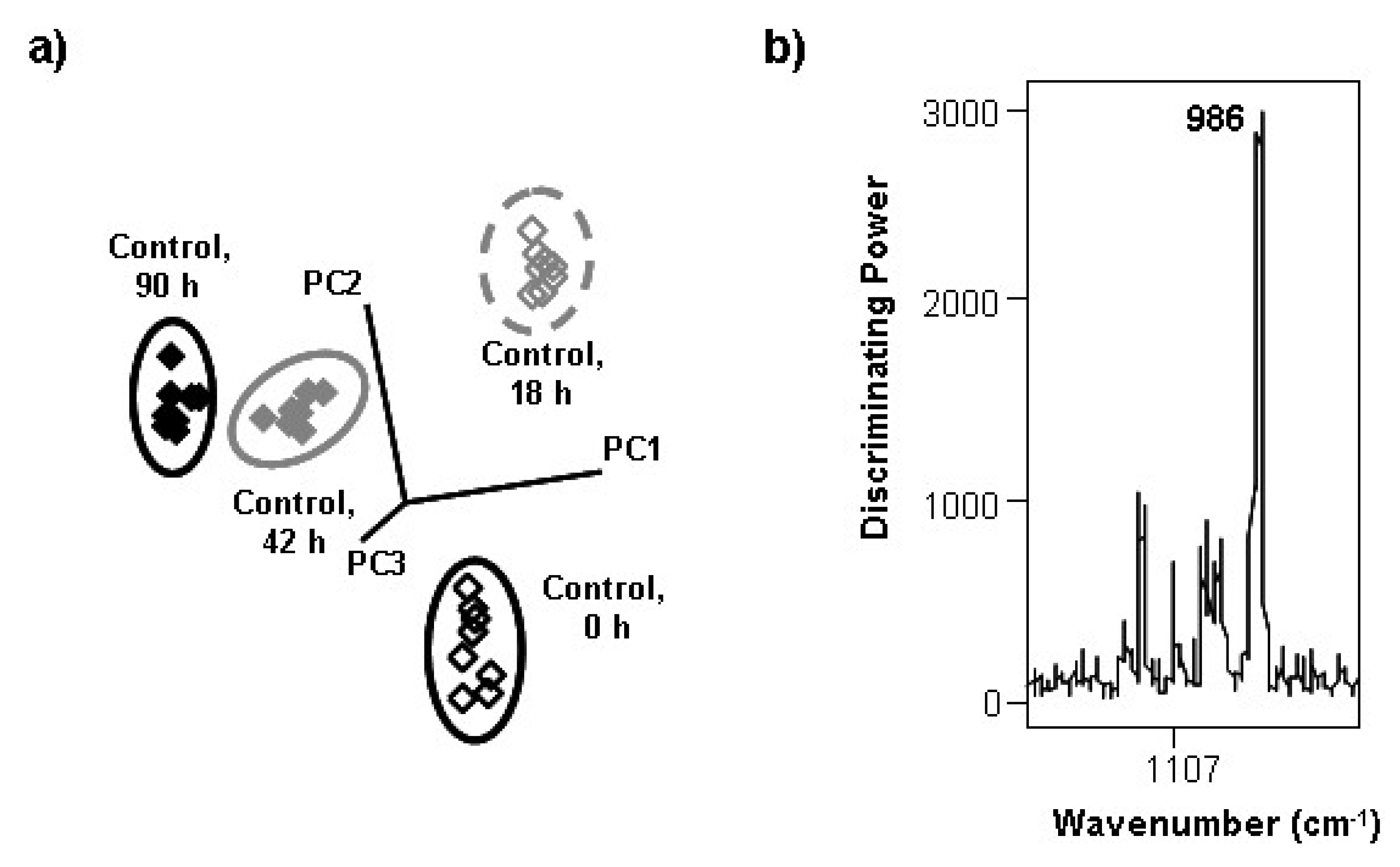
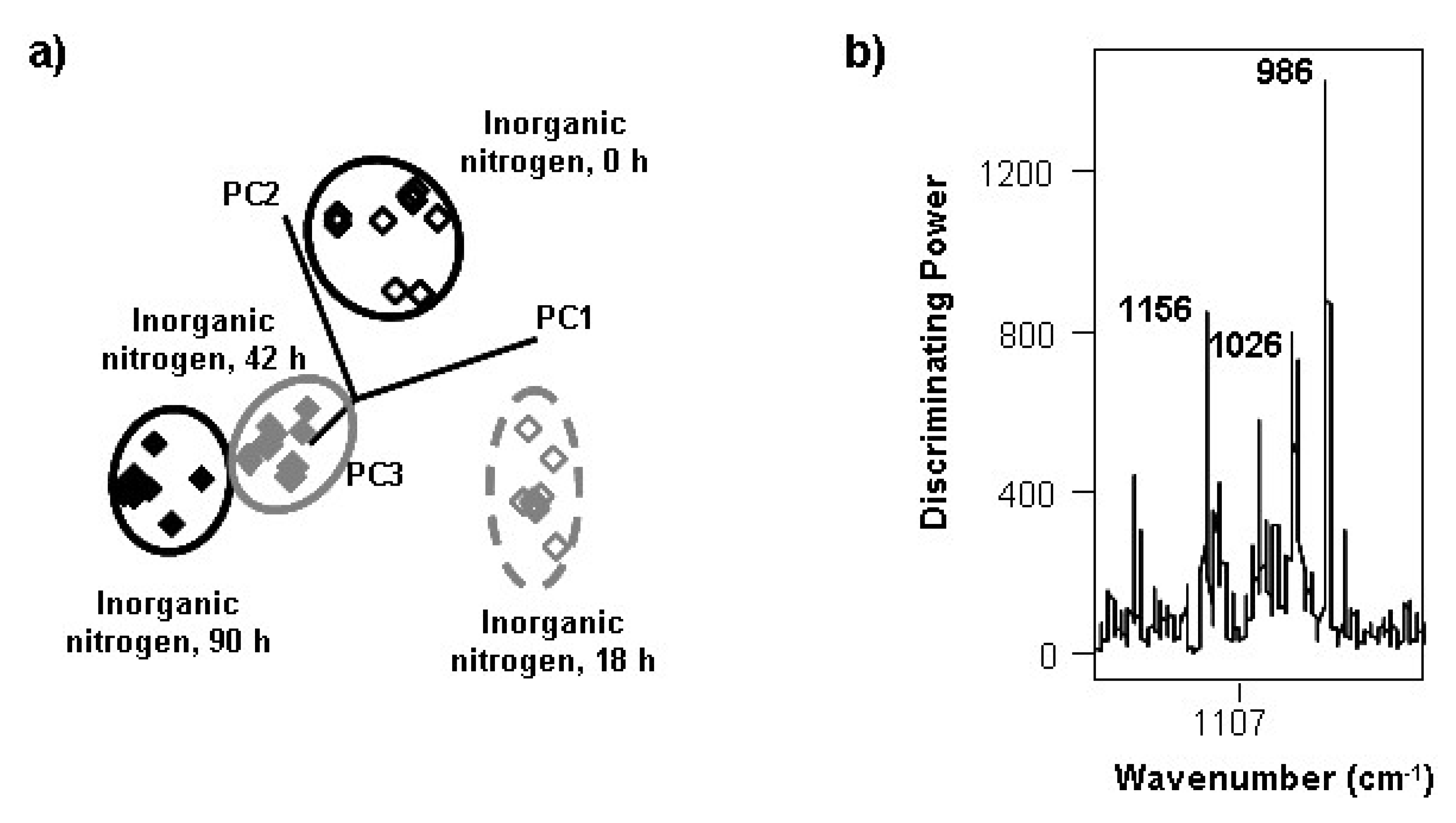
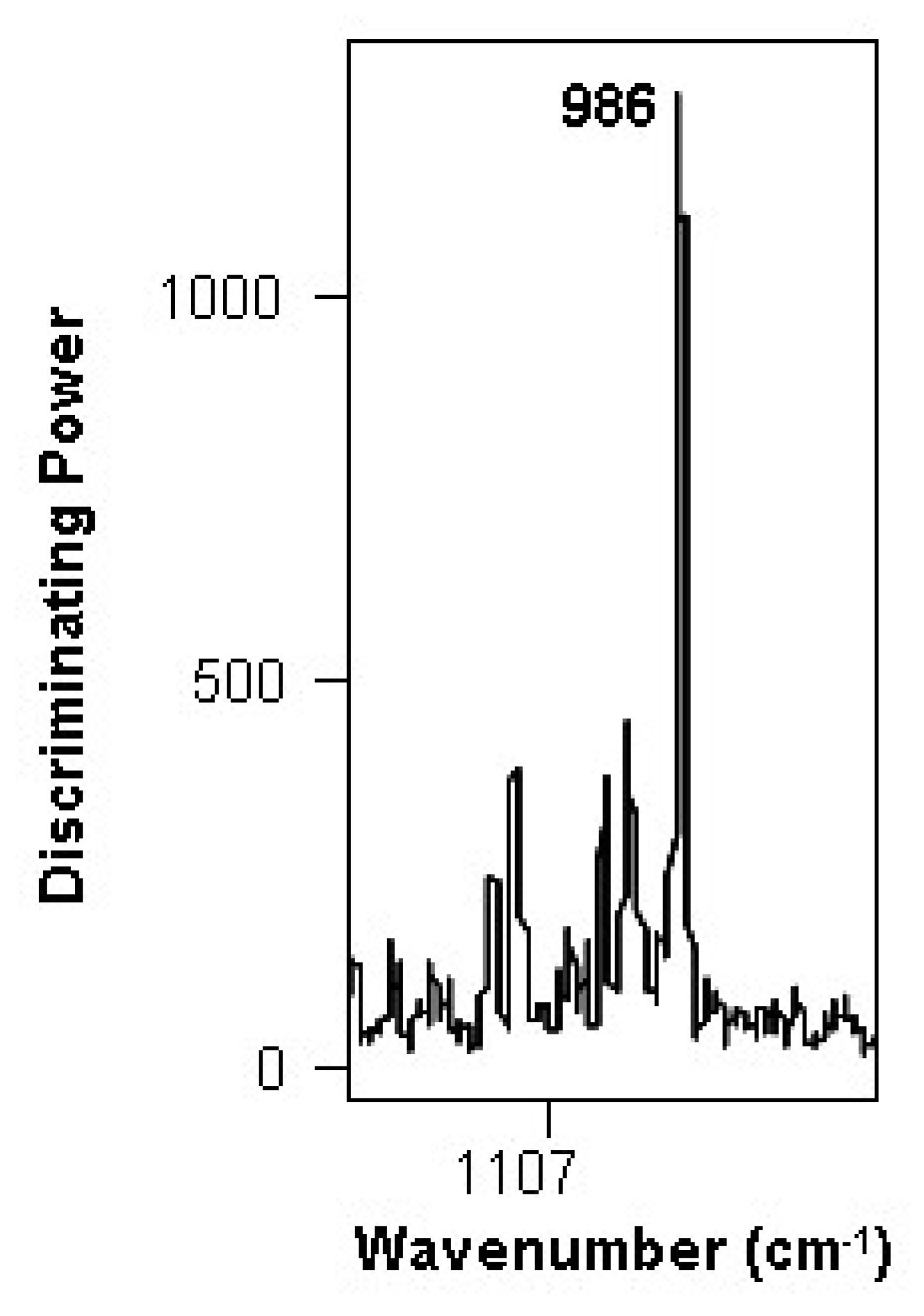
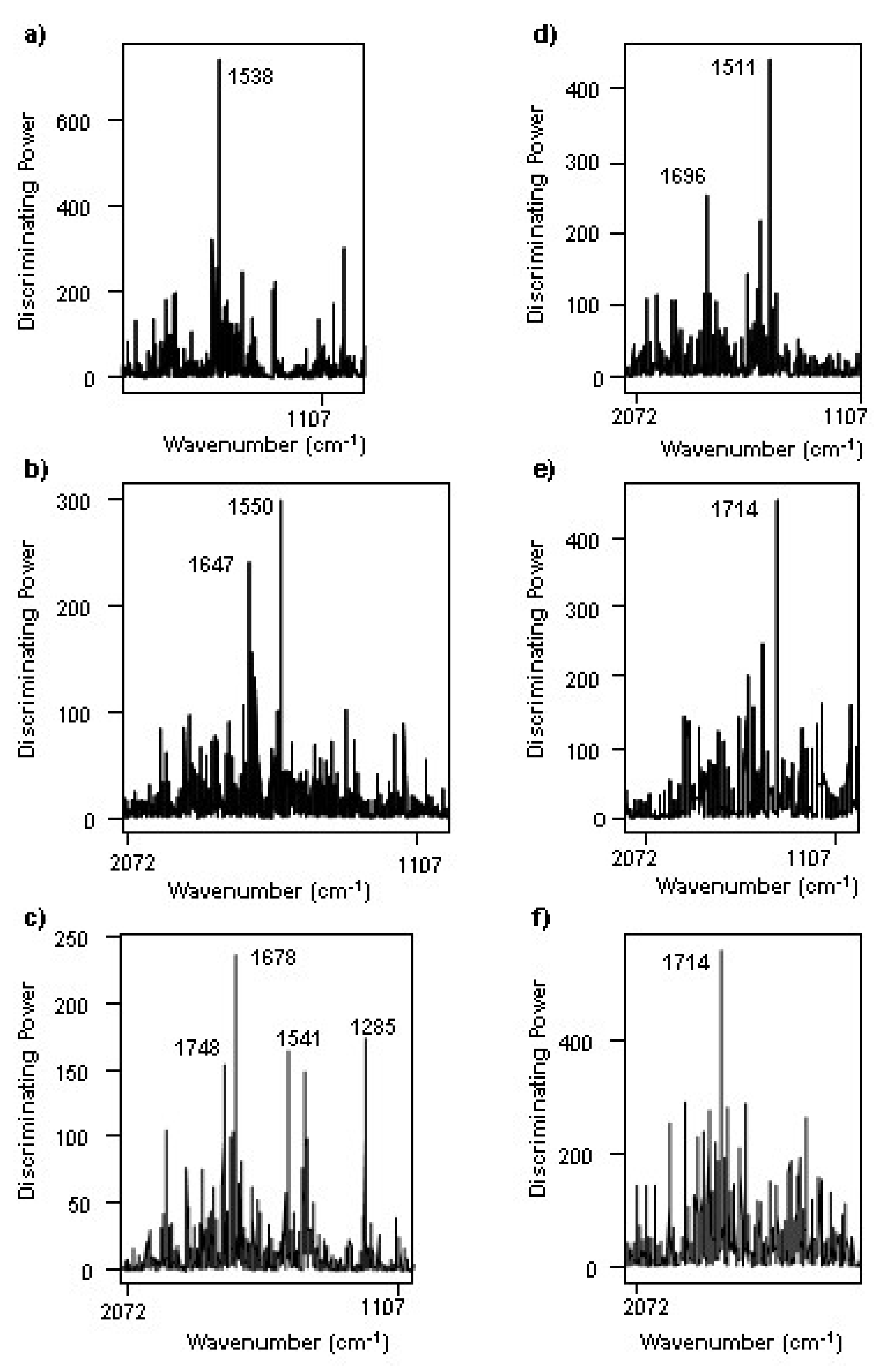
3.2. Changes during the Alcoholic Fermentation by ATR-FTIR
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kamiloglu, S. Authenticity and traceability in beverages. Food Chem. 2019, 277, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Dordevic, N.; Camin, F.; Marianella, R.M.; Postma, G.J.; Buydens, L.M.C.; Wehrens, R. Detecting the addition of sugar and water to wine. Aust. J. Grape Wine Res. 2013, 19, 324–330. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Pérez-Mestre, C.; Ferreras-Charro, R.; Rivero, F.J.; Heredia, F.J.; Escribano-Bailón, M.T. Addition of Mannoproteins and/or Seeds during Winemaking and Their Effects on Pigment Composition and Color Stability. J. Agric. Food Chem. 2019, 67, 4031–4042. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Gallego, R.; Puxeu, M.; Nart, E.; Martín, L.; Andorrà, I. Evaluation of Tempranillo and Albariño SO2-free wines produced by different chemical alternatives and winemaking procedures. Food Res. Int. 2017, 102, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.T.; Zhang, Y.S.; Wen, X.; Song, X.W.; Meng, D.; Li, B.J.; Wang, M.Y.; Tao, Y.Q.; Zhao, H.; Guan, W.Q.; et al. The glycerol and ethanol production kinetics in low-temperature wine fermentation using Saccharomyces cerevisiae yeast strains. Int. J. Food Sci. Technol. 2019, 54, 102–110. [Google Scholar] [CrossRef]
- Miller, K.V.; Oberholster, A.; Block, D.E. Predicting the impact of red winemaking practices using a reactor engineering model. Am. J. Enol. Vitic. 2019, 70, 162–168. [Google Scholar] [CrossRef]
- Ortiz-Tovar, G.; Minebois, R.; Barrio, E.; Querol, A.; Pérez-Torrado, R. Aroma production and fermentation performance of S. cerevisiae × S. kudriavzevii natural hybrids under cold oenological conditions. Int. J. Food Microbiol. 2019, 297, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Bely, M.; Sablayrolles, J.M.; Barre, P. Description of alcoholic fermentation kinetics: Its variability and significance. Am. J. Enol. Vitic. 1990, 41, 319–324. [Google Scholar]
- Bezenger, M.-C.; Navarro, J.-M. Alcoholic fermentation: Model accounting for initial nitrogen influence. Biotechnol. Bioeng. 1988, 31, 747–749. [Google Scholar] [CrossRef]
- Manginot, C.; Roustan, J.L.; Sablayrolles, J.M. Nitrogen demand of different yeast strains during alcoholic fermentation. Importance of the stationary phase. Enzym. Microb. Technol. 1998, 23, 511–517. [Google Scholar] [CrossRef]
- Amerine, M.A.; Berg, H.W.; Kunkee, R.E.; Ough, C.S.; Singleton, V.L.; Webb, A.D. The Technology of Wine Making, 4th ed.; AVI Publishing Co.: Westport, CT, USA, 1980. [Google Scholar]
- Torrea, D.; Varela, C.; Ugliano, M.; Ancin-Azpilicueta, C.; Leigh Francis, I.; Henschke, P.A. Comparison of inorganic and organic nitrogen supplementation of grape juice—Effect on volatile composition and aroma profile of a Chardonnay wine fermented with Saccharomyces cerevisiae yeast. Food Chem. 2011, 127, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.; Tourdot-Maréchal, R.; Sparrow, C.; Morge, C.; Alexandre, H. Influence of nitrogen status in wine alcoholic fermentation. Food Microbiol. 2019, 83, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Jiranek, V.; Langridge, P.; Henschke, P.A. Amino acid and ammonium Utilization by Saccharomyces cerevisiae wine yeasts from a chemically defined medium. Am. J. Enol. Vitic. 1995, 46, 75–83. [Google Scholar]
- Bisson, L.F. Stuck and sluggish fermentations. Am. J. Enol. Vitic. 1999, 50, 107–119. [Google Scholar]
- Spiropoulos, A.; Tanaka, J.; Flerianos, I.; Bisson, L.F. Characterization of Hydrogen Sulfide Formation in Commercial and Natural Wine Isolates of Saccharomyces. Am. J. Enol. Vitic. 2000, 51, 233–248. [Google Scholar]
- Bely, M.; Sablayrolles, J.M.; Barre, P. Automatic detection of assimilable nitrogen deficiencies during alcoholic fermentation in oenological conditions. J. Ferment. Bioeng. 1990, 70, 246–252. [Google Scholar] [CrossRef]
- Sablayrolles, J.-M.; Dubois, C.; Manginot, C.; Roustan, J.-L.; Barre, P. Effectiveness of combined ammoniacal nitrogen and oxygen additions for completion of sluggish and stuck wine fermentations. J. Ferment. Bioeng. 1996, 82, 377–381. [Google Scholar] [CrossRef]
- Vilanova, M.; Siebert, T.E.; Varela, C.; Pretorius, I.S.; Henschke, P.A. Effect of ammonium nitrogen supplementation of grape juice on wine volatiles and non-volatiles composition of the aromatic grape variety Albariño. Food Chem. 2012, 133, 124–131. [Google Scholar] [CrossRef]
- Bely, M.; Salmon, J.M.; Barre, P. Assimilable nitrotgen addition and hexose transport system activity during enological fermentation. J. Inst. Brew. 1994, 100, 279–282. [Google Scholar] [CrossRef]
- Bisson, L.F. Influence of nitrogen on yeast and fermentation of grapes. In Proceedings of the International Symposium on Nitrogen in Grapes and Wine, Seattle, WA, USA, 18–19 June 1991; pp. 78–89. [Google Scholar]
- Seguinot, P.; Rollero, S.; Sanchez, I.; Sablayrolles, J.M.; Ortiz-Julien, A.; Camarasa, C.; Mouret, J.R. Impact of the timing and the nature of nitrogen additions on the production kinetics of fermentative aromas by Saccharomyces cerevisiae during winemaking fermentation in synthetic media. Food Microbiol. 2018, 76, 29–39. [Google Scholar] [CrossRef] [PubMed]
- OIV. List of OIV Admitted Compounds and Their Status as Additives and Processing Aids and the Use Levels or Residual Limits; OIV: Paris, France, 2017. [Google Scholar]
- Aleixandre-Tudo, J.L.; Nieuwoudt, H.; Aleixandre, J.L.; du Toit, W. Chemometric compositional analysis of phenolic compounds in fermenting samples and wines using different infrared spectroscopy techniques. Talanta 2018, 176, 526–536. [Google Scholar] [CrossRef] [PubMed]
- OIV. Compendium of International Methods of Wine and Must Analysis; OIV-MA-ANNEX-C; OIV: Paris, France, 2016; Volume 2, p. 2. [Google Scholar]
- Ricardo-da-Silva, J.M.; Cheynier, V.; Samsom, A.; Bourzeix, M. Effect of Pomace Contact, Carbonic Maceration, and Hyperoxidation on the Procyanidin Composition of Grenache blanc Wines. Am. J. Enol. Vitic. 1993, 44, 168–172. [Google Scholar]
- Bisson, L.F.; Butzke, C.E. Diagnosis and rectification of stuck and sluggish fermentations. Am. J. Enol. Vitic. 2000, 51, 168–177. [Google Scholar]
- Gobbi, M.; Comitini, F.; D’Ignazi, G.; Ciani, M. Effects of nutrient supplementation on fermentation kinetics, H2S evolution, and aroma profile in Verdicchio DOC wine production. Eur. Food Res. Technol. 2013, 236, 145–154. [Google Scholar] [CrossRef]
- Sockalingum, G.D.; Manfait, M.; Belarbi, A.; Galichet, A. FTIR spectroscopic analysis of Saccharomyces cerevisiae cell walls: Study of an anomalous strain exhibiting a pink-colored cell phenotype. FEMS Microbiol. Lett. 2001, 197, 179–186. [Google Scholar] [CrossRef]
- Cavagna, M.; Dell’Anna, R.; Monti, F.; Rossi, F.; Torriani, S. Use of ATR-FTIR Microspectroscopy to Monitor Autolysis of Saccharomyces cerevisiae Cells in a Base Wine. J. Agric. Food Chem. 2010, 58, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Puxeu, M.; Andorra, I.; De Lamo-Castellví, S. Monitoring Saccharomyces cerevisiae Grape Must Fermentation Process by Attenuated Total Reflectance Spectroscopy. Food Bioprocess Technol. 2015, 8, 637–646. [Google Scholar] [CrossRef]
- Dunn, W.J.; Wold, S. SIMCA Pattern Recognition and Classification. In Chemometric Methods in Molecular Design; van de Waterbeemd, H., Ed.; VCH Publishers, Inc: New York, NY, USA, 1995; Volume 1, pp. 179–193. [Google Scholar]
- Hellingwerf, K.; Mol, P.; Klis, F.M.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef]
- Kapteyn, J.C.; Van Den Ende, H.; Klis, F.M. The contribution of cell wall proteins to the organization of the yeast cell wall. Biochim. Biophys. Acta Gen. Subj. 1999, 1426, 373–383. [Google Scholar] [CrossRef]
- Klis, F.M.; Boorsma, A.; De Groot, P.W.J. Cell wall construction in Saccharomyces cerevisiae. Yeast 2006, 23, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Maquelin, K.; Kirschner, C.; Choo-Smith, L.P.; van den Braak, N.; Endtz, H.P.; Naumann, D.; Puppels, G.J. Identification of medically relevant microorganisms by vibrational spectroscopy. J. Microbiol. Methods 2002, 51, 255–271. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Verstrepen, K.J.; Van Laere, S.D.M.; Voet, A.R.D.; Van Dijck, P.; Delvaux, F.R.; Thevelein, J.M. The Saccharomyces cerevisiae EHT1 and EEB1 genes encode novel enzymes with medium-chain fatty acid ethyl ester synthesis and hydrolysis capacity. J. Biol. Chem. 2006, 281, 4446–4456. [Google Scholar] [CrossRef] [PubMed]
- Boido, E.; Medina, K.; Farina, L.; Carrau, F.M.; Henschke, P.A.; Dellacassa, E. Production of fermentation aroma compounds by Saccharomyces cerevisiae wine yeasts: Effects of yeast assimilable nitrogen on two model strains. FEMS Yeast Res. 2008, 8, 1196–1207. [Google Scholar] [CrossRef]
- Lilly, M.; Lambrechts, M.G.; Pretorius, I.S. Effect of Increased Yeast Alcohol Acetyltransferase Activity on Flavor Profiles of Wine and Distillates. Appl. Environ. Microbiol. 2000, 66, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Swiegers, J.H.; Pretorius, I.S. Yeast Modulation of Wine Flavor. Adv. Appl. Microbiol. 2005, 57, 131–175. [Google Scholar] [PubMed]
- Saerens, S.M.G.; Delvaux, F.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters Affecting Ethyl Ester Production by Saccharomyces cerevisiae during Fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.C.; Wolff, S.R.; Bisson, L.F.; Ebeler, S.E. Yeast Strain and Nitrogen Supplementation: Dynamics of Volatile Ester Production in Chardonnay Juice Fermentations. Am. J. Enol. Vitic. 2007, 58, 470–483. [Google Scholar]
- Ugliano, M.; Siebert, T.; Mercurio, M.; Capone, D.; Henschke, P.A. Volatile and Color Composition of Young and Model-Aged Shiraz Wines as Affected by Diammonium Phosphate Supplementation before Alcoholic Fermentation. J. Agric. Food Chem. 2008, 56, 9175–9182. [Google Scholar] [CrossRef]
- Vilanova, M.; Ugliano, M.; Varela, C.; Siebert, T.; Pretorius, I.S.; Henschke, P.A. Assimilable nitrogen utilisation and production of volatile and non-volatile compounds in chemically defined medium by Saccharomyces cerevisiae wine yeasts. Appl. Microbiol. Biotechnol. 2007, 77, 145–157. [Google Scholar] [CrossRef]

| Initial Must | Mean ± SD |
|---|---|
| Brix degree (°Bx) | 20.3 ± 0.2 |
| Alcoholic potential strength (% vol.) | 11.8 ± 0.2 |
| YAN (mg/L) | 170 ± 6 |
| pH | 3.1 ± 0.1 |
| Total acidity (g/L) | 5.5 ± 0.1 |
| Turbidity (NTU *) | 18 ± 2 |
| Press yield (%) | 54 ± 0.7 |
| Clarification yield (%) | 81 ± 0.8 |
| Wines | Acetic Acid (g/L) | Alcohol (% vol.) |
|---|---|---|
| Control | 0.42 ± 0.03 a | 12.1 ± 0.1 b |
| Inorganic nitrogen | 0.38 ± 0.02 a | 12.3 ± 0.1 a |
| Organic nitrogen | 0.40 ± 0.02 a | 12.3 ± 0.1 a |
| Treatment | 18 h | 42 h | 90 h |
|---|---|---|---|
| Control | 162.1 ±1.5 a | 71.6 ± 1.5 c | 1.7 ± 0.5 e |
| Inorganic nitrogen | 164.7 ±1.5 ab | 56.0 ± 1.5 d | 0.0 ± 0.0 f |
| Organic nitrogen | 161.2 ± 0.7 b | 57.8 ± 1.5 d | 0.0 ± 0.0 f |
| Control | 0 h | 18 h | 42 h | 90 h |
| 0 h | 0.0 | |||
| 18 h | 13.3 | 0.0 | ||
| 42 h | 10.3 | 15.6 | 0.0 | |
| 90 h | 16.0 | 22.4 | 6.2 | 0.0 |
| Inorganic nitrogen | 0 h | 18h | 42h | 90 h |
| 0 h | 0.0 | |||
| 18 h | 7.9 | 0.0 | ||
| 42 h | 7.0 | 13.9 | 0.0 | |
| 90 h | 9.5 | 16.3 | 5.8 | 0.0 |
| Organic nitrogen | 0 h | 18 h | 42 h | 90 h |
| 0 h | 0.0 | |||
| 18 h | 4.7 | 0.0 | ||
| 42 h | 9.5 | 13.7 | 0.0 | |
| 90 h | 11.7 | 17.1 | 4.4 | 0.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puxeu, M.; Andorra, I.; De Lamo-Castellví, S.; Ferrer-Gallego, R. Determination of Nutrient Supplementation by Means of ATR-FTIR Spectroscopy during Wine Fermentation. Fermentation 2019, 5, 58. https://doi.org/10.3390/fermentation5030058
Puxeu M, Andorra I, De Lamo-Castellví S, Ferrer-Gallego R. Determination of Nutrient Supplementation by Means of ATR-FTIR Spectroscopy during Wine Fermentation. Fermentation. 2019; 5(3):58. https://doi.org/10.3390/fermentation5030058
Chicago/Turabian StylePuxeu, Miquel, Imma Andorra, Sílvia De Lamo-Castellví, and Raúl Ferrer-Gallego. 2019. "Determination of Nutrient Supplementation by Means of ATR-FTIR Spectroscopy during Wine Fermentation" Fermentation 5, no. 3: 58. https://doi.org/10.3390/fermentation5030058
APA StylePuxeu, M., Andorra, I., De Lamo-Castellví, S., & Ferrer-Gallego, R. (2019). Determination of Nutrient Supplementation by Means of ATR-FTIR Spectroscopy during Wine Fermentation. Fermentation, 5(3), 58. https://doi.org/10.3390/fermentation5030058