Rhodotorula mucilaginosa JAASSRY Alleviated Oxidative Damage in D-Galactose-Induced Aging Mice by Modulating the Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Materials
2.2. Strain Activation
2.3. Experimental Animal Grouping Design
2.4. Behavioral Tests
2.4.1. Tail Suspension Test
2.4.2. Grip Strength Measurement
2.4.3. Step-Down Test
2.5. Animal Tissue Collection
2.6. H&E Staining
2.7. Determination of Antioxidant Indices
2.8. Gut Microbiota Analysis
2.9. Statistical Analysis
3. Results
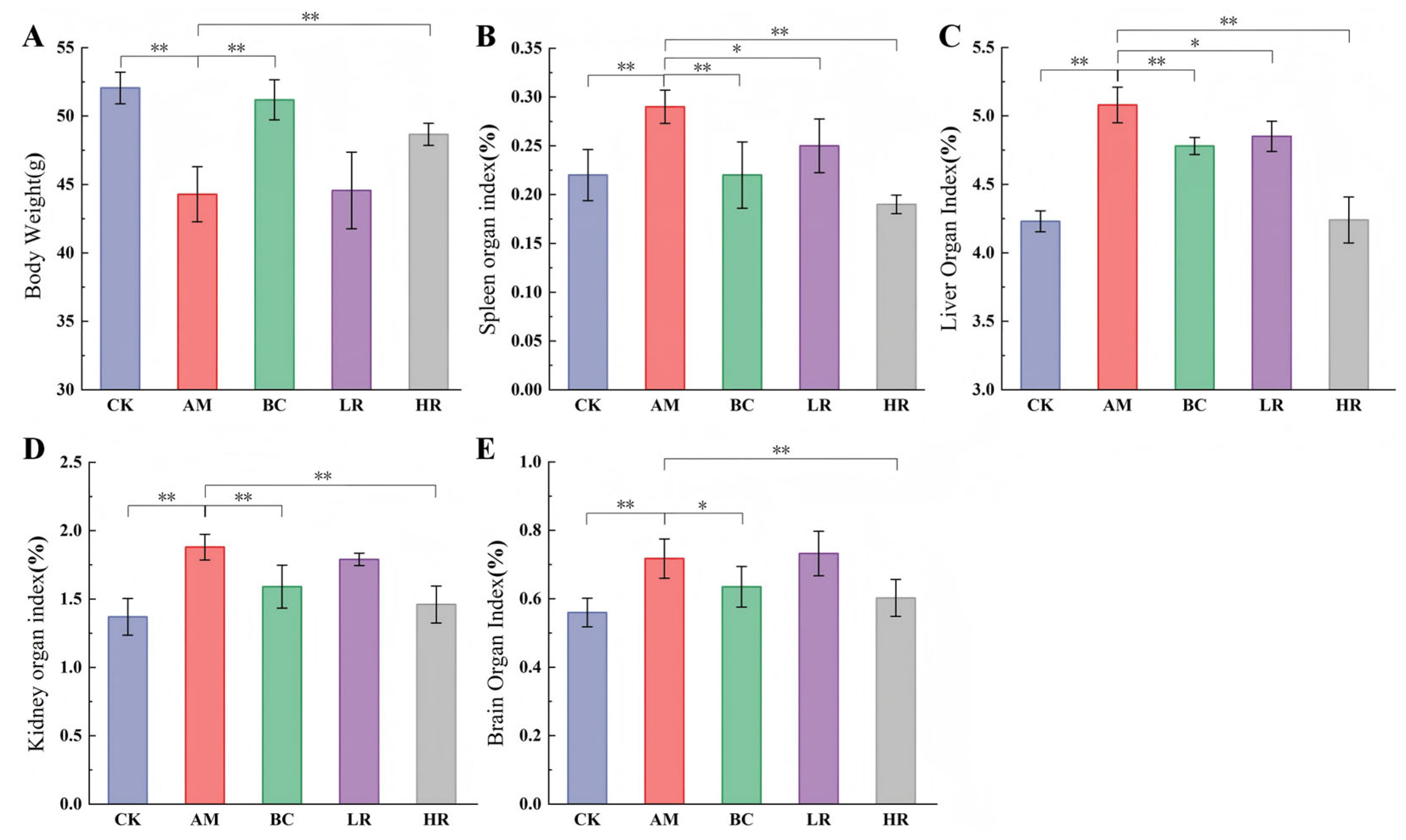
3.1. Effects of R. mucilaginosa JAASSRY on Body Weight and Organ Indices in Aging Mice
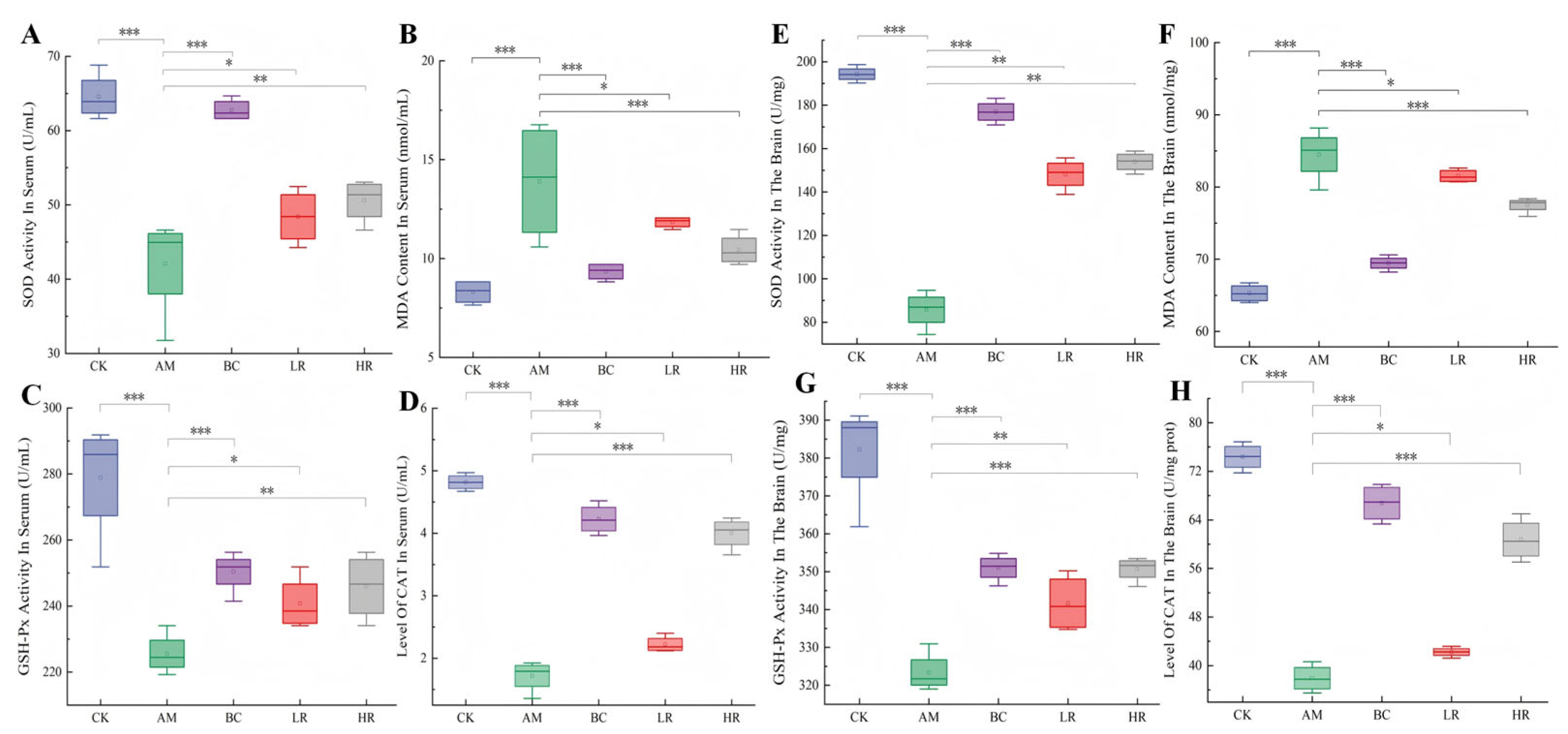
3.2. Effects of R. mucilaginosa JAASSRY on Serum and Cerebral Oxidative Stress Indicators in Aging Mice
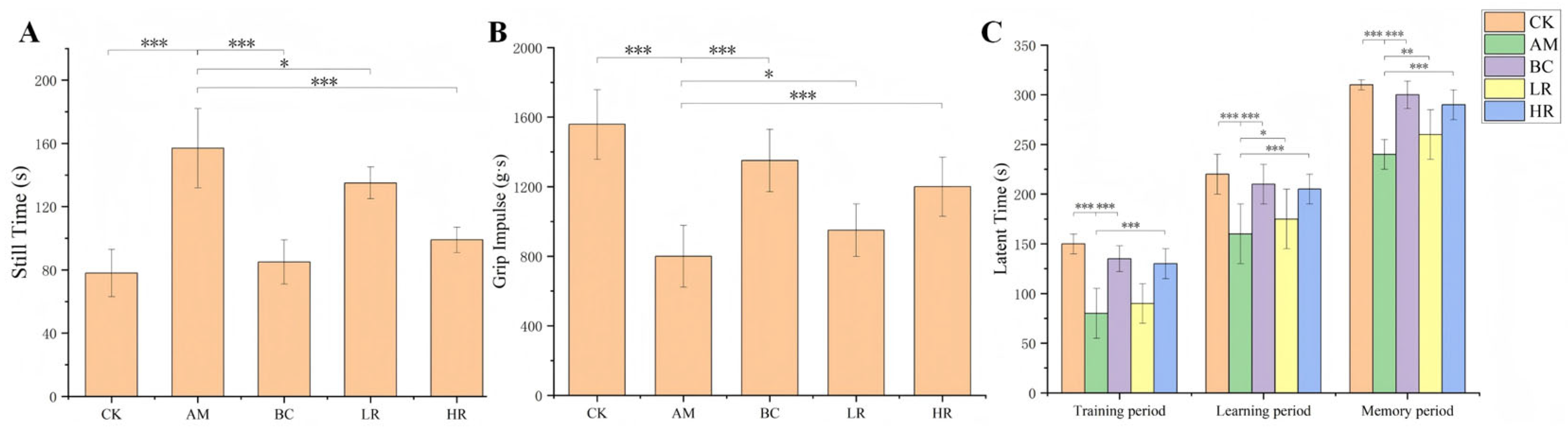
3.3. Effects of R. mucilaginosa JAASSRY on Behavioral Performance in Aging Mice
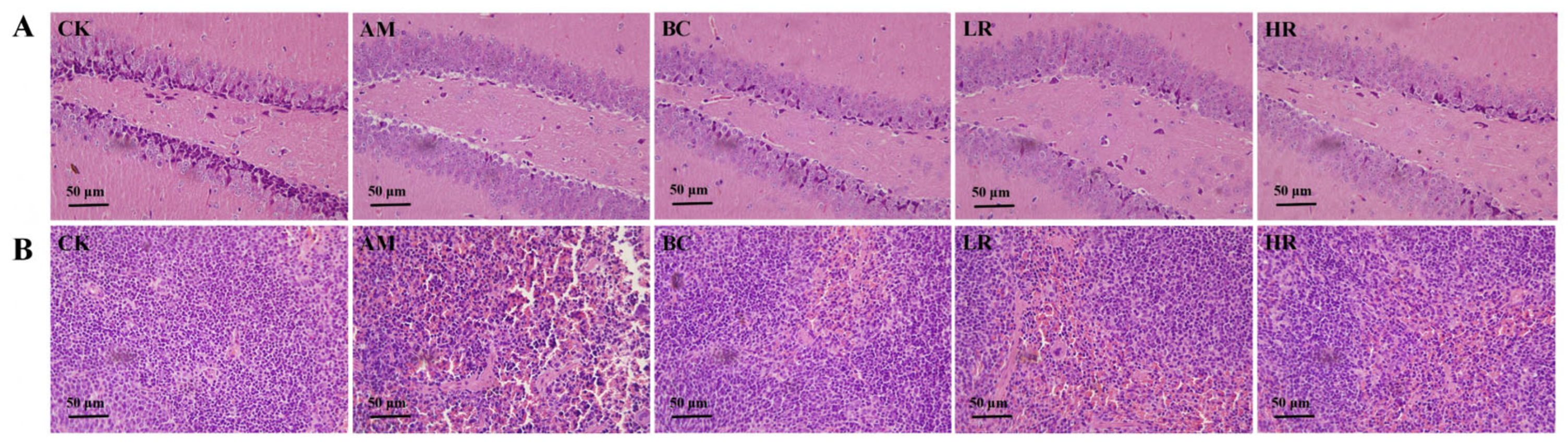
3.4. Effects of R. mucilaginosa JAASSRY on Histopathological Structure in Aging Mice
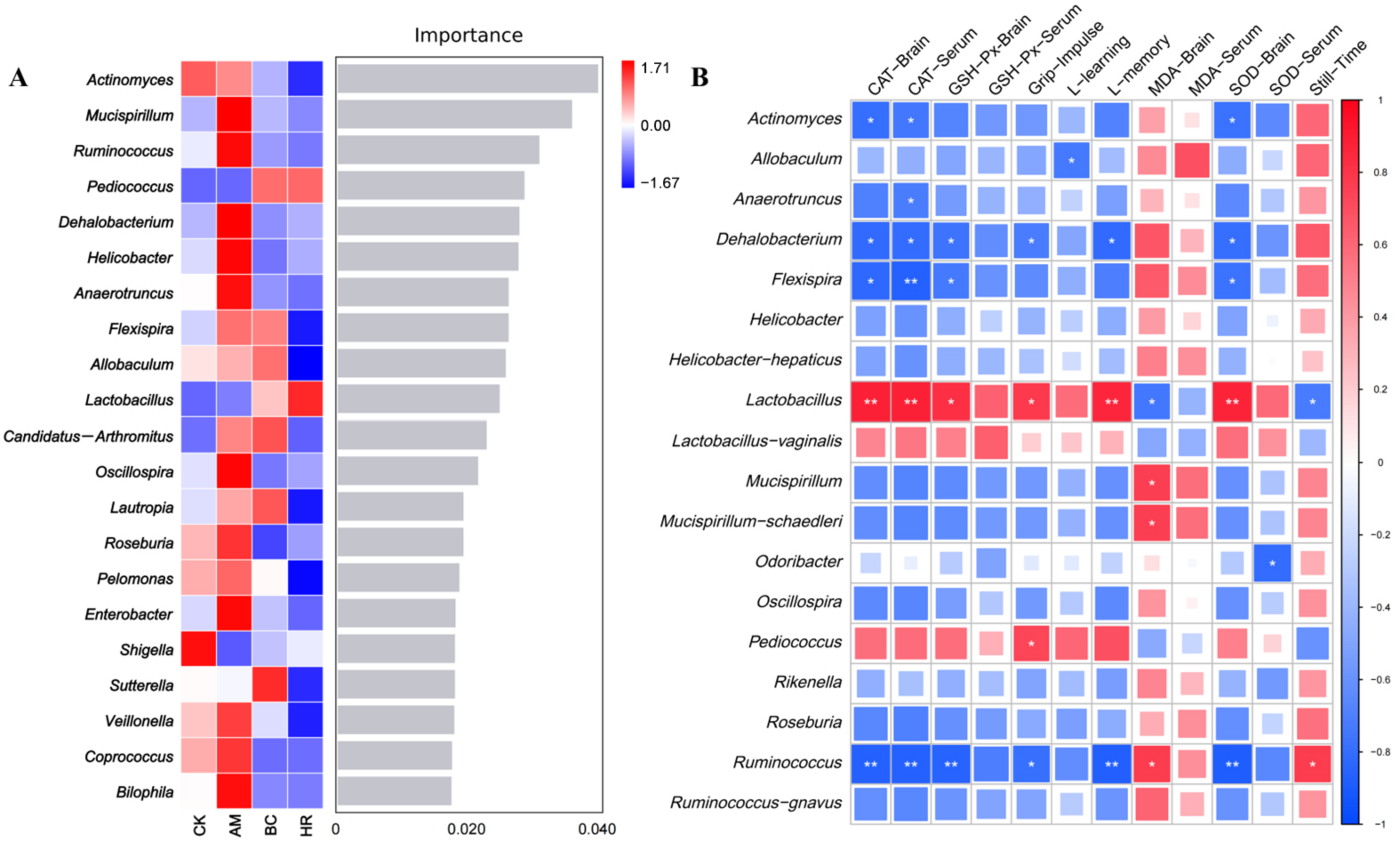
3.5. Effects of R. mucilaginosa JAASSRY on the Structure and Function of Gut Microbiota in Aging Mice
3.6. Correlation Analysis Between Gut Microbiota Changes and Antioxidant Indicators
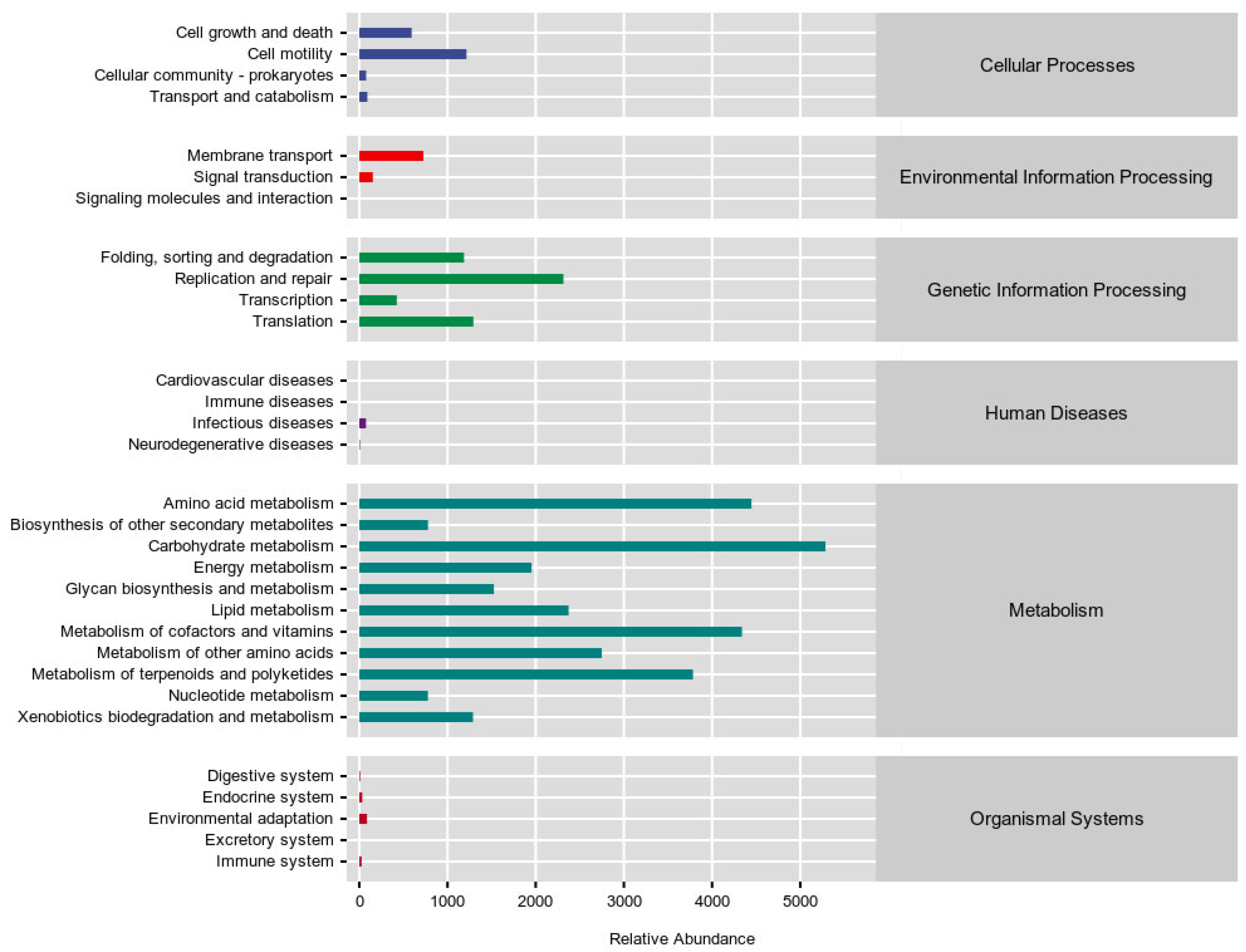
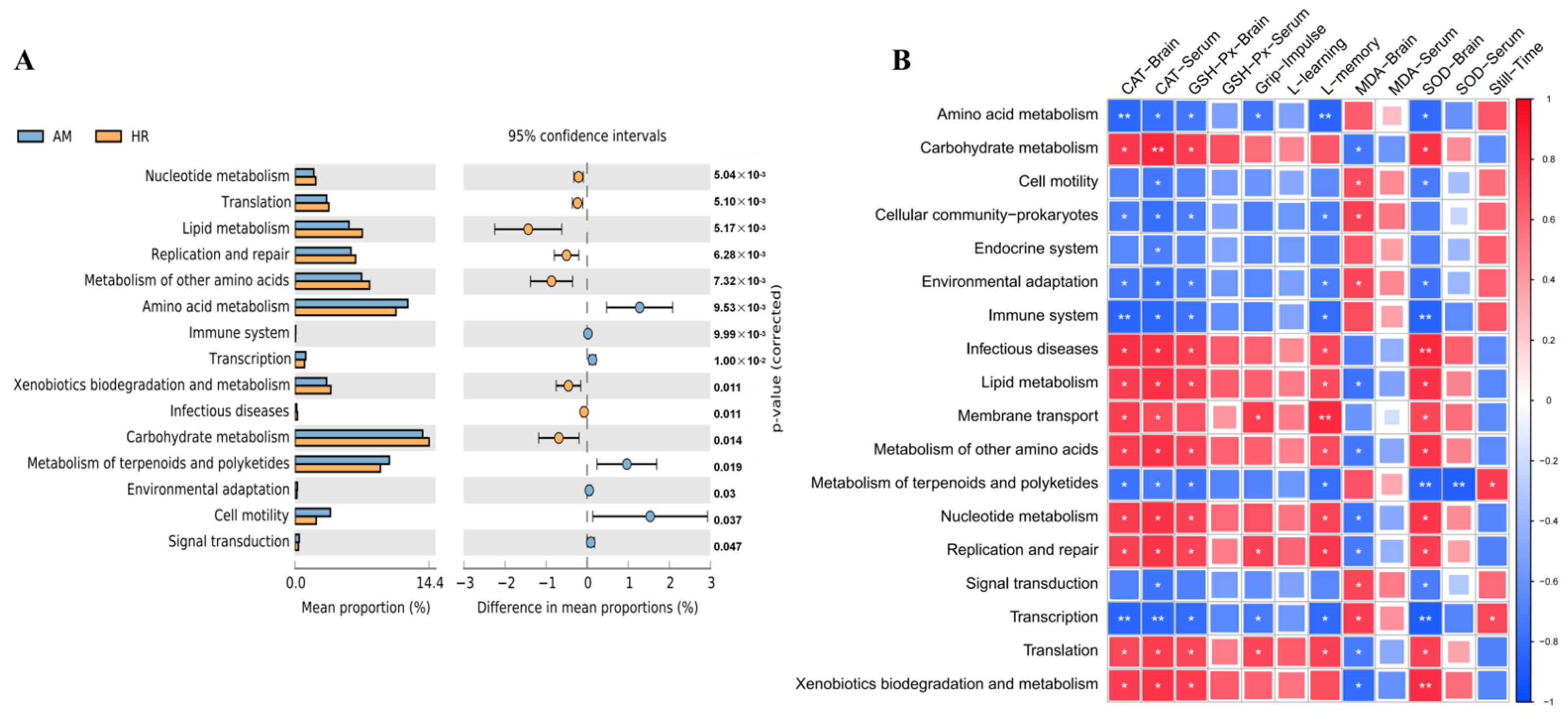
3.7. Effects of R. mucilaginosa JAASSRY on Metabolic Function of Gut Microbiota in Aging Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SOD | superoxide dismutase |
| MDA | malondialdehyde |
| CAT | catalase |
| GSH-Px | glutathione peroxidase |
Appendix A
References
- Sailwal, M.; Mishra, P.; Bhaskar, T.; Pandey, R.; Ghosh, D. Time-resolved transcriptomic profile of oleaginous yeast Rhodotorula mucilaginosa during lipid and carotenoids accumulation on glycerol. Bioresour. Technol. 2023, 384, 129379. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lyu, L.; Xue, H.; Shah, A.M.; Zhao, Z.K. Engineering a Non-Model Yeast Rhodotorula mucilaginosa for Terpenoids Synthesis. Synth. Syst. Biotechnol. 2024, 9, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Mattos, M.V.C.d.V.d.; Michelon, M.; Burkert, J.F.d.M. Production and stability of food-grade liposomes containing microbial carotenoids from Rhodotorula mucilaginosa. Food Struct. 2022, 33, 100282. [Google Scholar] [CrossRef]
- Mosqueda-Martínez, E.; Chiquete-Félix, N.; Castañeda-Tamez, P.; Ricardez-García, C.; Gutiérrez-Aguilar, M.; Uribe-Carvajal, S.; Mendez-Romero, O. In Rhodotorula mucilaginosa, active oxidative metabolism increases carotenoids to inactivate excess reactive oxygen species. Front. Fungal Biol. 2024, 5, 1378590. [Google Scholar] [CrossRef]
- Gentina, J.C.; Ah-Hen, K.S.; Alvarado, R.; Stevenson, J.; Briceño, E.; Montenegro, O.; González-Esparza, A. Survival of Spray-Dried Rhodotorula mucilaginosa Isolated from Natural Microbiota of Murta Berries and Antagonistic Effect on Botrytis cinerea. Food Technol. Biotechnol. 2019, 57, 222–229. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, E.; Li, X.; Xie, Y.; Tong, T.; Wang, J.; Zhang, Q. Effects of dietary marine red yeast supplementation on growth performance, antioxidant, immunity, lipid metabolism and mTOR signaling pathway in Juvenile Tilapia (Oreochromis niloticus). Aquac. Rep. 2024, 37, 102196. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Y.; Luo, K.; Zhang, S.; Wei, C.; Wang, L.; Qiu, Y.; Tian, X. ‘Biotic’ potential of the red yeast Rhodotorula mucilaginosa strain JM-01 on the growth, shell pigmentation, and immune defense attributes of the shrimp, Penaeus vannamei. Aquaculture 2023, 572, 739543. [Google Scholar] [CrossRef]
- Jati, I.R.A.P.; Darmoatmodjo, L.M.Y.D.; Suseno, T.I.P.; Ristiarini, S.; Wibowo, C. Effect of Processing on Bioactive Compounds, Antioxidant Activity, Physicochemical, and Sensory Properties of Orange Sweet Potato, Red Rice, and Their Application for Flake Products. Plants 2022, 11, 440. [Google Scholar] [CrossRef]
- Garcia-Cortes, A.; Garcia-Vásquez, J.A.; Aranguren, Y.; Ramirez-Castrillon, M. Pigment Production Improvement in Rhodotorula mucilaginosa AJB01 Using Design of Experiments. Microorganisms 2021, 9, 387. [Google Scholar] [CrossRef]
- Bo, S.; Ni, X.; Guo, J.; Liu, Z.; Wang, X.; Sheng, Y.; Zhang, G.; Yang, J. Carotenoid Biosynthesis: Genome-Wide Profiling, Pathway Identification in Rhodotorula glutinis X-20, and High-Level Production. Front. Nutr. 2022, 9, 918240. [Google Scholar] [CrossRef]
- Li, C.; Xie, Z.; Zhao, D.; Li, B.; Wang, D.; Chang, L.; Feng, F.; Zheng, L.; Wang, X.; Shao, M.; et al. Multi-omics analysis provides insights into the enhancement of β-carotene and torularhodin production in oleaginous red yeast Sporobolomyces pararoseus under H2O2-induced oxidative stress. LWT-Food Sci. Technol. 2024, 197, 115947. [Google Scholar] [CrossRef]
- Guo, B.; Guo, Q.; Wang, Z.; Shao, J.-B.; Liu, K.; Du, Z.-D.; Gong, S.-S. D-Galactose-induced oxidative stress and mitochondrial dysfunction in the cochlear basilar membrane: An in vitro aging model. Biogerontology 2020, 21, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Pantiya, P.; Thonusin, C.; Ongnok, B.; Chunchai, T.; Kongkaew, A.; Nawara, W.; Arunsak, B.; Chattipakorn, N.; Chattipakorn, S.C. Long-term D-galactose Administration Mimics Natural Aging in Rat’s Hippocampus. Alzheimer’s Dement. 2023, 19, e073540. [Google Scholar] [CrossRef]
- Lee, R.; Lee, W.-Y.; Park, H.-J. Effects of Melatonin on Liver of D-Galactose-Induced Aged Mouse Model. Curr. Issues Mol. Biol. 2023, 45, 8412–8426. [Google Scholar] [CrossRef]
- Sumbalová, Z.; Uličná, O.; Kucharská, J.; Rausová, Z.; Vančová, O.; Melicherčík, Ľ.; Tvrdík, T.; Nemec, M.; Kašparová, S. D-galactose-induced aging in rats—The effect of metformin on bioenergetics of brain, skeletal muscle and liver. Exp. Gerontol. 2022, 163, 111770. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Yun, Y.; Xue, J.; Sun, M.; Kim, S. D-galactose induces astrocytic aging and contributes to astrocytoma progression and chemoresistance via cellular senescence. Mol. Med. Rep. 2019, 20, 4111–4118. [Google Scholar] [CrossRef]
- Wang, L.; Li, A.; Zhang, X.; Iqbal, M.; Aabdin, Z.U.; Xu, M.; Mo, Q.; Li, J. Effect of Bacillus subtilis isolated from yaks on D-galactose-induced oxidative stress and hepatic damage in mice. Front. Microbiol. 2025, 16, 1550556. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhao, T.; Li, M.; Wang, Y.; Cao, J.; Liu, Y.; Wang, Z.; Cheng, G. Protective Effect of Que Zui Tea on d-Galactose-Induced Oxidative Stress Damage in Mice via Regulating SIRT1/Nrf2 Signaling Pathway. Molecules 2024, 29, 1384. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, N.; Srivastava, A.K.; He, G.; Tai, Z.; Wang, Z.; Yang, S.; Xie, X.; Li, X. Superoxide dismutase positively regulates Cu/Zn toxicity tolerance in Sorghum bicolor by interacting with Cu chaperone for superoxide dismutase. J. Hazard. Mater. 2024, 480, 135828. [Google Scholar] [CrossRef]
- Barbosa, M.R.; Sampaio, I.H.; Teodoro, B.G.; Sousa, T.A.; Zoppi, C.C.; Queiroz, A.L.; Passos, M.A.; Alberici, L.C.; Teixeira, F.R.; Manfiolli, A.O.; et al. Hydrogen peroxide production regulates the mitochondrial function in insulin resistant muscle cells: Effect of catalase overexpression. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2013, 1832, 1591–1604. [Google Scholar] [CrossRef]
- Liu, C.; Yan, Q.; Gao, C.; Lin, L.; Wei, J. Study on antioxidant effect of recombinant glutathione peroxidase 1. Int. J. Biol. Macromol. 2021, 170, 503–513. [Google Scholar] [CrossRef]
- Toto, A.; Wild, P.; Graille, M.; Turcu, V.; Crézé, C.; Hemmendinger, M.; Sauvain, J.-J.; Bergamaschi, E.; Guseva Canu, I.; Hopf, N.B. Urinary Malondialdehyde (MDA) Concentrations in the General Population—A Systematic Literature Review and Meta-Analysis. Toxics 2022, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Mazumder, P.M.; Banerjee, S. Vitamin K2 Protects Against D-Galactose Induced Ageing in Mice. Eur. J. Pharmacol. 2025, 990, 177277. [Google Scholar] [CrossRef] [PubMed]
- Csepanyi, E.; Czompa, A.; Haines, D.; Lekli, I.; Bakondi, E.; Balla, G.; Tosaki, A.; Bak, I. Cardiovascular Effects of Low Versus High-Dose Beta-Carotene in a Rat Model. Pharmacol. Res. 2015, 100, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.H.C.; Luo, J.K.; Wannan, C.M.J. Cognitive behavioral markers of neurodevelopmental trajectories in rodents. Transl. Psychiatry 2021, 11, 556. [Google Scholar] [CrossRef]
- Ma, S.; Ji, Z.; Zhang, B.; Geng, L.; Cai, Y.; Nie, C.; Li, J.; Zuo, Y.; Sun, Y.; Xu, G.; et al. Spatial transcriptomic landscape unveils immunoglobin-associated senescence as a hallmark of aging. Cell 2024, 187, 7025–7044.e34. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Jiang, Z. Cordycepin prevents radiation ulcer by inhibiting cell senescence via NRF2 and AMPK in rodents. Nat. Commun. 2019, 10, 2538. [Google Scholar] [CrossRef]
- Mossad, O.; Batut, B.; Yilmaz, B. Gut microbiota drives age-related oxidative stress and mitochondrial damage in microglia via the metabolite N6-carboxymethyllysine. Nat. Neurosci. 2022, 25, 295–305. [Google Scholar] [CrossRef]
- Li, C.; Wu, J.; Dong, Q.; Ma, J.; Gao, H.; Liu, G.; Chen, Y.; Ning, J.; Lv, X.; Zhang, M.; et al. The crosstalk between oxidative stress and DNA damage induces neural stem cell senescence by HO-1/PARP1 non-canonical pathway. Free Radic. Biol. Med. 2024, 223, 443–457. [Google Scholar] [CrossRef]
- Xiong, W. Nobiletin Mitigates D-Galactose-Induced Memory Impairment via Improving Hippocampal Neurogenesis in Mice. Nutrients 2023, 15, 2228. [Google Scholar] [CrossRef]
- Hu, T.; Chen, R.; Qian, Y.; Ye, K.; Long, X.; Park, K.-Y.; Zhao, X. Antioxidant effect of Lactobacillus fermentum HFY02-fermented soy milk on D-galactose-induced aging mouse model. Food Sci. Hum. Wellness 2022, 11, 1362–1372. [Google Scholar] [CrossRef]
- Niu, A.; Bian, W.-P.; Feng, S.-L.; Pu, S.-Y.; Wei, X.-Y.; Yang, Y.-F.; Song, L.-Y.; Pei, D.-S. Role of manganese superoxide dismutase (Mn-SOD) against Cr(III)-induced toxicity in bacteria. J. Hazard. Mater. 2021, 403, 123604. [Google Scholar] [CrossRef] [PubMed]
- Tumilaar, S.G.; Hutabarat, G.S.; Hardianto, A.; Kurnia, D. Computational Studies of Allylpyrocatechol from Piper betle L. as Inhibitor Against Superoxide Dismutase, Catalase, and Glutathione peroxidase as Antioxidant Enzyme. Lett. Drug Des. Discov. 2022, 21, 559–567. [Google Scholar] [CrossRef]
- Seyfi Zouleh, R.; Rahimnejad, M.; Najafpour-Darzi, G.; Sabour, D.; Almeida, J.M.S.; Brett, C.M.A. A catalase enzyme biosensor for hydrogen peroxide at a poly(safranine T)-ternary deep eutectic solvent and carbon nanotube modified electrode. Microchem. J. 2023, 195, 109475. [Google Scholar] [CrossRef]
- Prabowo, R.; Mariana, S. Measurement of malondialdehyde (MDA) level and superoxide dismutase (SOD) activity in the pancreatic tissue after the intervention of orange water kefir in the hyperlipidemic rats model. Int. J. Surg. 2022, 100, 106527. [Google Scholar] [CrossRef]
- Cao, L.; Zhao, J.; Ma, L.; Chen, J.; Xu, J.; Rahman, S.U.; Feng, S.; Li, Y.; Wu, J.; Wang, X. Lycopene Attenuates Zearalenone-Induced Oxidative Damage of Piglet Sertoli Cells Through the Nuclear Factor Erythroid-2 Related Factor 2 Signaling Pathway. Ecotoxicol. Environ. Saf. 2021, 225, 112737. [Google Scholar] [CrossRef]
- Ho, D.V.; Suryajaya, K.G.; Manh, K.; Duong, A.N.; Chan, J.Y. Characterization of NFE2L1-616, an Isoform of Nuclear Factor-Erythroid-2 Related Transcription Factor-1 That Activates Antioxidant Response Element-Regulated Genes. Sci. Rep. 2023, 13, 19900. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, L.; Bhat, O.M.; Lohner, H.; Li, P.-L. Differential Effects of Short Chain Fatty Acids on Endothelial Nlrp3 Inflammasome Activation and Neointima Formation: Antioxidant Action of Butyrate. Redox Biol. 2018, 16, 21–31. [Google Scholar] [CrossRef]
- Kang, K. Rhodotorula mucilaginosa ZTHY2 Attenuates Cyclophosphamide-Induced Immunosuppression in Mice. Animals 2023, 13, 3376. [Google Scholar] [CrossRef]
- Mu, X.Y.; Zhang, Y.Y.; Li, J.; Xia, J.Y.; Chen, X.B.; Jing, P.W.; Song, X.Y.; Wang, L.; Wang, Y.P. Angelica Sinensis Polysaccharide Prevents Hematopoietic Stem Cells Senescence in D-Galactose-Induced Aging Mouse Model. Stem Cells Int. 2017, 2017, 3508907. [Google Scholar] [CrossRef]
- Koizumi, H. Impact of the contraction of medial forearm muscles during grip tasks in different forearm positions on medial support at the elbow joint. J. Electromyogr. Kinesiol. 2023, 71, 102783. [Google Scholar] [CrossRef] [PubMed]
- Fan, S. Targeting the mTOR-mitochondrial function axis: Calcitriol attenuates sarcopenic obesity with lipid dysregulation etiology. Free Radic. Biol. Med. 2026, 242, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Altarejos, P.; Sanchez-Huertas, C.; Moreno-Manzano, V.; Felipo, V. OS150—Extracellular vesicles from mesenchimal stem cells reduce neuroinflammation in hippocampus and restore cognitive function in hyperammonemic rats. J. Hepatol. 2022, 77, S103–S104. [Google Scholar] [CrossRef]
- Lee, S.G. Impacts of Aging and Blackcurrant Supplementation on the Gut Microbiome Profile of Female Mice (P20-031-19). Curr. Dev. Nutr. 2019, 3, nzz040.P20-031-19. [Google Scholar] [CrossRef]
- Hor, Y.-Y. Lactobacillus sp. improved microbiota and metabolite profiles of aging rats. Pharmacol. Res. 2019, 146, 104312. [Google Scholar] [CrossRef]
- Conley, M.N. Aging and serum MCP-1 are associated with gut microbiome composition in a murine model. PeerJ 2016, 4, e1854. [Google Scholar] [CrossRef]
- Xiong, W. Probiotic Fermentation of Kelp Enzymatic Hydrolysate Promoted its Anti-Aging Activity in D-Galactose-Induced Aging Mice by Modulating Gut Microbiota. Mol. Nutr. Food Res. 2023, 67, 2200766. [Google Scholar] [CrossRef]
- Zheng, S. Helicobacter pylori-positive chronic atrophic gastritis and cellular senescence. Helicobacter 2023, 28, e12944. [Google Scholar] [CrossRef]
- Waragai, Y.; Kuroda, M.; Kodama, K.; Kanno, Y.; Miyata, M. Su1395 Effects of Helicobacter Pylori Eradication for Elderly Patients. Gastroenterology 2020, 158, S-575. [Google Scholar] [CrossRef]
- Wang, G.; Cao, Y.; Xu, C.; Zhang, S.; Huang, Y.; Zhang, S.; Bao, W. Comprehensive transcriptomic and metabolomic analysis of porcine intestinal epithelial cells after PDCoV infection. Front. Vet. Sci. 2024, 11, 1359547. [Google Scholar] [CrossRef]
- Frampton, J.; Murphy, K.G.; Frost, G. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef]
- Huang, Z.; Ma, Y.; Xie, Y.; Zhao, D.; Li, C. Carrageenan in meat: Improvement in lipid metabolism due to Sirtuin1-mediated fatty acid oxidation and inhibited lipid bioavailability. Food Funct. 2023, 14, 5404–5416. [Google Scholar] [CrossRef]
- Mu, Y.; Huang, J.; Zhou, R.; Zhang, S.; Qin, H.; Tang, H.; Pan, Q.; Tang, H. Bioaugmented Daqu-induced variation in community succession rate strengthens the interaction and metabolic function of microbiota during strong-flavor Baijiu fermentation. LWT-Food Sci. Technol. 2023, 182, 114806. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
An, F.; Feng, Y.; Li, D.; Hua, M.; Wang, X.; Xu, X.; He, Y.; Miao, X.; Sun, M.; Niu, H.; et al. Rhodotorula mucilaginosa JAASSRY Alleviated Oxidative Damage in D-Galactose-Induced Aging Mice by Modulating the Gut Microbiota. Fermentation 2026, 12, 24. https://doi.org/10.3390/fermentation12010024
An F, Feng Y, Li D, Hua M, Wang X, Xu X, He Y, Miao X, Sun M, Niu H, et al. Rhodotorula mucilaginosa JAASSRY Alleviated Oxidative Damage in D-Galactose-Induced Aging Mice by Modulating the Gut Microbiota. Fermentation. 2026; 12(1):24. https://doi.org/10.3390/fermentation12010024
Chicago/Turabian StyleAn, Fenghao, Yanchun Feng, Da Li, Mei Hua, Xiuquan Wang, Xifei Xu, Yuguang He, Xinyu Miao, Mubai Sun, Honghong Niu, and et al. 2026. "Rhodotorula mucilaginosa JAASSRY Alleviated Oxidative Damage in D-Galactose-Induced Aging Mice by Modulating the Gut Microbiota" Fermentation 12, no. 1: 24. https://doi.org/10.3390/fermentation12010024
APA StyleAn, F., Feng, Y., Li, D., Hua, M., Wang, X., Xu, X., He, Y., Miao, X., Sun, M., Niu, H., Xu, H., & Wang, J. (2026). Rhodotorula mucilaginosa JAASSRY Alleviated Oxidative Damage in D-Galactose-Induced Aging Mice by Modulating the Gut Microbiota. Fermentation, 12(1), 24. https://doi.org/10.3390/fermentation12010024





