Biochemical Production and Separation of Carboxylic Acids for Biorefinery Applications
Abstract
:1. Introduction
2. Carboxylic Acid: Formation and Applications
2.1. Current State of the Art
2.2. Challenges and Considerations for the Production of Carboxylic Acids from Lignocellulosic Biomass
3. Biochemical Routes to Carboxylic Acid Production
3.1. Microbes Used for Acetic Acid Production
3.2. Microbes Used for Butyric Acid Production
3.3. Microbes Used for Propionic Acid Production
3.4. Microbes Used for Lactic Acid Production
3.5. Disadvantages of Pure Microbial Cultures in Biorefineries
3.6. Mixed Bacterial Consortia for Carboxylic Acid Production
4. Product Separation and Purification
4.1. Separation Using Ion Exchange Resins
4.2. Separation Using Solvent Extraction
4.3. Effect of Product Concentration on Separation Processes
4.4. Separation Using Electrodialysis
5. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lange, J.-P. Lignocellulose conversion: An introduction to chemistry, process and economics. Biofuels Bioprod. Bioref 2007, 1, 39–48. [Google Scholar] [CrossRef]
- Baumann, I.; Westermann, P. Microbial production of short chain fatty acids from lignocellulosic biomass: Current processes and market. BioMed Res. Intl. 2016, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Worrell, E.; Phylipsen, D.; Einstein, D.; Martin, N. Energy Use and Energy Intensity of the US Chemical Industry. US DOE Report LBNL-44314; 2000. Available online: http://ateam.lbl.gov/PUBS/doc/LBNL-44314.pdf (accessed on 8 November 2016).
- Micromarket monitor. North America Acetic Acid Market by Application (Vinyl Acetate Monomer (VAM), Purified Terephthalic Acid (PTA), Acetic Anhydride, Ester Solvents & Others) & by Country–Trends & Forecasts to 2019. Report Code AC 1086. 2015. Available online: http://www.micromarketmonitor.com/market/north-america-acetic-acid-9541396535.html?utm_source=NL-NAAAM&utm_medium=NL-NAAAM&utm_campaign=NL-NAAAM (accessed on 25 May 2015).
- Renewable Chemicals Market–Alcohols (Ethanol, Methanol), Biopolymers (Starch Blends, Regenerated Cellulose, PBS, bio-PET, PLA, PHA, bio-PE, and Others), Platform Chemicals & Others-Global Trends & Forecast to 2020. Report Code CH 2063. 2015. Available online: http://www.marketsandmarkets.com/Market-Reports/renewable-chemical-274.html (accessed on 1 October 2015).
- Brindle, F. American chemistry council: Global chemical production looks positive. Energy Glob. 2016. Available online: https://www.energyglobal.com/downstream/petrochemicals/13042016/american-chemistry-council-positive-growth-cpri-global-chemical-production-3023/ (accessed on 13 April 2016).
- Metzger, J.O.; Huttermann, A. Sustainable global energy supply based on lignocellulosic biomass from afforestation of degraded areas. Naturwissenschaften 2009, 96, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: a review of product properties and effects of pyrolysis parameters. Ren. Sust. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical biomass gasification: A review of the current status of the technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015, 178, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Dasgupta, D.; Agrawal, D.; Kaul, S.; Adhikari, D.K.; Kurmi, A.K.; Arya, P.K.; Bangwal, D.; Negi, M.S. Fuels and chemicals from lignocellulosic biomass: An integrated biorefinery approach. Energy Fuels 2015, 29, 3149–3157. [Google Scholar] [CrossRef]
- Liao, J.C.; Mi, L.; Pontrelli, S.; Luo, S. Fuelling the future: Microbial engineering for the production of sustainable biofuels. Nat. Rev. Microbiol. 2016, 14, 288–304. [Google Scholar] [CrossRef] [PubMed]
- Murali, N.; Fernandez, S.; Ahring, B.K. Fermentation of wet-exploded corn stover for the production of volatile fatty acids. Bioresour. Technol. 2017, 227, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Raposo, F.; Borja, R.; Cacho, J.A.; Mumme, J.; Orupold, K.; Esteves, S.; Nogruel-Arias, J.; Picard, S.; Nielfa, A.; Scherer, P.; et al. First international comparative study of volatile fatty acids in aqueous samples by chromatographic techniques: Evaluating sources of error. Trends Anal. Chem. 2013, 51, 127–143. [Google Scholar] [CrossRef]
- Agler, M.T.; Wrenn, B.A.; Zinder, S.H.; Angenent, L.T. Waste to bioproducts conversion with undefined mixed cultures: the carboxylate platform. Trends Biotechnol. 2011, 29, 70–78. [Google Scholar] [CrossRef] [PubMed]
- GBIR Research. Acetic Acid Global Market to 2020-Surge in VAM and PTA Sectors in Asia Pacific to Drive Global demand. Report Code GBICH0082MR. 2013. Available online: http://www.gbiresearch.com/report-store/market-reports/archive/acetic-acid-global-market-to-2020-surge-in-vam-and-pta-sectors-in-asia-pacific-to-drive-global-demand (accessed on 1 February 2013).
- Yoneda, N.; Kusano, S.; Yasui, M.; Pujado, P.; Wilcher, S. Recent advances in processes and catalysts for the production of acetic acid. Appl. Catal. A 2001, 221, 253–265. [Google Scholar] [CrossRef]
- Wood, B.J.B. Microbiology of Fermented Foods, 2nd ed.; Springer: New York, NY, USA, 1998. [Google Scholar]
- Sengun, I.Y.; Karabiyikli, S. Importance of acetic acid bacteria in food industry. Food Control 2011, 22, 647–656. [Google Scholar] [CrossRef]
- Chavez, K.L.; Hess, D.W. A novel method of etching copper oxide using acetic acid. J. Electrochem. Soc. 2001, 148, G640–G643. [Google Scholar] [CrossRef]
- Gandini, A. Polymers from renewable resources: a challenge for the future of macromolecular materials. Macromolecules 2008, 41, 9491–9504. [Google Scholar] [CrossRef]
- Ganster, J.; Fink, H.-P. Cellulose and cellulose acetate. In Bio-Based Plastics: Materials and Applications, 1st ed.; Kabasci, S., Ed.; Wiley: West Sussex, UK, 2014; pp. 35–62. [Google Scholar]
- Weissermel, K.; Arpe, H.-J. Industrial Organic Chemistry, 4th ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Luck, E.; Jager, M. Propionic acid. In Antimicrobial Food Additives, 2nd ed.; Luck, E., Jager, M., Eds.; Springer: Berlin, Germany, 1997; pp. 145–151. [Google Scholar]
- Ali, S.H.; Tarakmah, A.; Merchant, S.Q.; Al-Sahhaf, T. Synthesis of esters: Development of the rate expression for the Dowex 50 Wx8-400 catalyzed esterification of propionic acid with 1-propanol. Chem. Eng. Sci. 2007, 62, 3197–3217. [Google Scholar] [CrossRef]
- El-Shahawy, T.A.E.-G. Chemicals with a natural reference for controlling water hyacinth, Eichhornia crassipes (Mart.) Solms. J. Plant Protec. Res. 2015, 55, 294–300. [Google Scholar] [CrossRef]
- Ihre, H.; Hult, A.; Soderlind, E. Synthesis, characterization and 1H NMR self-diffusion studies of dendritic aliphatic polyesters based on 2,2-bis (hydroxymethyl) propionic acid and 1,1,1-Tris(hydroxyphenyl)ethane. J. Am. Chem. Soc. 1996, 118, 6388–6395. [Google Scholar] [CrossRef]
- Propionic Acid & Derivatives Market by Applications (Animal Feed & Grain Preservatives, Food Preservatives, Herbicides, Cellulose Acetate Propionate) & Geography–Global Trends & Forecasts to 2018. Report Code CH 1533. 2013. Available online: http://www.marketsandmarkets.com/PressReleases/propionic-acid-derivatives.asp (accessed on 1 July 2013).
- Playne, M.J. Propionic and butyric acids. In Comprehensive Biotechnology; Moo-Young, M., Ed.; Pergamon Press: Oxford, UK, 1985. [Google Scholar]
- Jha, A.K.; Li, J.; Yuan, Y.; Baral, N.; Ai, B. A review on bio-butyric acid production and its optimization. Int. J. Agric. Biol. 2014, 16, 1019–1024. [Google Scholar]
- Entin-Meer, M.; Rephaeli, A.; Yang, X.; Nudelman, A.; VandenBerg, S.R.; Haas-Kogan, D.A. Butyric acid prodrugs are histone deacetylase inhibtiors that show antineoplastic activity and radiosensitizing capacity in the treatment of malignant gliomas. Mol. Cancer Ther. 2005, 4, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.W.; Yamazaki, H. Natural flavours production: A biotechnological approach. Trends Biotechnol. 1986, 4, 264–268. [Google Scholar] [CrossRef]
- Butyric Acid Market by Application (Animal Feed, Chemical Intermediate, Foods & Flavors, Pharmaceuticals, Perfumes, Others), by Type (Synthetic Butyric Acid, Renewable Butyric Acd) by Geography (APAC, North America, Europe, ROW)—Global Analysis and Forecast to 2020. Report Code CH 3662. 2015. Available online: http://www.marketsandmarkets.com/Market-Reports/butyric-acid-market-76962011.html (accessed on 8 January 2015).
- Holten, C.H.; Muller, A.; Rehbinder, D. Lactic Acid; International Research Association; Verlag Chemie: Copenhagen, Denmark, 1971. [Google Scholar]
- John, R.P.; Nampoothiri, K.M.; Pandey, A. Fermentative production of lactic acid from biomass: An overview on process developments and future perspectives. Appl. Microb. Biotechnol. 2007, 74, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Tsai, S.-P.; Bonsignore, P.; Moon, S.-H.; Frank, J.R. Technological and economical of poly (lactic acid) and lactic acid derivatives. FEMS Mircob. Rev. 1995, 16, 221–231. [Google Scholar] [CrossRef]
- Datta, R.; Henry, M. Lactic acid: Recent advances in products, processes and technologies—A review. J. Chem. Technol. Biotechnol. 2006, 81, 1119–1129. [Google Scholar] [CrossRef]
- Lactic Acid Market by Application (Biodegradable Polymer, Food & Beverage, Personal Care & Pharmaceutical) & Polylactic Acid Market by Application (Packaging, Agriculture, Automobile, Electronics, Textile), & by Geography-Global Trends & Forecasts to 2020. 2015. Available online: http://www.rnrmarketresearch.com/lactic-acid-market-by-application-biodegradable-polymer-food-beverage-personal-care-pharmaceutical-polylactic-acid-market-by-application-packaging-agriculture-automobile-electronics-tex-market-report.html (accessed on 12 March 2015).
- Dence, C.W.; Lin, S.Y. General structural features of lignin. In Methods in Lignin Chemisry; Lin, S.Y., Dence, C.W., Eds.; Springer: Berlin/Heidelberg, German, 1992. [Google Scholar]
- Cao, S.; Pu, Y.; Studer, M.; Wyman, C.; Ragauskas, A.J. Chemical transformations of Populus trichocarpa during dilute acid pretreatment. RSC Adv. 2012, 2, 10925–10936. [Google Scholar] [CrossRef]
- Tejado, A.; Pena, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol-formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Ahring, B.K.; Biswas, R.; Ahamed, A.; Teller, P.J.; Uellendahl, H. Making lignin accessible for anaerobic digestion by wet-explosion pretreatment. Bioresour. Technol. 2015, 175, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Schiel-Bengelsdorf, B.; Durre, P. Pathway engineering and synthetic biology using acetogens. FEBS Lett. 2012, 586, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood-Ljundahl pathway of CO2 fixation. Biochim. Biophys. Acta. 2008, 1784, 1873–1898. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.J.; Liu, H.; Nie, Y.Q.; Zeng, R.J.; Du, G.C.; Chen, J.; Yu, H.Q. Coupling glucose fermentation and homoacetogenesis for elevated acetate production: experimental and mathematical approaches. Biotechnol. Bioeng. 2010, 108, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Schuchmann, K.; Muller, V. Autotrophy at the thermodynamic limit of life a model for energy conservation in acetogenic bacteria. Natur. Rev. Microbiol. 2014, 12, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Zengler, K.; Edwards, E.A.; Mahadevan, R.; Stephanopoulos, G. Investigating Moorella thermoacetica metabolism with a genome-scale constraint-based metabolic model. Integr. Biol. 2015, 7, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Drake, H.L.; Daniel, S.L. Physiology of the thermophilic acetogen Moorella thermoacetica. Res. Microbiol. 2004, 155, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Isern, N.G.; Ewing, R.J.; Liyu, A.V.; Sears, J.A.; Knapp, H.; Iversen, J.; Sisk, D.R.; Ahring, B.K.; Majors, P.D. New generation NMR bioreactor coupled with high-resolution NMR spectroscopy leads to novel discoveries in Moorella thermoacetica metabolic profiles. Envrion. Biotechnol. 2014, 98, 8367–8375. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, N.; Kim, J.S.; Lee, Y.Y. Fermentation of xylose into acetic acid by Clostridium thermoaceticum. J. Appl. Biochem. Biotechnol. 2001, 367–376. [Google Scholar] [CrossRef]
- Ehsanipour, M.; Suko, A.V.; Bura, R. Fermentation of lignocellulosic sugars to acetic acid by Moorella thermoacetica. J. Ind. Microbiol. Biotechnol. 2016, 43, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Gaddy, J.L.; Clausen, E.C. Clostridium ljungdahlii, an anaerobic ethanol and acetate producing microorganism. U.S. Patent 5,173,429, 1992. [Google Scholar]
- Phillips, J.R.; Clausen, E.C.; Gaddy, J.L. Synthesis gas as substrate for the biological production of fuels and chemicals. Appl. Biochem. Biotechnol. 1994, 45, 145–157. [Google Scholar] [CrossRef]
- Heise, R.; Muller, V.; Gottschalk, G. Sodium dependence of acetate formation by the acetogenic bacterium Acetobacterium wooddii. J. Bacteriol. 1989, 171, 5473–5478. [Google Scholar] [CrossRef] [PubMed]
- Muller, V.; Bowien, S. Differential effects of sodium ions on motility in the homoacetogeniic bacteria Acetobacterium wooddii and Sporomusa sphaeroides. Arch. Microbiol. 1995, 164, 363–369. [Google Scholar] [CrossRef]
- Tracy, B.P.; Jones, S.W.; Fast, A.G.; Indurthi, D.C.; Papoutsakis, E.T. Clostridia: The importance of their exceptional substrate and metabolite diversity for biofuel and biorefinery applications. Curr. Opin. Biotechnol. 2012, 23, 364–381. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Ljungdahl, L.G. Electron-transport system in acetogens. In Biochemistry and Physiology of Anaerobic Bacteria; Ljungdahl, L.G., Adams, M.W., Barton, L., Ferry, J.G., Johnson, M.K., Eds.; Springer: New York, NY, USA, 2003. [Google Scholar]
- Huang, H.; Wang, S.; Moll, J.; Thauer, R.K. Electron bifurcation involved in the energy metabolism of the acetoggenic bacterium Moorella thermoacetica growing on glucose or H2 plus CO2. J. Bacteriol. 2012, 194, 3689–3699. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.; Wang, S.; Huang, H.; Kahnt, J.; Thauer, R.K. Evidence for a hexaheterometric methylenetetrahydrofolate reductase in Moorella thermoacetica. J. Bacteriol. 2014, 196, 3303–3314. [Google Scholar] [CrossRef] [PubMed]
- Kopke, M.; Held, C.; Hujer, S.; Liesegang, H.; Wiezer, A.; Wollherr, A.; Ehrenreich, A.; Liebl, W.; Gottschalk, G.; Durre, P. Clostridium ljungdahlii represents a microbial production platform based on syngas. Proc. Natl. Acad. Sci. USA 2010, 107, 13087–13092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, H.; Yang, F.; Ma, Y. Current progress on butyric acid production by fermentation. Curr. Microbiol. 2009, 59, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, S.T. Effects of pH on metabolic pathway shift in fermentation of xylose by Clostridium tyrobutyricum. J. Biotechnol. 2004, 110, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, Y.; Yang, S.T. Construction and characterization of ack deleted mutant of Clostridium tyrobutyricum for enhanced butyric acid and hydrogen production. Biotechnol. Prog. 2006, 22, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Lutke-Eversloh, T.; Bahl, H. Metabolic engineering of Clostridium acetobutylicum: Recent advances to improve butanol production. Curr. Opin. Biotechnol. 2011, 22, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jang, Y.S.; Choi, S.J.; Im, J.A.; Song, H.; Cho, J.H.; Seung, D.Y.; Papoutsakis, T.; Bennett, G.N.; Lee, S.Y. Metabolic engineering of Clostridium acetobutylicum ATCC 824 for isopropanol-butanol-ethanol fermentation. App. Environ. Microbiol. 2012, 78, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Wiegel, J.; Kuk, S.U.; Kohring, G.W. Clostridium thermobutyricum sp. nov., a moderate thermophile isolated from a cellulolytic culture, that produces butyrate as the major product. Int. J. Sys. Bacteriol. 1989, 39, 199–204. [Google Scholar] [CrossRef]
- Zhang, C.H.; Ma, Y.J.; Yang, F.X.; Liu, W.; Zhang, Y.-D. Optimization of medium composition for butyric acid production by Clostridium thermobutyricum using response surface methodology. Bioresour. Technol. 2009, 100, 4284–4288. [Google Scholar] [CrossRef] [PubMed]
- Bahl, H.; Gottschalk, G. Parameters affecting solvent production by Clostridium acetobutylicum in continuous culture. Biotechnol. Bioeng. Symp. 1984, 14, 215–233. [Google Scholar]
- Vandak, D.; Tomaska, M.; Zigova, J.; Sturdik, E. Effect of growth supplements and whey pretreatment on butyric-acid production by Clostridium butyricum. World J. Microbiol. Biotechnol. 1995, 11, 363. [Google Scholar] [CrossRef] [PubMed]
- Ueki, A.; Akasaka, H.; Suzuki, D.; Ueki, K. Paludibacter propionicigenes gen. nov., sp. nov., a novel strictly anaerobic, gram-negative, propionate-producing bacterium isolated from plant residue in irrigated rice-field soil in Japan. Int. J. Sys. Evol. Microbiol. 2006, 56, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Narihiro, T.; Kim, N.K.; Mei, R.; Nobu, M.K.; Liu, W.-T. Microbial community analysis of anaerobic reactors treating soft drink wastewater. PLoS ONE 2015, 10, e0119131. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.L.; Kuang, X.Z.; Shi, X.S.; Yuan, X.Z.; Guo, R.B. Paludibacter jiangxiensis sp. nov., a strictly anaerobic, propionate-producing bacterium isolated from rice paddy field. Arch. Microbiol. 2014, 196, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Oshio, S.; Tahata, I.; Minato, H. Effect of diets differing in ratios of roughage to concentrate on microflora in the rumen of heifers. J. Gen. Appl. Microbiol. 1987, 33, 99–111. [Google Scholar] [CrossRef]
- Paynter, M.J.; Elsdent, S.R. Mechanism of propionate formation by Selenomonas ruminatium, a rumen microorganism. Microbiology 1970, 61, 1–7. [Google Scholar]
- Akedo, M.; Cooney, C.L.; Sinskey, A.J. Direct demonstration of lactate-acrylate interconversion in Clostridium propionicum. Natur. Biotechnol. 1983, 1, 791–794. [Google Scholar] [CrossRef]
- Johns, A.T. The mechanism of propionic acid formation by Clostridium propionicum. J. Gen. Microbiol. 1952, 6, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.A.; Stowers, C.C.; Pham, V.; Cox, B.M. The production of propionic acid, propanol and propylene via sugar fermentation: an industrial perspective on the progress, technical challenges and future outlook. Green Chem. 2014, 16, 1066–1076. [Google Scholar] [CrossRef]
- Himmi, E.H.; Bories, A.; Boussaid, A.; Hassani, L. Propionic acid fermentation of glycerol and glucose by Propionibacterium acidipropionici and Propionibacterium freudenreichii ssp. shermanii. Appl. Microbiol. Biotechnol. 2000, 53, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Stine, A.; Zhang, M.; Ro, S.; Clendennen, S.; Shelton, M.C.; Tyo, K.E.J.; Broadbelt, L.J. Exploring de novo metabolic pathways from pyruvate to propionic acid. Biotechnol. Prog. 2016, 32, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, W.H.; Wood, B.J.B. Lactic Acid Bacteria—Biodiversity and Taxonomy; John Wiley & Sons, Wiley Blackwell Publishing: Oxford, UK, 2014. [Google Scholar]
- Claesson, M.J.; van Sinderen, D.; O’Toole, P.W. The genus Lactobacillus–a genomic basis for understanding its diversity. FEMS Microbiol. Lett. 2007, 269, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Garrett, B.G.; Srinivas, K.; Ahring, B.K. Performance and stability of Amberlite IRA-67 ion exchange resin for product extraction and pH control during homolactic fermentation of corn stover sugars. Biochem. Eng. J. 2015, 94, 1–8. [Google Scholar] [CrossRef]
- Nolasco-Hipolito, C.; Matsunaka, T.; Kobayashi, G.; Sonomoto, K.; Ishizaki, A. Synchronized fresh cell bioreactor system for continuous l-(+)-lactic acid production using Lactococcus lactis IO-1 in hydrolysed sago starch. J. Biosci. Bioeng. 2002, 93, 281–287. [Google Scholar] [CrossRef]
- Kadam, S.R.; Patil, S.S.; Bastawde, K.B.; Khire, J.M.; Gokhale, D.V. Strain improvement of Lactobacillus delbruecki NCIM 2365 for lactic acid production. Proc. Biochem. 2006, 41, 120–126. [Google Scholar] [CrossRef]
- Tango, M.; Ghaly, A. A continuous lactic acid production system using an immobilized packed bed of Lactobacillus helveticus. Appl. Microbiol. Biotechnol. 2002, 58, 712–720. [Google Scholar] [PubMed]
- Hujanen, M.; Linko, S.; Linko, Y.-Y.; Leisola, M. Optimization of media and cultivation conditions for L (+)(S)-lactic acid production by Lactobacillus casei NRRL B-441. Appl. Microbiol. Biotechnol. 2001, 56, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Romero-Garcia, S.; Hernandez-Bustos, C.; Merino, E.; Gosset, G.; Martinez, A. Homolactic fermentation from glucose and cellobiose using Bacillus subtilis. Microb. Cell Fac. 2009, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Payot, T.; Chemaly, Z.; Fick, M. Lactic acid production by Bacillus coagulans–kinetic studies and optimization of culture medium for batch and continuous fermentations. Enzym. Microb. Technol. 1999, 24, 191–199. [Google Scholar] [CrossRef]
- Pol, E.C.; Eggink, G.; Weusthuis, R.A. Production of l(+)-lactic acid from acid pretreated sugarcane bagasse using Bacillus coagulans DSM2314 in a simultaneous saccharification and fermentation strategy. Biotechnol. Biofuels 2016, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- Ahring, B.K.; Traverso, J.J.; Murali, N.; Srinivas, K. Continuous fermentation of clarified corn stover hydrolysate for the production of lactic acid at high yield and productivity. Biochem. Engg. J. 2016, 109, 162–169. [Google Scholar] [CrossRef]
- Zhou, X.; Ye, L.; Wu, J.C. Efficient production of L-lactic acid by newly isolated thermophilic Bacillus coagulans WCP 10–4 with high glucose tolerance. Appl. Microbiol. Biotechnol. 2013, 97, 4309–4314. [Google Scholar] [CrossRef] [PubMed]
- Baranofsky, J.J.; Schreurs, W.J.; Kashket, E.R. Uncoupling by acetic acid limits growth of and acetogenesis by Clostridium thermoaceticum. Appl. Environ. Microbiol. 1984, 48, 1134–1139. [Google Scholar]
- Schwartz, R.D.; Keller, F.A. Isolation of a strain of Clostridium thermoaceticum capable of growth and acetic acid production at pH 4.5. Appl. Environ. Microbiol. 1982, 43, 117–123. [Google Scholar] [PubMed]
- Paredes, C.J.; Alsaker, K.V.; Papoutsakis, E.T. A comparative genomic view of clostridial sporulation and physiology. Nat. Rev. Microbiol. 2005, 3, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Bumbaca, D.; Kosman, J.; Setlow, P.; Jedrzejas, M.J. Structure of a protein-DNA complex essential for DNS protection in spores of Bacillus spores. Proc. Nat. Acad. Sci. USA 2008, 105, 2806–2811. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zheng, H.; Gu, Y.; Zhao, J.; Zhang, W.; Yang, Y.; Wang, S.; Zhao, G.; Yang, S.; Jiang, W. Comparative genomic and transcriptomic analysis revealed genetic characteristics related to solvent formation and xylose utilization in Clostridium acetobutylicum EA 2018. BMC Genom. 2011, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Jarboe, L.R. Metabolic engineering of biocatalysts for carboxylic acids production. Comput. Struct. Biotechnol. J. 2012, 3, e201210011. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Chokhawala, H.A.; Nadler, D.C.; Blanch, H.W.; Clark, D.S. Binding modules alter the activity of chimeric cellulases: Effects of biomass pretreatment and enzyme source. Biotechnol. Bioeng. 2010, 107, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Hackmann, T.J.; Spain, J.N. Invited review: Ruminant ecology and evolution: Perspectives useful to ruminant livestock research and production. J. Dairy Sci. 2010, 93, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, J.; Forbes, J.M.; France, J. Quantitative Aspects of Ruminant Digestion and Metabolism; CABI Publishing: Cambridge, MA, USA, 1994. [Google Scholar]
- Saleem, F.; Bouatra, S.; Guo, A.C.; Psychogios, N.; Mandal, R.; Dunn, S.M.; Ametaj, B.N.; Wishart, D.S. The bovine ruminal fluid metabolome. Metabolomics 2013, 9, 360–378. [Google Scholar] [CrossRef]
- Ahring, B.K. Perspectives for anaerobic digestion. Adv. Biochem. Eng. Biotechnol. 2003, 81, 1–30. [Google Scholar] [PubMed]
- Mah, R.A.; Ward, D.M.; Baresi, L.; Glass, T.L. Biogenesis of methane. Annu. Rev. Microbiol. 1977, 31, 309–341. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Schulman, M.D.; Valentino, D. Factors influencing rumen fermentation: Effect of hydrogen on formation of propionate. J. Dairy Sci. 1976, 59, 1444–1451. [Google Scholar] [CrossRef]
- Miller, T.L.; Wolin, M.J. Inhibition of growth of methane-producing bacteria of the ruminant forestomach by hydroxymethylglutaryl~ SCoA reductase inhibitors. J. Dairy Sci. 2001, 84, 1445–1448. [Google Scholar] [CrossRef]
- Van Kessel, J.A.S.; Russell, J.B. The effect of pH on ruminal methanogenesis. FEMS Microbiol. Ecol. 1996, 20, 205–210. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, J.; Meng, L. Effects of volatile fatty acid concentrations on methane yield and methanogenic bacteria. Biomass Bioenergy 2009, 33, 848–853. [Google Scholar] [CrossRef]
- Hess, M.; Sczyrba, A.; Egan, R.; Kim, T.W.; Chokhawala, H.; Schroth, G.; Luo, S.; Clark, D.S.; Chen, F.; Zhang, T.; et al. Metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science 2011, 331, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Weimer, P.J. Manipulating ruminal fermentation: a microbial ecological perspective. J. Anim. Sci. 1998, 76, 3114–3122. [Google Scholar] [CrossRef] [PubMed]
- Perret, S.; Casalot, L.; Fierobe, H.-P.; Tardif, C.; Sabathe, F.; Belaich, J.-P.; Belaich, A. Production of heterologous and chimeric scaffoldins by Clostridium acetobutylicum ATCC 824. J. Bacteriol. 2004, 186, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Le Van, T.D.; Robinson, J.A.; Ralph, J.; Greening, R.C.; Smolenski, W.J.; Leedle, J.A.; Schaefer, D.M. Assessment of reductive acetogenesis with indigenous ruminal bacterium populations and Acetitomaculum ruminis. Appl. Environ. Microbiol. 1998, 64, 3429–3436. [Google Scholar] [PubMed]
- Lopez, S.; Valdes, C.; Newbold, C.J.; Wallace, R.J. Influence of sodium fumarate addition on rumen fermentation in vitro. Br. J. Nutr. 1999, 81, 59–64. [Google Scholar] [PubMed]
- Christy, P.M.; Gopinath, L.R.; Divya, D. A review on anaerobic decomposition and enhancement of biogas production through enzymes and microorganisms. Ren. Sus. Energ. Rev. 2014, 34, 167–173. [Google Scholar] [CrossRef]
- Rieu-Lesme, F.; Morva, B.; Collins, M.D.; Fonty, G.; Willems, A. A new H2/CO2-using acetogenic bacterium from the rumen: Description of Ruminococcus schinkii sp. nov. FEMS Microbiol. Lett. 2006, 140, 281–286. [Google Scholar]
- Leedle, J.A.; Greening, R.C. Postprandial changes in methanogenic and acidogenic bacteria in the rumens of steers fed high- or low-forage diets once daily. Appl. Environ. Microbiol. 1988, 54, 502–506. [Google Scholar] [PubMed]
- Davidson, C.A.; Rehberger, T.G. Characterization of Propionibacterium isolated from rumen of lactating dairy cows. Proc. Am. Soc. Microbiol. 1995, 1, 6. [Google Scholar]
- Russell, J.R.; Hino, T. Regulation of lactate production in Streptococcus bovis: A spiraling effect that contributes to rumen acidosis. J. Dairy Sci. 1985, 68, 1712–1721. [Google Scholar] [CrossRef]
- Wells, J.E.; Krause, D.O.; Callaway, T.R.; Russell, J.B. A bacteriocin-mediated antagonism by ruminal lactobacilli against Streptococcus bovis. FEMS Microbiol. Ecol. 1997, 22, 237–243. [Google Scholar] [CrossRef]
- Sharpe, M.E.; Latham, M.J.; Garvie, E.I.; Zirngibl, J.; Kandler, O. Two new species of Lactobacillus isolated from the bovine rumen, Lactobacillus ruminis sp. nov. and Lactobacillus vitulinus sp. nov. J. Gen. Microbiol. 1973, 77, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.G.; Withers, S.E. Changes in the rumen microbial population and its activities during the refaunation period after the reintroduction of ciliate protozoa into the rumen of defaunated sheep. Can. J. Microbiol. 1993, 39, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Hobson, P.N.; Stewart, C.S. The rumen microbial ecosystem; Blackie academic & professional, Chapman & Hall: London, UK, 1997. [Google Scholar]
- Bryant, M.P. Bacterial species of the rumen. Bacteriol. Rev. 1959, 23, 125–153. [Google Scholar] [PubMed]
- Doetsch, R.N.; Howard, B.H.; Mann, S.O. Physiological factors in the production of an iodophilic polysaccharide from pentose by a sheep rumen bacterium. J. Gen. Microbiol. 1957, 16, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Bryant, M.P.; Small, N. Characteristics of two new genera of anaerobic curved rods isolated from the rumen of cattle. J. Bacteriol. 1956, 72, 22–26. [Google Scholar] [PubMed]
- Dehority, B.A. Characterization of several bovine rumen bacteria isolated with a xylan medium. J. Bacteriol. 1966, 91, 1724–1729. [Google Scholar] [PubMed]
- Pind, P.F.; Angelidaki, I.; Ahring, B.K. Dynamics of the anaerobic process: effects of volatile fatty acids. Biotechnol. Bioeng. 2003, 82, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Ahring, B.K.; Sandberg, M.; Angelidaki, I. Volatile fatty acids as indicators of process imbalance in anaerobic digestors. Appl. Microbiol. Biotechnol. 1995, 43, 559–565. [Google Scholar] [CrossRef]
- Ahring, B.K. Status on science and application of thermophilic anaerobic digestion. Water Sci. Technol. 1994, 30, 241–249. [Google Scholar]
- Ahring, B.K.; Wstermann, P. Kinetics of butyrate, acetate, and hydrogen metabolism in a thermophilic, anaerobic, butyrate-degrading triculture. Appl. Environ. Microbiol. 1987, 53, 434–439. [Google Scholar] [PubMed]
- Angelidaki, I.; Ahring, B.K. Establishment and characterization of an anaerobic thermophilic 55 (deg) C enrichment culture degrading long-chain fatty acids. Appl. Environ. Microbiol. 1995, 61, 2442–2445. [Google Scholar] [PubMed]
- Baroi, G.N.; Skiadas, I.V.; Westermann, P.; Gavala, H.N. Continuous fermentation of wheat straw hydrolysate by Clostridium tyrobutyircum with in-situ acids removal. Wast. Biomass Valor. 2015, 6, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Zhang, X.; Feng, H.; Xu, T. In-situ combination of fermentation and electrodialysis with bipolar membranes for the production of lactic acid: continuous operation. Bioresour. Technol. 2013, 147, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.T.; Matsuoka, H.; Toda, K. Production and recovery of propionic and acetic acids in electrodialysis culture of Propionibacterium shermanii. J. Ferm. Bioeng. 1993, 75, 276–282. [Google Scholar] [CrossRef]
- Jones, R.J.; Massanet-Nicolau, J.; Guwy, A.; Premier, G.C.; Dinsdale, R.M.; Reilly, M. Removal and recovery of inhibitory volatile fatty acids from mixed acid fermentations by conventional electrodialysis. Bioresour. Technol. 2015, 189, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Zigova, J.; Sturdik, E.; Vandak, D.; Schlosser, S. Butyric acid production by Clostridium butyricum with integrated extraction and pertraction. Proc. Biochem. 1999, 34, 835–843. [Google Scholar] [CrossRef]
- Tik, N.; Bayraktar, E.; Mehmetoglu, U. In situ reactive extraction of lactic acid from fermentation media. J. Chem. Technol. Biotechnol. 2001, 76, 764–768. [Google Scholar] [CrossRef]
- Jin, Z.; Yang, S.-T. Extractive fermentation for enhanced propionic acid production from lactose by Propionibacterium acidipropionici. Biotechnol. Prog. 1998, 14, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Lee, Y.Y. Membrane-mediated extractive fermentation for lactic acid production from cellulosic biomass. Appl. Biochem. Biotechnol. 1997, 63, 435. [Google Scholar] [CrossRef] [PubMed]
- Von Frieling, P.; Schugerl, K. Recovery of lactic acid from aqueous model solutions and fermentation broths. Proc. Biochem. 1999, 34, 685–696. [Google Scholar] [CrossRef]
- Nelson, R.S.; Peterson, D.J.; Karp, E.M.; Beckham, G.T.; Salvachua, D. Mixed carboxylic acid production by Megasphaera elsdenii from glucose and lignocellulosic hydrolysate. Fermentation 2017, 3, 10. [Google Scholar] [CrossRef]
- Ataei, S.A.; Vasheghani-Farahani, E.J. In situ separation of lactic acid from fermentation broth using ion exchange resins. J. Ind. Microbiol. Biotechnol. 2008, 35, 1229–1233. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, J.M.; Aldavero, M. Production of L-lactic acid by Lactobacillus delbrueckii in chemostat culture using an ion exchange resins system. J. Chem. Technol. Biotechnol. 1999, 74, 627–634. [Google Scholar] [CrossRef]
- Bishai, M.; de, S.; Adhikari, B.; Banerjee, R. A platform technology of recovery of lactic acid from a fermentation broth of novel substrate Zizyphus oenophlia. Biotech 2015, 5, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, A.; Bonk, F.; Bastidas-Oyandel, J.-R.; Schmidt, J.E. Recovery of carboxylic acids produced during dark fermentation of food waste by adsorption on Amberlite IRA-67 and activated carbon. Bioresour. Technol. 2016, 217, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.; Woodley, J.M.; Lilly, M.D. In-situ product removal as a tool for bioprocessing. Biotechnol. 1993, 11, 1007–1012. [Google Scholar] [CrossRef]
- Weil, J.R.; Dien, B.; Bothast, R.; Hendrickson, R.; Mosier, N.S.; Ladisch, M.R. Removal of fermentation inhibitors formed during pretreatment of biomass by polymeric adsorbents. Ind. Eng. Chem. Res. 2002, 41, 6132–6138. [Google Scholar] [CrossRef]
- Xu, T. Ion exchange membranes: State of their development and perspective. J. Membr. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Gonzalez, M.I.; Alvarez, S.; Riera, F.A.; Alvarez, R. Purification of lactic acid from fermentation broths by ion-exchange resins. Ind. Eng. Chem. Res. 2006, 45, 3242–3247. [Google Scholar] [CrossRef]
- Dethe, M.J.; Marathe, K.V.; Gaikar, V.G. Adsorption of lactic acid on weak base polymeric resins. J. Separ. Sci. Technol. 2006, 41, 2947–2971. [Google Scholar] [CrossRef]
- Uslu, H.; Inci, I.; Bayazit, S.S. Adsorption equilibrium data for acetic acid and glycolic acid onto Amberlite IRA-67. J. Chem. Eng. Data 2010, 55, 1295–1299. [Google Scholar] [CrossRef]
- Uslu, H.; Inci, I.; Bayazit, S.S.; Demir, G. Comparison of solid-liquid equilibrium data for the adsorption of propionic acid and tartaric acid from aqueous solution onto Amberlite IRA-67. Ind. Eng. Chem. Res. 2009, 48, 7767–7772. [Google Scholar] [CrossRef]
- Yang, S.T.; White, S.A.; Hsu, S.T. Extraction of carboxylic acids with tertiary and quarternary amines: effect of pH. Ind. Eng. Chem. Res. 1991, 30, 1335–1342. [Google Scholar] [CrossRef]
- Keshav, A.; Wasewar, K.L.; Chand, S. Reactive extraction of propionic acid using tri-n-octylamine, tri-n-butyl phosphate and aliquat 336 in sunflower oil as diluent. J. Chem. Technol. Biotechnol. 2009, 84, 484–489. [Google Scholar] [CrossRef]
- Vane, L.M. A review of pervaporation of product recovery from biomass fermentation processes. J. Chem. Technol. Biotechnol. 2005, 80, 603–629. [Google Scholar] [CrossRef]
- Cockrem, M.C.M. Process for Recovering Organic Acids From Aqueous Salt Solutions. U.S. Patent 5,522,995, 1996. [Google Scholar]
- Garrett, B.G.; Srinivas, K.; Ahring, B.K. Design and optimization of a semi-continuous high pressure carbon dioxide extraction system for acetic acid. J. Supercrit. Fluids 2014, 95, 243–251. [Google Scholar] [CrossRef]
- Huang, C.; Xu, T.; Zhang, Y.; Xue, Y.; Chen, G. Application of electrodialysis to the production of organic acids: state-of-the-art and recent developments. J. Membr. Sci. 2007, 288, 1–12. [Google Scholar] [CrossRef]
- Akerberg, C.; Zacchi, G. An economic evaluation of the fermentative production of lactic acid from wheat flour. Bioresour. Technol. 2000, 75, 119–126. [Google Scholar] [CrossRef]
- Habova, V.; Melzoch, K.; Rychtera, M.; Sekavova, B. Electrodialysis as a useful technique for lactic acid separation from a model solution and a fermentation broth. Desal. 2004, 162, 361–372. [Google Scholar] [CrossRef]
- Lee, E.G.; Moon, S.H.; Chang, Y.K.; Yoo, I.-K.; Chang, H.N. Lactic acid recovery using two-stage electrodialysis and its modelling. J. Membr. Sci. 1998, 145, 53–66. [Google Scholar]
- Prado-Rubio, O.A.; Jorgensen, S.B.; Jonsson, G. Reverse electro-enhanced dialysis for lactate recovery from a fermentation broth. J. Membr. Sci. 2011, 374, 20–32. [Google Scholar] [CrossRef]
- Garde, A. Production of Lactic Acid From Renewable Resources Using Electrodialysis for Product Recovery. Ph.D. Thesis, Technical University of Denmark, Copenhagen, Denmark, 2002. [Google Scholar]
- Dwidar, M.; Lee, S.; Mitchell, R.J. The production of biofuels from carbonated beverages. Appl. Energy 2012, 100, 47–51. [Google Scholar] [CrossRef]
- Boaro, A.A.; Kim, Y.M.; Konopka, A.E.; Callister, S.J.; Ahring, B.K. Integrated omics analysis for studying the microbial community response to a pH perturbation of a cellulose-degrading bioreactor culture. FEMS Microbiol. Ecol. 2014, 90, 802–815. [Google Scholar] [CrossRef] [PubMed]
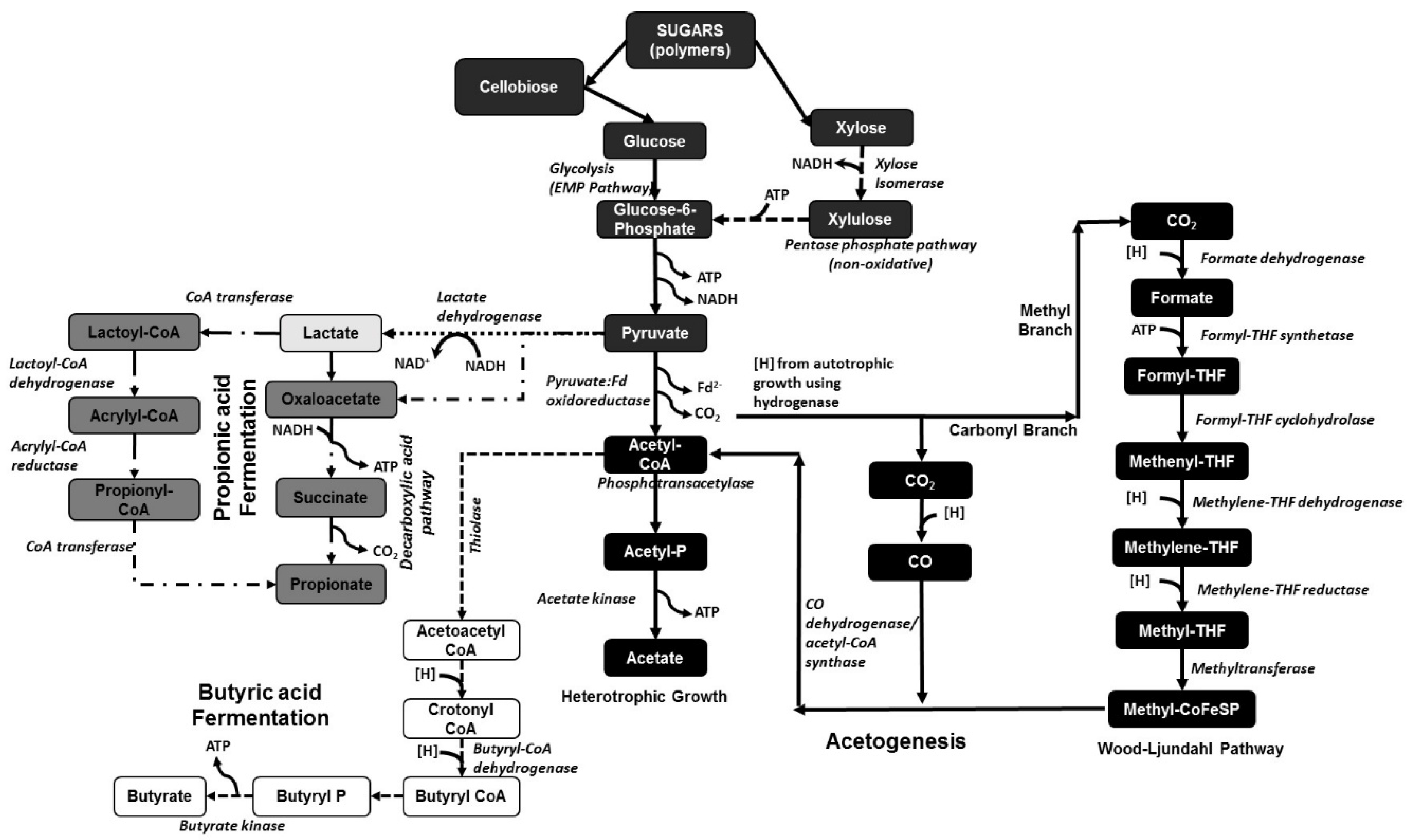
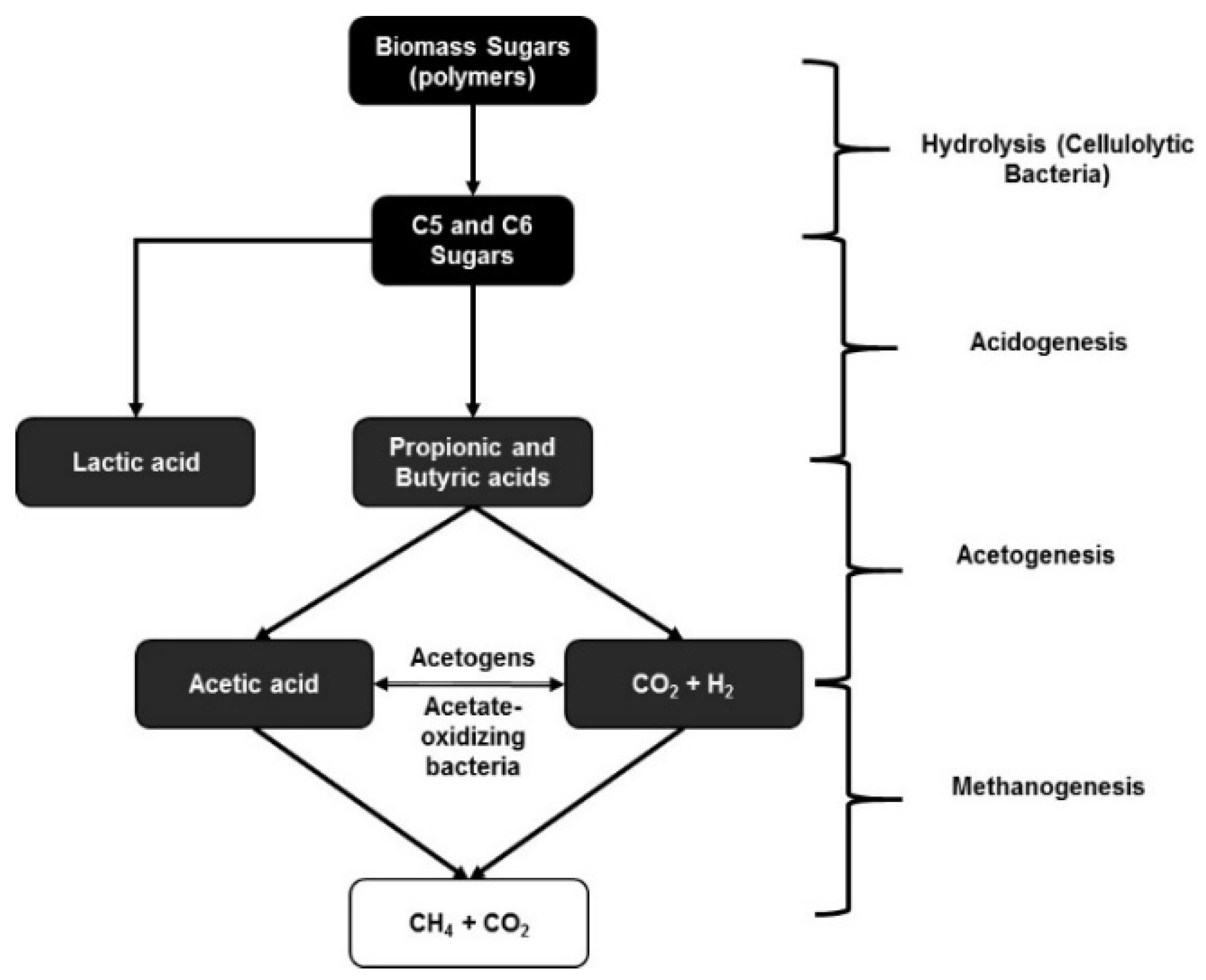
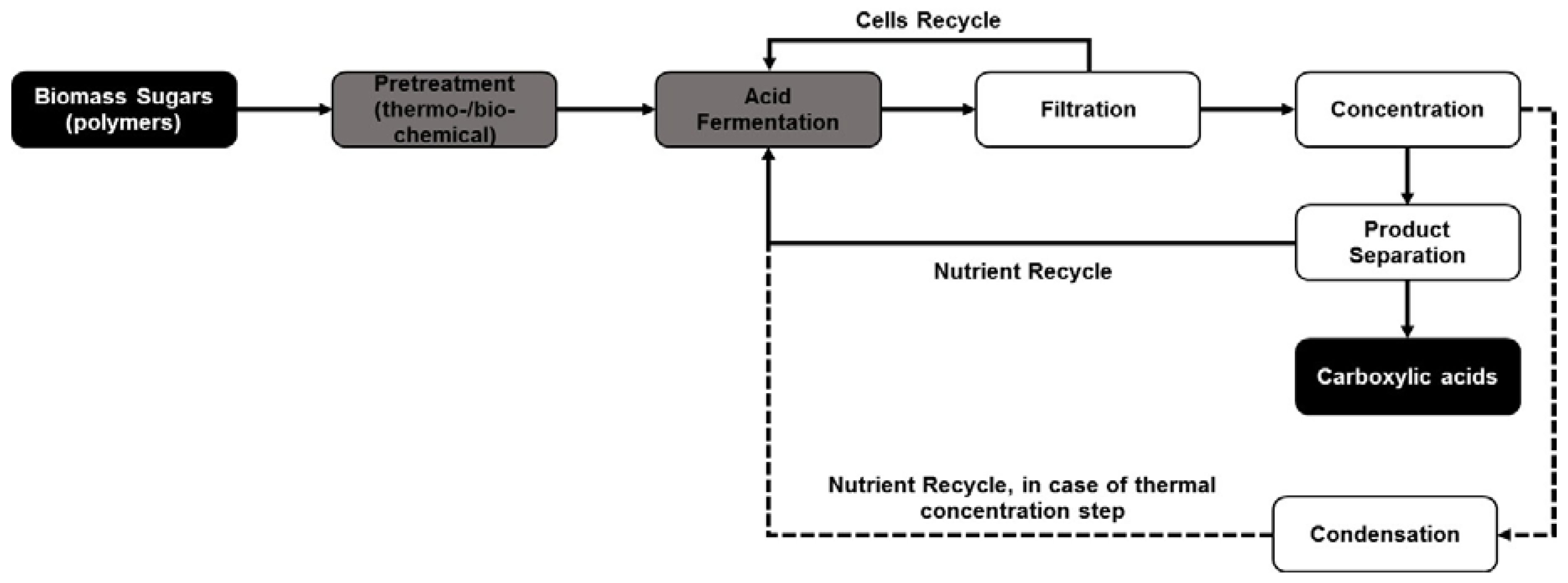
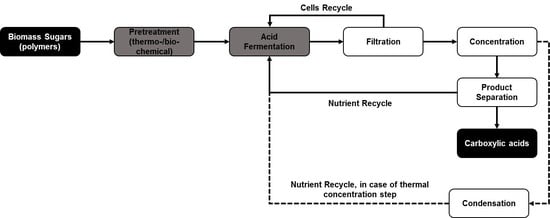
| Substrate | Fermentation | Product | Type of Separation | Optimized Conditions and Process Efficiency |
|---|---|---|---|---|
| In situ separation using electrodialysis | ||||
| Wheat Straw [132] | Continuous with C. tyrobutyricum | Butyric acid | Reverse electro-enhanced electrodialysis (REED) | 19- and 53-fold higher sugar consumption in presence of REED resulting in butyric acid. Yield as high as 0.45 g/g sugars |
| Glucose [133] | Continuous with L. plantarum | Lactic acid | Bipolar electrodialysis | Lactate recovery of 69.5% (1.32 mol/L lactate) with current density of 40 mA/cm2 |
| Whey [134] | Batch with P. shermanii | Propionic and acetic acid | Electrodialysis | Increased acid yield by 1.4-fold and 1.31-fold for propionic and acetic acids, respectively, when compared to controls. |
| Sucrose and grass [135] | Fed-Batch with anaerobic sludge | Acetic and butyric acid | Electrodialysis | Up to 99% VFA removal from fermentation broth within 60 min containing 1.2 g/L initial VFAs |
| In situ separation using reactive extraction | ||||
| Sucrose [136] | Fed-Batch with C. tyrobutyricum | Butyric acid | Pertraction using 20% w/w Hostarex A327 in oleyl alcohol | 0.30 g butyrate/g sugar with productivity of 0.21 g/L/h (pH 5.2 at 37 °C) |
| Glucose [137] | Batch with immobilized L. delbrueckii | Lactic acid | Alamine-336 in oleyl alcohol | Maximum yield of 25.5 g/L with Alamine-336 together with immobilized cells with 15% v/v sunflower oil (Vor/Vaq = 0.5 at 37 °C) |
| Lactose [138] | Hollow-fiber membrane extractor (Fed-batch) with P. acidipropionici ATCC 4875 | Propionic acid | Adogen 283 (ditridecylamine) in oleyl alcohol | 0.66 g propionate/g substrate with product concentration of 75 g/L and purity of ~90% (pH 5.3) |
| Switchgrass [139] | Hollow-fiber membrane extractor (Fed-batch) with L. delbruecki | Lactic acid | Alamine 336 in oleyl alcohol with kerosene as diluent (20:40:40 wt%) | Lactate yield of 67% that of theoretical maximum (pH 5.0 at 43 °C) |
| Glucose [140] | Batch with L. salivarius | Lactic acid | Hoe F 2562, Cyanex 923 and Hostarex A327 with isodecanol and kerosene | Lactic acid yield as high as 87.5% with 10 wt % Hostarex A327 and 81% with 40 wt % Cyanex 927 |
| Corn Stover [141] | Fed-Batch with Megasphaera elsdenii | Butyric and hexanoic acids | Pertraction with oleyl alcohol and 10% (v/v) trioctylamine | Carboxylic acid productivities were found to be increased by 3-fold for pertractive fermentation system when compared to batch and glucose conversion rates was also higher by ~3-fold |
| In situ separation using ion exchange resins | ||||
| Whey [142] | Batch with L. casei | Lactic acid | Amberlite IRA-400 (Cl-) | Maximum concentration of 37.4 g/L with yield of 0.85 g lactate/g substrate and productivity of 0.984 g/L/h (pH 6.1 and 37 °C) |
| Corn Stover [82] | Fed-Batch with B. coagulans | Lactic acid | Amberlite IRA-67 | 0.94 g lactate/g biomass sugars obtained with productivity of 0.33 g/L/h (pH 5.5 at 50 °C) |
| Beet Molasses [143] | Continuous with L. delbrueckii | Lactic acid | Amberlite IRA-420 combined with Amberlite IR-120 | Maximum lactate yield of 0.91 g/g sucrose at dilution rate of 0.1 h-1 (pH 6 at 49 °C) |
| Zizylhus oenoplia [144] | Batch with L. amylophilus GV6 | Lactic acid | Amberlite IRA-96 combined with Amberlite IR-120 | Maximum lactate recovery of 98.9% with optical purity of 99.17%. Maximum acid loading around 210.46 mg/g bead |
| Synthetic food waste [145] | Batch with mixed culture from food waste | Lactic, acetic and butyric acids | Amberlite IRA-67 | Lactic, acetic and butyric acid loadings onto the resin of 84, 20.5, and 50.7 mg/g resin, respectively, with acid removal of around 75% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murali, N.; Srinivas, K.; Ahring, B.K. Biochemical Production and Separation of Carboxylic Acids for Biorefinery Applications. Fermentation 2017, 3, 22. https://doi.org/10.3390/fermentation3020022
Murali N, Srinivas K, Ahring BK. Biochemical Production and Separation of Carboxylic Acids for Biorefinery Applications. Fermentation. 2017; 3(2):22. https://doi.org/10.3390/fermentation3020022
Chicago/Turabian StyleMurali, Nanditha, Keerthi Srinivas, and Birgitte K. Ahring. 2017. "Biochemical Production and Separation of Carboxylic Acids for Biorefinery Applications" Fermentation 3, no. 2: 22. https://doi.org/10.3390/fermentation3020022
APA StyleMurali, N., Srinivas, K., & Ahring, B. K. (2017). Biochemical Production and Separation of Carboxylic Acids for Biorefinery Applications. Fermentation, 3(2), 22. https://doi.org/10.3390/fermentation3020022