Dry Anaerobic Digestion of Food and Paper Industry Wastes at Different Solid Contents
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Substrates
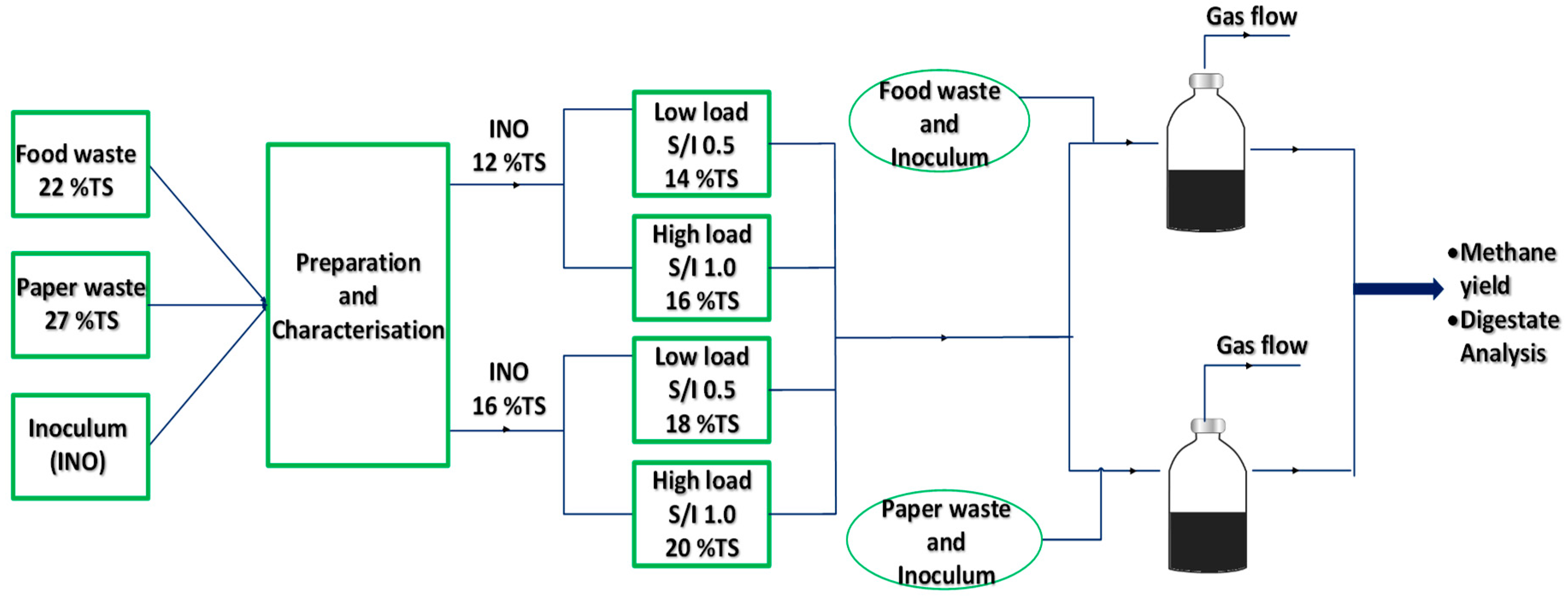
2.2. Dry Anaerobic Digestion Assays
2.3. Analytical Methods
2.4. Digestate Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Synthetic Food Waste, Paper Waste and Inoculum
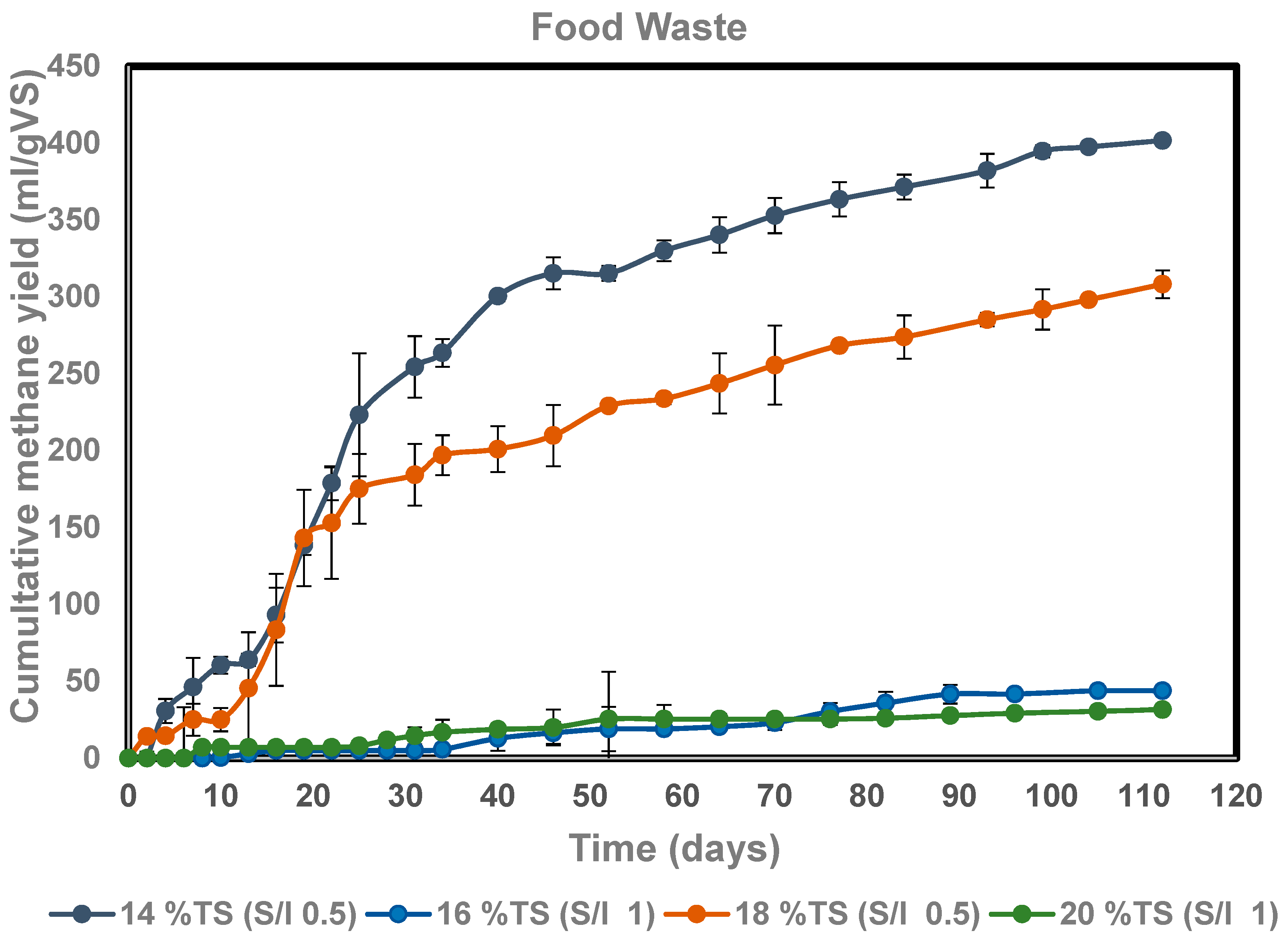
3.2. Dry Anaerobic Digestion of Food Waste
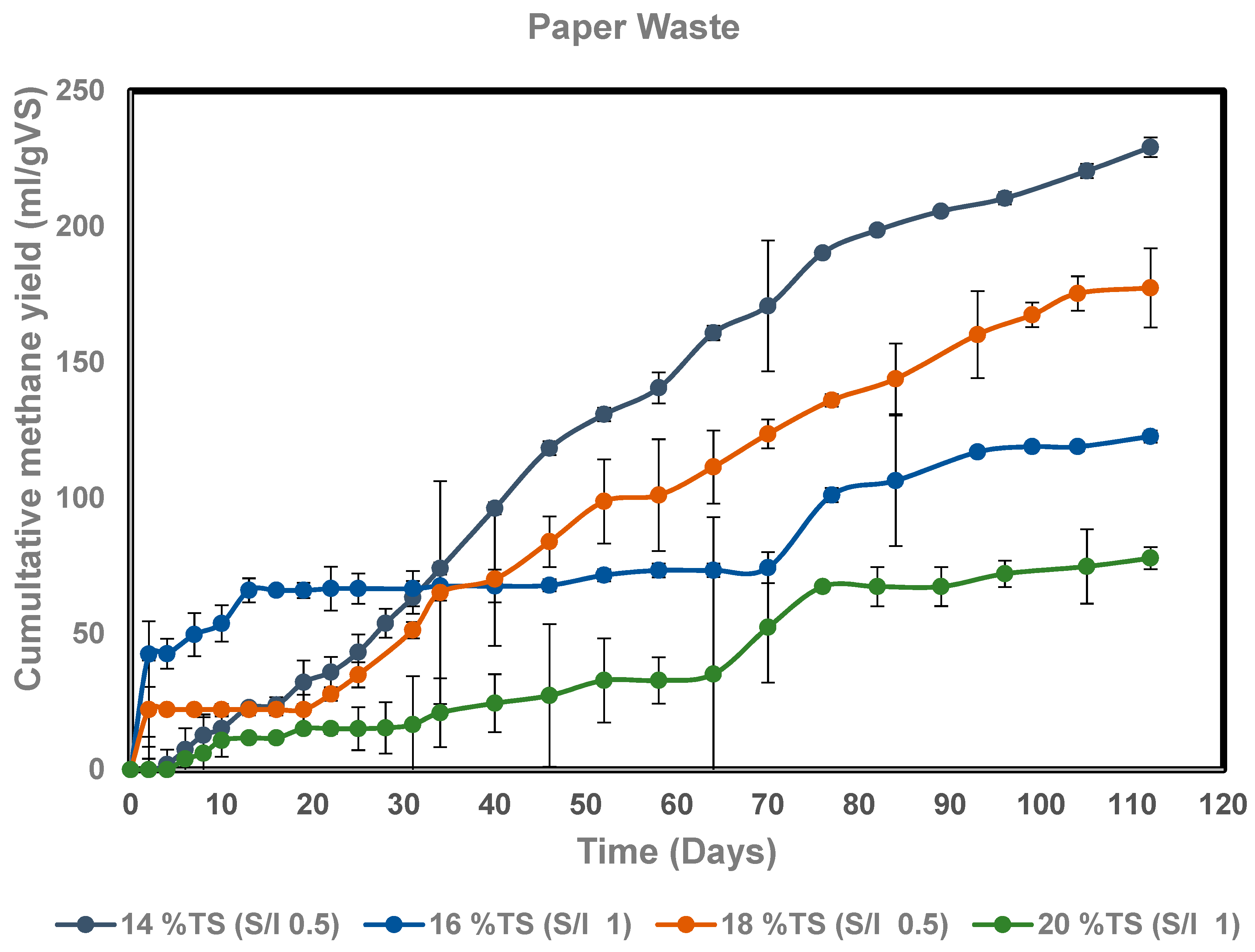
3.3. Dry Anaerobic Digestion of Paper Waste
3.4. Digestate Quality
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Matheri, A.N.; Sethunya, V.L.; Belaid, M.; Muzenda, E. Analysis of the biogas productivity from dry anaerobic digestion of organic fraction of municipal solid waste. Renew. Sust. Energ. Rev. 2018, 81, 2328–2334. [Google Scholar] [CrossRef]
- Woon, K.S.; Lo, I.M. A proposed framework of food waste collection and recycling for renewable biogas fuel production in Hong Kong. Waste Manag. 2016, 47, 3–10. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Esposito, G.; Yeh, D.H.; Lens, P.N.L. Enhanced anaerobic digestion of food waste by supplementing trace elements: Role of Selenium (VI) and Iron (II). Front. Environ. Sci. 2016, 4, 8. [Google Scholar] [CrossRef]
- Lou, X.; Nair, J. The impact of landfilling and composting on greenhouse gas emissions—A review. Bioresour. Technol. 2009, 100, 3792–3798. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Alaswad, A.; El-Hassan, Z.; Olabi, A.G. Mechanical pretreatment of waste paper for biogas production. Waste Manag. 2017, 68, 157–164. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, X.; Shang, D.; Bao, D.; Zhang, S.; Zheng, T. Energetic-environmental-economic assessment of the biogas system with three utilization pathways: Combined heat and power, biomethane and fuel cell. Bioresour. Technol. 2016, 214, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Makádi, M.; Tomócsik, A.; Orosz, V. Digestate: A New Nutrient Source—Review, Biogas, Sunil Kumar. IntechOpen, 2012. Available online: https://www.intechopen.com/books/biogas/digestate-a-new-nutrient-source-review (accessed on 14 March 2012). [CrossRef]
- Li, Y.; Park, S.Y.; Zhu, J. Solid-state anaerobic digestion for methane production from organic waste. Renew. Sust. Energ. Rev. 2011, 15, 821–826. [Google Scholar] [CrossRef]
- Bolzonella, D.; Innocenti, L.; Pavan, P.; Traverso, P. Cecchi, F. Semi-dry thermophilic anaerobic digestion of the organic fraction of municipal solid waste: Focusing on the start-up phase. Bioresour. Technol. 2003, 86, 123–129. [Google Scholar] [CrossRef]
- Yi, J.; Dong, B.; Jin, J.; Dai, X. Effect of increasing total solids contents on anaerobic digestion of food waste under mesophilic conditions: Performance and microbial characteristics analysis. PLoS ONE 2014, 9, e102548. [Google Scholar] [CrossRef] [PubMed]
- Watkins, K. Human Development Report 2006—Beyond Scarcity: Power, Poverty and the Global Water Crisis. In UNDP Human Development Reports; United Nations Development Programme (UNDP): New York, NY, USA, 2006. [Google Scholar]
- Karthikeyan, O.P.; Visvanathan, C. Bio-energy recovery from high-solid organic substrates by dry anaerobic bio-conversion processes: A review. Rev. Environ. Sci. Bio-Technol. 2013, 12, 257–284. [Google Scholar] [CrossRef]
- Rapport, J.; Zhang, R.; Jenkins, B.M.; Williams, R.B. Current anaerobic digestion technologies used for treatment of municipal organic solid waste. Calif. Integr. Waste Manag. Board 2008, 35–36. [Google Scholar]
- Veluchamy, C.; Kalamdhad, A.S. Influence of pretreatment techniques on anaerobic digestion of pulp and paper mill sludge: A review. Bioresour. Technol. 2017, 245, 1206–1219. [Google Scholar] [CrossRef] [PubMed]
- Vandevivere, P.; De Baere, L.; Verstraete, W. Biomethanization of the Organic Fraction of Municipal Solid Wastes; Iwa Publishing: London, UK, 2003; pp. 111–140. [Google Scholar]
- Kamali, M.; Gameiro, T.; Costa, M.E.V.; Capela, I. Anaerobic digestion of pulp and paper mill wastes–An overview of the developments and improvement opportunities. Chem. Eng. J. 2016, 298, 162–182. [Google Scholar] [CrossRef]
- Kamali, M.; Khodaparast, Z. Review on recent developments on pulp and paper mill wastewater treatment. Ecotox. Environ. Safe. 2015, 114, 326–342. [Google Scholar] [CrossRef]
- FAO. Global Food Losses and Food Waste—Extent, Causes and Prevention. Available online: http://www.fao.org/docrep/014/mb060e/mb060e.pdf (accessed on 20 September 2016).
- Zhang, R.H.; El-Mashad, H.M.; Hartman, K.; Wang, F.Y.; Liu, G.Q.; Choate, C.; Gamble, P. Characterization of food waste as feedstock for anaerobic digestion. Bioresour. Technol. 2007, 98, 929–935. [Google Scholar] [CrossRef]
- Ariunbaatar, J. Methods to Enhance Anaerobic Digestion of Food Waste; Université Paris-Est: Paris, France, 2014. [Google Scholar]
- Demirel, B.; Scherer, P. Trace element requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenerg. 2011, 35, 992–998. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, Z.; Huang, Z.-L.; Dong, M.; Yu, X.-L.; Ning, P. A new strategy for co-composting dairy manure with rice straw: Addition of different inocula at three stages of composting. Waste Manag. 2015, 40, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Appels, L.; Baeyens, J.; Degreve, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Banks, C.J.; Chesshire, M.; Heaven, S.; Arnold, R. Anaerobic digestion of source-segregated domestic food waste: Performance assessment by mass and energy balance. Bioresour. Technol. 2011, 102, 612–620. [Google Scholar] [CrossRef]
- Charles, W.; Walker, L.; Cord-Ruwisch, R. Effect of pre-aeration and inoculum on the start-up of batch thermophilic anaerobic digestion of municipal solid waste. Bioresour. Technol. 2009, 100, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Chynoweth, D.P.; Owens, J.M.; Legrand, R. Renewable methane from anaerobic digestion of biomass. Renew. Energy 2001, 22, 1–8. [Google Scholar] [CrossRef]
- Hartmann, H.; Ahring, B.K. Anaerobic digestion of the organic fraction of municipal solid waste: Influence of co-digestion with manure. Water Res. 2005, 39, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.L.; Feng, Y.Z.; Wang, X.J.; Ren, G.X. Review on research achievements of biogas from anaerobic digestion. Renew. Sust. Energ. Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sust. Energ. Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Panico, A.; d’Antonio, G.; Esposito, G.; Frunzo, L.; Iodice, P.; Pirozzi, F. The effect of substrate-bulk interaction on hydrolysis modeling in anaerobic digestion process. Sustainability 2014, 6, 8348–8363. [Google Scholar] [CrossRef]
- Teghammar, A.; Yngvesson, J.; Lundin, M.; Taherzadeh, M.J.; Horvath, I.S. Pretreatment of paper tube residuals for improved biogas production. Bioresour. Technol. 2010, 101, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Apha, A. WPCF, Standard Methods for the Examination of Water and Wastewater; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 1995. [Google Scholar]
- Ferree, M.A.; Shannon, R.D. Evaluation of a second derivative UV/visible spectroscopy technique for nitrate and total nitrogen analysis of wastewater samples. Water Res. 2001, 35, 327–332. [Google Scholar] [CrossRef]
- Haug, R. The Practical Handbook of Compost Engineering; Lewis Publishers: Boca Raton, FL, USA, 1993. [Google Scholar]
- Birke, M.; Reimann, C.; Demetriades, A.; Rauch, U.; Lorenz, H.; Harazim, B.; Glatte, W. Determination of major and trace elements in European bottled mineral water—analytical methods. J. Geochem. Explor. 2010, 107, 217–226. [Google Scholar] [CrossRef]
- Patinvoh, R.J.; Mehrjerdi, A.K.; Horváth, I.S.; Taherzadeh, M.J. Dry fermentation of manure with straw in continuous plug flow reactor: Reactor development and process stability at different loading rates. Bioresour. Technol. 2017, 224, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Habiba, L.; Hassib, B.; Moktar, H. Improvement of activated sludge stabilisation and filterability during anaerobic digestion by fruit and vegetable waste addition. Bioresour. Technol. 2009, 100, 1555–1560. [Google Scholar] [CrossRef]
- Patinvoh, R.J.; Osadolor, O.A.; Horváth, I.S.; Taherzadeh, M.J. Cost effective dry anaerobic digestion in textile bioreactors: Experimental and economic evaluation. Bioresour. Technol. 2017, 245, 549–559. [Google Scholar] [CrossRef]
- Al Seadi, T.; Lukehurst, C. Quality management of digestate from biogas plants used as fertiliser. IEA Bioenergy 2012, 37, 40. [Google Scholar]
- Capson-Tojo, G.; Rouez, M.; Crest, M.; Steyer, J.-P.; Delgenes, J.-P.; Escudie, R. Food waste valorization via anaerobic processes: A review. Rev. Environ. Sci. Bio-Technol. 2016, 15, 499–547. [Google Scholar] [CrossRef]
- De Vrieze, J.; Hennebel, T.; Boon, N.; Verstraete, W. Methanosarcina: The rediscovered methanogen for heavy duty biomethanation. Bioresour. Technol. 2012, 112, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Nagao, N.; Tajima, N.; Niwa, C.; Matsuyama, T.; Toda, T. The effect of the labile organic fraction in food waste and the substrate/inoculum ratio on anaerobic digestion for a reliable methane yield. Bioresour. Technol. 2014, 157, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, W.; Gao, X.; Zhou, Y.; Shen, R. Effect of thermal pretreatment on the physical and chemical properties of municipal biomass waste. Waste Manage. 2012, 32, 249–255. [Google Scholar] [CrossRef]
- Meyer, T.; Edwards, E.A. Anaerobic digestion of pulp and paper mill wastewater and sludge. Water Res. 2014, 65, 321–349. [Google Scholar] [CrossRef]
- Kabir, M.M.; Taherzadeh, M.J.; Sárvári Horváth, I. Dry anaerobic digestion of lignocellulosic and protein residues. BRJ 2015, 2, 309–316. [Google Scholar] [CrossRef][Green Version]
| Parameters | Food Waste | Paper Waste | Inoculum, High TS | Inoculum, Low TS |
|---|---|---|---|---|
| Total solids (%) | 21.90 ± 0.15 | 27.33 ± 0.05 | 15.91 ± 2.77 | 11.91 ± 0.26 |
| Volatile solids (% TS) a | 96.76 ± 0.10 | 99.46 ± 0.03 | 77.10 ± 2.35 | 69.42 ± 1.62 |
| Moisture (%) | 78.10 ± 0.15 | 72.66 ± 0.15 | 84.09 ± 2.77 | 88.09 ± 0.26 |
| Ash (%) a | 3.24 ± 0.10 | 0.54 ± 0.03 | 22.39 ± 2.35 | 30.58 ± 1.62 |
| Total organic carbon (% TS) a | 53.76 ± 0.06 | 55.26 ± 0.02 | 42.84 ± 1.30 | 38.57 ± 0.90 |
| Kjeldahl Nitrogen (% TS) a | 2.929 ± 0.38 | 0.069 ± 0.01 | 3.02 ± 0.11 | 3.02 ± 0.11 |
| C/N ratio | 18.67 ± 3.45 | 807 ± 25.46 | 14.22 ± 1.34 | 12.80 ± 1.07 |
| Bulk density (kg/ m3) | 1090.4 ± 15.99 | 432.9 ± 6.35 | 1016.8 ± 17.65 | 1016.8 ± 17.65 |
| Protein content (%) a | 18.31 ± 0.38 | 0.43 ± 0.01 | 18.88 ± 0.14 | 18.88 ± 0.14 |
| pH | 5.34 ± 000 | ND | 8.42 ± 000 | 8.42 ± 000 |
| Macro Elements | g/kg of Dry Matter | g/kg of Fresh Mass |
| Calcium | 52.7 | 6.11 |
| Potassium | 19.2 | 2.23 |
| Magnesium | 2.55 | 0.30 |
| Natrium | 15.0 | 1.74 |
| Phosphorus | 10.8 | 1.25 |
| Sulphur | 5.33 | 0.62 |
| Micro/Trace Elements | mg/kg of Dry Matter | mg/kg of Fresh Mass |
| Aluminum | 1700 | 197 |
| Arsenic | * < 15.0 | * < 1.74 |
| Boron | 20.0 | 2.32 |
| Barium | 49.2 | 5.71 |
| Cadmium | 0.28 | 0.03 |
| Cobalt | 5.31 | 0.62 |
| Chrome | 5.95 | 0.69 |
| Copper | 30.2 | 3.50 |
| Iron | 4500 | 522 |
| Mercury | * < 4.20 | * < 0.49 |
| Lithium | 1.47 | 0.17 |
| Manganese | 201 | 23.3 |
| Molybdenum | 3.26 | 0.38 |
| Niobium | * < 0.30 | * < 0.03 |
| Nickel | 6.59 | 0.76 |
| Lead | 2.96 | 0.34 |
| Selenium | * < 15.0 | * < 1.74 |
| Silicon | 486 | 56.4 |
| Tin | 2.34 | 0.27 |
| Strontium | 142 | 16.5 |
| Vanadium | 2.27 | 0.26 |
| Zinc | 95.1 | 11.0 |
| 14%TS (S/I 0.5) | pH | VFA (g/l) | NH4 - N (mg/l) |
|---|---|---|---|
| Food waste | 8.14 ± 0.03 | 1.03 ± 0.93 | 4200.00 ± 283.00 |
| Paper waste | 7.74 ± 0.43 | 0.38 ± 0.13 | 3250.00 ± 100.00 |
| 18%TS (S/I 0.5) | |||
| Food waste | 8.11 ± 0.08 | 0 | 540.00 ± 30.00 |
| Paper waste | 8.11 ± 0.07 | 0 | 363.33 ± 15.28 |
| 16%TS (S/I 1) | |||
| Food waste | 6.88 ± 1.19 | 7.48 ± 4.52 | 4600 ± 566.00 |
| Paper waste | 7.27 ± 1.27 | 0.29 ± 0.04 | 2900.00 ± 100.00 |
| 20%TS (S/I 1) | |||
| Food waste | 5.92 ± 0.07 | 10.42 ± 4.16 | 6700 ± 892 |
| Paper waste | 6.36 ± 1.60 | 0.52 ± 0.19 | 3400 ± 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansson, A.T.; Patinvoh, R.J.; Sárvári Horváth, I.; Taherzadeh, M.J. Dry Anaerobic Digestion of Food and Paper Industry Wastes at Different Solid Contents. Fermentation 2019, 5, 40. https://doi.org/10.3390/fermentation5020040
Jansson AT, Patinvoh RJ, Sárvári Horváth I, Taherzadeh MJ. Dry Anaerobic Digestion of Food and Paper Industry Wastes at Different Solid Contents. Fermentation. 2019; 5(2):40. https://doi.org/10.3390/fermentation5020040
Chicago/Turabian StyleJansson, Anette T., Regina J. Patinvoh, IIona Sárvári Horváth, and Mohammad J. Taherzadeh. 2019. "Dry Anaerobic Digestion of Food and Paper Industry Wastes at Different Solid Contents" Fermentation 5, no. 2: 40. https://doi.org/10.3390/fermentation5020040
APA StyleJansson, A. T., Patinvoh, R. J., Sárvári Horváth, I., & Taherzadeh, M. J. (2019). Dry Anaerobic Digestion of Food and Paper Industry Wastes at Different Solid Contents. Fermentation, 5(2), 40. https://doi.org/10.3390/fermentation5020040






