Functional Role of Probiotics and Prebiotics on Skin Health and Disease
Abstract
1. Introduction
2. Probiotics and Prebiotics on Skin Health
3. Probiotics and Prebiotics on Skin Disease
3.1. Dermatities
3.1.1. Atopic Dermatitis
3.1.2. Allergic Contact Dermatitis
3.2. Skin Infections
3.2.1. Wounds
3.2.2. Acne
3.3. Psoriasis
3.4. Photoaging
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McGovern, P.E.; Zhang, J.; Tang, J.; Zhang, Z.; Hall, G.R.; Moreau, R.A.; Nunez, A.; Butrym, E.D.; Richards, M.P.; Wang, C.-S.; et al. Fermented beverages of pre- and proto-historic China. Proc. Natl. Acad. Sci. USA 2004, 101, 17593–17598. [Google Scholar] [CrossRef] [PubMed]
- Sicard, D.; Legras, J.L. Bread, beer and wine: Yeast domestication in the Saccharomyces sensu stricto complex. C. R. Biol. 2011, 334, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Ozen, M.; Dinleyici, E.C. The history of probiotics: The untold story. Benef. Microbes 2015, 6, 159–165. [Google Scholar] [CrossRef]
- Isolauri, E. Probiotics in human disease. Am. J. Clin. Nutr. 2001, 73, 1142–1146. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Zakharova, I.; Dmitrieva, Y. Oligosaccharides in infant formula: More evidence to validate the role of prebiotics. Br. J. Nutr. 2015, 113, 1339–1344. [Google Scholar] [CrossRef]
- Gordon, S. Ellie Metchnikoff: Father of natural immunity. Eur. J. Immunol. 2008, 38, 3257–3264. [Google Scholar] [CrossRef]
- Collins, M.D.; Phillips, B.A.; Zanoni, P. Deozyribonucleic acid homology studies of lactobacillus casei, Lactobacillus paracasei sp. nov., subsp. paracasei and subsp. tolerans, and Lactobacillus rhamnosus sp. nov., comb. nov. Int. J. Syst. Bacteriol. 1989, 39, 105–118. [Google Scholar] [CrossRef]
- Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; Guidelines for the Evaluation of Probiotics in Food: London, ON, Canada, 2002; pp. 1–11.
- Gasbarrini, G.; Bonvicini, F.; Gramenzi, A. Probiotics History. J. Clin. Gastroenterol. 2016, 50, S116–S119. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Salminen, S.; Isolauri, E. Probiotics: An overview of beneficial effect. Antonie Van Leeuwenhoek 2002, 82, 279–289. [Google Scholar] [CrossRef]
- Britti, M.S.; Roselli, M.; Finamore, A.; Merendino, N.; Mengheri, E. Regulation of immune response at intestinal and peripheral sites by probiotics. Biologia (Bratislava) 2006, 61, 735–740. [Google Scholar] [CrossRef]
- Chen, C.C.; Allan Walker, W. Probiotics and the mechanism of necrotizing enterocolitis. Semin. Pediatr. Surg. 2013, 22, 94–100. [Google Scholar] [CrossRef]
- Bansal, S.; Mangal, M.; Sharma, S.K.; Gupta, R.K. Non-dairy Based Probiotics: A Healthy Treat for Intestine. Crit. Rev. Food Sci. Nutr. 2016, 56, 1856–1867. [Google Scholar] [CrossRef]
- Wang, X.; Farnell, Y.Z.; Peebles, E.D.; Kiess, A.S.; Wamsley, K.G.; Zhai, W. Effects of prebiotics, probiotics, and their combination on growth performance, small intestine morphology, and resident Lactobacillus of male broilers. Poult. Sci. 2016, 95, 1332–1340. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, A.D.; Paz, M.L.; Leoni, J.; Maglio, D.H. Message in a bottle: Dialog between intestine and skin modulated by probiotics. Int. J. Mol. Sci. 2017, 18, 1067. [Google Scholar] [CrossRef]
- Thomas, C.M.; Versalovic, J. Probiotics-host communication. Gut Microbes 2010, 1, 148–163. [Google Scholar] [CrossRef]
- Mori, N.; Kano, M.; Masuoka, N.; Konno, T.; Suzuki, Y.; Miyazaki, K.; Ueki, Y. Effect of probiotic and prebiotic fermented milk on skin and intestinal conditions in healthy young female students. Biosci. Microbiota Food Health 2016, 35, 105–112. [Google Scholar] [CrossRef]
- Yamada, T.; Nagata, S.; Kondo, S.; Bian, L.; Wang, C.; Asahara, T.; Ohta, T.; Nomoto, K.; Yamashiro, Y. [Effect of Continuous Fermented Milk Intake Containing Lactobacillus casei Strain Shirota on Fever in Mass Infectious Gastroenteritis Rest Home Outbreak]. Kansenshogaku Zasshi 2009, 83, 31–35. [Google Scholar] [CrossRef]
- White, J.S.; Hoper, M.; Parks, R.W.; Clements, W.D.; Diamond, T.; Bengmark, S. The probiotic bacterium Lactobacillus plantarum species 299 reduces intestinal permeability in experimental biliary obstruction. Lett. Appl. Microbiol. 2006, 42, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Anukam, K.C.; Hayes, K.; Summers, K.; Reid, G. Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 may help downregulate TNF-alpha, IL-6, IL-8, IL-10 and IL-12 (p70) in the neurogenic bladder of spinal cord injured patient with urinary tract infections: A two-case study. Adv. Urol. 2009, 680363. [Google Scholar]
- Bennett, R.G.; Gorbach, S.L.; Greenough, W.B.; Bartlett, J.G. Treatment of Relapsing Clostridium difficile Diarrhea with Lactobacillus GG. Nutr. Today Suppl. 1996, 31, 35–38. [Google Scholar] [CrossRef]
- Amara, A.A.; Shibl, A. Role of Probiotics in health improvement, infection control and disease treatment and management. Saudi Pharm. J. 2015, 23, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Boudeau, J.; Glasser, A.L.; Julien, S.; Colombel, J.F.; Darfeuille-Michaud, A. Inhibitory effect of probiotic Escherichia coli strain Nissle 1917 on adhesion to and invasion of intestinal epithelial cells by adherent-invasive E.coli strains isolated from patients with Crohn’s disease. Aliment. Pharm. Ther. 2003, 18, 45–56. [Google Scholar] [CrossRef]
- Mego, M.; Májek, J.; Končeková, R.; Ebringer, L.; Čierniková, S.; Rauko, P.; Kovac, M.; Trupl, J.; Slezak, P.; Zajac, V. Intramucosal bacteria in colon cancer and their elimination by probiotic strain Enterococcus faecium M-74 with organic selenium. Folia Microbiol. 2005, 50, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Thirabunyanon, M.; Boonprasom, P.; Niamsup, P. Probiotic potential of lactic acid bacteria isolated from fermented dairy milks on antiproliferation of colon cancer cells. Biotechnol. Lett. 2009, 31, 571–576. [Google Scholar] [CrossRef]
- Abdin, A.A.; Saeid, E.M. An experimental study on ulcerative colitis as a potential target for probiotic therapy by Lactobacillus acidophilus with or without “olsalazine”. J. Crohn’s Colitis 2008, 2, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Imaoka, A.; Shima, T.; Kato, K.; Mizuno, S.; Uehara, T.; Matsumoto, S.; Setoyama, H.; Hara, T.; Umesaki, Y. Anti-inflammatory activity of probiotic Bifidobacterium: Enhancement of IL-10 production in peripheral blood mononuclear cells from ulcerative colitis patients and inhibition of IL-8 secretion in HT-29 cells. World J. Gastroenterol. 2008, 14, 2511–2516. [Google Scholar] [CrossRef]
- Fujiya, M.; Musch, M.W.; Nakagawa, Y.; Hu, S.; Alverdy, J.; Kohgo, Y.; Schneewind, O.; Jabri, B.; Chang, E.B. The Bacillus subtilis Quorum-Sensing Molecule CSF Contributes to Intestinal Homeostasis via OCTN2, a Host Cell Membrane Transporter. Cell Host Microbe 2007, 1, 299–308. [Google Scholar] [CrossRef]
- Kojima, K.; Musch, M.W.; Ren, H.; Boone, D.L.; Hendrickson, B.A.; Ma, A.; Chang, E.B. Enteric flora and lymphocyte-derived cytokines determine expression of heat shock proteins in mouse colonic epithelial cells. Gastroenterology 2003, 124, 1395–1407. [Google Scholar] [CrossRef]
- Tao, Y.; Drabik, K.A.; Waypa, T.S.; Musch, M.W.; Alverdy, J.C.; Schneewind, O.; Chang, E.B.; Petrof, E.O. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 2006, 290, C1018–C1030. [Google Scholar] [CrossRef]
- Petrof, E.O.; Kojima, K.; Ropeleski, M.J.; Musch, M.W.; Tao, Y.; De Simone, C.; Cheng, E.B. Probiotics inhibit nuclear factor-κB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology 2004, 127, 1474–1487. [Google Scholar] [CrossRef]
- Neish, A.S.; Gewirtz, A.T.; Zeng, H.; Young, A.N.; Hobert, M.E.; Karmali, V.; Rao, A.S.; Madara, J.L. Prokaryotic Regulation of Epithelial Responses by Inhibition of IκΒ-α Ubiquitination. Science 2000, 289, 1560–1563. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Forsythe, P.; Bienenstock, J. Live Lactobacillus reuteri Is Essential for the Inhibitory Effect on Tumor Necrosis Factor Alpha-Induced Interleukin-8 Expression. Infect Immun 2004, 72, 5308–5314. [Google Scholar] [CrossRef]
- Tien, M.T.; Girardin, S.E.; Regnault, B.; Le Bourhis, L.; Dillies, M.A.; Coppee, J.Y.; Bourdet-Sicard, R.; Sansonetti, P.J.; Pedron, T. Anti-Inflammatory Effect of Lactobacillus casei on Shigella- Infected Human Intestinal Epithelial Cells. J. Immunol. 2009, 176, 1228–1237. [Google Scholar] [CrossRef]
- Frick, J.S.; Schenk, K.; Quitadamo, M.; Kahl, F.; Köberle, M.; Bohn, E.; Aepfelbacher, M.; Autenrieth, I.B. Lactobacillus fermentum attenuates the proinflammatory effect of Yersinia enterocolitica on human epithelial cells. Inflamm. Bowel. Dis. 2007, 13, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Bai, A.P.; Ouyang, Q.; Zhang, W.; Wang, C.H.; Li, S.F. Probiotics inhibit TNF-alpha-induced interleukin-8 secretion of HT29 cells. World J. Gastroenterol. 2004, 10, 455–457. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humará, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Haller, D.; Russo, M.P.; Balfour-Sartor, R.; Jobin, C. IKKβ and phosphatidylinositol 3-kinase/Akt participate in non-pathogenic gram-negative enteric bacteria-induced RelA phosphorylation and NF-κB activation in both primary and intestinal epithelial cell lines. J. Biol. Chem. 2002, 277, 38168–38178. [Google Scholar] [CrossRef]
- Ruiz, P.A.; Hoffmann, M.; Szcesny, S.; Blaut, M.; Haller, D. Innate mechanisms for Bifidobacterium lactis to activate transient pro-inflammatory host responses in intestinal epithelial cells after the colonization of germ-free rats. Immunology 2005, 115, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Jijon, H.; Backer, J.; Diaz, H.; Yeung, H.; Thiel, D.; McKaigney, C.; De Simone, C.; Madsen, K. DNA from probiotic bacteria modulates murine and human epithelial and immune function. Gastroenterology 2004, 126, 1358–1373. [Google Scholar] [CrossRef]
- Resta-Lenert, S.; Barrett, K.E. Probiotics and commensals reverse TNF-α- and IFN-γ-induced dysfunction in human intestinal epithelial cells. Gastroenterology 2006, 130, 731–746. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.; Campbell, J.I.; King, T.P.; Grant, G.; Jansson, E.A.; Coutts, A.G.; Petterson, S.; Conway, S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shutting of PPAR-γ and ReIA. Nat. Immunol. 2004, 5, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Are, A.; Aronsson, L.; Wang, S.; Greicius, G.; Lee, Y.K.; Gustafsson, J.A.; Petterson, S.; Arulampalam, V. Enterococcus faecalis from newborn babies regulate endogenous PPAR activity and IL-10 levels in colonic epithelial cells. Proc. Natl. Acad. Sci. USA 2008, 105, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Ewaschuk, J.B.; Walker, J.W.; Diaz, H.; Madsen, K.L. Bioproduction of Conjugated Linoleic Acid by Probiotic Bacteria Occurs. J. Nutr. 2006, 136, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, L.R.; Small, J.; Hoerr, R.A.; Bostwick, E.F.; Maines, L.; Koltun, W.A. In vitro and in vivo effects of the probiotic Escherichia coli strain M-17: Immunomodulation and attenuation of murine colitis. Br. J. Nutr. 2008, 100, 530–541. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J. Biol. Chem. 2002, 277, 50959–50965. [Google Scholar] [CrossRef]
- Yan, F.; Cao, H.; Cover, T.; Whitehead, R.; Washington, M.K.; Polk, D.B. Soluble Proteins Produced by Probiotic Bacteria Regulate Intestinal Epithelial Cell Survival and Growth. Gastroenterology 2007, 132, 562–575. [Google Scholar] [CrossRef]
- Watanabe, T.; Nishio, H.; Tanigawa, T.; Yamagami, H.; Okazaki, H.; Watanabe, K.; Tominaga, K.; Fujiwara, Y.; Oshitani, N.; Asahara, T.; et al. Probiotic Lactobacillus casei strain Shirota prevents indomethacin-induced small intestinal injury: Involvement of lactic acid Probiotic Lactobacillus casei strain Shirota prevents indomethacin-induced small intestinal injury: Involvement of lactic acid. Am. J. Physiol Gastrointest Liver Physiol. 2009, 297, 506–513. [Google Scholar] [CrossRef]
- Sougioultzis, S.; Simeonidis, S.; Bhaskar, K.R.; Chen, X.; Anton, P.M.; Keates, S.; Pothoulakis, C.; Kelly, C.P. Saccharomyces boulardii produces a soluble anti-inflammatory factor that inhibits NF-κB-mediated IL-8 gene expression. Biochem. Biophys Res. Commun. 2006, 343, 69–76. [Google Scholar] [CrossRef]
- Ménard, S.; Candalh, C.; Bambou, J.C.; Terpend, K.; Cerf-Bensussan, N.; Heyman, M. Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut 2004, 53, 821–828. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, N.R.; Gim, M.G.; Lee, J.M.; Lee, S.Y.; Ko, M.Y.; Kim, J.Y.; Han, S.H.; Chung, D.K. Lipoteichoic Acid Isolated from Lactobacillus plantarum Inhibits Lipopolysaccharide-Induced TNF-Production in THP-1 Cells and Endotoxin Shock in Mice. J. Immunol. 2008, 180, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Matsuguchi, T.; Takagi, A.; Matsuzaki, T.; Nagaoka, M.; Ishikawa, K.; Yokokura, T.; Yoskikai, Y. Lipoteichoic Acids from Lactobacillus Strains Elicit Strong Tumor Necrosis Factor Alpha-Inducing Activities in Macrophages through Toll-Like Receptor 2. Clin. Diagn Lab. Immunol. 2003, 10, 259–266. [Google Scholar] [CrossRef]
- Kim, S.O.; Sheikh, H.I.; Ha, S.D.; Martins, A.; Reid, G. G-CSF-mediated inhibition of JNK is a key mechanism for Lactobacillus rhamnosus-induced suppression of TNF production in macrophages. Cell Microbiol. 2006, 8, 1958–1971. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J.; Watts, D.H.; Mehlin, C.; Headley, C.M. Lactobacilli and vaginal host defense: Activation of the human immunodeficiency virus type 1 long terminal repeat, cytokine production, and NF-kappaB. J. Infect. Dis. 1999, 179, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Lehtonen, A.; Ilkka, J.; Matikainen, S. Lactobacilli and Streptococci Activate NF-κΒ and STAT Signaling Pathways in Human Macrophages. J. Immunol. 2000, 164, 3733–3740. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Hsieh, Y.J.; Liao, K.W.; Peng, K.C. Preferential promotion of apoptosis of monocytes by Lactobacillus casei rhamnosus soluble factors. Clin. Nutr. 2010, 29, 131–140. [Google Scholar] [CrossRef]
- Iyer, C.; Kosters, A.; Sethi, G.; Kunnumakkara, A.B.; Aggarwal, B.B.; Versalovic, J. Probiotic Lactobacillus reuteri promotes TNF-induced apoptosis in human myeloid leukemia-derived cells by modulation of NF-κB and MAPK signalling. Cell Microbiol. 2008, 10, 1442–1452. [Google Scholar] [CrossRef]
- Horinaka, M.; Yoshida, T.; Kishi, A.; Akatani, K.; Yasuda, T.; Kouhara, J.; Wakada, M.; Sakai, T. Lactobacillus strains induce TRAIL production and facilitate natural killer activity against cancer cells. FEBS Lett. 2010, 584, 577–582. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Tang, L. Cancer-preventive isothiocyanates: Dichotomous modulators of oxidative stress. Free. Radic. Biol. Med. 2005, 38, 70–77. [Google Scholar] [CrossRef]
- Lee, D.E.; Huh, C.S.; Ra, J.; Choi, I.D.; Jeong, J.W.; Kim, S.H.; Ryu, J.H.; Seo, Y.K.; Koh, J.S.; Lee, J.H.; et al. Clinical evidence of effects of Lactobacillus plantarum HY7714 on skin aging: A randomized, double blind, placebo-controlled study. J. Microbiol Biotechnol. 2015, 25, 2160–2168. [Google Scholar] [CrossRef]
- Pite, H. Effect of probiotic mix (Bifidobacterium bifidum, Bifidobacterium Lactis, Lactobacillus acidophilus) in the primary prevention of eczema: A double-blind, randomized, placebo -controlled trial. Rev. Port. Imunoalergol. 2010, 18, 385–386. [Google Scholar]
- Brouwer, M.L.; Wolt-Plompen, S.A.; Dubios, A.E.; van der Heide, S.; Jansen, D.F.; Hoijer, M.A.; Kauffman, H.F.; Duiverman, E.J. No effects of probiotics on atopic dermatitis in infancy: A randomized placebo-controlled trial. Clin. Exp. Allergy 2006, 36, 899–906. [Google Scholar] [CrossRef]
- Weston, S.; Halbert, A.; Richmond, P.; Prescott, S.L. Effects of probiotics on atopic dermatitis: A randomised controlled trial. Arch. Dis. Child. 2005, 90, 892–897. [Google Scholar] [CrossRef]
- Rosenfeldt, V.; Benfeldt, E.; Valerius, N.H.; Pærregaard, A.; Michaelsen, K.F. Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. J. Pediatr. 2004, 145, 612–616. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Salminen, S.; Poussa, T.; Isolauri, E. Probiotics during the first 7 years of life: A cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2007, 119, 1019–1021. [Google Scholar] [CrossRef]
- Cho, S.H.; Strickland, I.; Boguniewicz, M.; Leung, D.Y. Fibronectin and fibrinogen contribute to the enhanced binding of Staphylococcus aureus to atopic skin. J. Allergy Clin. Immunol. 2001, 108, 269–274. [Google Scholar] [CrossRef]
- Taylor, A.L.; Dunstan, J.A.; Prescott, S.L. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: A randomized controlled trial. J. Allergy Clin. Immunol. 2007, 119, 184–191. [Google Scholar] [CrossRef]
- Lee, J.; Seto, D.; Bielory, L. Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J. Allergy Clin. Immunol. 2008, 121, 116–121. [Google Scholar] [CrossRef]
- Odamaki, T.; Iwabuchi, N.; Xiao, J. Effects and Mechanisms of Probiotics on the Prevention and Treatment of Allergic Rhinitis. In Lactic Acid Bacteria and Bifidobacteria: Current Progress in Advanced Research, 1st ed.; Sonomoto, K., Yokota, A., Eds.; Caiser Academic Press: Norfolk, UK, 2011; pp. 239–251. [Google Scholar]
- Nogueira, J.C.; Gonçalves, M.C. Probiotics in allergic rhinitis. Braz. J. Otorhinolaryngol. 2011, 77, 129–134. [Google Scholar] [CrossRef]
- Jebur, M.S. Therapeutic efficacy of Lactobacillus acidophilus against bacterial isolates from burn wounds. N. Am. J. Med. Sci. 2010, 2, 586–591. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Croxatto, A.; Righi, E.; Guery, B. How to manage Pseudomonas aeruginosa infections. Drugs Context. 2018, 7, 212527. [Google Scholar] [CrossRef]
- Defez, C.; Fabbro-Peray, P.; Bouziges, N.; Gouby, A.; Mahamat, A.; Daurès, J.P.; Sotto, A. Risk factors for multidrug-resistant Pseudomonas aeruginosa nosocomial infection. J. Hosp. Infect. 2004, 57, 209–216. [Google Scholar] [CrossRef]
- Livermore, D.M. Multiple Mechanisms of Antimicrobial Resistance in Pseudomonas aeruginosa: Our Worst Nightmare? Clin. Infect. Dis. 2002, 34, 634–640. [Google Scholar] [CrossRef]
- Peral, M.C.; Rachid, M.M.; Gobbato, N.M.; Huaman-Martinez, M.A.; Valdez, J.C. Interleukin-8 production by polymorphonuclear leukocytes from patients with chronic infected leg ulcers treated with Lactobacillus plantarum. Clin. Microbiol. Infect. 2010, 16, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Sonal Sekhar, M.; Unnikrishnan, M.K.; Vijayanarayana, K.; Rodrigues, G.S.; Mukhopadhyay, C. Topical application/formulation of probiotics: Will it be a novel treatment approach for diabetic foot ulcer? Med. Hypotheses 2014, 82, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Peral, M.C.; Huaman Martinez, M.A.; Valdez, J.C. Bacteriotherapy with Lactobacillus plantarum in burns. Int. Wound J. 2009, 6, 73–81. [Google Scholar] [CrossRef]
- Atalan, G.; Demirkan, I.; Yaman, H.; Cihan, M.; Onder, F.; Sozmen, M. Effect of topical kefir application on open wound healing on in vivo study. Kafkas Univ. Vet Fak. Dderg. 2003, 9, 43–47. [Google Scholar]
- Frei, R.; Akdis, M.; O’Mahony, L. Prebiotics, probiotics, synbiotics, and the immune system: Experimental data and clinical evidence. Curr. Opin. Gastroenterol. 2015, 31, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics- a review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Scholz-Ahrens, K.E.; Adolphi, B.; Rochat, F.; Barclay, D.V.; de Vrese, M.; Açil, Y.; Schrezenmeir, J. Effects of probiotics, prebiotics, and synbiotics on mineral metabolism in ovariectomized rats—Impact of bacterial mass, intestinal absorptive area and reduction of bone turn-over. NFS J. 2016, 3, 41–50. [Google Scholar] [CrossRef]
- Hutkins, R.W.; Krumbeck, J.A.; Bindels, L.B.; Cani, P.D.; Fahey, G.; Goh, Y.J.; Hamaker, B.; Martens, E.C.; Mills, D.A.; Rastal, R.A.; et al. Prebiotics: Why definitions matter. Curr. Opin. Biotechnol. 2016, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Schley, P.D.; Field, C.J. The immune-enhancing effects of dietary fibres and prebiotics. Br. J. Nutr. 2002, 87, S221–S230. [Google Scholar] [CrossRef]
- Yamada, K.; Tokunaga, Y.; Ikeda, A.; Ohkura, K.; Mamiya, S.; Kaku, S.; Sugano, M.; Tachibana, H. Dietary effect of guar gum and its partially hydrolyzed product on the lipid metabolism and immune function of Sprague-Dawley rats. Biosci. Biotechnol. Biochem. 1999, 2163–2167. [Google Scholar] [CrossRef]
- Yun, C.H.; Estrada, A.; Van Kessel, A.; Gajadhar, A.; Redmond, M.; Laarveld, B. Immunomodulatory effects of oat beta-glucan administered intragastrically or parenterally on mice infected with Eimeria vermiformis. Microbiol. Immunol. 1998, 42, 457–465. [Google Scholar]
- de Preter, V.; Geboes, K.; Verbrugghe, K.; de Vuyst, L.; Vanhoutte, T.; Huys, G.; Swings, J.; Pot, B.; Verbeke, K. The in vivo use of the stable isotope-labelled biomarkers lactose-[N]ureide and [H4]tyrosine to assess the effects of pro- and prebiotics on the intestinal flora of healthy human volunteers. Br. J. Nutr. 2004, 92, 439–446. [Google Scholar] [CrossRef]
- Cogen, A.L.; Nizetà, V.; Gallo, R.L. Skin microbiota: A source of disease or defence? Br. J. Dermatol. 2008, 158, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A. The skin microbiome: Potential for novel diagnostic and therapeutic approaches to cutaneous disease. Semin. Cutan. Med. Surg. 2014, 33, 98–103. [Google Scholar] [CrossRef]
- Kano, M.; Masuoka, N.; Kaga, C.; Sugimoto, S.; Iizuka, R.; Manabe, K.; Sone, T.; Oeda, K.; Nonaka, C.; Miyazaki, K.; et al. Consecutive Intake of Fermented Milk Containing Bifidobacterium breve Strain Yakult and Galacto-oligosaccharides Benefits Skin Condition in Healthy Adult Women. Biosci. Microb. Food Health 2013, 32, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.-H.; Park, J.-A.; Kang, S.-M. Effects of Lactobacillus reuteri Intake to Facial Skin Condition of Women. J. Kor. Soc. Cosm. 2018, 24, 661–670. [Google Scholar]
- Kimoto-Nira, H.; Aoki, R.; Sasaki, K.; Suzuki, C.; Mizumachi, K. Oral intake of heat-killed cells of lactococcus lactis strain h61 promotes skin health in women. J. Nutr. Sci. 2012, 1, e18. [Google Scholar] [CrossRef]
- Lee, J.B.; Suk, J.H.; Kang, S.M. Effect of Lactobacillus rhamnosus KCTC 5033 on the Appearance of Facial Skin due to the Ingestion of Probiotics and Paraprobiotics. J. Investig. Cosmetol. 2018, 14, 287–296. [Google Scholar]
- McPherson, T. Current understanding in pathogenesis of atopic dermatitis. Ind. J. Dermatol. 2016, 61, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka-Operacz, M.; Sadowska-Przytocka, A. Probiotics for the prevention of atopic dermatitis and other allergic diseases: What are the real facts? Alergol. Pol. Pol. J. Allergol. 2017, 4, 89–92. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Kirjavainen, P.; Eerola, E.; Kero, P.; Salminen, S.; Isolauri, E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J. Allergy Clin. Immunol. 2001, 107, 129–134. [Google Scholar] [CrossRef]
- Prince, T.; Mcbain, A.J.; O’Neill, C.A. Lactobacillus reuteri protects epidermal keratinocytes from Staphylococcus aureus-induced cell death by competitive exclusion. Appl. Environ. Microbiol. 2012, 78, 5119–5126. [Google Scholar] [CrossRef]
- Jung, G.W.; Tse, J.E.; Guiha, I.; Rao, J. Prospective, Randomized, Open-Label Trial Comparing the Safety, Efficacy, and Tolerability of an Acne Treatment Regimen with and without a Probiotic Supplement and Minocycline in Subjects with Mild to Moderate Acne. J. Cutan. Med. Surg. 2013, 17, 114–122. [Google Scholar] [CrossRef]
- Vijayashankar, M.; Raghunath, N. Pustular psoriasis responding to Probiotics—A new insight. Our Dermatol. Online 2012, 3, 326–328. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Khan, T.M.; Duncan, S.H.; Harmsen, H.J.M.; Garcia-Gil, L.J.; Flint, H.J. Cultured representatives of two major phylogroups of human colonic Faecalibacterium prausnitzii can utilize pectin, uronic acids, and host-derived substrates for growth. Appl. Environ. Microbiol. 2012, 78, 420–428. [Google Scholar] [CrossRef]
- Eppinga, H.; Weilard, C.J.S.; Thio, H.B.; van der Wounde, C.J.; Nijsten, T.E.C.; Peppelenbosch, M.P. Similar depletion of protective Faecalibacterium prausnitzii in psoriasis and inflammatory bowel disease, but not in hidradenitis suppurativa. J. Crohns Colitis 2016, 10, 1067–1075. [Google Scholar] [CrossRef]
- Jones, M.; Ganopolsky, J.G.; Labbe, A.; Gilardino, M.; Wahl, C.; Martoni, C.; Prakash, S. Novel nitric oxide producing probiotic wound healing patch: Preparation and in vivo analysis in a New Zealand white rabbit model of ischaemic and infected wounds. Int. Wound J. 2012, 9, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Ridnour, L.A.; Espey, M.G.; Wink, D.A.; Roberts, D.D. Nitric oxide in wound-healing. Microsurgery 2005, 25, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.; Saio, M.; Yamashita, H.; Tanaka, H. Lactobacillus acidophilus Strain L-92 Induces CD4 CD25 Foxp3 Regulatory T Cells and Suppresses Allergic Contact Dermatitis. Biol. Pharm. Bull. 2012, 35, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Karska-Wysocki, B.; Bazo, M.; Smoragiewicz, W. Antibacterial activity of Lactobacillus acidophilus and Lactobacillus casei against methicillin-resistant Staphylococcus aureus (MRSA). Microbiol. Res. 2010, 165, 674–686. [Google Scholar] [CrossRef]
- Chapat, L.; Chemin, K.; Dubois, B.; Bourdet-Sicard, R.; Kaiserlian, D. Lactobacillus casei reduces CD8+T cell-mediated skin inflammation. Eur. J. Immunol. 2004, 3, 2520–2528. [Google Scholar] [CrossRef] [PubMed]
- Hacini-Rachinel, F.; Gheit, H.; Le Luduec, J.B.; Dif, F.; Nancey, S.; Kaiserlian, D. Oral probiotic control skin inflammation by acting on both effector and regulatory T cells. PLoS ONE 2009, 4, e4903. [Google Scholar] [CrossRef]
- Watanabe, J.; Sasajima, N.; Aramaki, A.; Sonoyama, K. Consumption of fructo-oligosaccharide reduces 2,4-dinitrofluorobenzene-induced contact hypersensitivity in mice. Br. J. Nutr. 2008, 100, 339–346. [Google Scholar] [CrossRef][Green Version]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viabil. 2017, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Codoner, F.M.; Ramirez-Bosca, A.; Climent, E.; Carrion-Gutierrez, M.; Guerrero, M.; Perez-Orquin, J.M.; Horga de la Parte, J.; Genoves, S.; Ramon, D.; Navarro-Lopez, V.; et al. Gut microbial composition in patients with psoriasis. Sci. Rep. 2018, 8, 3812. [Google Scholar] [CrossRef]
- Groeger, D.; O’Mahony, L.; Murphy, E.F.; Bourke, J.F.; Dinan, T.G.; Kiely, B.; Shanahan, F.; Quigley, E.M. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes 2013, 4, 325–339. [Google Scholar] [CrossRef]
- Wang, Y.; Kuo, S.; Shu, M.; Yu, J.; Huang, S.; Dai, A.; Two, A.; Gallo, R.L.; Huang, C.M. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: Implications of probiotics in acne vulgaris. Appl. Microbiol. Biotechnol. 2014, 98, 411–424. [Google Scholar] [CrossRef]
- Kang, B.S.; Seo, J.G.; Lee, G.S.; Kim, J.H.; Kim, S.Y.; Han, Y.W.; Kang, H.; Kim, H.O.; rhee, J.H.; Chung, M.J.; et al. Antimicrobial activity of enterocins from Enterococcus faecalis SL-5 against Propionibacterium acnes, the causative agent in acne vulgaris, and its therapeutic effect. J. Microbiol. 2009, 47, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Weise, C.; Zhu, Y.; Ernst, D.; Ku, A.A.; Worm, M. Oral administration of Escherichia coli Nissle 1917 prevents allergen-induced dermatitis in mice. Exp. Dermatol. 2011, 20, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Huseini, H.F.; Rahimzadeh, G.; Fazeli, M.R.; Mehrazma, M.; Salehi, M. Evaluation of wound healing activities of kefir products. Burns 2012, 38, 719–723. [Google Scholar] [CrossRef]
- Rodrigues, K.L.; Gaudino Caputo, L.R.; Tavares Carvalho, J.C.; Evangelista, J.; Schneedorf, J.M. Antimicrobial and healing activity of kefir and kefiran extract. Int. J. Antimicrob Agents 2005, 25, 404–408. [Google Scholar] [CrossRef]
- Woodfolk, J.A. T-cell responses to allergens. J. Allergy Clin. Immunol. 2007, 119, 280–294. [Google Scholar] [CrossRef]
- Al-Ghazzewi, F.H.; Tester, R.F. Effect of konjac glucomannan hydrolysates and probiotics on the growth of the skin bacterium Propionibacterium acnes in vitro. Int. J. Cosmet. Sci. 2010, 32, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Bateni, E.; Tester, R.; Al-Ghazzewi, F.; Bateni, S.; Alvani, K.; Piggott, J. The Use of Konjac Glucomannan Hydrolysates (GMH) to Improve the Health of the Skin and Reduce Acne Vulgaris. Am. J. Dermatol. Venereol. 2013, 2, 10–14. [Google Scholar]
- Daehn, I.S.; Varelias, A.; Rayner, T.E. Sodium butyrate induced keratinocyte apoptosis. Apoptosis 2006, 11, 1379–1390. [Google Scholar] [CrossRef]
- Staiano-Coico, L.; Khandke, L.; Krane, J.F.; Sharif, S.; Gottlieb, A.B.; Krueger, J.G.; Heim, L.; Rigas, B.; Higgins, P.J. TGF-α and TGF-β expression during sodium-N-butyrate-induced differentiation of human keratinocytes: Evidence for subpopulation-specific up-regulation of TGF-β mRNA in suprabasal cells. Exp. Cell Res. 1990, 191, 286–291. [Google Scholar] [CrossRef]
- Elder, J.T.; Zhao, X. Evidence for local control of gene expression in the epidermal differentiation complex. Exp. Dermatol. 2002, 11, 406–412. [Google Scholar] [CrossRef]
- Leon Carrion, S.; Sutter, C.H.; Sutter, T.R. Combined treatment with sodium butyrate and PD153035 enhances keratinocyte differentiation. Exp. Dermatol. 2014, 23, 211–214. [Google Scholar] [CrossRef]
- Sugimoto, S.; Ishii, Y.; Izawa, N.; Masuoka, N.; Kano, M.; Sone, T.; Chiba, K.; Miyazaki, K.; Ishikawa, F. Photoprotective effects of Bifidobacterium breve supplementation against skin damage induced by ultraviolet irradiation in hairless mice. Photodermatol. Photoimmunol. Photomed. 2012, 28, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Murata, M.; Iwabuchi, N.; Odamaki, T.; Wakabayashi, H.; Yamauchi, K.; Abe, F.; Xiao, J.Z. Effect of Bifidobacterium breve B-3 on skin photoaging induced by chronic UV irradiation in mice. Benef. Microbes 2015, 6, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, M. The physiology of wound healing. J. Wound Care 2000, 9, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Rieger, S.; Zhao, H.; Martin, P.; Abe, K.; Lisse, T.S. The role of nuclear hormone receptors in cutaneous wound repair. Cell Biochem. Funct. 2015, 33, 1–13. [Google Scholar] [CrossRef]
- Canesso, M.C.; Vieira, A.T.; Castro, T.B.; Schirmer, B.G.; Cisalpino, D.; Martins, F.S.; Rachid, M.A.; Nicoli, J.R.; Teixeira, M.M.; Barcelos, L.S. Skin Wound Healing Is Accelerated and Scarless in the Absence of Commensal Microbiota. J. Immunol. 2014, 193, 5171–5180. [Google Scholar] [CrossRef]
- Robson, M.C. Wound infection: A failure of wound healing caused by an imbalance of bacteria. Surg. Clin. N. Am. 1997, 77, 637–650. [Google Scholar] [CrossRef]
- Oryan, A.; Jalili, M.; Kamali, A.; Nikahval, B. The concurrent use of probiotic microorganism and collagen hydrogel/scaffold enhances burn wound healing: An in vivo evaluation. Burns 2018, 44, 1775–1786. [Google Scholar] [CrossRef]
- Sikorska, H.; Smoragiewicz, W. Role of probiotics in the prevention and treatment of meticillin-resistant Staphylococcus aureus infections. Int. J. Antimicrob. Agents 2013, 42, 475–481. [Google Scholar] [CrossRef]
- Codex Alimentarius. Codex Standards for fermented milks. In Milk and Milk Products, 2nd ed.; The European Community and its Member States (ECMS): Queenstown, New Zealand, 2011; pp. 6–16. [Google Scholar]
- Farnworth, E.R. Kefir a complex probiotic. Food Sci. Technol. Bull. Funct. Foods 2006, 2, 1–17. [Google Scholar] [CrossRef]
- Satir, G.; Guzel-Seydim, Z.B. How kefir fermentation can affect product composition? Small Rumin. Res. 2016, 134, 1–7. [Google Scholar] [CrossRef]
- Irigoyen, A.; Arana, I.; Castiella, M.; Torre, P.; Ibáñez, F.C. Microbiological, physicochemical, and sensory characteristics of kefir during storage. Food Chem. 2005, 90, 613–620. [Google Scholar] [CrossRef]
- Chen, H.C.; Wang, S.Y.; Chen, M.J. Microbiological study of lactic acid bacteria in kefir grains by culture-dependent and culture-independent methods. Food Microbiol. 2008, 25, 492–501. [Google Scholar] [CrossRef]
- Rahimzadeh, G.; Fazeli, M.R.; Mozafari, A.N.; Mesbahi, M. Evaluation of anti-microbial activity and wound healing of kefir. Int. J. Pharm. Sci. Res. 2015, 6, 286–293. [Google Scholar]
- Serafini, F.; Turroni, F.; Ruas-Madiedo, P.; Lugli, G.A.; Milani, C.; Duranti, S.; Zamboni, N.; Bottachini, F.; van Sinderen, D.; Margolles, A.; et al. Kefir fermented milk and kefiran promote growth of Bifidobacterium bifidum PRL2010 and modulate its gene expression. Int. J. Food Microbiol. 2014, 178, 50–59. [Google Scholar] [CrossRef]
- Tsiouris, C.G.; Kelesi, M.; Vasilopoulos, G.; Kalemikerakis, I.; Papageorgiou, E.G. The efficacy of probiotics as pharmacological treatment of cutaneous wounds: Meta-analysis of animal studies. Eur. J. Pharm. Sci. 2017, 104, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Drust, B.; Cable, N.T.; Reilly, T. Investigation of the effects of the pre-cooling on the physiological responses to soccer-specific intermittent exercise. Eur. J. Appl. Physiol. Occup. Physiol. 2000, 81, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Tett, A.; Pasolli, E.; Farina, S.; Truong, D.T.; Asnicar, F.; Zolfo, M.; Beghini, F.; Armanini, F.; Jousson, O.; De Sanctis, V.; et al. Unexplored diversity and strain-level structure of the skin microbiome associated with psoriasis. NPJ Biofilms Microbiomes 2017, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Chang, HW.; Yan, D.; Singh, R.; Liu, J.; Lu, X.; Ucmak, D.; Lee, K.; Afifi, L.; Fedrosh, D.; Leech, J.; Vasquez, K.S.; et al. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome 2018, 6, 154. [Google Scholar] [CrossRef]
- Eppinga, H.; Thio, H.B.; Schreurs, M.W.J.; Blakaj, B.; Tahitu, R.I.; Konstantinov, S.R.; Peppelenbosch, M.P.; Fuhler, G.M. Depletion of Saccharomyces cerevisiae in psoriasis patients restored by Dimethylfumarate therapy (DMF). PLoS ONE 2017, 12, e0176955. [Google Scholar] [CrossRef]
- Thio, H.H. The microbiome in psoriasis and psoriatic arthritis: The skin perspective. J. Rheumatol. Suppl. 2018, 94, 30–31. [Google Scholar] [PubMed]
- Manning, T.S.; Gibson, G.R. Microbial-gut interactions in health and disease. Prebiotics. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 287–298. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, D.E.; Park, S.D.; Kim, Y.-T.; Kim, Y.J.; Jeong, J.W.; Jeng, S.S.; Ahn, Y.T.; Sim, J.H.; Huh, C.S.; et al. Oral administration of lactobacillus plantarum HY7714 protects hairless mouse against ultraviolet B-induced photoaging. J. Microbiol. Biotechnol. 2014, 24, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Ra, J.; Lee, D.E.; Kim, S.H.; Jeong, J.W.; Ku, H.K.; Kim, T.Y.; Choi, I.D.; Jeung, W.; Sim, J.H.; Ahn, Y.T. Effect of oral administration of Lactobacillus plantarum HY7714 on epidermal hydration in ultraviolet B-irradiated hairless mice. J Microbiol. Biotechnol. 2014, 24, 1736–1743. [Google Scholar] [CrossRef]
- Hong, K.B.; Jeong, M.; Han, K.S.; Hwan Kim, J.; Park, Y.; Suh, H.J. Photoprotective effects of galacto-oligosaccharide and/or Bifidobacterium longum supplementation against skin damage induced by ultraviolet irradiation in hairless mice. Int. J. Food Sci. Nutr. 2015, 66, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Moczar, E.; Yvetter, S.G.; Robert, L.; Robert, A. Use of Oligosaccharides in the Prevention and Treatment of the Aging of Tissues. U.S. Patent No. 5910490, 16 August 1999. [Google Scholar]
- Strickland, M.F.; Pelley, P.R.; Kripke, L.M. Cytoprotective Oligosaccharide from Aloe Preventing Damage to the Skin Immune System by UV Radiation. U.S. Patent No. 5824659, 20 October 1998. [Google Scholar]
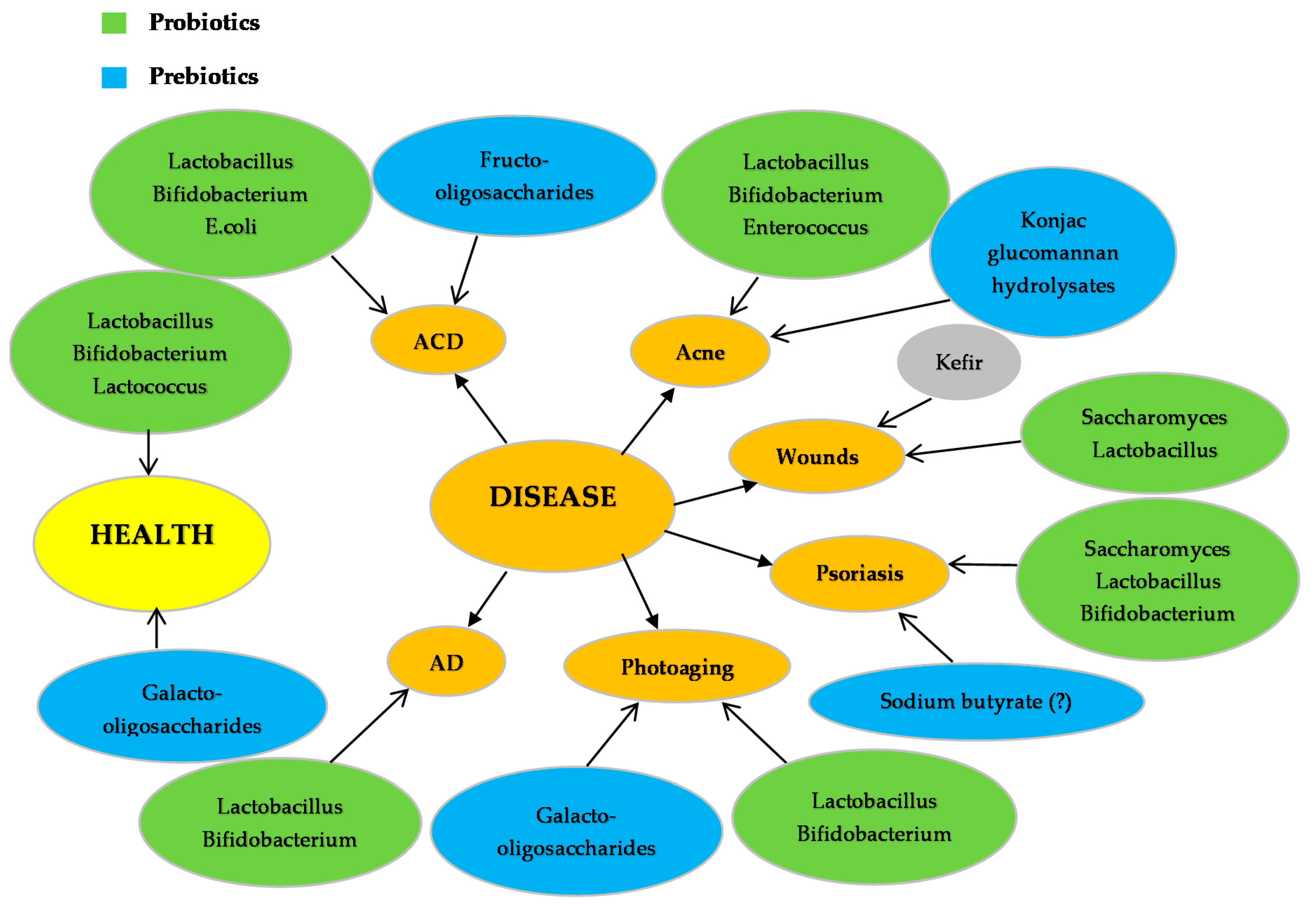
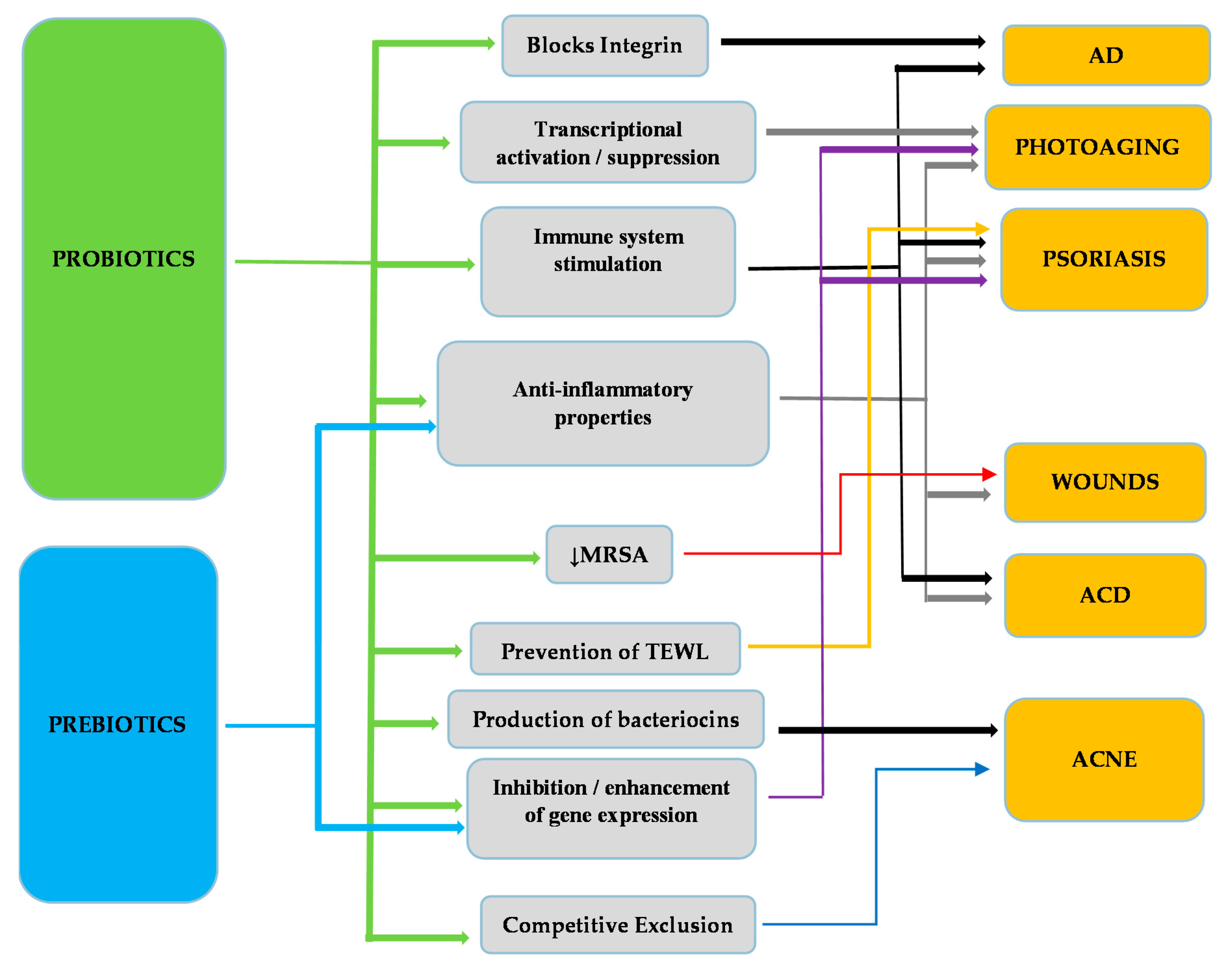
| Probiotics | Disease | Function | Reference |
|---|---|---|---|
| L. rhamnosus | AD 1 | Improvement of severity of eczema, reduction of risk of AD development in infants | [65,66] |
| L. reuteri | AD Infections (S. aureus) | Improvement of eczema. Blocks integrin, Reduces cell death due to S. aureus infection | [65,97] |
| L. delbrueckii subspecies bulgaricus | Acne | Improvement of Acne symptoms (Acne Vulgaris) | [98] |
| L. sporogenes | Psoriasis | Improvement of symptoms, reduction of blood sugar levels and fever | [99] |
| L. plantarum | Photoaging | Inhibition of MMP-1, MMP-2, MMP-9 and MMP-13 2, enhancement of procollagen expression, inhibition of phosphorylation of Jun N-terminal kinase, increase of palmitoytransferase mRNA levels, decrease of ceramide mRNA levels, reduction of wrinkles and epidermal thickness | [100,101] |
| L. fermentum | Infections (wounds) | Production of gNO 3, increases productions of IL-1 4 and TGF-β 5 cytokines | [102,103] |
| L. acidophilus | AD ACD 6 Infections (S. aureus) Acne | Reduction of Ig-E 7, reduction of eczema, Increase of TGF-β, Foxp3 8, IFN-γ 9 and IL-10 10 expression, Inhibition of S. aureus infection, reduction of acne symptoms | [62,98,104,105] |
| L. casei L. salivarius | ACD Infections (MRSA) 11 | Reduction of skin inflammation, inhibition of IFN-γ, CD8+ T cells, increase in IL-10 production, activation of CD4+CD25+ T cells, inhibition of MRSA | [105,106,107] |
| B. bifidum | AD Acne | Reduction of Ig-E, reduction of development of AD in infants, reduction of Acne Vulgaris symptoms | [62,98] |
| B. lactis | AD | Reduction of Ig-E, reduction of development of AD in infants. | [62] |
| B. pseudolongum | ACD | Reduction of allergic reaction on mice | [108] |
| B. longum | Photoaging | Prevention of TEWL 12, reduction of skin erythema, increase of mRNA expression of CD44, TIMP-113 and Col114. | [109] |
| B. breve strain Yakult | Photoaging | Prevention of loss of elasticity, suppression of elastase, activation of IL-1β | [38,110] |
| B. infantis | Psoriasis | Reduction of plasma TNF-α15, increase of IL-6 | [111] |
| S. epidermidis | Acne | Growth inhibition of Propionibacterium acnes and Acne Vulgaris by competitive exclusion | [112] |
| E. faecalis | Acne | Reduction of inflammation areas, production of bacteriocins | [113] |
| E. coli Nissle 1917 | ACD | Increase of TGF-β, Foxp3, IFN-γ and IL-10 expression | [114] |
| Kefir grains | Infections | Production of antimicrobial substances (lactic acid, acetic acid, hydrogen peroxide, bacteriocins), Healing of P. aeruginosa infected wounds, Inhibition of S. aureus, S. salivarius, S. pyogenes, P. aeruginosa, C. albicans, S. tympimurium, L. monocytogenes and E. coli growth | [115,116] |
| Prebiotics | Disease | Function | Reference |
|---|---|---|---|
| Fructo-oligosaccharides | ACD | Reduction of allergic reaction. | [108] |
| Konjac glucomannan hydrolysates (GMH) | Acne | Inhibition of Acne Vulgaris and P. acnes, growth enhancement of lactic acid bacteria. | [118,119] |
| Galacto-oligosaccharides | Photoaging | Prevention of 1 TEWL, reduction of skin erythema, increase of mRNA expression of CD44, 2TIMP-1 and 3Col1. | [109] |
| Sodium Butyrate (?) | Psoriasis | Increases Fas, 4 TGF-β and p52 | [120,121,122,123] |
| Oligo-saccharides | Photoaging | Modulation of the expression of elastase-type proteases through elastin receptors | [124,125] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lolou, V.; Panayiotidis, M.I. Functional Role of Probiotics and Prebiotics on Skin Health and Disease. Fermentation 2019, 5, 41. https://doi.org/10.3390/fermentation5020041
Lolou V, Panayiotidis MI. Functional Role of Probiotics and Prebiotics on Skin Health and Disease. Fermentation. 2019; 5(2):41. https://doi.org/10.3390/fermentation5020041
Chicago/Turabian StyleLolou, Vasiliki, and Mihalis I. Panayiotidis. 2019. "Functional Role of Probiotics and Prebiotics on Skin Health and Disease" Fermentation 5, no. 2: 41. https://doi.org/10.3390/fermentation5020041
APA StyleLolou, V., & Panayiotidis, M. I. (2019). Functional Role of Probiotics and Prebiotics on Skin Health and Disease. Fermentation, 5(2), 41. https://doi.org/10.3390/fermentation5020041






