Mixed Carboxylic Acid Production by Megasphaera elsdenii from Glucose and Lignocellulosic Hydrolysate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrolysate Preparation
2.2. Microorganism and Growth Media
2.3. Seed Culture Preparation
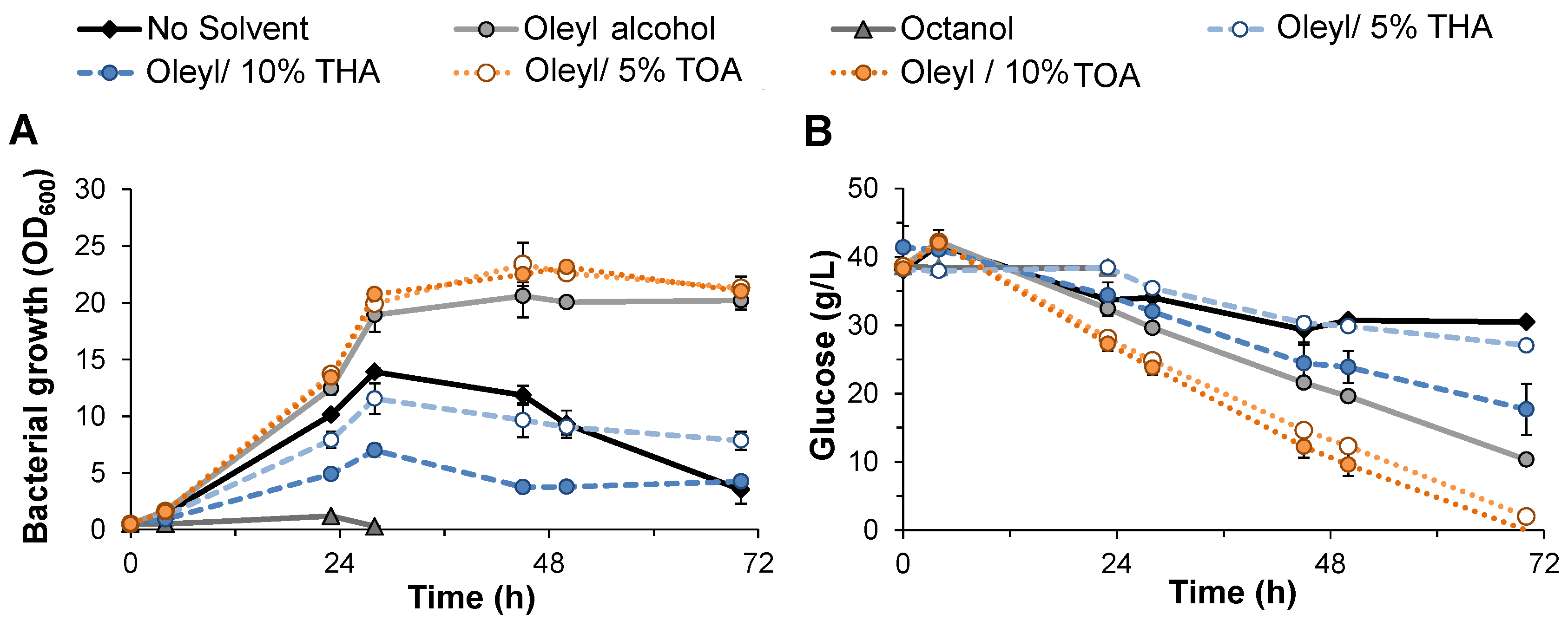
2.4. Fermentation in Serum Bottles to Test the Effect of Organic Solvents on M. elsdenii Growth
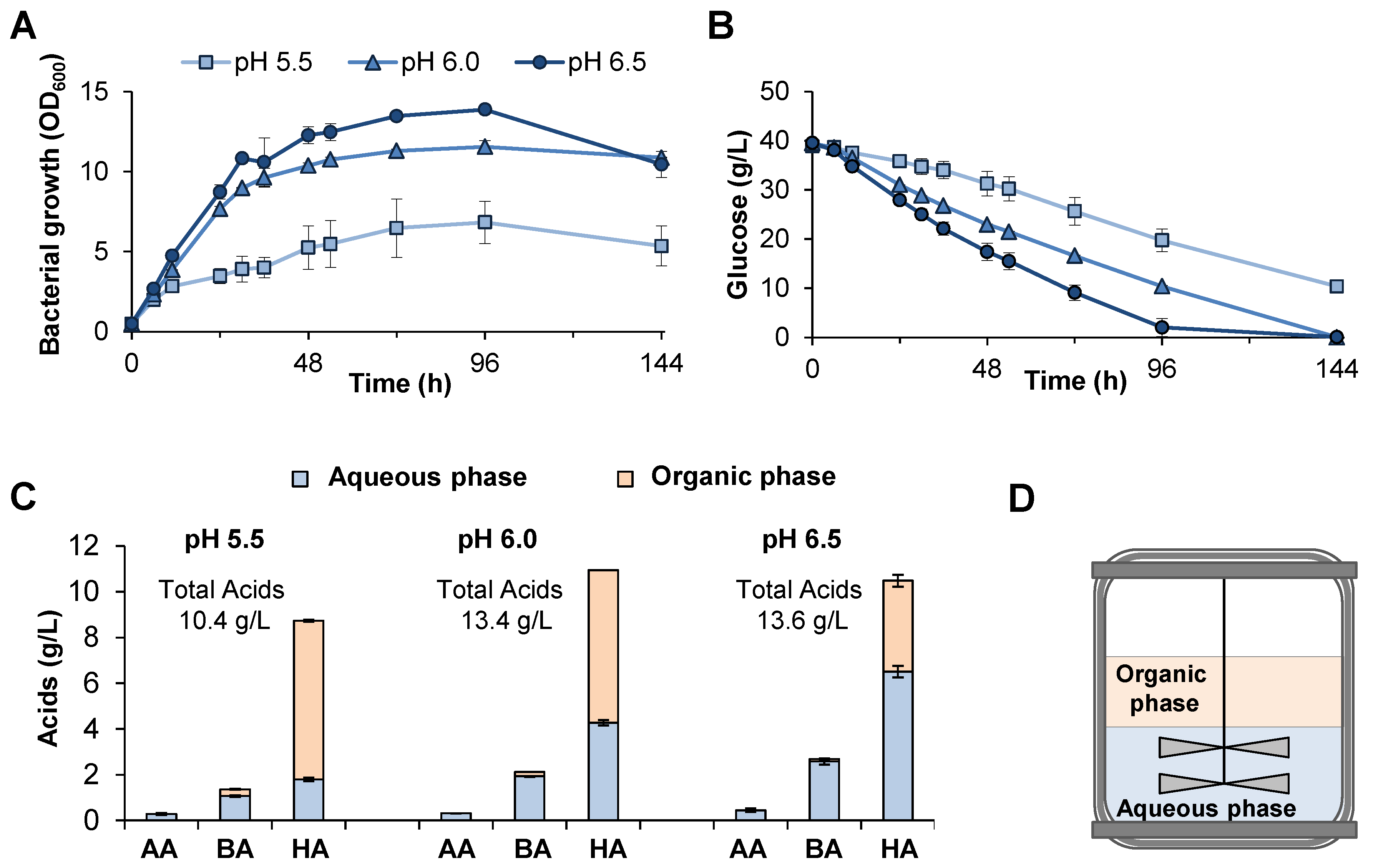
2.5. Batch Extractive Fermentations at Different pHs
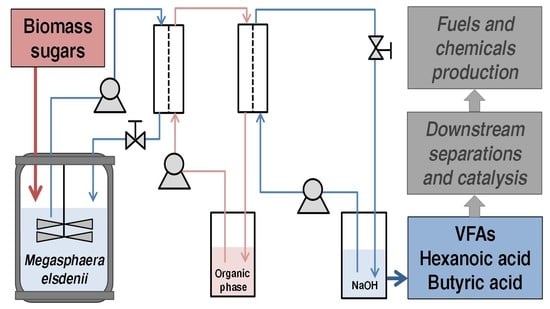
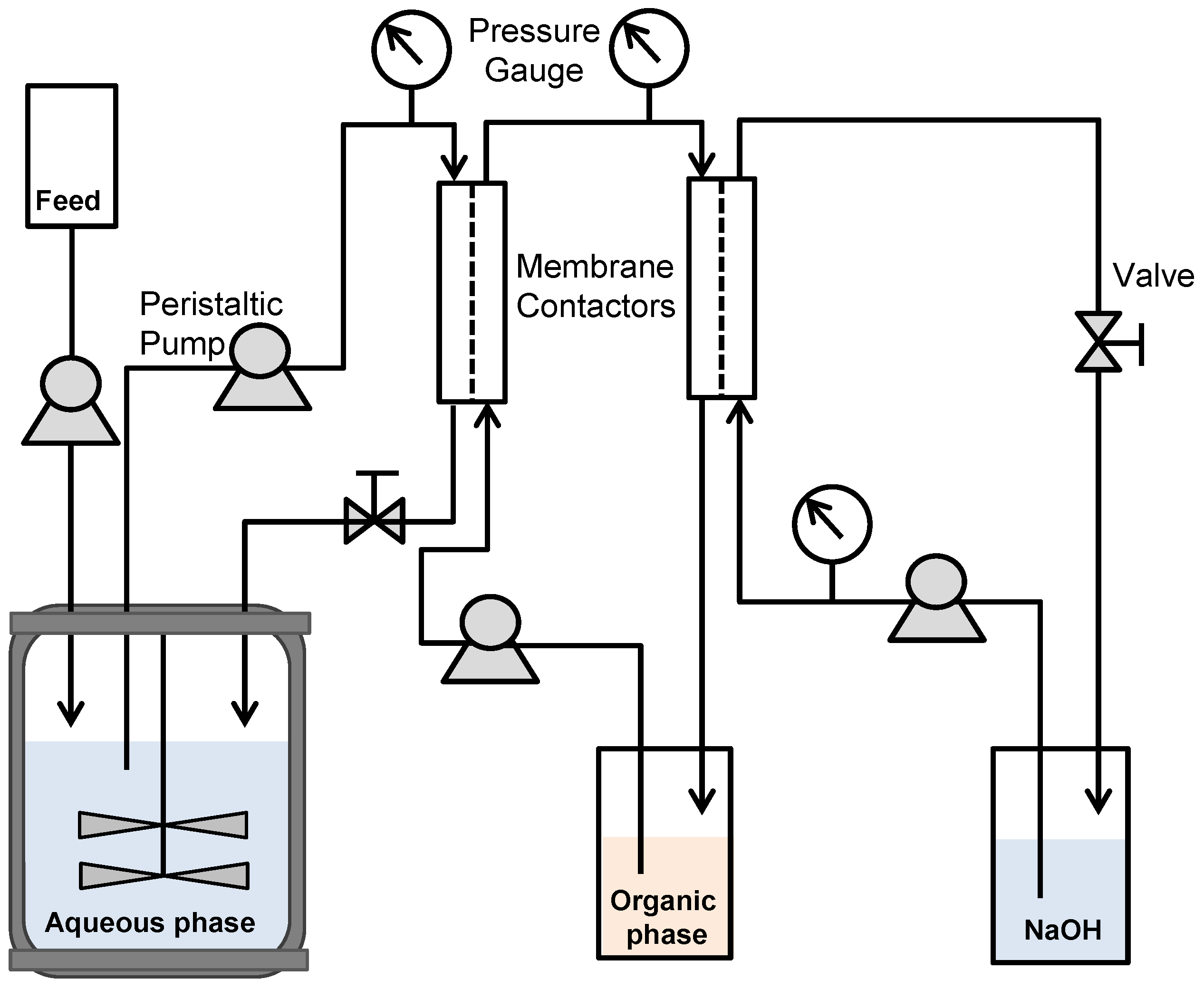
2.6. Fed-Batch Pertractive Fermentations
2.7. M. elsdenii Fermentations on Biomass Hydrolysate
2.8. Analytical Methods
2.9. Yields, Productivities, Carbon Yields, and Mass Balance Calculations
3. Results
3.1. Evaluation of Solvent Compatibility for M. elsdenii Growth and Glucose Utilization
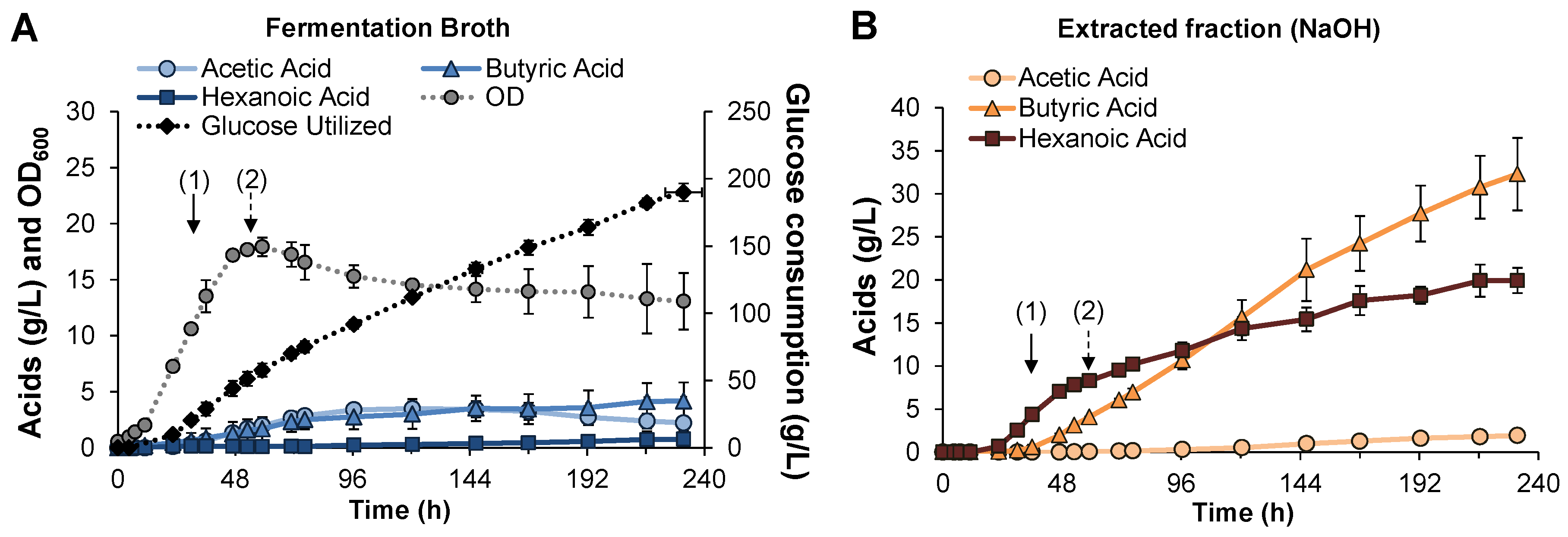
3.2. Batch Liquid–Liquid Extractive Fermentation at Different pHs
3.3. Fed-Batch Pertractive Fermentation
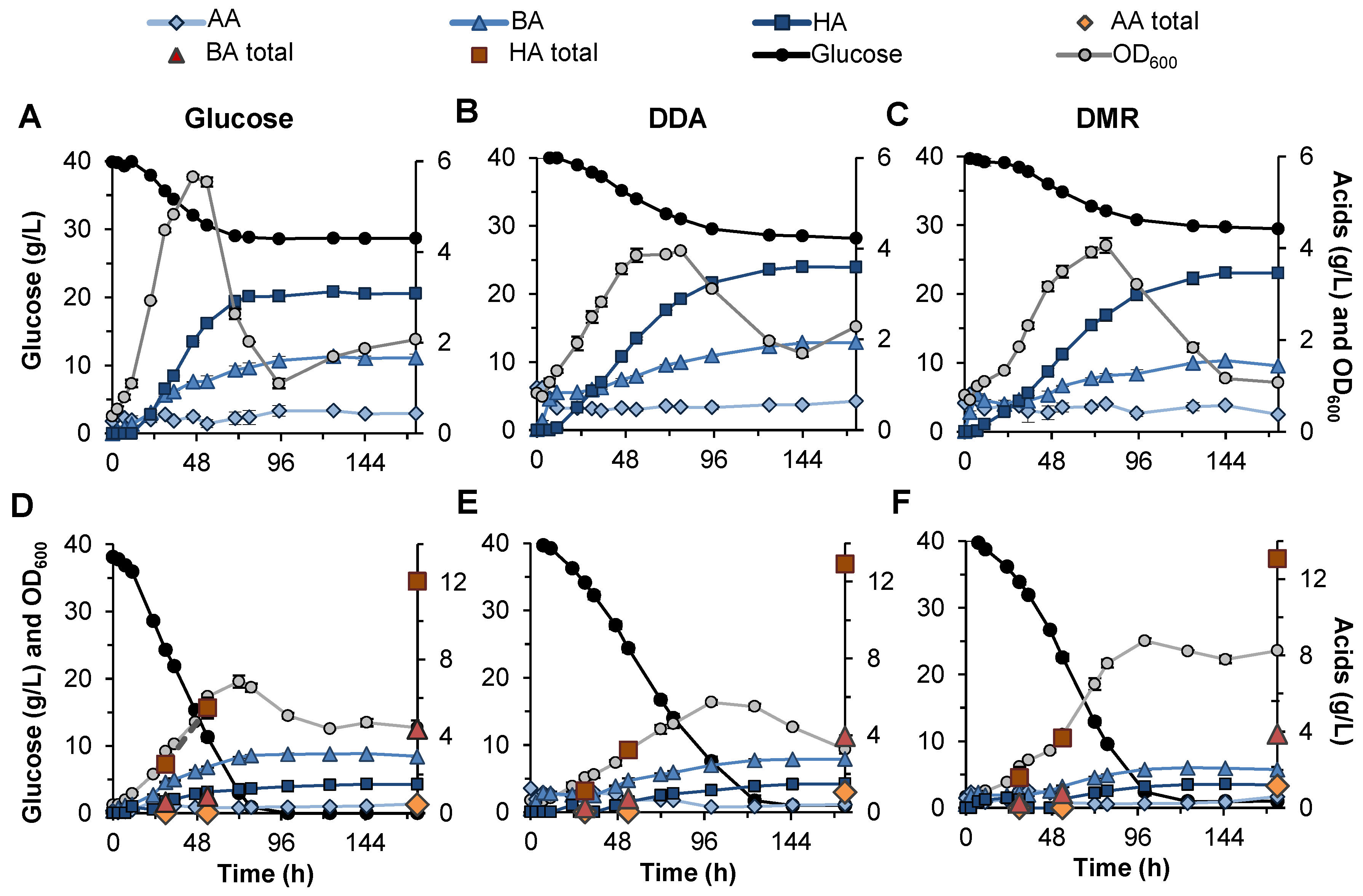
3.4. Fermentation of Biomass Sugars with and without Acid Extraction
3.4.1. Non-Extracted Fermentation of Biomass Sugars
3.4.2. Extracted Fermentation of Biomass Sugars
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Agler, M.T.; Wrenn, B.A.; Zinder, S.H.; Angenent, L.T. Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends Biotechnol. 2011, 29, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Holtzapple, M.T.; Granda, C.B. Carboxylate platform: The MixAlco process Part 1: Comparison of three biomass conversion platforms. Appl. Biochem. Biotechnol. 2009, 156, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Kucek, L.A.; Spirito, C.M.; Angenent, L.T. High n-caprylate productivities and specificities from dilute ethanol and acetate: Chain elongation with microbiomes to upgrade products from syngas fermentation. Energy Environ. Sci. 2016, 9, 3482–3494. [Google Scholar] [CrossRef]
- Angenent, L.T.; Richter, H.; Buckel, W.; Spirito, C.M.; Steinbusch, K.J.J.; Plugge, C.M.; Strik, D.P.B.T.B.; Grootscholten, T.I.M.; Buisman, C.J.N.; Hamelers, H.V.M. Chain elongation with reactor microbiomes: Open-culture biotechnology to produce biochemicals. Environ. Sci. Technol. 2016, 50, 2796–2810. [Google Scholar] [CrossRef] [PubMed]
- Renz, M. Ketonization of carboxylic acids by decarboxylation: Mechanism and scope. Eur. J. Org. Chem. 2005, 2005, 979–988. [Google Scholar] [CrossRef]
- Gaertner, C.A.; Serrano-Ruiz, J.C.; Braden, D.J.; Dumesic, J.A. Catalytic coupling of carboxylic acids by ketonization as a processing step in biomass conversion. J. Catal. 2009, 266, 71–78. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; West, R.M.; Dumesic, J.A. Catalytic conversion of renewable biomass resources to fuels and chemicals. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 79–100. [Google Scholar] [CrossRef] [PubMed]
- Nilges, P.; dos Santos, T.R.; Harnisch, F.; Schroder, U. Electrochemistry for biofuel generation: Electrochemical conversion of levulinic acid to octane. Energy Environ. Sci. 2012, 5, 5231–5235. [Google Scholar] [CrossRef]
- Straathof, A.J.J. Transformation of biomass into commodity chemicals using enzymes or cells. Chem. Rev. 2014, 114, 1871–1908. [Google Scholar] [CrossRef] [PubMed]
- Dwidar, M.; Park, J.-Y.; Mitchell, R.J.; Sang, B.-I. The future of butyric acid in industry. Sci. World J. 2012, 2012, 471417. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, S.T. Extractive fermentation for butyric acid production from glucose by Clostridium tyrobutyricum. Biotechnol. Bioeng. 2003, 82, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-T.; Yu, M.; Chang, W.-L.; Tang, I.C. Anaerobic fermentations for the production of acetic and butyric acids. In Bioprocessing Technologies in Biorefinery for Sustainable Production of Fuels, Chemicals, and Polymers; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 351–374. [Google Scholar]
- Weimer, P.J.; Stevenson, D.M. Isolation, characterization, and quantification of Clostridium kluyveri from the bovine rumen. Appl. Microbiol. Biotechnol. 2012, 94, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.S.; Moon, C.; Kim, B.-C.; Kim, H.; Um, Y.; Sang, B.-I. In situ extractive fermentation for the production of hexanoic acid from galactitol by Clostridium sp. BS-1. Enzyme Microb. Technol. 2013, 53, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Kenealy, W.R.; Cao, Y.; Weimer, P.J. Production of caproic acid by cocultures of ruminal cellulolytic bacteria and Clostridium kluyveri grown on cellulose and ethanol. Appl. Microbiol. Biotechnol. 1995, 44, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Thanakoses, P.; Black, A.S.; Holtzapple, M.T. Fermentation of corn stover to carboxylic acids. Biotechnol. Bioeng. 2003, 83, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Thanakoses, P.; Mostafa, N.A.A.; Holtzapple, M.T. Conversion of sugarcane bagasse to carboxylic acids using a mixed culture of mesophilic microorganisms. Appl. Biochem. Biotechnol. 2003, 107, 523–546. [Google Scholar] [CrossRef]
- Weimer, P.J.; Nerdahl, M.; Brandl, D.J. Production of medium-chain volatile fatty acids by mixed ruminal microorganisms is enhanced by ethanol in co-culture with Clostridium kluyveri. Bioresour. Technol. 2015, 175, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Steinbusch, K.J.J.; Hamelers, H.V.M.; Plugge, C.M.; Buisman, C.J.N. Biological formation of caproate and caprylate from acetate: Fuel and chemical production from low grade biomass. Energy Environ. Sci. 2011, 4, 216–224. [Google Scholar] [CrossRef]
- Choi, K.; Jeon, B.S.; Kim, B.C.; Oh, M.K.; Um, Y.; Sang, B.I. In situ biphasic extractive fermentation for hexanoic acid production from sucrose by Megasphaera elsdenii NCIMB 702410. Appl. Biochem. Biotechnol. 2013, 171, 1094–1107. [Google Scholar] [CrossRef] [PubMed]
- Roddick, F.A.; Britz, M.L. Production of hexanoic acid by free and immobilised cells of Megasphaera elsdenii: Influence of in-situ product removal using ion exchange resin. J. Chem. Technol. Biotechnol. 1997, 69, 383–391. [Google Scholar] [CrossRef]
- Grootscholten, T.I.; Steinbusch, K.J.; Hamelers, H.V.; Buisman, C.J. Improving medium chain fatty acid productivity using chain elongation by reducing the hydraulic retention time in an upflow anaerobic filter. Bioresour. Technol. 2013, 136, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Elsden, S.R.; Volcani, B.E.; Gilchrist, F.M.C.; Lewis, D. Properties of a fatty acid forming organism isolated from the rumen of sheep. J. Bacteriol. 1956, 72, 681–689. [Google Scholar] [PubMed]
- Jeon, B.S.; Choi, O.; Um, Y.; Sang, B.I. Production of medium-chain carboxylic acids by Megasphaera sp. MH with supplemental electron acceptors. Biotechnol. Biofuels 2016, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Agler, M.T.; Spirito, C.M.; Usack, J.G.; Werner, J.J.; Angenent, L.T. Chain elongation with reactor microbiomes: Upgrading dilute ethanol to medium-chain carboxylates. Energy Environ. Sci. 2012, 5, 8189–8192. [Google Scholar] [CrossRef]
- Jarboe, L.R.; Royce, L.A.; Liu, P. Understanding biocatalyst inhibition by carboxylic acids. Front. Microbiol. 2013, 4, 272. [Google Scholar] [CrossRef] [PubMed]
- Roddick, F.A.; Britz, M.L. Influence of product removal on volatile fatty acid production by Megasphaera elsdenii. In Proceedings of the VIIth Australian Biotechnology Conference, Melbourne, Australia, 25–28 August 1986; pp. 386–389.
- Li, X.; Swan, J.E.; Nair, G.R.; Langdon, A.G. Preparation of volatile fatty acid (VFA) calcium salts by anaerobic digestion of glucose. Biotechnol. Appl. Biochem. 2015, 62, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Kurzrock, T.; Weuster-Botz, D. New reactive extraction systems for separation of bio-succinic acid. Bioprocess Biosyst. Eng. 2011, 34, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Yang, S.-T. Extractive fermentation for enhanced propionic acid production from lactose by Propionibacterium acidipropionici. Biotechnol. Prog. 1998, 14, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.K.; Hong, W.H.; Han, D.H. Application of reactive extraction to recovery of carboxylic acids. Biotechnol. Bioprocess Eng. 2001, 6, 386. [Google Scholar] [CrossRef]
- Schell, D.J.; Dowe, N.; Chapeaux, A.; Nelson, R.S.; Jennings, E.W. Accounting for all sugars produced during integrated production of ethanol from lignocellulosic biomass. Bioresour. Technol. 2016, 205, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kuhn, E.; Jennings, E.W.; Nelson, R.; Tao, L.; Zhang, M.; Tucker, M.P. DMR (deacetylation and mechanical refining) processing of corn stover achieves high monomeric sugar concentrations (230 g·L−1) during enzymatic hydrolysis and high ethanol concentrations (>10% v/v) during fermentation without hydrolysate purification or concentration. Energy Environ. Sci. 2016, 9, 1237–1245. [Google Scholar]
- Zigová, J.; Šturdı́k, E.; Vandák, D.; Schlosser, Š. Butyric acid production by Clostridium butyricum with integrated extraction and pertraction. Process Biochem. 1999, 34, 835–843. [Google Scholar] [CrossRef]
- Miyazaki, K.; Hino, T.; Itabashi, H. Effects of extracellular pH on the intracellular pH, membrane potential, and growth yield of Megasphaera elsdenii in relation to the influence of monensin, ethanol, and acetate. J. Gen. Appl. Microbiol. 1991, 37, 415–422. [Google Scholar] [CrossRef]
- Huang, C.B.; Alimova, Y.; Myers, T.M.; Ebersole, J.L. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 2011, 56, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B. Another explanation for the toxicity of fermentation acids at low pH: Anion accumulation versus uncoupling. J. Appl. Bacteriol. 1992, 73, 363–370. [Google Scholar] [CrossRef]
- Ge, S.; Usack, J.G.; Spirito, C.M.; Angenent, L.T. Long-term n-caproic acid production from yeast-fermentation beer in an anaerobic bioreactor with continuous product extraction. Environ. Sci. Technol. 2015, 49, 8012–8021. [Google Scholar] [CrossRef] [PubMed]
- Franden, M.A.; Pilath, H.M.; Mohagheghi, A.; Pienkos, P.T.; Zhang, M. Inhibition of growth of Zymomonas mobilis by model compounds found in lignocellulosic hydrolysates. Biotechnol. Biofuels 2013, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Weimer, P.J.; Moen, G.N. Quantitative analysis of growth and volatile fatty acid production by the anaerobic ruminal bacterium Megasphaera elsdenii T81. Appl. Microbiol. Biotechnol. 2013, 97, 4075–4081. [Google Scholar] [CrossRef] [PubMed]
- Grzenia, D.L.; Wickramasinghe, S.R.; Schell, D.J. Fermentation of reactive-membrane-extracted and ammonium-hydroxide-conditioned dilute-acid-pretreated corn stover. Appl. Biochem. Biotechnol. 2012, 166, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, C.; Gao, C.; Xu, P. Efficient utilization of hemicellulose hydrolysate for propionic acid production using Propionibacterium acidipropionici. Bioresour. Technol. 2012, 114, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Taherzadeh, M.J.; Gustafsson, L.; Niklasson, C.; Lidén, G. Conversion of furfural in aerobic and anaerobic batch fermentation of glucose by Saccharomyces cerevisiae. J. Biosci. Bioeng. 1999, 87, 169–174. [Google Scholar] [CrossRef]
- Gutiérrez, T.; Buszko, M.L.; Ingram, L.O.; Preston, J.F. Reduction of furfural to furfuryl alcohol by ethanologenic strains of bacteria and its effect on ethanol production from xylose. Biotechnol. Fuel Chem. 2002, 98, 327–340. [Google Scholar]
- Salvachúa, D.; Mohagheghi, A.; Smith, H.; Bradfield, M.F.A.; Nicol, W.; Black, B.A.; Biddy, M.J.; Dowe, N.; Beckham, G.T. Succinic acid production on xylose-enriched biorefinery streams by Actinobacillus succinogenes in batch fermentation. Biotechnol. Biofuels 2016, 9, 28. [Google Scholar] [CrossRef] [PubMed]
| Biomass Stream | Glucose (g/L) | Xylose (g/L) | Galactose (g/L) | Arabinose (g/L) | Acetic Acid (g/L) | Lactic Acid (g/L) | HMF * (g/L) | Furfural (g/L) |
|---|---|---|---|---|---|---|---|---|
| DDA | 85.9 | 52.3 | 3.1 | 7.8 | 1.9 | 0.0 | 0.1 | 1.0 |
| DMR | 68.0 | 48.9 | 0.8 | 3.6 | 0.8 | 2.8 | 0.0 | 0.0 |
| Condition | Total Titer 1 | Total Yield 1 | AA Yield 2 | BA Yield | HA Yield | Productivity 1,3 | Carbon Yield 1 | CMB 4 |
|---|---|---|---|---|---|---|---|---|
| (g/L) | (g/g) | (g/g) | (g/g) | (g/g) | (g/L/h) | (mol/mol) | (%) | |
| Liquid–liquid batch pH 5.5 | 10.4 ± 0.1 | 0.36 ± 0.00 | 0.01 ± 0.00 | 0.05 ± 0.00 | 0.31 ± 0.00 | 0.07 ± 0.00 | 0.55 ± 0.01 | 94 ± 1 |
| Liquid–liquid batch pH 6.0 | 13.4 ± 0.2 | 0.34 ± 0.01 | 0.01 ± 0.00 | 0.05 ± 0.00 | 0.28 ± 0.01 | 0.09 ± 0.00 | 0.51 ± 0.01 | 92 ± 1 |
| Liquid–liquid batch pH 6.5 | 13.6 ± 0.2 | 0.35 ± 0.01 | 0.01 ± 0.00 | 0.07 ± 0.01 | 0.27 ± 0.00 | 0.09 ± 0.00 | 0.52 ± 0.01 | 92 ± 1 |
| Pertractive Fed-batch | 61.3 ± 3.6 | 0.32 ± 0.01 | 0.02 ± 0.00 | 0.19 ± 0.01 | 0.11 ± 0.00 | 0.26 ± 0.01 | 0.63 ± 0.02 | 98 ± 1 |
| Glucose 5 | 5.2 ± 0.3 | 0.44 ± 0.00 | 0.01 ± 0.01 | 0.15 ± 0.01 | 0.28 ± 0.02 | 0.03 ± 0.00 | 0.65 ± 0.01 | 103 ± 1 |
| DDA 5 | 6.1 ± 0.0 | 0.43 ± 0.02 | −0.02 ± 0.00 | 0.16 ± 0.01 | 0.30 ± 0.01 | 0.04 ± 0.00 | 0.65 ± 0.02 | 104 ± 2 |
| DMR 5 | 5.3 ± 0.01 | 0.37 ± 0.03 | −0.02 ± 0.02 | 0.11 ± 0.00 | 0.27 ± 0.00 | 0.04 ± 0.00 | 0.56 ± 0.03 | 91 ± 3 |
| Extracted Glucose 5 | 16.9 ± 0.1 | 0.44 ± 0.00 | 0.01 ± 0.00 | 0.11 ± 0.01 | 0.32 ± 0.01 | 0.10 ± 0.00 | 0.65 ± 0.00 | 108 ± 0 |
| Extracted DDA 5 | 17.8 ± 0.4 | 0.42 ± 0.01 | −0.01 ± 0.01 | 0.10 ± 0.00 | 0.33 ± 0.00 | 0.10 ± 0.00 | 0.63 ± 0.01 | 103 ± 1 |
| Extracted DMR 5 | 18.1 ± 0.1 | 0.44 ± 0.04 | 0.03 ± 0.00 | 0.10 ± 0.03 | 0.32 ± 0.02 | 0.10 ± 0.00 | 0.66 ± 0.06 | 115 ± 7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelson, R.S.; Peterson, D.J.; Karp, E.M.; Beckham, G.T.; Salvachúa, D. Mixed Carboxylic Acid Production by Megasphaera elsdenii from Glucose and Lignocellulosic Hydrolysate. Fermentation 2017, 3, 10. https://doi.org/10.3390/fermentation3010010
Nelson RS, Peterson DJ, Karp EM, Beckham GT, Salvachúa D. Mixed Carboxylic Acid Production by Megasphaera elsdenii from Glucose and Lignocellulosic Hydrolysate. Fermentation. 2017; 3(1):10. https://doi.org/10.3390/fermentation3010010
Chicago/Turabian StyleNelson, Robert S., Darren J. Peterson, Eric M. Karp, Gregg T. Beckham, and Davinia Salvachúa. 2017. "Mixed Carboxylic Acid Production by Megasphaera elsdenii from Glucose and Lignocellulosic Hydrolysate" Fermentation 3, no. 1: 10. https://doi.org/10.3390/fermentation3010010
APA StyleNelson, R. S., Peterson, D. J., Karp, E. M., Beckham, G. T., & Salvachúa, D. (2017). Mixed Carboxylic Acid Production by Megasphaera elsdenii from Glucose and Lignocellulosic Hydrolysate. Fermentation, 3(1), 10. https://doi.org/10.3390/fermentation3010010