Process Development for Enhanced 2,3-Butanediol Production by Paenibacillus polymyxa DSM 365
Abstract
:1. Introduction
2. Methodology
2.1. Experimental Methods
2.1.1. Microorganism and Culture Preparation
2.1.2. Batch and Fed-Batch Fermentations
2.1.3. Analytical Methods
2.2. Experimental Design and Data Analysis
2.2.1. Plackett-Burman Design
2.2.2. Path of Steepest Ascent
2.2.3. Box-Behnken Design and Response Surface Methodology
3. Results and Discussion
3.1. Plackett-Burman Design
3.2. Path of Steepest Ascent Design
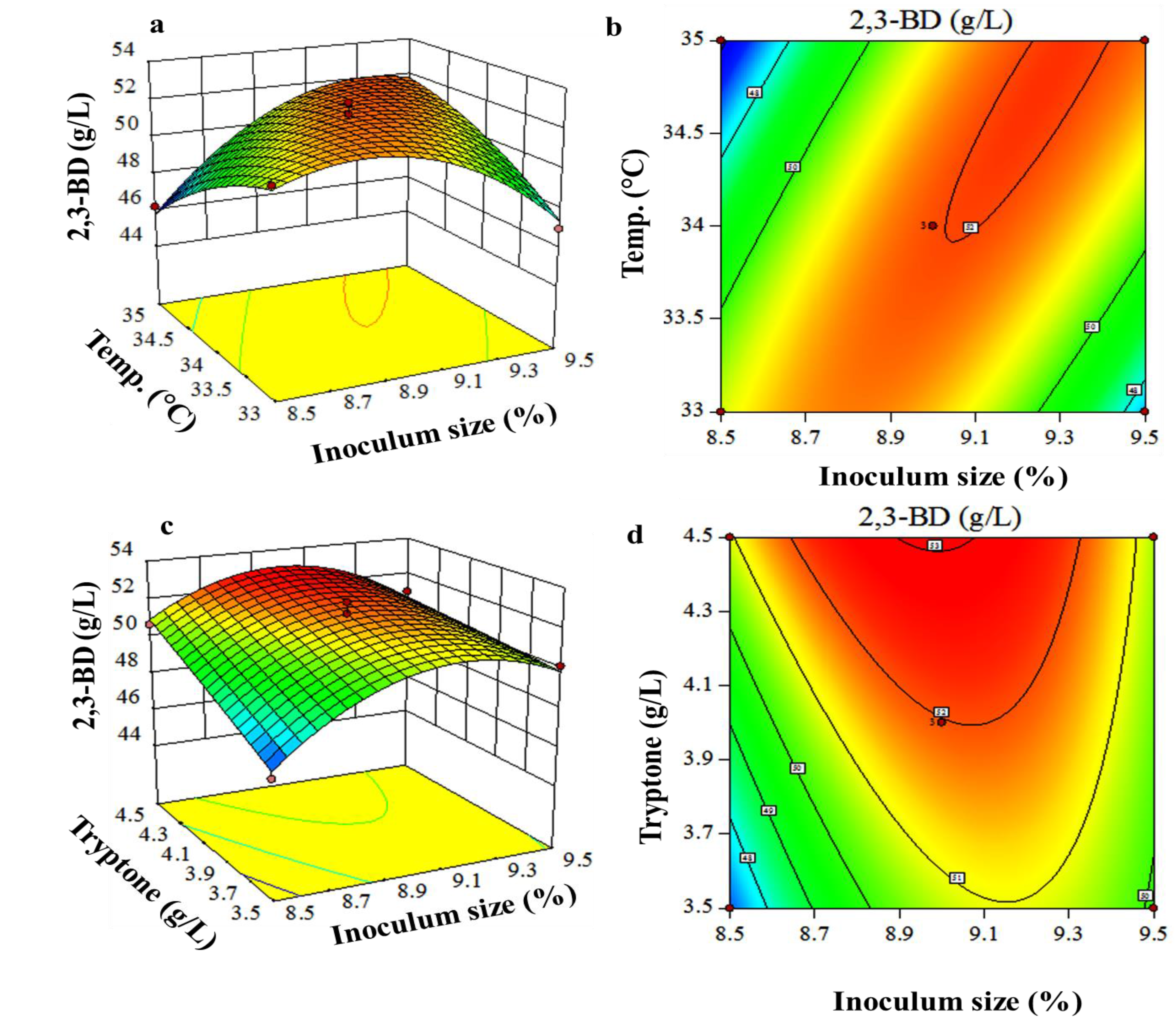
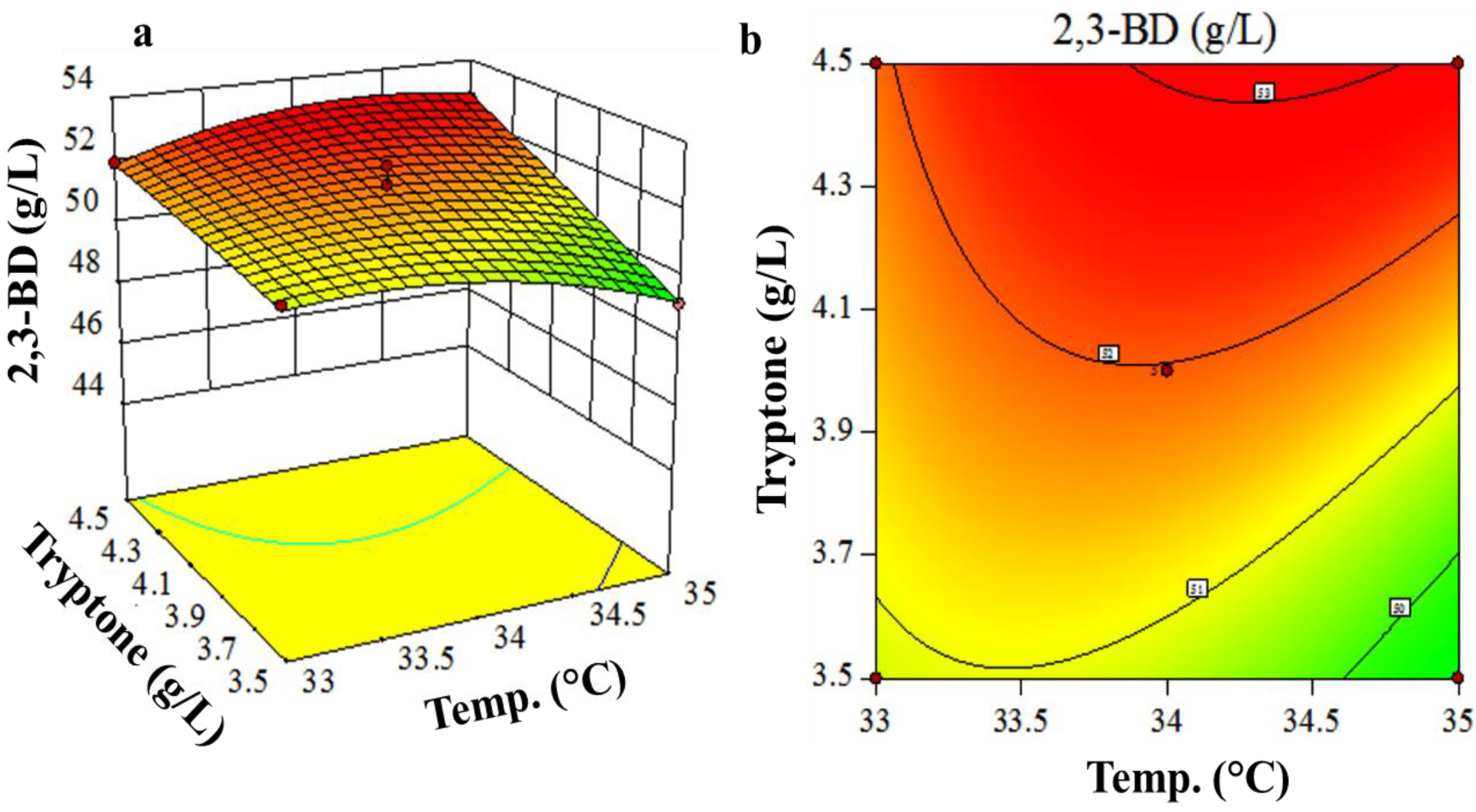
3.3. Box-Behnken Design and Response Surface Methodology
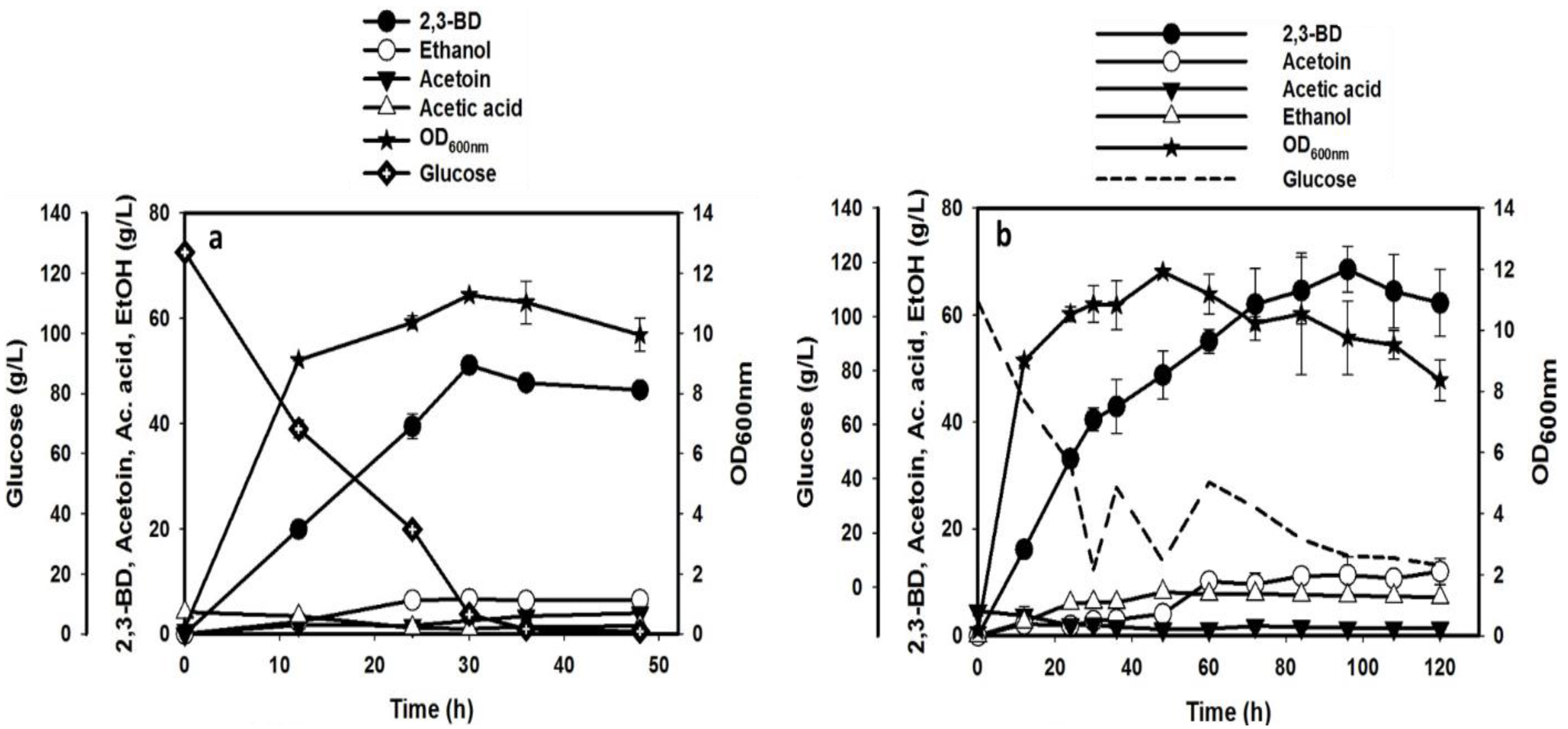
3.4. Experimental Validation of the Optimized Medium and Conditions in Batch and Fed-Batch Fermentations
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Celinska, E.; Grajet, W. Biotechnological production of 2,3-butanediol-current states and prospects. Biotechnol. Adv. 2009, 27, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Soltys, K.A.; Batta, A.K.; Koneru, B. Successful nonfreezing, subzero preservation of rat liver with 2,3-butanediol and type I antifreeze protein. J. Surg. Res. 2001, 96, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Bartowsky, E.J.; Henschke, P.A. The ′buttery′ attribute of wine–diacetyl–desirability, spoilage and beyond. Int. J. Food Microbiol. 2004, 96, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.L.; Robertson, N.C. Oxidation of Hydrocarbons. U.S. Patent US2689253 A, 1954. [Google Scholar]
- O’Neil, M.J. The Merck Index-An Encyclopedia of Chemicals, Drugs, and Biologicals, 13th ed.; Merck and Co., Inc.: Whitehouse Station, NJ, USA, 2001. [Google Scholar]
- Garg, S.K.; Jain, A. Fermentative production of 2,3-butanediol: A review. Bioresour. Technol. 1995, 51, 103–109. [Google Scholar] [CrossRef]
- Okonkwo, C.C.; Ujor, V.; Ezeji, T.C. Investigation of relationships between 2,3-butanediol toxicity and production during growth of Paenibacillus polymyxa. New Biotechnol. 2017, 34, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Häßler, T.; Schieder, D.; Pfaller, R.; Faulstich, M.; Sieber, V. Enhanced fed-batch fermentation of 2,3-butanediol by Paenibacillus polymyxa DSM 365. Bioresour. Technol. 2012, 124, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, V.; de Vicente, G.; Morán, B.; Rojas, A.; Segarra, S.; Montesinos, A.; Tortajada, M.; Ramón, D.; Ladero, M.; Santos, V.E. Novel biocatalysts for glycerol conversion into 2,3-butanediol. Process Biochem. 2016, 51, 740–748. [Google Scholar] [CrossRef]
- Kim, T.; Cho, S.; Woo, H.M.; Lee, S.M.; Lee, J.; Um, Y.; Seo, J.H. High production of 2,3-butanediol from glycerol without 1,3-propanediol formation by Raoultella ornithinolytica B6. Appl. Microbiol. Biotechnol. 2017, 101, 2821–2830. [Google Scholar] [CrossRef] [PubMed]
- Petrov, K.; Petrova, P. Enhanced production of 2,3-butanediol from glycerol by forced pH fluctuations. Appl. Microbiol. Biotechnol. 2010, 87, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Biebl, H.; Zeng, A.P.; Menzel, K.; Deckwer, W.D. Fermentation of glycerol to 1,3-propanediol and 2,3-butanediol by Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. 1998, 50, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.C.C. Glycerol dissimilation and its regulation in bacteria. Annu. Rev. Microbiol. 1976, 30, 535–578. [Google Scholar] [CrossRef] [PubMed]
- Neijssel, O.M.; Hueting, S.; Crabbendam, K.J.; Tempest, D. Dual pathways of glycerol assimilation in Klebsiella aerogenes NCIB 418. Arch. Microbiol. 1975, 104, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Voloch, M.; Ladish, M.R.; Rodwell, V.W.; Tsao, G.T. Reduction of acetoin to 2,3-butanediol in Klebsiella pneumoniae: A new model. Biotechnol. Bioeng. 1983, 26, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, H.; Li, Q.-J.; Feng, X.-H.; Li, S. Optimization of medium for one-step fermentation of inulin extract from Jerusalem artichoke tubers using Paenibacillus polymyxa ZJ-9 to produce R,R-2,3-butanediol. Bioresour. Technol. 2010, 101, 7076–7082. [Google Scholar] [CrossRef] [PubMed]
- Anvari, M.; Motlagh, R.S. Enhancement of 2,3-butanediol production by Klebsiella oxytoca PTCC 1402. BioMed Res. Int. Available online: https://www.mysciencework.com/publication/show/16bdb9aba87406e5a516be552043648a (accessed on 8 January 2017).
- Zhang, L.; Yang, Y.; Sun, J.; Shen, Y.; Wei, D.; Zhu, J.; Chu, J. Microbial production of 2,3-butanediol by a mutagenized strain of Serratia marcescens H30. Bioresour. Technol. 2010, 101, 1961–1967. [Google Scholar] [CrossRef] [PubMed]
- Perego, P.; Converti, A.; del Borghi, M. Effects of temperature, inoculum size and starch hydrolyzate concentration on butanediol production by Bacillus licheniformis. Bioresour. Technol. 2003, 89, 25–131. [Google Scholar] [CrossRef]
- Marwoto, B.; Nakashimada, Y.; Kakizono, T.; Nishio, N. Enhancement of (R,R)-2,3-butanediol production from xylose by Paenibacillus polymyxa at elevated temperatures. Biotechnol. Lett. 2002, 24, 109–114. [Google Scholar] [CrossRef]
- Khuri, A.I.; Mukhopadhyay, S. Response surface methodology. Comp. Stat. 2010, 2, 128–149. [Google Scholar] [CrossRef]
- Box, G.E.P.; Hunter, J.S.; Hunter, W.G. Statistics for Experimenters; John Wiley and Sons: New York, NY, USA, 1978. [Google Scholar]
- Chang, Y.; Huang, J.; Lee, C.; Shih, I.; Tzeng, Y. Use of response surface methodology to optimize culture medium for production of lovastatin by Monascus ruber. Enzyme Microbial Technol. 2002, 30, 889–894. [Google Scholar] [CrossRef]
- Kwak, J.S. Application of Taguchi and response surface methodologies for geometric error in surface grinding process. Int. J. Machine Tools Manuf. 2005, 45, 327–334. [Google Scholar] [CrossRef]
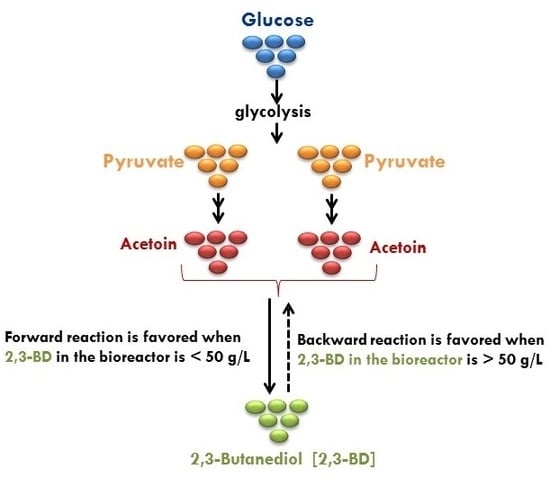
- Xiao, Z.; Xu, P. Acetoin metabolism in bacteria. Crit. Rev. Microbiol. 2007, 33, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, W.; Ma, Y.; Zheng, A. Medium optimization and proteome analysis of (R,R)-2,3-butanediol production by Paenibacillus polymyxa ATCC 12321. Appl. Microbiol. Biotechnol. 2013, 97, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Nilegaonkar, S.; Bhosale, S.B.; Kshirsagar, D.C.; Kapidi, A.H. Production of 2,3-butanediol from glucose by Bacillus licheniformis. World J. Microbiol. Biotechnol. 1992, 8, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.K.; Saddler, J.N. Fed-batch approach to production of 2,3-butanediol by Klebsiella pneumoniae grown on high substrate concentrations. Appl. Environ. Microbiol. 1983, 46, 630–635. [Google Scholar] [PubMed]
- Jyothi, A.N.; Sasikiran, K.; Nambisan, B.; Balagopalan, C. Optimisation of glutamic acid production from cassava starch factory residues using Brevibacterium divaricatum. Process Biochem. 2005, 40, 3576–3579. [Google Scholar] [CrossRef]
- Muthukumar, M.; Mohan, D.; Rajendran, M. Optimization of mix proportions of mineral aggregates using Box Behnken designs of experiments. Cem. Concr. Compos. 2003, 25, 751–758. [Google Scholar] [CrossRef]
- Bas, D.; Boyaci, I.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Liu, J.; Luo, J.; Ye, H.; Sun, Y.; Lu, Z.; Zeng, X. Medium optimization and structural characterization of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydr. Polym. 2010, 79, 206–213. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Tanyildizi, M.S.; Ozer, D.; Elibol, M. Optimization of α-amylase by Bacillus sp. using response surface methodology. Process Biochem. 2005, 40, 2291–2296. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; John Wiley & Sons, Inc.: New York, NY, USA, 2009. [Google Scholar]
- Dai, J.-J.; Cheng, J.-S.; Liang, Y.-Q.; Jiang, T.; Yuan, Y.-J. Regulation of extracellular oxidoreduction potential enhanced (R,R)-2,3-butanediol production by Paenibacillus polymyxa CJX518. Bioresour. Technol. 2014, 167, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Adlakha, N.; Yazdani, S.S. Efficient production of (R,R)-2,3-butanediol from cellulosic hydrolysate using Paenibacillus polymyxa ICGEB2008. J. Ind. Microbiol. Biotechnol. 2015, 42, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, Y.; Gao, J.; Xu, H.; Cao, C.; Xue, F.; Ding, G.; Peng, Y. Introduction of the exogenous NADH coenzyme regeneration system and its influence on intracellular metabolic flux of Paenibacillus polymyxa. Bioresour. Technol. 2016, 201, 319–328. [Google Scholar] [CrossRef] [PubMed]
| Factor | Low Level (−1) | High Level (+1) | % Contribution | t Value | p Value |
|---|---|---|---|---|---|
| X1: Inoculum size (%) | 6 | 10 | 39.56 | 10.29 | 0.0260 * |
| X2: Temperature (°C) | 35 | 37 | 23.23 | −7.88 | 0.0348 * |
| X3: CH3COONH4 (g/L) | 3 | 5 | 4.87 | 3.61 | 0.0753 |
| X4: (NH4)2SO4 (g/L) | 2 | 4 | 9.30 | 4.99 | 0.0595 |
| X5: Glycerol (g/L) | 5 | 10 | 0.29 | 0.89 | 0.2112 |
| X6: Yeast extract (g/L) | 5 | 7 | 4.51 | 3.47 | 0.1174 |
| X7: Tryptone (g/L) | 5 | 7 | 18.23 | −6.99 | 0.0377 * |
| Run | Factors | 2,3-BD (g/L) | ||
|---|---|---|---|---|
| Inoculum Size (%) | Temperature (°C) | Tryptone (g/L) | ||
| 1 | 8.0 | 36 | 5.0 | 46.56 |
| 2 | 8.5 | 35 | 4.5 | 47.87 |
| 3 | 9.0 | 34 | 4.0 | 51.96 |
| 4 | 9.5 | 33 | 3.5 | 48.26 |
| 5 | 10 | 32 | 3.0 | 45.50 |
| Run | Coded Values | Actual Values | 2,3-BD (g/L) | ||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X7 | X1 (%) | X2 (°C) | X7 (g/L) | ||
| 1 | −1 | 0 | +1 | 8.5 | 34 | 4.5 | 50.65 |
| 2 | +1 | 0 | +1 | 9.5 | 34 | 4.5 | 50.99 |
| 3 | −1 | +1 | 0 | 8.5 | 35 | 4.0 | 46.20 |
| 4 | 0 | 0 | 0 | 9.0 | 34 | 4.0 | 52.56 |
| 5 | 0 | −1 | −1 | 9.0 | 33 | 3.5 | 50.98 |
| 6 | −1 | −1 | 0 | 8.5 | 33 | 4.0 | 51.04 |
| 7 | 0 | 0 | 0 | 9.0 | 34 | 4.0 | 51.98 |
| 8 | +1 | −1 | 0 | 9.5 | 33 | 4.0 | 46.99 |
| 9 | 0 | +1 | +1 | 9.0 | 35 | 4.5 | 52.70 |
| 10 | +1 | 0 | −1 | 9.5 | 34 | 3.5 | 50.13 |
| 11 | +1 | +1 | 0 | 9.5 | 35 | 4.0 | 51.58 |
| 12 | 0 | +1 | −1 | 9.0 | 35 | 3.5 | 49.18 |
| 13 | 0 | 0 | 0 | 9.0 | 34 | 4.0 | 51.37 |
| 14 | 0 | −1 | +1 | 9.0 | 33 | 4.5 | 51.94 |
| 15 | −1 | 0 | −1 | 8.5 | 34 | 3.5 | 46.65 |
| Factors | Sum of Squares | Degree of Freedom | Mean of Squares | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 61.55 | 9 | 6.84 | 21.65 | 0.0017 * |
| X1 | 3.32 | 1 | 3.32 | 10.50 | 0.0230 * |
| X2 | 0.21 | 1 | 0.21 | 0.66 | 0.4539 |
| X7 | 10.90 | 1 | 10.90 | 34.52 | 0.0020 * |
| X1 X2 | 22.23 | 1 | 22.23 | 70.38 | 0.0004 * |
| X1 X7 | 2.46 | 1 | 2.46 | 7.80 | 0.0383 * |
| X2 X7 | 1.64 | 1 | 1.64 | 5.19 | 0.0718 |
| X12 | 19.64 | 1 | 19.64 | 62.18 | 0.0005 * |
| X22 | 1.87 | 1 | 1.87 | 5.91 | 0.0592 |
| X72 | 0.013 | 1 | 0.013 | 0.040 | 0.8487 |
| Residual | 1.58 | 5 | 0.32 | ||
| Lack of fit | 0.87 | 3 | 0.29 | 0.82 | 0.5904 |
| Pure error | 0.71 | 2 | 0.35 | ||
| Cor. Total | 63.13 | 14 |
| Product Profile | Batch Fermentation | Fed-Batch Fermentation | ||||
|---|---|---|---|---|---|---|
| Max. conc. (g/L) | Yield (g/g) | Productivity (g/L/h) | Max. conc. (g/L) | Yield (g/g) | Productivity (g/L/h) | |
| 2,3-BD | 51.10 ± 0.61 | 0.42 ± 0.01 | 1.70 ± 0.02 | 68.54 ± 4.25 | 0.34 ± 0.03 | 0.70 ± 0.04 |
| Ethanol | 6.64 ± 0.19 | 0.06 ± 0.00 | 0.22 ± 0.01 | 8.15 ± 0.51 | 0.06 ± 0.00 | 0.17 ± 0.01 |
| Acetoin | 3.96 ± 0.08 | 0.03 ± 0.00 | 0.08 ± 0.00 | 12.01 ± 2.43 | 0.05 ± 0.01 | 0.10 ± 0.02 |
| Acetic acid | 1.51 ± 0.07 | 0.01 ± 0.00 | 0.03 ± 0.00 | 1.82 ± 0.71 | 0.01 ± 0.00 | 0.03 ± 0.01 |
| EPS | 4.97 ± 0.15 | 0.04 ± 0.00 | 0.41 ± 0.01 | 4.69 ± 0.02 | 0.02 ± 0.00 | 0.39 ± 0.00 |
| Glucose consumed | 120.54 ± 1.55 | N/A | N/A | 199.97 ± 8.53 | N/A | N/A |
| Carbon Source | 2,3-BD (g/L) | 2,3-BD Yield (g/g) | 2,3-BD Prod. (g/L/h) | GO | ONS (g/L) | PP | FM | Ref. |
|---|---|---|---|---|---|---|---|---|
| Glucose | 51.10 ± 0.61 | 0.42 ± 0.01 | 1.70 ± 0.02 | 11.27 ± 0.06 | YE, 5; tryp., 3.5 | PP DSM 365 | B | This work |
| Glucose | 68.54 ± 4.25 | 0.34 ± 0.03 | 0.70 ± 0.04 | 11.92 ± 0.11 | YE, 5; tryp., 3.5 | PP DSM 365 | FB | This work |
| Raw inulin extract from Jerusalem artichoke tubers | 37.57 ± 0.32 | 0.51 | 0.89 | 26.79 ± 0.35 * | YE, 3; pep, 2 | PP ZJ-9 | B | [16] |
| Glucose | 71.71 | ND | 1.33 | 13 | YE, 10 | PP CJX518 | FB | [36] |
| Sucrose | 111 | ND | 2.06 | 23 † | YE, 60 | PP DSM 365 | FB | [8] |
| Glucose | 16.50 | 0.33 | 2.01 | 9.5 | YE, 15 | PP ICGEB2008 | B | [37] |
| Inulin | 51.3 | ND | ND | NS | YE, 6; Pep., 3 | PP ZJ-9 (XG-1) | FB | [38] |
| Inulin | 36.8 | ND | ND | 11 † | YE, 6; Pep., 3 | PP ZJ-9 (XG-1) | B | [38] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okonkwo, C.C.; Ujor, V.C.; Mishra, P.K.; Ezeji, T.C. Process Development for Enhanced 2,3-Butanediol Production by Paenibacillus polymyxa DSM 365. Fermentation 2017, 3, 18. https://doi.org/10.3390/fermentation3020018
Okonkwo CC, Ujor VC, Mishra PK, Ezeji TC. Process Development for Enhanced 2,3-Butanediol Production by Paenibacillus polymyxa DSM 365. Fermentation. 2017; 3(2):18. https://doi.org/10.3390/fermentation3020018
Chicago/Turabian StyleOkonkwo, Christopher Chukwudi, Victor C. Ujor, Pankaj K. Mishra, and Thaddeus Chukwuemeka Ezeji. 2017. "Process Development for Enhanced 2,3-Butanediol Production by Paenibacillus polymyxa DSM 365" Fermentation 3, no. 2: 18. https://doi.org/10.3390/fermentation3020018
APA StyleOkonkwo, C. C., Ujor, V. C., Mishra, P. K., & Ezeji, T. C. (2017). Process Development for Enhanced 2,3-Butanediol Production by Paenibacillus polymyxa DSM 365. Fermentation, 3(2), 18. https://doi.org/10.3390/fermentation3020018