Lactic Acid Bacteria-Derived Exopolysaccharides: Dual Roles as Functional Ingredients and Fermentation Agents in Food Applications
Abstract
1. Introduction
2. Exopolysaccharide-Producing Lactic Acid Bacteria
2.1. Genera and Species Involved in EPS Production
2.2. EPS Types and Biosynthesis
| EPS | Linkage | Monomer Units | Branching | Charge | Reference |
|---|---|---|---|---|---|
| Dextran | α(1→6); α(1→3) | Glucose | Branched | Neutral | [13] |
| Reuteran | α(1→6); α(1→4) | ||||
| Mutan | α(1→3) | Linear | |||
| Alternan | α(1→6); α(1→3) | ||||
| β-D-glucan | α(1→3) | ||||
| Levan | β(2→6); β(2→1) | Fructose | Branched | Neutral | |
| Kefiran | Glucose, galactose | Branched | Neutral | ||
| BC-25 EPS from Lactobacillus plantarum BC-25 | (1→2)-linked Man, (1→2,6)-linked Glc, (2→6)-linked Man, and (2→6)-linked Gal | Mannose, galactose, glucose | Branched | ND | [25] |
| LgEPS from Lactobacillus gasseri FR4 | 1,6 linked-α-D-Glcp; 1,4 linked-α-D-Galp, 1,3,4 linked-α-D-Manp, 1,3 linked-α-L-Rhap, 1,4 linked-α-L-Fucp, 1,4 linked-β-D-Glcp, and β-D-Galp-1 | Glucose, galactose, mannose, rhamnose, fucose | Branched | ND | [26] |
| R-17-EPS from Lactobacillus pentosus LZ-R-17 | →2)-α-D-Galp-(1→4)-β-D-Glcp-(1→4)-β-D-Glcp-(1→4)-β-D-Glcp-(1→ | Galactose, glucose | Linear | ND | [27] |
| LPE-1 EPS from L. plantarum AR307 | Backbone: 1,4-β-D-Glcp, 1,4-α-D-Glcp, and 1,4,6-β-D-Galp; branched 1,6-β-D-Galp | Glucose, galactose | Branched | ND | [28] |
| EPS-T1 from L. plantarum T1 | 1,4-linked Glcp and 1,6-linked Galp | Glucose, galactose | Branched | ND | [29] |
2.3. EPS Yield, Culture Conditions, and Composition
2.3.1. Effect of Carbon Sources
2.3.2. Stress Factors’ Impact
2.3.3. Co-Culture Effects on EPS Production
2.3.4. Temperature Effect on EPS Production
| Lactic Acid Bacteria | EPS Type | Culture Conditions | Yield | Reference |
|---|---|---|---|---|
| Lactobacillus rhamnosus RW-9595M | Hetero-EPS (glucose, galactose, and rhamnose) | Supplemented whey permeate (5% (w/w) whey permeate, MgSO4·7H2O, MnSO4·H2O, Tween 80, corn steep liquor, and yeast extract) at 37 °C for 7 h and 200 rpm | 2350 mg/L | [53] |
| Weissella cibaria WC4 and Lactobacillus plantarum PL9 | Glucan (homo EPS constituted of glucose) | MRS broth supplemented with 292 mM sucrose incubated at 30 °C for 1 day Wheat flour (312 g), water (137.5 mL), sucrose (50 g), and cellular suspension (50 mL) incubated at 30 °C for 24 h | 3.88 and 3.14 mg/mL, respectively, in MRS broth W. cibaria produces 2500 mg/kg glucan in dough | [54] |
| Streptococcus thermophilus NIZO0131, NIZO2104, and Lactobacillus delbrueckii subsp. bulgaricus ATCC 11842, NCIMB 702074, and DGCC 291 | Hetero-EPS NIZO0131: galactose/rhamnose NIZO2104: galactose/ribose/N-acetyl-galactosamine ATCC 11842: galactose/glucose NCIMB 702074: galactose/glucose DGCC 291: galactose/glucose | Reconstituted skim milk at 12% incubated at 37 °C for 16 h For S. thermophilus strains, a subcultivation at 42 °C for 6 h was applied | NIZO0131: 78 mg/L NIZO2104: 45 mg/L ATCC 11842: 60 mg/L NCIMB 702074: 36 mg/L DGCC 291: 38 mg/L | [55] |
| W. cibaria MG1 | Dextran (homo-EPS constituted of glucose) | MRS broth added with sucrose (10%), incubated at 30 °C for 72 h Wort (9% final extract content) added with 5 or 10% sucrose, incubated at 30 °C for 72 h | 36,400 mg powder/L in MRS+Suc, 8600 mg powder/L in wort + 5% Suc, and 14,400 mg powder/L in wort with 10% Suc | [56] |
| L. plantarum 162 R, Leuconostoc mesenteroides N6, and the mixture | Not reported | Sucuk sausage (beef meat, tail fat, salt, garlic, red pepper, powdered black pepper, cumin, allspice) incubated at 18 °C for 12 days | 9.79, 18.60, and 17.56 mg/kg dry matter for L. plantarum, Ln. mesenteroides, and the mixture, respectively. Sucuk fermented spontaneously did not detect EPS | [49] |
| Lactobacillus kefiranofaciens DN1 | Hetero-EPS DN1 (rhamnose, arabinose, galactose, glucose, and mannose) | MRS broth (20 g/L glucose) and supplemented with glucose (40, 60, or 80 g/L) | 1380 mg powder/L in MRS broth and 2260 mg/L for MRS + 60 g/L glucose | [57] |
| L. rhamnosus ZY | Hetero-EPS ZY (fructose, galactose, glucose, fucose, rhamnose, and mannose) | MRS broth supplemented with H2H2 or/and CaCl2 at 37 °C under anaerobic conditions | 342.8 mg powder/L in MRS broth at 24 h, 567 mg powder/L in MRS broth + 3 mM H2O2 after 24 h, 2203.5 mg powder/L in MRS broth + 10 mM CaCl2 after 12 h, and 2498.5 mg powder/L in MRS broth + 3 mM H2O2 + 10 mM CaCl2 and 12 h | [42] |
| L. plantarum BR2 | Hetero-EPS (glucose and mannose) | EPS production medium (yeast extract 4, lactose 4, Tween 80 0.1, sodium acetate 0.5, and ammonium sulfate 0.5 g/100 mL), incubate at 37 °C for 72 h | 2800 mg powder/L | [58] |
| L. plantarum NR 104573.1 and Pediococcus pentosaceus NR 042058.1 from wheat bran sourdough | ---- | MRS broth supplemented with 10% glucose at 37 °C for 24 h | 408 and 263 mg/L | [59] |
| Weissella confusa OF126 | Dextran (homo-EPS constituted of glucose) | MRS broth + 10 g/L sucrose incubated at 30 °C for 24 h and 170 rpm | 2000 mg/L | [40] |
| Leuconostoc citreum B-2 | Highly branched dextran (homo-EPS constituted of glucose) | MRS broth with 75 g/L sucrose incubated at 30 °C for 48 h and 80 rpm/min | 28,300 mg powder/L | [24] |
| Lactobacillus gasseri FR4 | Hetero LgEPS (glucose, mannose, galactose, rhamnose, and fucose) | MRS broth (glucose substituted by sucrose) added with 2% sucrose | 7200 mg powder/L | [26] |
| W. confusa PP29 | Dextran (homo-EPS constituted of glucose) | MRS I: MRS broth added with fructose (40 g/L) and glucose (40 g/L) MRS II: MRS broth plus 80 g/L sucrose MRS III: MRS broth and 80 g/L sucrose dissolved in UHT milk Culture media were incubated at 33 °C for 48 h under agitation at 100 rpm | 2800 mg powder/L MRS I, 5180 mg powder/L MRS II, and 17,400 mg powder/L MRS III | [60] |
| W. cibaria SJ14 | Hetero-EPS (mannose, glucose, galactose, arabinose, xylose, and rhamnose) | Semi-defined medium (MRS modified) incubated at 30 °C for 34 h | 331.47 mg/L | [61] |
| Lactobacillus sanfranciscensis Ls-1001 | Glucan (homo EPS constituted of glucose) | MRS broth, carbon source replaced by maltose, incubated at 30 °C for 24 h | 190.3 mg/L | [39] |
| Fructilactobacillus sanfranciscensis Ls5 | Hetero-EPS (glucose and mannose) | MRS broth, carbon source replaced by maltose, incubated at 30 °C for 24 h | 202.3 mg/L | [62] |
| L. rhamnosus EM1107, Lactobacillus mucosae CNPC007, L. plantarum CNPC003 | For L. plantarum: Hetero-EPS (mannose, glucose, and galactose), the composition was similar despite the carbon source | MRS broth or MRS broth carbon source replaced by fructooligosaccharide (FOS, Orafti®), incubated at 37 °C for 24 h | In MRS broth, EPS production was 167.6, 153.2, and 378 mg/L, respectively. In MRS containing FOS, EPS was 356.8, 345.7, and 568.4 mg/L, respectively | [63] |
| Leuconostoc pseudomesenteroides JF17 | Dextran (homo EPS constituted of glucose) | MRS broth added with 18% sucrose, pH 7.3 at 20 °C for 48 h | 53,770 mg/L | [64] |
| Ln. pseudomesenteroides DSM 20193 and W. confusa Ck15 | Dextran (homo EPS constituted of glucose) | Chickpea flour (28 g), sucrose (2 g), water (70 mL), incubated at 30 °C for 24 h | 1.18% and 1.49%, respectively | [65] |
| W. confusa C19 | Dextran | MRS agar and cereal (rice, oat, wheat, and maize) extract (ratio cereal and water 1:10) in proportion 1:1 was added with sucrose (5%), incubated at 37 °C for 3 days | 21,900 mg/L, 20,900 mg/L, 19,100 mg/L, 18,500 mg/L for rice, wheat, maize, and oat medium, respectively | [66] |
| Lactobacillus reuteri E81 | Glucan (homo EPS constituted of glucose) | Wheat dough yield of 200 added with 15% sucrose, incubated for 24 h | 15,200 mg/kgdry sourdough | [67] |
| Lactobacillus paracasei H9 | Hetero-EPS (mannose, glucose, galactose) | Milk incubated for 8 h, at 37 °C, and inoculum size 14% | 932 mg/L | [48] |
| Lactobacillus fermentum S1 | Hetero-EPS S1 (glucose, galactose, mannose, arabinose) | Liquid medium (glucose (20 g), ammonium citrate (5 g), soya peptone (10 g), yeast extract (6 g), MnSO4 (0.05 g), FeSO4 (0.04 g), MgSO4 (0.2 g), and Tween 80 (1 mL)) incubated at 33 °C for 24 h | 668 mg/L | [68] |
| Lactobacillus pentosus LZ-R-17 | Hetero-R-17-EPS (galactose and glucose) | Milk incubated at 37 °C for 24 h | 185.2 mg/L | [27] |
| P. acidilactici M76 | ---- | Black raspberry beverage (30 °Brix) incubated at 25–30 °C for 3–15 days | 1620 mg/L (at 25 °C and 3 days) | [47] |
| W. confusa QS813 | Dextran (homo EPS constituted of glucose) | Red bean dough yield of 250 added with 10% sucrose, incubated at 30 °C for 24 h | 18,680 mg/kgsourdough | [69] |
| W. confusa XG-3 | Dextran (homo EPS constituted of glucose) | Optimized medium (sucrose 80.1 g/L, beef extract 8 g/L, casein peptone 5 g/L, yeast extract 10 g/L, sodium acetate 3.7 g/L, ammonium citrate 3 g/L, K2HPO4 4 g/L, and Tween 80 2 mL/L adjusted to pH 5.8), incubated at 30 °C for 72 h and 120 rpm | 97,500 mg powder/L | [52] |
| Lactobacillus curvatus SJTUF 62116 | Hetero-EPS 1 (glucose and mannose) | MRS broth cultivated at 30 °C for 24 h | 283.5 mg powder/L, | [70] |
| Lactiplantibacillus plantarum T1, CL80, CSK, S-1A | Hetero-EPS T1, -EPS CL80, -EPS CSK, and EPS S-1A (mannose, rhamnose, glucose, and galactose) | Inoculate in milk at 108 CFU/mL and incubated at 37 °C | 385 mg powder/L, 336 mg powder/L, 157 mg powder/L, and 98 mg powder/L | [71] |
| Lpb. plantarum T1 | Hetero-EPS T1 (glucose and galactose) | MRS broth at 37 °C for 30 h | 249 mg powder/L | [29] |
| Enterococcus sp. BE11 | Hetero-EPS BE11 (L-rhamnopyranose, D-arabinose, D-galactopyranose, D-glucuronic acid, D-glucopyranose) | MRS broth supplemented with 1% sucrose at 37 °C for 48 h | 173 mg powder/L | [72] |
| Lactococcus lactis subsp. diacetylactis RBL 37 | ---- | Modified MRS broth replaced carbon source with 20% sucrose, the cells were grown until DO600 0.5–2.0 | 274.3 mg/L | [73] |
| Lactobacillus acidophilus LAC-1 | ---- | Whey and whey supplemented with 2% lactose incubated for 48–72 h under anaerobic conditions | 2172 and 2168 mgdry EPS/L | [38] |
| Lpb. plantarum ITD-ZM-101 and Lc. lactis ITD-ZM-106 | ---- | Brain heart infusion broth containing 15 g/L of dried agave bagasse or agave leaves, incubated at 37 °C, 120 rpm for 120 h | 147.2 and 130 mg/L for agave leaves | [74] |
3. Techno-Functional Properties of LAB Exopolysaccharides in Food Systems
| Lactic Acid Bacteria | EPS Type | Food Product | Techno-Functional Properties | Quality Improvement | Reference |
|---|---|---|---|---|---|
| Lactobacillus sanfranciscensis TMW 1.392 | Levan (homo-EPS) | Dough/bread | Water absorption, bread volume, and crumb firmness | Bread texture and quality | [75] |
| Streptococcus thermophilus NIZO2104 and Lactobacillus delbrueckii subsp. bulgaricus DGCC 291 | Hetero-EPS NIZO2104: galactose/ribose/N-acetyl-galactosamine DGCC 291: galactose/glucose | Fermented milk | Gel formation, viscosity, and water-holding capacity | Firmness, apparent viscosity, and reduced syneresis | [55] |
| Lactobacillus curvatus TMW 1.624 | Dextran (homo-EPS) | Gluten-free bread | Increase viscosity, gas retention, and water-holding capacity | Bread texture and volume Shelf life by retarding bread staling | [76] |
| Weissella cibaria MG1 | Dextran (homo-EPS) | Malt fermented beverage | Viscosity improver | Beverage stability Desirable body | [56] |
| Lactobacillus plantarum 162 R, Leuconostoc mesenteroides N6, and the mixture. | Not reported | Sucuk (Turkish-type fermented sausage) | Gel formation and retention water | Texture | [49] |
| Leuconostoc citreum B-2 Leuconostoc pseudomesenteriodes JF17 | Highly branched dextran (homo-EPS) Dextran (homo-EPS) | ---- | Water-holding capacity | Binding and stabilizing agent of water | [24,64] |
| Lactobacillus reuteri E81 | Glucan (homo EPS constituted of glucose) | Dough/wheat bread | Dough viscoelasticity and retention water | Hardness of fresh bread | [67] |
| Leuconostoc lactis L2 | EPS-L2 (homo EPS constituted of glucose) | Fermented milk | Gel formation, viscosity | Gel stability, texture | [41] |
| Weissella confusa QS813 | Dextran (homo EPS constituted of glucose) | Red bean sourdough and gluten-red bean dough | Water binding capacity and reduced water distribution Cryoprotective on gluten protein matrix | Quality of frozen gluten-red bean dough during freeze–thaw cycles | [69] |
| Lactiplantibacillus plantarum T1 and CL80 | Hetero-EPS T1 and hetero-EPS S-1A | Fermented milk | Viscosity enhancer Water-holding capacity | Gel stability Texture (less hardness and increasing cohesiveness and gumminess) | [71] |
| Lpb. plantarum CSK | Hetero-EPS CSK (glucose and galactose) | Soymilk fermented | Gel formation, viscosity, water-holding capacity | Gel stability, texture, shelf life, and reducing syneresis | [18] |
4. Health-Promoting Potential of LAB Exopolysaccharides
| Intervention Type and Time | Lactic Acid Bacteria or Food Intake | Primary Effect of EPS | Main Findings | Reference |
|---|---|---|---|---|
| Randomized controlled study. Simultaneous comparative study in men (>40 from Funagata or >60 years from Arita) for 8 weeks for Funagata and 12 weeks for Arita. The effect on immune system parameters in the elderly and preventive effects against respiratory tract infections (common cold and influenza virus) were investigated | Lactobacillus delbrueckii subsp. bulgaricus OLL1073R-1 produced immunostimulatory EPS and Streptococcus thermophilus OLS3059 Yogurt containing (36.5–68 mg/kg EPS) or milk group 90 g yogurt or 100 mL milk per day | Immunomodulatory activity | The risk of catching the common cold or influenza virus infection was lower in yogurt groups from both places. Lymphocyte blastoid transformation induced by Con A increased in yogurt group from Funagata. Natural killer activity in the low-activity subjects improved to normal values in subjects’ intake yogurt from both places. Thus, yogurt reduced the risk of respiratory infections. The score for eye/nose/throat was higher for yogurt group. Also, yogurt improved the quality-of-life score of the elderly. | [82] |
| Randomized control pre-test–post-test design on diabetes mellitus (DM) outpatients in various hospitals to investigate the biomolecular nature of the glycemic status of Type 2 DM 30 days | Clear kefir and control group 200 mL/day | Antioxidant activity | HbA1c was significantly reduced in delta level, and insulin was reduced in the groups that consumed clear kefir. | [83] |
| Randomized, double-blind, controlled trial in men for 12 weeks to investigate the effect of summer heat fatigue | L. bulgaricus OLL1073R-1 produced immunostimulatory EPS and S. thermophilus OLS3059 Yogurt containing (2.9 mg/100 mL EPS) or placebo (acidified yogurt) 100 mL per day | Antioxidant activity and radical-scavenging activity | The visual analogue scale (VAS) scores for “general malaise”, “feeling languid”, “fatigue”, and “psychological stress” were significantly lower after 12 weeks in the study group. The blood pressure was reduced in the yogurt + EPS group after 4 weeks. The autonomic nervous system balance was better maintained in yogurt + EPS group, relieving the physical and mental disorders induced by seasonal changes. | [84] |
| Randomized controlled open-label study in women healthcare workers for 16 weeks The effects of influenza infection during winter were studied | L. bulgaricus OLL1073R-1 produced immunostimulatory EPS and S. thermophilus 112 mL of yogurt per day | Immunomodulatory activity | The production of IFN-γ (immunobiological market) increased in the intervention group. The influenza A or common cold cumulative incidence rate was similar in both groups. | [81] |
| Randomized, double-blind, parallel, and placebo-controlled in the elderly with limited activity and a mostly sedentary life, resident in nursing homes 12 weeks of intervention to evaluate the saliva flow rate, the total amount of salivary IgA, and the amount of the influenza virus-bound salivary IgA | L. bulgaricus OLL1073R-1 produced immunostimulatory EPS Yogurt with EPS and yogurt fermented with L. bulgaricus OLL1256 (placebo) 100 g yogurt containing EPS or placebo yogurt daily | Immunomodulatory activity | Influenza virus A subtype H3N2-bound IgA in saliva was higher in yogurt EPS compared with placebo. | [85] |
| Randomized crossover method Adult males participated in the study to evaluate the carotene absorption during co-ingestion with yogurt 2 weeks | L. bulgaricus OLL1256 and S. thermophilus OLS3059 EPS produced strains 100 g yogurt containing (90 μg/g) + 100 g carrot juice concentrate/tomato paste/spinach paste or 100 g water + 100 g carrot juice concentrate/tomato paste/spinach paste | Diffusion mechanisms (emulsifier, dispersion stabilizer, and prolong carotenoid contact with adsorbing membranes) | Higher β-carotene, α-carotene, lycopene and incremental area under the concentration-time curve for β-carotene, α-carotene, and retinyl palmitate, and lycopene concentration for the plasma triacylglycerol-rich lipoprotein fraction at 4, 6, and 8 h were recorded when pastes were intake with yogurt. Lutein concentration increased in total plasma after 2, 4, 6, and 8 h when spinach paste was consumed with yogurt. Thus, yogurt enhanced the bioavailability and absorption of dietary carotenoids in humans. | [86] |
| Randomized controlled study in women healthcare workers for 16 weeks The impact on psychological quality was assessed | L. bulgaricus OLL1073R-1 produced immunostimulatory EPS and S. thermophilus 112 mL of yogurt per day | Antioxidant activity | The scores of Pittsburgh Sleep Quality Index, the General Health and Vitality from the eight-item Short Form Health Survey (subjective quality of life), and the constipation of the Gastrointestinal Symptom Rating Scale were improved after 16 weeks of yogurt intake. | [87] |
| Randomized, double-blind, placebo-controlled study in adults with perennial allergy symptoms to study allergic conditions 12 weeks | Capsules containing 260 mg of dried pineapple juice fermented with Lactobacillus paracasei IJH-SONE68 heat treated, and dextrin or capsules of dextrin as a placebo Four capsules per day | Anti-inflammatory | Head dullness, watery eyes, frequency of nose-blowing, and sneezing symptoms decreased in the study group. Also, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and cholinesterase (serum liver function indices) in serum decline in the study group. | [88] |
| Randomized, double-blind, placebo-controlled trial in overweight adults to investigate the effect on obesity indices, anti-inflammatory, and other obesity-related factors 12 weeks | Capsules containing 260 mg of dried pineapple juice fermented with L. paracasei IJH-SONE68 heat treated, and dextrin or capsules of dextrin as placebo Four capsules per day | Anti-inflammatory and hypotriglyceridemia | Serum triglyceride and serum liver function indices (aspartate aminotransferase and alanine aminotransferase) levels reduced in the study group. Anaerostipes genus increased while Veillonella decreased from human microbiota in the study group. | [89] |
| Trial 1: Randomized, double-blind, placebo-controlled, vaccinated male university students Trial 2: Randomized, double-blind, placebo-controlled vaccinated healthy 25- to 59-year-old adults | L. bulgaricus OLL1073R-1 produced immunostimulatory EPS and S. thermophilus OLS3059 Yogurt containing (3.3 mg EPS) or placebo (acidified yogurt) 112 mL per day | Immunomodulatory activity (act as a B-cell mitogen) | The daily intake of yogurt + EPS augmented the serum antibody titers against the seasonal influenza vaccine. Trial 1: The geometric mean titer (GMT) of the H3N2 and B viruses were significantly higher in the yogurt group fulfilled the EMA criteria of seroprotection, improving the vaccine immunogenicity leading to enhance protection against influenza infection. Trial 2: The GMT of the H1N1 and B viruses was significantly higher in yogurt group, indicating yogurt intake improved the vaccine immunogenicity via serum antibodies production. | [90] |
| Randomized, double-blind, placebo-controlled human study for 4 weeks to evaluate the effect on functional constipation | Weissella confusa VP30 Pasteurized fermented milk containing 3.52 EPS g/L (control) or 39.2 g/L EPS 200 mL of fermented milk was intake daily | Dietary fiber | Defecation frequency and fecal volume increased while stool hardness and the score sum of symptoms (difficulty, flatulence, pain, bloating, severity) reduced when fermented milk with EPS was consumed for 4 weeks. Regarding laboratory analysis, fecal water content increased in fermented milk + EPS. Weight loss or reduction was also observed in fermented milk + EPS. | [91] |
5. Food Applications of LAB Exopolysaccharides
5.1. Direct Addition of Purified EPS to Food Products
| Application Area | Function of EPS | Food Examples | Relevant EPS Types/Sources | Reference |
|---|---|---|---|---|
| Baked Goods (wheat-based) | Improve dough rheology, moisture retention, and softness | Bread, rolls, cakes | Dextran (Weissella cibaria), kefiran | [94,95,96] |
| Gluten-Free Products | Mimic gluten structure, enhance volume, delay staling | Gluten-free bread, muffins, pizza bases | Dextran, β-glucans, heteropolysaccharides | [14,99] |
| Fermented Beverages | Improve mouthfeel, suspension, viscosity | Soy yogurt, oat drinks, kefir | Kefiran, dextran | [34,78] |
| Dairy Products | Enhance creaminess, reduce syneresis, stabilize emulsions | Yogurt, cream cheese, dairy emulsions | EPS from Leuconostoc pseudomesenteroides, Leuconostoc mesenteroides F27, Streptococcus thermophilus, Lactobacillus delbrueckii subsp. bulgaricus, Weissella confusa | [13,99,100] |
| Emulsified Foods | Stabilize oil droplets, reduce interfacial tension | Salad dressings, emulsifier for low-fat mayonnaise | Heteropolysaccharides from L. plantarum, Leuconostoc lactis GW-6 | [101,102,103] |
| Frozen Desserts | Inhibit ice recrystallization, improve texture | Low-fat ice cream, frozen yogurt | Dextran, kefiran, EPS from Leuconostoc citreum-BMS | [79,99,104] |
| Edible Films and Coatings | Moisture/O2 barrier, antimicrobial or antioxidant carrier | Fresh produce, cheese slices, minimally processed foods | Kefiran, dextran, composite blends with proteins/lipids | [105,106,107,108] |
5.1.1. Milk Products
5.1.2. Bakery and Gluten-Free Products
5.1.3. Beverages and Emulsions
5.1.4. Films and Coatings
5.2. In Situ Production of EPS in Fermented Foods
5.2.1. Dairy Products
5.2.2. Meat Products
5.2.3. Bakery Products
5.2.4. Beverage Products
| Type of Food | LAB Strain | Improvements in the Product Due to EPS | Reference |
|---|---|---|---|
| Dairy products | |||
| Reduced-fat yogurts | Levilactobacillus brevis UCLM-Lb47, Leuconostoc mesenteroides subsp. mesenteroides 6F6-12 and Ln. mesenteroides subsp. mesenteroides 2F6-9 | Increased water-holding capacity. Higher EPS levels. Greater mouthfeel viscosity. A possible alternative to the use of hydrocolloids or gums in reduced-fat yogurts. | [115] |
| Fermented milk (yogurt type) | Lactobacillus helveticus LH18 | Increases the product’s consistency. Enhances water-holding capacity. Reduces syneresis. Improves the overall texture of the product. | [7] |
| Fermented milk (yogurt type) | Streptococcus thermophilus (capsular exopolysaccharide producer) and Lactococcus lactis (non-capsular exopolysaccharide producer) | The combination of both LAB strains improves the protein network structure, resulting in smaller pores, reduced syneresis, and enhanced gel stability. | [116] |
| Dairy model system simulating yogurt conditions | Lactobacillus delbrueckii subsp. bulgaricus 210R and S. thermophilus NIZO 2104 S. thermophilus HC15 and L. bulgaricus DGCC 291 | Linear, stiff, and negatively charged EPS likely enhanced gel stiffness (elastic modulus) through electrostatic interactions with caseins and contributed to increased viscosity. Neutral and stiff EPS increased viscosity by promoting water retention and increasing the bulk volume; however, their effect on gel stiffness was likely limited due to thermodynamic incompatibility. | [117] |
| Requeson-Type Cheese | L. bulgaricus NCFB 2772 and S. thermophilus SY-102 | The co-culture exhibited the highest EPS production compared to the monocultures. Enhanced water retention in the co-culture cheese, resulting in increased cohesiveness and reduced hardness of the product. Fermentation with LAB and the production of EPS nearly doubled the cheese yield. | [118] |
| Cream cheese | Lc. lactis LL-1 (ropy EPS producer) Lc. lactis LL-2A (capsular EPS producer) Lc. lactis LL-2 (non-ropy EPS producer) | The presence of ropy EPS resulted in higher yield stress and creaminess. Capsular EPS presumably leads to higher serum retention of cheese after curd homogenization at 0.05 and 15 MPa. Higher firmness and serum retention after curd homogenization at higher pressure. Suggested conditions to obtain a cream cheese with greater creaminess, firmness, and whey retention were 0.05/15 MPa for ropy and capsular EPS; 15/30 MPa for non-ropy EPS. | [119] |
| Low-fat Cheddar cheese | Lactobacillus plantarum JLK0142 | Enhancement of the ripening properties. Improved moisture retention. Improved textural and sensory properties (appearance, flavor, and overall acceptance). | [120] |
| Meat products | |||
| Cooked ham model systems | Homopolysaccharides producer (Lactobacillus curvatus TMW 1.624 and Lactobacillus sakei TMW 1.411) Heteropolysaccharides producer (L. plantarum TMW 1.1478 and TMW 1.25) | Homo-EPS-producing strains L. sakei 1.411 and L. curvatus 1.624, along with hetero-PS-producing strains L. plantarum 1.1478 and 1.25, were able to synthesize EPS not only at 15 °C but also at 2 °C within the initial 10–24 h of storage, a feature crucial for the tumbling stage in cooked ham manufacturing. Enhanced water retention in the ham. The study did not include an in-depth evaluation of the cooked ham’s quality or sensory attributes. | [50] |
| Fermented sausages (salami) | L. plantarum TMW 1.1478 | The EPS were predominantly formed during the first 72 h of fermentation at 24 °C. The sausage fermented with the EPS-producing LAB exhibited a softer texture, which is atypical for this type of product. No alterations or adverse effects were detected in the flavor of the final product. Therefore, the application of this LAB strain could represent a potential alternative for the development of spreadable fermented meat products. | [121] |
| Fat-reduced raw fermented sausages (Teewurst) | Homopolysaccharides producer (L. curvatus TMW 1.1928 and L. sakei TMW 1.411) Heteropolysaccharides producer (L. plantarum TMW 1.1478) | The homopolysaccharide-producing strains reduced the hardness of the fat-reduced sausages. Homopolysaccharides LAB were rated softer and more spreadable than the corresponding control samples. The presence of EPS from LAB did not negatively influence the taste of the products. | [122] |
| Bakery products | |||
| Sourdough bread | Weissella cibaria FAFU821 | Enhanced viscoelasticity of sourdough. Improved bread moisture retention by increasing the water-holding capacity. Reduces the hardness of bread. Increased the volatile profile of bread, including linoleic acid ethyl ester and acetic acid. | [94] |
| Sourdough bread | Lactobacillus reuteri E81 | In situ α-glucan production enhanced dough elasticity. No significant changes were observed in the bread’s textural characteristics. | [67] |
| Sorghum sourdough bread (gluten-free) | Dextran-forming W. cibaria MG1 Reuteran producing L. reuteri VIP Fructan-forming L. reuteri Y2 | The three types of EPS generated during sourdough fermentation contributed to a softer crumb in both fresh and stored sorghum bread. Dextran demonstrated the most significant effect on extending shelf life, reducing firmness in bread. All three strains synthesized oligosaccharides during sorghum sourdough fermentation, enhancing the nutritional value of gluten-free sorghum bread. | [127] |
| Chickpea sourdough | Weissella confusa Ck15 | The production of EPS increased the dough viscosity. The researchers did not perform texture analysis on the bread. | [65] |
| Buckwheat bread | W. cibaria NC516.11 | Improve the rheological properties and viscoelastic properties of sourdough. W. cibaria NC516.11 significantly improved the texture of the bread and reduced the hardness and moisture loss during storage. | [126] |
| Chinese steamed bread | W. confusa QS813 + sucrose addition. | The overall quality of the bread improved with the addition of the LAB strain and sucrose. The presence of EPS positively influenced dough behavior and bread quality. | [124] |
| Beverage products | |||
| Coconut water-based beverage | L. plantarum SVP2. | A non-dairy functional beverage enriched with exopolysaccharides and exhibiting probiotic benefits was successfully developed. During 7 days of refrigerated storage, the EPS content, pH, and bacterial viability remained nearly unchanged. The beverage exhibited moderate acceptance, with texture and flavor (sweet–sour) receiving favorable evaluations. | [128] |
| Fermented soymilk | W. confusa wild-type or sac mutant | Fermentation of soymilk with either the W. confusa wild-type or its sac mutant resulted in notable improvements in water-holding capacity and viscosity, indicating their potential. The production of EPS in fermented plant-based alternatives represents a promising strategy for achieving textural properties comparable to those of conventional dairy products. | [129] |
6. Challenges and Limitations in the Application of LAB Exopolysaccharides
6.1. Low and Variable Yields
6.2. Structural Heterogeneity and Lack of Standardization
6.3. Extraction and Purification Challenges
6.4. Functional Variability in Food Systems
6.5. Regulatory Status, Labeling Barrier, and Limited Clinical Evidence for Health Claims
6.6. Knowledge Gaps and Research Needs
| Knowledge Gap | Description | Impact on EPS Application | Proposed Research/Technological Approaches | References |
|---|---|---|---|---|
| Structure–function relationships | Limited understanding of how the EPS molecular structure affects techno-functional and bioactive properties | Inconsistent functionality in foods and limited health applications | Integrate glycomics with functional assays; develop predictive models | [1,138,165] |
| Low and variable EPS yields | EPS production is strain- and condition-dependent; yields are often too low for industrial scale | Limits commercial feasibility and product consistency | Metabolic engineering, precision fermentation, adaptive evolution | [19,166] |
| Matrix interactions | Poor understanding of EPS behavior in complex food matrices, especially non-dairy | Reduced efficacy and unpredictable sensory effects | Systematic studies in diverse food matrices; multi-omics integration | [43,167] |
| Limited clinical evidence | Few human trials validating the health benefits of specific EPS | Restricts regulatory approval and consumer trust | Biomarker development | [1] |
| Regulatory fragmentation | Disparate regulations hinder global commercialization | Delays market entry and innovation | Harmonize standards and definitions; foster collaboration among agencies | [1,159,163] |
7. Future Perspectives
7.1. Precision Fermentation and Strain Engineering
| Strategy/Technology | Key Features | Advantages | Challenges | References |
|---|---|---|---|---|
| Metabolic engineering | Genetic modification of EPS biosynthetic pathways (e.g., glycosyltransferases) | Improved yield; customized EPS structure | Regulatory hurdles; strain stability | [19,166] |
| Adaptive laboratory evolution | Non-GMO selection under stress to improve traits | Regulatory-friendly, natural adaptations | Slow process; unpredictable results | [1] |
| CRISPR-Cas genome editing | Precise gene knock-ins/knockouts without off-targets | Targeted control, high specificity | Consumer perception; GMO classification issues | [171,172,173] |
| Synthetic biology and modular design | Integration of novel or heterologous EPS gene clusters | Novel EPS production; programmable structures | Requires deep pathway knowledge | [166,171] |
| Precision fermentation platforms | Real-time control of culture conditions and feeding strategies | Scalable, consistent production; structural tuning | High capital and technical complexity | [34,111,155,174,175] |
7.2. Multi-Omics for Structure–Function Insights
7.3. Functional EPS in Symbiotic and Personalized Nutrition
7.4. Regulatory Harmonization and Clean-Label Strategies
8. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, A.; Prajapat, J.B. Food and Health Applications of Exopolysaccharides Produced by Lactic Acid Bacteria. Adv. Dairy Res. 2013, 1, 1000107. [Google Scholar] [CrossRef]
- Korcz, E.; Varga, L. Exopolysaccharides from Lactic Acid Bacteria: Techno-Functional Application in the Food Industry. Trends Food Sci. Technol. 2021, 110, 375–384. [Google Scholar] [CrossRef]
- Kaur, N.; Dey, P. Bacterial Exopolysaccharides as Emerging Bioactive Macromolecules: From Fundamentals to Applications. Res. Microbiol. 2023, 174, 104024. [Google Scholar] [CrossRef]
- Nguyen, P.-T.; Nguyen, T.-T.; Bui, D.-C.; Hong, P.-T.; Hoang, Q.-K.; Nguyen, H.-T. Exopolysaccharide Production by Lactic Acid Bacteria: The Manipulation of Environmental Stresses for Industrial Applications. AIMS Microbiol. 2020, 6, 451–469. [Google Scholar] [CrossRef] [PubMed]
- Salimi, F.; Farrokh, P. Recent Advances in the Biological Activities of Microbial Exopolysaccharides. World J. Microbiol. Biotechnol. 2023, 39, 213. [Google Scholar] [CrossRef]
- Karabekmez Erdem, T.; Dilşad Tatar, H.; Ayman, S.; Gezginç, Y. Exopolysaccharides from Lactic Acid Bacteria: A Review on Functions, Biosynthesis and Applications in Food Industry. Turk. J. Agric.-Food Sci. Technol. 2023, 11, 414–423. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, S.; Tang, H.; Evivie, S.E.; Guo, Z.; Li, B. Effect of Exopolysaccharides Yield and Addition Concentration of Lactobacillus Helveticus on the Processing Characteristics of Fermented Milk and Its Mechanism. Int. J. Biol. Macromol. 2024, 260, 129480. [Google Scholar] [CrossRef]
- Prete, R.; Alam, M.K.; Perpetuini, G.; Perla, C.; Pittia, P.; Corsetti, A. Lactic Acid Bacteria Exopolysaccharides Producers: A Sustainable Tool for Functional Foods. Foods 2021, 10, 1653. [Google Scholar] [CrossRef]
- Sørensen, H.M.; Rochfort, K.D.; Maye, S.; MacLeod, G.; Brabazon, D.; Loscher, C.; Freeland, B. Exopolysaccharides of Lactic Acid Bacteria: Production, Purification and Health Benefits towards Functional Food. Nutrients 2022, 14, 2938. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.; Lopes Neto, J.H.P.; Cardarelli, H.R. Exopolysaccharides Produced by Lactobacillus plantarum: Technological Properties, Biological Activity, and Potential Application in the Food Industry. Ann. Microbiol. 2019, 69, 321–328. [Google Scholar] [CrossRef]
- Bibi, A.; Xiong, Y.; Rajoka, M.S.R.; Mehwish, H.M.; Radicetti, E.; Umair, M.; Shoukat, M.; Khan, M.K.I.; Aadil, R.M. Recent Advances in the Production of Exopolysaccharide (EPS) from Lactobacillus spp. and Its Application in the Food Industry: A Review. Sustainability 2021, 13, 12429. [Google Scholar] [CrossRef]
- Pourjafar, H.; Ansari, F.; Sadeghi, A.; Samakkhah, S.A.; Jafari, S.M. Functional and Health-Promoting Properties of Probiotics’ Exopolysaccharides; Isolation, Characterization, and Applications in the Food Industry. Crit. Rev. Food Sci. Nutr. 2023, 63, 8194–8225. [Google Scholar] [CrossRef]
- Nguyen, H.-T.; Pham, T.-T.; Nguyen, P.-T.; Le-Buanec, H.; Rabetafika, H.N.; Razafindralambo, H.L. Advances in Microbial Exopolysaccharides: Present and Future Applications. Biomolecules 2024, 14, 1162. [Google Scholar] [CrossRef]
- Liang, S.; Wang, X.; Li, C.; Liu, L. Biological Activity of Lactic Acid Bacteria Exopolysaccharides and Their Applications in the Food and Pharmaceutical Industries. Foods 2024, 13, 1621. [Google Scholar] [CrossRef]
- Weber, G.; Broich, W. Shelf Life Extension of Cultured Dairy Foods. Cult. Dairy Prod. J. 1986, 21, 19–21. [Google Scholar]
- Mani-López, E.; Arrioja-Bretón, D.; López-Malo, A. The Impacts of Antimicrobial and Antifungal Activity of Cell-free Supernatants from Lactic Acid Bacteria in Vitro and Foods. Compr. Rev. Food Sci. Food Saf. 2022, 21, 604–641. [Google Scholar] [CrossRef]
- Wingender, J.; Neu, T.R.; Flemming, H.-C. (Eds.) Microbial Extracellular Polymeric Substances; Springer: Berlin/Heidelberg, Germany, 1999; ISBN 978-3-642-64277-7. [Google Scholar]
- Liu, S.; Wang, Y.; Lin, T.; Zhang, X.; Lin, Y.; Xu, C.; Ma, K.; Zhang, C.; Geng, S.; Mahsa, G.C.; et al. Investigating the Role of the Exopolysaccharide Gene Cluster in Lactiplantibacillus plantarum CSK: Phenotypic Modulation and Its Effect on the Rheological Properties of Fermented Soymilk Gel. ACS Food Sci. Technol. 2025, 5, 2718–2730. [Google Scholar] [CrossRef]
- Zeidan, A.A.; Poulsen, V.K.; Janzen, T.; Buldo, P.; Derkx, P.M.F.; Øregaard, G.; Neves, A.R. Polysaccharide Production by Lactic Acid Bacteria: From Genes to Industrial Applications. FEMS Microbiol. Rev. 2017, 41, S168–S200. [Google Scholar] [CrossRef] [PubMed]
- Berthold-Pluta, A.M.; St.Pluta, A.; Garbowska, M.; Stasiak-Różańska, L. Exopolysaccharide-Producing Lactic Acid Bacteria–Health-Promoting Properties And Application In The Dairy Industry. Postępy Mikrobiol.-Adv. Microbiol. 2019, 58, 191–204. [Google Scholar] [CrossRef]
- Ishtiaq, N.; Ahmed, S. Probiotic Potential and Antimicrobial Efficacy of Exopolysaccharide-Producing Lactic Acid Bacteria Isolated from Yoghurt. Explor. Foods Foodomics 2025, 3, 101075. [Google Scholar] [CrossRef]
- Torres-Rodríguez, I.; Rodríguez-Alegría, M.E.; Miranda-Molina, A.; Giles-Gómez, M.; Conca Morales, R.; López-Munguía, A.; Bolívar, F.; Escalante, A. Screening and Characterization of Extracellular Polysaccharides Produced by Leuconostoc Kimchii Isolated from Traditional Fermented Pulque Beverage. SpringerPlus 2014, 3, 583. [Google Scholar] [CrossRef]
- Mårtensson, O.; Dueñas-Chasco, M.; Irastorza, A.; Öste, R.; Holst, O. Comparison of Growth Characteristics and Exopolysaccharide Formation of Two Lactic Acid Bacteria Strains, Pediococcus Damnosus 2.6 and Lactobacillus brevis G-77, in an Oat-Based, Nondairy Medium. LWT-Food Sci. Technol. 2003, 36, 353–357. [Google Scholar] [CrossRef]
- Feng, F.; Zhou, Q.; Yang, Y.; Zhao, F.; Du, R.; Han, Y.; Xiao, H.; Zhou, Z. Characterization of Highly Branched Dextran Produced by Leuconostoc Citreum B-2 from Pineapple Fermented Product. Int. J. Biol. Macromol. 2018, 113, 45–50. [Google Scholar] [CrossRef]
- Zhou, K.; Zeng, Y.; Yang, M.; Chen, S.; He, L.; Ao, X.; Zou, L.; Liu, S. Production, Purification and Structural Study of an Exopolysaccharide from Lactobacillus plantarum BC-25. Carbohydr. Polym. 2016, 144, 205–214. [Google Scholar] [CrossRef]
- Rani, R.P.; Anandharaj, M.; David Ravindran, A. Characterization of a Novel Exopolysaccharide Produced by Lactobacillus gasseri FR4 and Demonstration of Its in Vitro Biological Properties. Int. J. Biol. Macromol. 2018, 109, 772–783. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Yang, L.; Zhao, X.; Ma, K.; Chen, X.; Zhang, C.; Wang, G.; Dong, M.; Rui, X.; Zhang, Q.; et al. Isolation, Purification, Characterization and Immunostimulatory Activity of an Exopolysaccharide Produced by Lactobacillus pentosus LZ-R-17 Isolated from Tibetan Kefir. Int. J. Biol. Macromol. 2020, 158, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, H.; Lai, P.F.H.; Xiong, Z.; Ai, L. Structure Characterization of a Pyruvated Exopolysaccharide from Lactobacillus plantarum AR307. Int. J. Biol. Macromol. 2021, 178, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Zhao, X.; Ma, K.; Zhang, C.; Wang, G.; Tian, J.; Wang, X.; Xiao, L.; Li, W. A Novel Viscous Hydrophilic Colloidal Polysaccharide Produced by Lactiplantibacillus plantarum T1: Structural Characterization, Rheological Behavior and Biological Activity. Process Biochem. 2023, 131, 101–113. [Google Scholar] [CrossRef]
- Kumari, M.; Kumar, R.; Singh, D.; Bhatt, S.; Gupta, M. Physiological and Genomic Characterization of an Exopolysaccharide-Producing Weissella cibaria CH2 from Cheese of the Western Himalayas. Food Biosci. 2020, 35, 100570. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Li, D.; Tian, J.; Xiao, L.; Kwok, L.-Y.; Li, W.; Sun, Z. Structure Characterization, Antioxidant Capacity, Rheological Characteristics and Expression of Biosynthetic Genes of Exopolysaccharides Produced by Lactococcus lactis Subsp. lactis IMAU11823. Food Chem. 2022, 384, 132566. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, Y.; Wang, H.; Zhang, H.; Chen, W.; Lu, W. Lactic Acid Bacteria-Derived Exopolysaccharide: Formation, Immunomodulatory Ability, Health Effects, and Structure-Function Relationship. Microbiol. Res. 2023, 274, 127432. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, S.; Liang, S.; Xiang, F.; Wang, X.; Lian, H.; Li, B.; Liu, F. Exopolysaccharides of Lactic Acid Bacteria: Structure, Biological Activity, Structure-Activity Relationship, and Application in the Food Industry: A Review. Int. J. Biol. Macromol. 2024, 257, 128733. [Google Scholar] [CrossRef]
- Zang, J.; Yan, B.; Liu, Z.; Tang, D.; Liu, Y.; Chen, J.; Yin, Z. Current State, Challenges and Future Orientations of the Applications of Lactic Acid Bacteria Exopolysaccharide in Foods. Food Microbiol. 2025, 126, 104678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, D.; Yang, H.; Jiao, X.; Zhou, R.; Zheng, J.; Wu, C. Lactic Acid Bacteria-Derived Exopolysaccharide: Biosynthesis and Antibacterial Characterization. Trends Food Sci. Technol. 2025, 160, 105033. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, G.; Tian, Y. Biological Activities and Applications of Exopolysaccharides Produced by Lactic Acid Bacteria: A Mini-Review. World J. Microbiol. Biotechnol. 2023, 39, 155. [Google Scholar] [CrossRef]
- Zisu, B.; Shah, N.P. Effects of pH, Temperature, Supplementation with Whey Protein Concentrate, and Adjunct Cultures on the Production of Exopolysaccharides by Streptococcus thermophilus 1275. J. Dairy Sci. 2003, 86, 3405–3415. [Google Scholar] [CrossRef]
- Pintado, A.I.E.; Barbosa, C.C.R.; Pintado, M.E.; Malcata, F.X.; Gomes, A.M.P. Efficient Screening and Enhanced Exopolysaccharide Production by Functional Lactic Acid Bacteria (LAB) in Lactose Supplemented Media. Appl. Microbiol. Theory Technol. 2024, 5, 37–50. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, W.; Sun, L.; Sadiq, F.A.; Yang, Y.; Gao, J.; Sang, Y. Preparation Screening, Production Optimization and Characterization of Exopolysaccharides Produced by Lactobacillus sanfranciscensis Ls-1001 Isolated from Chinese Traditional Sourdough. Int. J. Biol. Macromol. 2019, 139, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Adesulu-Dahunsi, A.T.; Sanni, A.I.; Jeyaram, K.; Ojediran, J.O.; Ogunsakin, A.O.; Banwo, K. Extracellular Polysaccharide from Weissella confusa OF126: Production, Optimization, and Characterization. Int. J. Biol. Macromol. 2018, 111, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Guo, S.; Ping, W.; Zhao, D.; Ge, J. Optimization Production of Exopolysaccharide from Leuconostoc Lactis L2 and Its Partial Characterization. Int. J. Biol. Macromol. 2020, 159, 630–639. [Google Scholar] [CrossRef]
- Ng, I.-S.; Xue, C. Enhanced Exopolysaccharide Production and Biological Activity of Lactobacillus rhamnosus ZY with Calcium and Hydrogen Peroxide. Process Biochem. 2017, 52, 295–304. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, M.; Zhang, Y.; Zulewska, J.; Yang, Z. Calcium (Ca2+)-Regulated Exopolysaccharide Biosynthesis in Probiotic Lactobacillus plantarum K25 as Analyzed by an Omics Approach. J. Dairy Sci. 2021, 104, 2693–2708. [Google Scholar] [CrossRef]
- Bertsch, A.; Roy, D.; LaPointe, G. Enhanced Exopolysaccharide Production by Lactobacillus rhamnosus in Co-Culture with Saccharomyces Cerevisiae. Appl. Sci. 2019, 9, 4026. [Google Scholar] [CrossRef]
- Yu, Y.-J.; Chen, Z.; Chen, P.T.; Ng, I.-S. Production, Characterization and Antibacterial Activity of Exopolysaccharide from a Newly Isolated Weissella cibaria under Sucrose Effect. J. Biosci. Bioeng. 2018, 126, 769–777. [Google Scholar] [CrossRef]
- Kojic, M.; Vujcic, M.; Banina, A.; Cocconcelli, P.; Cerning, J.; Topisirovic, L. Analysis of Exopolysaccharide Production by Lactobacillus casei CG11, Isolated from Cheese. Appl. Environ. Microbiol. 1992, 58, 4086–4088. [Google Scholar] [CrossRef]
- Song, Y.-R.; Lee, C.-M.; Lee, S.-H.; Baik, S.-H. Evaluation of Probiotic Properties of Pediococcus Acidilactici M76 Producing Functional Exopolysaccharides and Its Lactic Acid Fermentation of Black Raspberry Extract. Microorganisms 2021, 9, 1364. [Google Scholar] [CrossRef]
- Li, X.-W.; Lv, S.; Shi, T.-T.; Liu, K.; Li, Q.-M.; Pan, L.-H.; Zha, X.-Q.; Luo, J.-P. Exopolysaccharides from Yoghurt Fermented by Lactobacillus paracasei: Production, Purification and Its Binding to Sodium Caseinate. Food Hydrocoll. 2020, 102, 105635. [Google Scholar] [CrossRef]
- Dertli, E.; Yilmaz, M.T.; Tatlisu, N.B.; Toker, O.S.; Cankurt, H.; Sagdic, O. Effects of in Situ Exopolysaccharide Production and Fermentation Conditions on Physicochemical, Microbiological, Textural and Microstructural Properties of Turkish-Type Fermented Sausage (Sucuk). Meat Sci. 2016, 121, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Hilbig, J.; Loeffler, M.; Herrmann, K.; Weiss, J. Application of Exopolysaccharide-Forming Lactic Acid Bacteria in Cooked Ham Model Systems. Food Res. Int. 2019, 119, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Ismail, B.; Nampoothiri, K.M. Production, Purification and Structural Characterization of an Exopolysaccharide Produced by a Probiotic Lactobacillus plantarum MTCC 9510. Arch. Microbiol. 2010, 192, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Jiang, J.; Liu, L.; Wang, S.; Ping, W.; Ge, J. Characterization of Exopolysaccharides Produced by Weissella confusa XG-3 and Their Potential Biotechnological Applications. Int. J. Biol. Macromol. 2021, 178, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Bergmaier, D.; Champagne, C.P.; Lacroix, C. Exopolysaccharide Production during Batch Cultures with Free and Immobilized Lactobacillus rhamnosus RW-9595M. J. Appl. Microbiol. 2003, 95, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; De Angelis, M.; Limitone, A.; Minervini, F.; Carnevali, P.; Corsetti, A.; Gaenzle, M.; Ciati, R.; Gobbetti, M. Glucan and Fructan Production by Sourdough Weissella cibaria and Lactobacillus plantarum. J. Agric. Food Chem. 2006, 54, 9873–9881. [Google Scholar] [CrossRef] [PubMed]
- Gentès, M.-C.; St-Gelais, D.; Turgeon, S.L. Gel Formation and Rheological Properties of Fermented Milk with in Situ Exopolysaccharide Production by Lactic Acid Bacteria. Dairy Sci. Technol. 2011, 91, 645–661. [Google Scholar] [CrossRef]
- Zannini, E.; Mauch, A.; Galle, S.; Gänzle, M.; Coffey, A.; Arendt, E.K.; Taylor, J.P.; Waters, D.M. Barley Malt Wort Fermentation by Exopolysaccharide-Forming Weissella cibaria MG1 for the Production of a Novel Beverage. J. Appl. Microbiol. 2013, 115, 1379–1387. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, D.-H.; Kang, I.-B.; Kim, H.; Song, K.-Y.; Kim, H.-S.; Seo, K.-H. Characterization and Antibacterial Activity of a Novel Exopolysaccharide Produced by Lactobacillus kefiranofaciens DN1 Isolated from Kefir. Food Control 2017, 78, 436–442. [Google Scholar] [CrossRef]
- Sasikumar, K.; Kozhummal Vaikkath, D.; Devendra, L.; Nampoothiri, K.M. An Exopolysaccharide (EPS) from a Lactobacillus plantarum BR2 with Potential Benefits for Making Functional Foods. Bioresour. Technol. 2017, 241, 1152–1156. [Google Scholar] [CrossRef]
- Abedfar, A.; Hosseininezhad, M.; Sadeghi, A.; Raeisi, M.; Feizy, J. Investigation on “Spontaneous Fermentation” and the Productivity of Microbial Exopolysaccharides by Lactobacillus plantarum and Pediococcus pentosaceus Isolated from Wheat Bran Sourdough. LWT 2018, 96, 686–693. [Google Scholar] [CrossRef]
- Rosca, I.; Petrovici, A.R.; Peptanariu, D.; Nicolescu, A.; Dodi, G.; Avadanei, M.; Ivanov, I.C.; Bostanaru, A.C.; Mares, M.; Ciolacu, D. Biosynthesis of Dextran by Weissella confusa and Its In Vitro Functional Characteristics. Int. J. Biol. Macromol. 2018, 107, 1765–1772. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, C.; Jia, S.; Wang, B.; Zhou, K.; Chen, S.; Yang, Y.; Liu, S. Purification, Characterization and Antioxidant Activity of the Exopolysaccharide from Weissella cibaria SJ14 Isolated from Sichuan Paocai. Int. J. Biol. Macromol. 2018, 115, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, H.; Wang, J.; Liang, J.; Li, J.; Jimenez, L.; Lejeune, P.; Zhou, S.; Zhang, G. Impact of Exopolysaccharides from Fructilactobacillus sanfranciscensis Ls5 on the Quality of Mantou: A Comparative Stude of Fermentation Conditions. Foods 2024, 13, 3611. [Google Scholar] [CrossRef]
- Bomfim, V.B.; Pereira Lopes Neto, J.H.; Leite, K.S.; De Andrade Vieira, É.; Iacomini, M.; Silva, C.M.; Olbrich Dos Santos, K.M.; Cardarelli, H.R. Partial Characterization and Antioxidant Activity of Exopolysaccharides Produced by Lactobacillus plantarum CNPC003. LWT 2020, 127, 109349. [Google Scholar] [CrossRef]
- Farinazzo, F.S.; Valente, L.J.; Almeida, M.B.; Simionato, A.S.; Carlos Fernandes, M.T.; Ishii Mauro, C.S.; Bosso Tomal, A.A.; Garcia, S. Characterization and Antioxidant Activity of an Exopolysaccharide Produced by Leuconostoc Pseudomesenteroides JF17 from Juçara Fruits (Euterpe Edulis Martius). Process Biochem. 2020, 91, 141–148. [Google Scholar] [CrossRef]
- Galli, V.; Venturi, M.; Coda, R.; Maina, N.H.; Granchi, L. Isolation and Characterization of Indigenous Weissella confusa for in Situ Bacterial Exopolysaccharides (EPS) Production in Chickpea Sourdough. Food Res. Int. 2020, 138, 109785. [Google Scholar] [CrossRef] [PubMed]
- Heperkan, Z.D.; Bolluk, M.; Bülbül, S. Structural Analysis and Properties of Dextran Produced by Weissella confusa and the Effect of Different Cereals on Its Rheological Characteristics. Int. J. Biol. Macromol. 2020, 143, 305–313. [Google Scholar] [CrossRef]
- İspirli, H.; Özmen, D.; Yılmaz, M.T.; Sağdıç, O.; Dertli, E. Impact of Glucan Type Exopolysaccharide (EPS) Production on Technological Characteristics of Sourdough Bread. Food Control 2020, 107, 106812. [Google Scholar] [CrossRef]
- Wang, K.; Niu, M.; Song, D.; Song, X.; Zhao, J.; Wu, Y.; Lu, B.; Niu, G. Preparation, Partial Characterization and Biological Activity of Exopolysaccharides Produced from Lactobacillus fermentum S1. J. Biosci. Bioeng. 2020, 129, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Omedi, J.O.; Zheng, J.; Huang, W.; Jia, C.; Zhou, L.; Zou, Q.; Li, N.; Gao, T. Exopolysaccharides in Sourdough Fermented by Weissella confusa QS813 Protected Protein Matrix and Quality of Frozen Gluten-Red Bean Dough during Freeze-Thaw Cycles. Food Biosci. 2021, 43, 101180. [Google Scholar] [CrossRef]
- Zhu, J.; Tan, Z.; Zhang, Z.; Shi, X. Characterization on Structure and Bioactivities of an Exopolysaccharide from Lactobacillus curvatus SJTUF 62116. Int. J. Biol. Macromol. 2022, 210, 504–517. [Google Scholar] [CrossRef]
- Zhang, X.; LaPointe, G.; Liu, Y.; Wang, X.; Xiao, L.; Zhao, X.; Li, W. Comparative Analysis of Exopolysaccharide-Producing Lactiplantibacillus plantarum with Ropy and Non-Ropy Phenotypes on the Gel Properties and Protein Conformation of Fermented Milk. Food Chem. 2023, 420, 136117. [Google Scholar] [CrossRef]
- Zaghloul, E.H.; Ibrahim, M.I.A.; Zaghloul, H.A.H. Antibacterial Activity of Exopolysaccharide Produced by Bee Gut-Resident Enterococcus Sp. BE11 against Marine Fish Pathogens. BMC Microbiol. 2023, 23, 231. [Google Scholar] [CrossRef]
- Dong, Y.; Ronholm, J.; Fliss, I.; Karboune, S. Screening of Lactic Acid Bacteria Strains for Potential Sourdough and Bread Applications: Enzyme Expression and Exopolysaccharide Production. Probiotics Antimicrob. Proteins 2024. [Google Scholar] [CrossRef]
- Vázquez-Vargas, C.C.; Cordero-Soto, I.N.; Flores-Maciel, H.A.; Lara-Ceniceros, T.E.; Gallegos-Infante, A.; González-Herrera, S.M.; Ochoa-Martínez, L.A.; Rutiaga-Quiñones, O.M. Bioproduction of Exopolysaccharides by Lactic Acid Bacteria Using Agave By-Products. Process Biochem. 2024, 146, 234–240. [Google Scholar] [CrossRef]
- Kaditzky, S.; Seitter, M.; Hertel, C.; Vogel, R.F. Performance of Lactobacillus sanfranciscensis TMW 1.392 and Its Levansucrase Deletion Mutant in Wheat Dough and Comparison of Their Impact on Bread Quality. Eur. Food Res. Technol. 2008, 227, 433–442. [Google Scholar] [CrossRef]
- Rühmkorf, C.; Jungkunz, S.; Wagner, M.; Vogel, R.F. Optimization of Homoexopolysaccharide Formation by Lactobacilli in Gluten-Free Sourdoughs. Food Microbiol. 2012, 32, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Daba, G.M.; Elnahas, M.O.; Elkhateeb, W.A. Contributions of Exopolysaccharides from Lactic Acid Bacteria as Biotechnological Tools in Food, Pharmaceutical, and Medical Applications. Int. J. Biol. Macromol. 2021, 173, 79–89. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Ye, T.; Zhao, L.; Zhang, X. Safety Evaluation of Weissella confusa SY628 and the Effect of Its Fermentation on the Taste and Quality of Soy Yogurt. Front. Microbiol. 2025, 16, 1567399. [Google Scholar] [CrossRef]
- Abid, Y.; Azabou, S.; Blecker, C.; Gharsallaoui, A.; Corsaro, M.M.; Besbes, S.; Attia, H. Rheological and Emulsifying Properties of an Exopolysaccharide Produced by Potential Probiotic Leuconostoc Citreum-BMS Strain. Carbohydr. Polym. 2021, 256, 117523. [Google Scholar] [CrossRef]
- Pocan, P.; Ilhan, E.; Oztop, M.H. Characterization of Emulsion Stabilization Properties of Gum Tragacanth, Xanthan Gum and Sucrose Monopalmitate: A Comparative Study. J. Food Sci. 2019, 84, 1087–1093. [Google Scholar] [CrossRef]
- Kinoshita, T.; Maruyama, K.; Suyama, K.; Nishijima, M.; Akamatsu, K.; Jogamoto, A.; Katakami, K.; Saito, I. The Effects of OLL1073R-1 Yogurt Intake on Influenza Incidence and Immunological Markers among Women Healthcare Workers: A Randomized Controlled Trial. Food Funct. 2019, 10, 8129–8136. [Google Scholar] [CrossRef]
- Makino, S.; Ikegami, S.; Kume, A.; Horiuchi, H.; Sasaki, H.; Orii, N. Reducing the Risk of Infection in the Elderly by Dietary Intake of Yoghurt Fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Br. J. Nutr. 2010, 104, 998–1006. [Google Scholar] [CrossRef]
- Judiono, J.; Hadisaputro, S.; Indranila, K.S.; Cahyono, B.; Suzery, M.; Widiastuti, Y.; Purnawan, A.I. Effects of Clear Kefir on Biomolecular Aspects of Glycemic Status of Type 2 Diabetes Mellitus (T2DM) Patients in Bandung, West Java [Study on Human Blood Glucose, c Peptide and Insulin]. Funct. Foods Health Dis. 2014, 4, 340–348. [Google Scholar] [CrossRef]
- Makino, S.; Hemmi, J.; Kano, H.; Kashiwagi, M.; Hojo, K.; Asami, Y. Anti-Fatigue Effects of Yogurt Fermented with Lactobacillus delbrueckii Subsp. Bulgaricus OLL1073R-1 in Healthy People Suffering from Summer Heat Fatigue: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 798. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Saruta, J.; Takahashi, T.; To, M.; Shimizu, T.; Hayashi, T.; Morozumi, T.; Kubota, N.; Kamata, Y.; Makino, S.; et al. Effect of Ingesting Yogurt Fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 on Influenza Virus-Bound Salivary IgA in Elderly Residents of Nursing Homes: A Randomized Controlled Trial. Acta Odontol. Scand. 2019, 77, 517–524. [Google Scholar] [CrossRef]
- Morifuji, M.; Ichikawa, S.; Kitade, M.; Fukasawa, T.; Asami, Y.; Manabe, Y.; Sugawara, T. Exopolysaccharides from Milk Fermented by Lactic Acid Bacteria Enhance Dietary Carotenoid Bioavailability in Humans in a Randomized Crossover Trial and in Rats. Am. J. Clin. Nutr. 2020, 111, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Maruyama, K.; Suyama, K.; Nishijima, M.; Akamatsu, K.; Jogamoto, A.; Katakami, K.; Saito, I. Consumption of OLL1073R-1 Yogurt Improves Psychological Quality of Life in Women Healthcare Workers: Secondary Analysis of a Randomized Controlled Trial. BMC Gastroenterol. 2021, 21, 237. [Google Scholar] [CrossRef]
- Noda, M.; Kanno, K.; Danshiitsoodol, N.; Higashikawa, F.; Sugiyama, M. Plant-Derived Lactobacillus paracasei IJH-SONE68 Improves Chronic Allergy Status: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2021, 13, 4022. [Google Scholar] [CrossRef]
- Danshiitsoodol, N.; Noda, M.; Kanno, K.; Uchida, T.; Sugiyama, M. Plant-Derived Lactobacillus paracasei IJH-SONE68 Improves the Gut Microbiota Associated with Hepatic Disorders: A Randomized, Double-Blind, and Placebo-Controlled Clinical Trial. Nutrients 2022, 14, 4492. [Google Scholar] [CrossRef] [PubMed]
- Hemmi, J.; Makino, S.; Yokoo, T.; Kano, H.; Asami, Y.; Takeda, K.; Suzuki, Y.; Kawai, S.; Nagaoka, I.; Sawaki, K.; et al. Consumption of Yogurt Fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 Augments Serum Antibody Titers against Seasonal Influenza Vaccine in Healthy Adults. Biosci. Microbiota Food Health 2023, 42, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Park, J.; Li, R.; Ji, G.E.; Johnston, T.V.; Choe, D.; Park, S.-H.; Park, M.S.; Ku, S. A Randomized, Double-Blind, Controlled Human Study: The Efficacy of Exopolysaccharides in Milk Fermented by Weissella confusa VP30 (VP30-EPS) to Ameliorate Functional Constipation. J. Funct. Foods 2023, 104, 105491. [Google Scholar] [CrossRef]
- Martínez-Burgos, W.J.; Ocán-Torres, D.Y.; Manzoki, M.C.; Scapini, T.; De Mello, A.F.M.; Pozzan, R.; Medeiros, A.B.P.; Vandenberghe, L.P.D.S.; Soccol, C.R. New Trends in Microbial Gums Production, Patented Technologies and Applications in Food Industry. Discov. Food 2024, 4, 49. [Google Scholar] [CrossRef]
- Zammouri, A.; Ziadi, M.; Gharsallaoui, A.; Fguiri, I.; Sbissi, I.; Hammadi, M.; Khorchani, T. Characterization of Novel Exopolysaccharides from Weissella cibaria and Lactococcus lactis Strains and Their Potential Application as Bio-Hydrocolloid Agents in Emulsion Stability. Fermentation 2024, 10, 532. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Wang, L.; Zheng, B.; Zhang, Y.; Pan, L. Weissella cibaria FAFU821 Improved Bread Quality Based on the Three-Dimensional Network Structure of Its Exopolysaccharide. Food Chem. 2025, 475, 143336. [Google Scholar] [CrossRef]
- Soleimanifard, M.; Faramarz, M.A.; Goudarz Najafian, K.C. Production of Kefiran in Kefir Grains and Its Effects on the Rheological Properties Low Protein Wheat Dough and Quality of France Bulky Bread. Adv. Crop Sci. Technol. 2015, 3, 1000190. [Google Scholar] [CrossRef]
- Hermann, M.; Kronseder, K.; Sorgend, J.; Ua-Arak, T.; Vogel, R.F. Functional Properties of Water Kefiran and Its Use as a Hydrocolloid in Baking. Eur. Food Res. Technol. 2016, 242, 337–344. [Google Scholar] [CrossRef]
- Moradi, Z.; Kalanpour, N. Kefiran, a Branched Polysaccharide: Preparation, Properties and Applications: A Review. Carbohydr. Polym. 2019, 223, 115100. [Google Scholar] [CrossRef] [PubMed]
- Gentry, B.; Cazón, P.; O’Brien, K. A Comprehensive Review of the Production, Beneficial Properties, and Applications of Kefiran, the Kefir Grain Exopolysaccharide. Int. Dairy. J. 2023, 144, 105691. [Google Scholar] [CrossRef]
- Liu, W.; Wei, Y.; Xiang, R.; Dong, B.; Yang, X. Lactic Acid Bacteria Exopolysaccharides Unveiling Multifaceted Insights from Structure to Application in Foods and Health Promotion. Foods 2025, 14, 823. [Google Scholar] [CrossRef]
- Ge, Z.; Wang, D.; Zhao, W.; Wang, P.; Zhao, X. Advancements in the Structure, Spatial Configuration, Functional Properties, and Dairy Applications of Lactic Acid Bacteria Exopolysaccharides. Food Rev. Int. 2025, 1–30. [Google Scholar] [CrossRef]
- Tarannum, N.; Hossain, T.J.; Ali, F.; Das, T.; Dhar, K.; Nafiz, I.H. Antioxidant, Antimicrobial and Emulsification Properties of Exopolysaccharides from Lactic Acid Bacteria of Bovine Milk: Insights from Biochemical and Genomic Analysis. LWT 2023, 186, 115263. [Google Scholar] [CrossRef]
- Yalmanci, D.; Dertli, E.; Tekin-Cakmak, Z.H.; Karasu, S. Utilization of Exopolysaccharide Produced by Leuconostoc Lactis GW-6 as an Emulsifier for Low-Fat Mayonnaise Production. Int. J. Biol. Macromol. 2023, 226, 772–779. [Google Scholar] [CrossRef]
- Mouro, C.; Gomes, A.P.; Gouveia, I.C. Microbial Exopolysaccharides: Structure, Diversity, Applications, and Future Frontiers in Sustainable Functional Materials. Polysaccharides 2024, 5, 241–287. [Google Scholar] [CrossRef]
- Nicolescu, C.M.; Bumbac, M.; Buruleanu, C.L.; Popescu, E.C.; Stanescu, S.G.; Georgescu, A.A.; Toma, S.M. Biopolymers Produced by Lactic Acid Bacteria: Characterization and Food Application. Polymers 2023, 15, 1539. [Google Scholar] [CrossRef]
- Motedayen, A.A.; Khodaiyan, F.; Salehi, E.A. Development and Characterisation of Composite Films Made of Kefiran and Starch. Food Chem. 2013, 136, 1231–1238. [Google Scholar] [CrossRef]
- Davidović, S.; Miljković, M.; Gordic, M.; Cabrera-Barjas, G.; Nesic, A.; Dimitrijević-Branković, S. Dextran-Based Edible Coatings to Prolong the Shelf Life of Blueberries. Polymers 2021, 13, 4252. [Google Scholar] [CrossRef]
- Zikmanis, P.; Juhņeviča-Radenkova, K.; Radenkovs, V.; Segliņa, D.; Krasnova, I.; Kolesovs, S.; Orlovskis, Z.; Šilaks, A.; Semjonovs, P. Microbial Polymers in Edible Films and Coatings of Garden Berry and Grape: Current and Prospective Use. Food Bioprocess. Technol. 2021, 14, 1432–1445. [Google Scholar] [CrossRef]
- Hasheminya, S.-M.; Dehghannya, J. Development and Characterization of Kefiran-Gelatin Bio-Nanocomposites Containing Zhumeria Majdae Essential Oil Nanoemulsion to Use as Active Food Packaging in Sponge Cakes. Int. J. Biol. Macromol. 2024, 279, 135120. [Google Scholar] [CrossRef]
- Mende, S.; Rohm, H.; Jaros, D. Influence of Exopolysaccharides on the Structure, Texture, Stability and Sensory Properties of Yoghurt and Related Products. Int. Dairy. J. 2016, 52, 57–71. [Google Scholar] [CrossRef]
- Lynch, K.M.; Coffey, A.; Arendt, E.K. Exopolysaccharide Producing Lactic Acid Bacteria: Their Techno-Functional Role and Potential Application in Gluten-Free Bread Products. Food Res. Int. 2018, 110, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Lin, T.; Shi, Y.; Lin, Y.; Xu, C.; Ma, K.; Zhang, C.; Rui, X.; Gan, D.; Li, W. Recent Advances in Exopolysaccharide–Protein Interactions in Fermented Dairy- and Plant-Based Yogurts: Mechanisms, Influencing Factors, Health Benefits, Analytical Techniques, and Future Directions. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70219. [Google Scholar] [CrossRef]
- Álvarez, A.; Manjarres, J.J.; Ramírez, C.; Bolívar, G. Use of an Exopolysaccharide-Based Edible Coating and Lactic Acid Bacteria with Antifungal Activity to Preserve the Postharvest Quality of Cherry Tomato. LWT 2021, 151, 112225. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Mehwish, H.M.; Hayat, H.F.; Hussain, N.; Sarwar, S.; Aslam, H.; Nadeem, A.; Shi, J. Characterization, the Antioxidant and Antimicrobial Activity of Exopolysaccharide Isolated from Poultry Origin Lactobacilli. Probiotics Antimicrob. Proteins 2019, 11, 1132–1142. [Google Scholar] [CrossRef]
- Patil, T.D.; Deshmukh, R.K.; Gaikwad, K.K. Microbial Exopolysaccharide-Based Flexible Bioactive Film with Murraya koenigii Berries’ Extract for Food Packaging: Physico-Chemical, Microstructural, Antioxidant, and Antimicrobial Properties. ACS Food Sci. Technol. 2025, 5, 695–708. [Google Scholar] [CrossRef]
- Ramos, I.M.; Seseña, S.; Poveda, J.M.; Palop, M.L. Screening of Lactic Acid Bacteria Strains to Improve the Properties of Non-Fat Set Yogurt by in Situ EPS Production. Food Bioprocess Technol. 2023, 16, 2541–2558. [Google Scholar] [CrossRef]
- Brüls, M.; Foroutanparsa, S.; Maljaars, C.E.P.; Olsthoorn, M.; Tas, R.P.; Voets, I.K. Investigating the Impact of Exopolysaccharides on Yogurt Network Mechanics and Syneresis through Quantitative Microstructural Analysis. Food Hydrocoll. 2024, 150, 109629. [Google Scholar] [CrossRef]
- Gentès, M.-C.; St-Gelais, D.; Turgeon, S.L. Exopolysaccharide–Milk Protein Interactions in a Dairy Model System Simulating Yoghurt Conditions. Dairy Sci. Technol. 2013, 93, 255–271. [Google Scholar] [CrossRef]
- Patlan-Velázquez, L.-F.; González-Olivares, L.-G.; García-Garibay, M.; Alatorre-Santamaría, S.; Gómez-Ruiz, L.; Rodríguez-Serrano, G.; Cruz-Guerrero, A. Effect of Biogenic Exopolysaccharides in Characteristics and Stability of a Novel Requeson-Type Cheese. Food Biosci. 2024, 59, 103896. [Google Scholar] [CrossRef]
- Surber, G.; Spiegel, T.; Dang, B.P.; Wolfschoon Pombo, A.; Rohm, H.; Jaros, D. Cream Cheese Made with Exopolysaccharide-Producing Lactococcus lactis: Impact of Strain and Curd Homogenization Pressure on Texture and Syneresis. J. Food Eng. 2021, 308, 110664. [Google Scholar] [CrossRef]
- Wang, J.; Wu, T.; Fang, X.; Yang, Z. Manufacture of Low-Fat. Cheddar Cheese by Exopolysaccharide-Producing Lactobacillus plantarum JLK0142 and Its Functional Properties. J. Dairy Sci. 2019, 102, 3825–3838. [Google Scholar] [CrossRef] [PubMed]
- Velasco, L.; Weiss, J.; Loeffler, M. Influence of Microbial In-Situ Heteropolysaccharide Production on Textural Properties of Raw Fermented Sausages (Salami). J. Food Sci. Technol. 2021, 58, 562–570. [Google Scholar] [CrossRef]
- Hilbig, J.; Gisder, J.; Prechtl, R.M.; Herrmann, K.; Weiss, J.; Loeffler, M. Influence of Exopolysaccharide-Producing Lactic Acid Bacteria on the Spreadability of Fat-Reduced Raw Fermented Sausages (Teewurst). Food Hydrocoll. 2019, 93, 422–431. [Google Scholar] [CrossRef]
- Hernández-Figueroa, R.H.; Mani-López, E.; Palou, E.; López-Malo, A. Sourdoughs as Natural Enhancers of Bread Quality and Shelf Life: A Review. Fermentation 2023, 10, 7. [Google Scholar] [CrossRef]
- Tang, X.; Liu, R.; Huang, W.; Zhang, B.; Wu, Y.; Zhuang, J.; Omedi, J.O.; Wang, F.; Zheng, J. Impact of in Situ Formed Exopolysaccharides on Dough Performance and Quality of Chinese Steamed Bread. LWT 2018, 96, 519–525. [Google Scholar] [CrossRef]
- Montemurro, M.; Pontonio, E.; Rizzello, C.G. Design of a “Clean-Label” Gluten-Free Bread to Meet Consumers Demand. Foods 2021, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yao, Y.; Li, J.; Ju, X.; Wang, L. Impact of Exopolysaccharides-Producing Lactic Acid Bacteria on the Chemical, Rheological Properties of Buckwheat Sourdough and the Quality of Buckwheat Bread. Food Chem. 2023, 425, 136369. [Google Scholar] [CrossRef]
- Galle, S.; Schwab, C.; Dal Bello, F.; Coffey, A.; Gänzle, M.G.; Arendt, E.K. Influence of In-Situ Synthesized Exopolysaccharides on the Quality of Gluten-Free Sorghum Sourdough Bread. Int. J. Food Microbiol. 2012, 155, 105–112. [Google Scholar] [CrossRef]
- Goveas, L.C.; Ashwath, K.S.; Nazerath, B.R.; Dsouza, O.; Ullekh; Umesh, A.; Muddappa, V.S. Development of Coconut Water-Based Exopolysaccharide Rich Functional Beverage by Fermentation with Probiotic Lactobacillus plantarum SVP2. Biocatal. Agric. Biotechnol. 2021, 34, 102030. [Google Scholar] [CrossRef]
- Huang, W.; Yang, S.; Wätjen, A.P.; Gumulya, Y.; Fernández-Pacheco, P.; Marcellin, E.; Prakash, S.; Bang-Berthelsen, C.H.; Turner, M.S. Isolation of an Exopolysaccharide-Producing Weissella confusa Strain from Lettuce and Exploring Its Application as a Texture Modifying Adjunct Culture in a Soy Milk Alternative. Int. J. Food Microbiol. 2025, 428, 110992. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Upadhyay, L.S.B. Microbial Exopolysaccharides: Synthesis Pathways, Types and Their Commercial Applications. Int. J. Biol. Macromol. 2020, 157, 577–583. [Google Scholar] [CrossRef]
- Loeffler, M.; Hilbig, J.; Velasco, L.; Weiss, J. Usage of in Situ Exopolysaccharide—Forming Lactic Acid Bacteria in Food Production: Meat Products—A New Field of Application? Compr. Rev. Food Sci. Food Saf. 2020, 19, 2932–2954. [Google Scholar] [CrossRef]
- Ruas-Madiedo, P.; Hugenholtz, J.; Zoon, P. An Overview of the Functionality of Exopolysaccharides Produced by Lactic Acid Bacteria. Int. Dairy J. 2002, 12, 163–171. [Google Scholar] [CrossRef]
- Ruas-Madiedo, P.; Salazar, N.; De Los Reyes-Gavilán, C.G. Exopolysaccharides Produced by Lactic Acid Bacteria in Food and Probiotic Applications. In Microb. Glycobiol.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 885–902. ISBN 978-0-12-374546-0. [Google Scholar]
- Abid, Y.; Casillo, A.; Gharsallah, H.; Joulak, I.; Lanzetta, R.; Corsaro, M.M.; Attia, H.; Azabou, S. Production and Structural Characterization of Exopolysaccharides from Newly Isolated Probiotic Lactic Acid Bacteria. Int. J. Biol. Macromol. 2018, 108, 719–728. [Google Scholar] [CrossRef]
- Zikmanis, P.; Brants, K.; Kolesovs, S.; Semjonovs, P. Extracellular Polysaccharides Produced by Bacteria of the Leuconostoc Genus. World J. Microbiol. Biotechnol. 2020, 36, 161. [Google Scholar] [CrossRef]
- Fuso, A.; Bancalari, E.; Castellone, V.; Caligiani, A.; Gatti, M.; Bottari, B. Feeding Lactic Acid Bacteria with Different Sugars: Effect on Exopolysaccharides (EPS) Production and Their Molecular Characteristics. Foods 2023, 12, 215. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Panesar, P.S.; Mehariya, S. Microbial Exopolysaccharides: Production and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2024; ISBN 978-1-00-334268-7. [Google Scholar]
- Sengupta, D.; Datta, S.; Biswas, D. Towards a Better Production of Bacterial Exopolysaccharides by Controlling Genetic as Well as Physico-Chemical Parameters. Appl. Microbiol. Biotechnol. 2018, 102, 1587–1598. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, G.; Ahmad, T.; Kaur, B.; Hakeem, K.R. Tailoring Cellular Metabolism in Lactic Acid Bacteria through Metabolic Engineering. J. Microbiol. Methods 2020, 170, 105862. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J. Bacterial Exopolysaccharides: Chemical Structures, Gene Clusters and Genetic Engineering. Int. J. Biol. Macromol. 2021, 173, 481–490. [Google Scholar] [CrossRef]
- Kong, L.; Xiong, Z.; Song, X.; Xia, Y.; Ai, L. CRISPR/dCas9-Based Metabolic Pathway Engineering for the Systematic Optimization of Exopolysaccharide Biosynthesis in Streptococcus Thermophilus. J. Dairy Sci. 2022, 105, 6499–6512. [Google Scholar] [CrossRef]
- Hu, G.; Hu, H.; Zhou, Z.; Aziz, T.; Yang, Z.; Yang, Z.; Alharbi, N.K.; Shami, A.; Al-Asmari, F.; AlQadeeb, H.; et al. Elucidating the Exopolysaccharide Biosynthesis in Pediococcus Acidilactici BCB1H Regulated by Iron (Fe2+) Using a Multi-Omics Approach. Int. J. Biol. Macromol. 2025, 309, 142915. [Google Scholar] [CrossRef]
- Caggianiello, G.; Kleerebezem, M.; Spano, G. Exopolysaccharides Produced by Lactic Acid Bacteria: From Health-Promoting Benefits to Stress Tolerance Mechanisms. Appl. Microbiol. Biotechnol. 2016, 100, 3877–3886. [Google Scholar] [CrossRef]
- Wu, J.; Han, X.; Ye, M.; Li, Y.; Wang, X.; Zhong, Q. Exopolysaccharides Synthesized by Lactic Acid Bacteria: Biosynthesis Pathway, Structure-Function Relationship, Structural Modification and Applicability. Crit. Rev. Food Sci. Nutr. 2023, 63, 7043–7064. [Google Scholar] [CrossRef]
- Bamigbade, G.; Ali, A.H.; Subhash, A.; Tamiello-Rosa, C.; Al Qudsi, F.R.; Esposito, G.; Hamed, F.; Liu, S.-Q.; Gan, R.-Y.; Abu-Jdayil, B.; et al. Structural Characterization, Biofunctionality, and Environmental Factors Impacting Rheological Properties of Exopolysaccharide Produced by Probiotic Lactococcus lactis C15. Sci. Rep. 2023, 13, 17888. [Google Scholar] [CrossRef]
- Yadav, M.K.; Song, J.H.; Vasquez, R.; Lee, J.S.; Kim, I.H.; Kang, D.-K. Methods for Detection, Extraction, Purification, and Characterization of Exopolysaccharides of Lactic Acid Bacteria—A Systematic Review. Foods 2024, 13, 3687. [Google Scholar] [CrossRef]
- Notararigo, S.; Nácher-Vázquez, M.; Ibarburu, I.; Werning, M.L.; De Palencia, P.F.; Dueñas, M.T.; Aznar, R.; López, P.; Prieto, A. Comparative Analysis of Production and Purification of Homo- and Hetero-Polysaccharides Produced by Lactic Acid Bacteria. Carbohydr. Polym. 2013, 93, 57–64. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Coffey, A.; Arendt, E.K. Lactic Acid Bacteria Exopolysaccharides in Foods and Beverages: Isolation, Properties, Characterization, and Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 155–176. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Bumbadia, M.; Singh, S.K. Prospects and Functionality of Bacterial Exopolysaccharides in Dairy Foods: A Review. J. Agrisearch 2022, 9, 1–5. Available online: https://journal.jsure.org.in/index.php/jas/article/view/984 (accessed on 17 August 2025). [CrossRef]
- Seneviratne, C.J.; Suriyanarayanan, T.; Widyarman, A.S.; Lee, L.S.; Lau, M.; Ching, J.; Delaney, C.; Ramage, G. Multi-Omics Tools for Studying Microbial Biofilms: Current Perspectives and Future Directions. Crit. Rev. Microbiol. 2020, 46, 759–778. [Google Scholar] [CrossRef]
- Flemming, H.-C. EPS—Then and Now. Microorganisms 2016, 4, 41. [Google Scholar] [CrossRef]
- Kavitake, D.; Kandasamy, S.; Devi, P.B.; Shetty, P.H. Recent Developments on Encapsulation of Lactic Acid Bacteria as Potential Starter Culture in Fermented Foods–A Review. Food Biosci. 2018, 21, 34–44. [Google Scholar] [CrossRef]
- Kumari, J.; Kumawat, R.; Prasanna, R.; Jothieswari, D.; Debnath, R.; Ikbal, A.M.A.; Palit, P.; Rawat, R.; Gopikrishna, K.; Tiwari, O.N. Microbial Exopolysaccharides: Classification, Biosynthetic Pathway, Industrial Extraction and Commercial Production to Unveil Its Bioprospection: A Comprehensive Review. Int. J. Biol. Macromol. 2025, 297, 139917. [Google Scholar] [CrossRef]
- Barcelos, M.C.S.; Vespermann, K.A.C.; Pelissari, F.M.; Molina, G. Current Status of Biotechnological Production and Applications of Microbial Exopolysaccharides. Crit. Rev. Food Sci. Nutr. 2020, 60, 1475–1495. [Google Scholar] [CrossRef]
- Zang, J.; Lin, T.; Xu, C.; Lin, Y.; Ma, K.; Zhang, C.; Rui, X.; Gan, D.; Li, W. Advances in Lactic Acid Bacteria and Their Metabolites in Fermented Foods: Mechanisms of Food Quality Enhancement, Gut Microbiota Modulation, and Future Prospects. Food Rev. Int. 2025, 1–35. [Google Scholar] [CrossRef]
- Obis, D.; Ouwehand, A. The Safety of Lactic Acid Bacteria for Use in Foods. In Lactic Acid Bacteria; Vinderola, C.G., Ouwehand, A., Salminen, S., Von Wright, A., Eds.; CRC Press: Boca Raton, FL, USA, 2024; ISBN 978-1-00-335207-5. [Google Scholar]
- EFSA Panel on Biological Hazards (BIOHAZ); Allende, A.; Alvarez-Ordóñez, A.; Bortolaia, V.; Bover-Cid, S.; De Cesare, A.; Dohmen, W.; Guillier, L.; Jacxsens, L.; Nauta, M.; et al. Update of the List of Qualified Presumption of Safety (QPS) Recommended Microbiological Agents Intentionally Added to Food or Feed as Notified to EFSA 22: Suitability of Taxonomic Units Notified to EFSA until March 2025. EFSA J. 2025, 23, e9510. [Google Scholar] [CrossRef]
- USDA Microorganisms & Microbial-Derived Ingredients Used in Food (Partial List). Available online: https://www.fda.gov/food/generally-recognized-safe-gras/microorganisms-microbial-derived-ingredients-used-food-partial-list (accessed on 5 September 2025).
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA). Guidance on the Scientific Requirements for Health Claims Related to Gut and Immune Function. EFSA J. 2011, 9, 1984. [Google Scholar] [CrossRef]
- Osemwegie, O.O.; Adetunji, C.O.; Ayeni, E.A.; Adejobi, O.I.; Arise, R.O.; Nwonuma, C.O.; Oghenekaro, A.O. Exopolysaccharides from Bacteria and Fungi: Current Status and Perspectives in Africa. Heliyon 2020, 6, e04205. [Google Scholar] [CrossRef]
- Patten, D.A.; Laws, A.P. Lactobacillus-Produced Exopolysaccharides and Their Potential Health Benefits: A Review. Benef. Microbes 2015, 6, 457–472. [Google Scholar] [CrossRef]
- Moscovici, M. Present and Future Medical Applications of Microbial Exopolysaccharides. Front. Microbiol. 2015, 6, 1012. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Guidance on the Scientific Requirements for an Application for Authorisation of a Novel Food in the Context of Regulation (EU) 2015/2283. EFSA J. 2024, 22, e8961. [Google Scholar] [CrossRef]
- Mohedano, M.L.; Zarour, K.; Diez-Ozaeta, I.; Dueñas, M.T.; López, P.; Russo, P. Recent Advances in Fermented Functional Foods. In Functional Foods of the Future; Gupta, V.K., Sharma, M., Gaur, S., Kuhad, R.C., Eds.; Royal Society of Chemistry: London, UK, 2025; pp. 30–61. ISBN 978-1-83767-000-0. [Google Scholar]
- Rühmann, B.; Schmid, J.; Sieber, V. Methods to Identify the Unexplored Diversity of Microbial Exopolysaccharides. Front. Microbiol. 2015, 6, 565. [Google Scholar] [CrossRef]
- Liu, J.; Chan, S.H.J.; Chen, J.; Solem, C.; Jensen, P.R. Systems Biology–A Guide for Understanding and Developing Improved Strains of Lactic Acid Bacteria. Front. Microbiol. 2019, 10, 876. [Google Scholar] [CrossRef]
- Wang, W.; Ju, Y.; Liu, N.; Shi, S.; Hao, L. Structural Characteristics of Microbial Exopolysaccharides in Association with Their Biological Activities: A Review. Chem. Biol. Technol. Agric. 2023, 10, 137. [Google Scholar] [CrossRef]
- Laws, A.; Gu, Y.; Marshall, V. Biosynthesis, Characterisation, and Design of Bacterial Exopolysaccharides from Lactic Acid Bacteria. Biotechnol. Adv. 2001, 19, 597–625. [Google Scholar] [CrossRef]
- Jolly, L.; Vincent, S.J.F.; Duboc, P.; Neeser, J.-R. Exploiting Exopolysaccharides from Lactic Acid Bacteria. Antonie Van Leeuwenhoek 2002, 82, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Taneja, N.K.; Raposo, A.; Alarifi, S.N.; Teixeira-Lemos, E.; Lima, M.J.; Gonçalves, J.C.; Dhewa, T. Exploring Prebiotic Properties and Its Probiotic Potential of New Formulations of Soy Milk-Derived Beverages. Front. Microbiol. 2024, 15, 1404907. [Google Scholar] [CrossRef]
- Mugwanda, K.; Hamese, S.; Van Zyl, W.F.; Prinsloo, E.; Du Plessis, M.; Dicks, L.M.T.; Thimiri Govinda Raj, D.B. Recent Advances in Genetic Tools for Engineering Probiotic Lactic Acid Bacteria. Biosci. Rep. 2023, 43, BSR20211299. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Guo, T.; Qiao, M. Current Application and Future Prospects of CRISPR-Cas in Lactic Acid Bacteria: A Review. Food Res. Int. 2025, 209, 116315. [Google Scholar] [CrossRef]
- Ayed, L.; M’hir, S.; Nuzzolese, D.; Di Cagno, R.; Filannino, P. Harnessing the Health and Techno-Functional Potential of Lactic Acid Bacteria: A Comprehensive Review. Foods 2024, 13, 1538. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, S. Exploring the Functionality of Microbes in Fermented Foods: Technological Advancements and Future Directions. Fermentation 2025, 11, 300. [Google Scholar] [CrossRef]
- Mirsalami, S.M.; Mirsalami, M. Advances in Genetically Engineered Microorganisms: Transforming Food Production through Precision Fermentation and Synthetic Biology. Future Foods 2025, 11, 100601. [Google Scholar] [CrossRef]
- Amenaghawon, A.N.; Igemhokhai, S.; Eshiemogie, S.A.; Ugbodu, F.; Evbarunegbe, N.I. Data-Driven Intelligent Modeling, Optimization, and Global Sensitivity Analysis of a Xanthan Gum Biosynthesis Process. Heliyon 2024, 10, e25432. [Google Scholar] [CrossRef]
- Akpoghelie, P.O.; Edo, G.I.; Ali, A.B.M.; Yousif, E.; Zainulabdeen, K.; Owheruo, J.O.; Isoje, E.F.; Igbuku, U.A.; Essaghah, A.E.A.; Makia, R.S.; et al. Lactic Acid Bacteria: Nature, Characterization, Mode of Action, Products and Applications. Process Biochem. 2025, 152, 1–28. [Google Scholar] [CrossRef]
- Murata, R.; Shim, Y.Y.; Kim, Y.J.; Reaney, M.J.T.; Tse, T.J. Exploring Lactic Acid Bacteria in Food, Human Health, and Agriculture. Crit. Rev. Food Sci. Nutr. 2025, 1–27. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, S.T.; Ross, R.P.; Stanton, C. The Progress of Multi-Omics Technologies: Determining Function in Lactic Acid Bacteria Using a Systems Level Approach. Front. Microbiol. 2020, 10, 3084. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, K.; Kline, K.; Renault, P.; Kok, J. Editorial: Omics and Systems Approaches to Study the Biology and Applications of Lactic Acid Bacteria. Front. Microbiol. 2020, 11, 1786. [Google Scholar] [CrossRef]
- Korcz, E.; Kerényi, Z.; Varga, L. Dietary Fibers, Prebiotics, and Exopolysaccharides Produced by Lactic Acid Bacteria: Potential Health Benefits with Special Regard to Cholesterol-Lowering Effects. Food Funct. 2018, 9, 3057–3068. [Google Scholar] [CrossRef] [PubMed]
- USDA Guidance for Industry: The Declaration of Certain Isolated or Synthetic Non-Digestible Carbohydrates as Dietary Fiber on Nutrition and Supplement Facts Labels. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-declaration-certain-isolated-or-synthetic-non-digestible-carbohydrates-dietary (accessed on 13 August 2025).
- Bajpai, V.K.; Rather, I.A.; Majumder, R.; Shukla, S.; Aeron, A.; Kim, K.; Kang, S.C.; Dubey, R.C.; Maheshwari, D.K.; Lim, J.; et al. Exopolysaccharide and Lactic Acid Bacteria: Perception, Functionality and Prospects. Bangladesh J. Pharmacol. 2015, 11, 1–23. [Google Scholar] [CrossRef]
- Olojede, A.O.; Sanni, A.I.; Banwo, K.; Michael, T. Improvement of Texture, Nutritional Qualities, and Consumers’ Perceptions of Sorghum-Based Sourdough Bread Made with Pediococcus pentosaceus and Weissella confusa Strains. Fermentation 2022, 8, 32. [Google Scholar] [CrossRef]
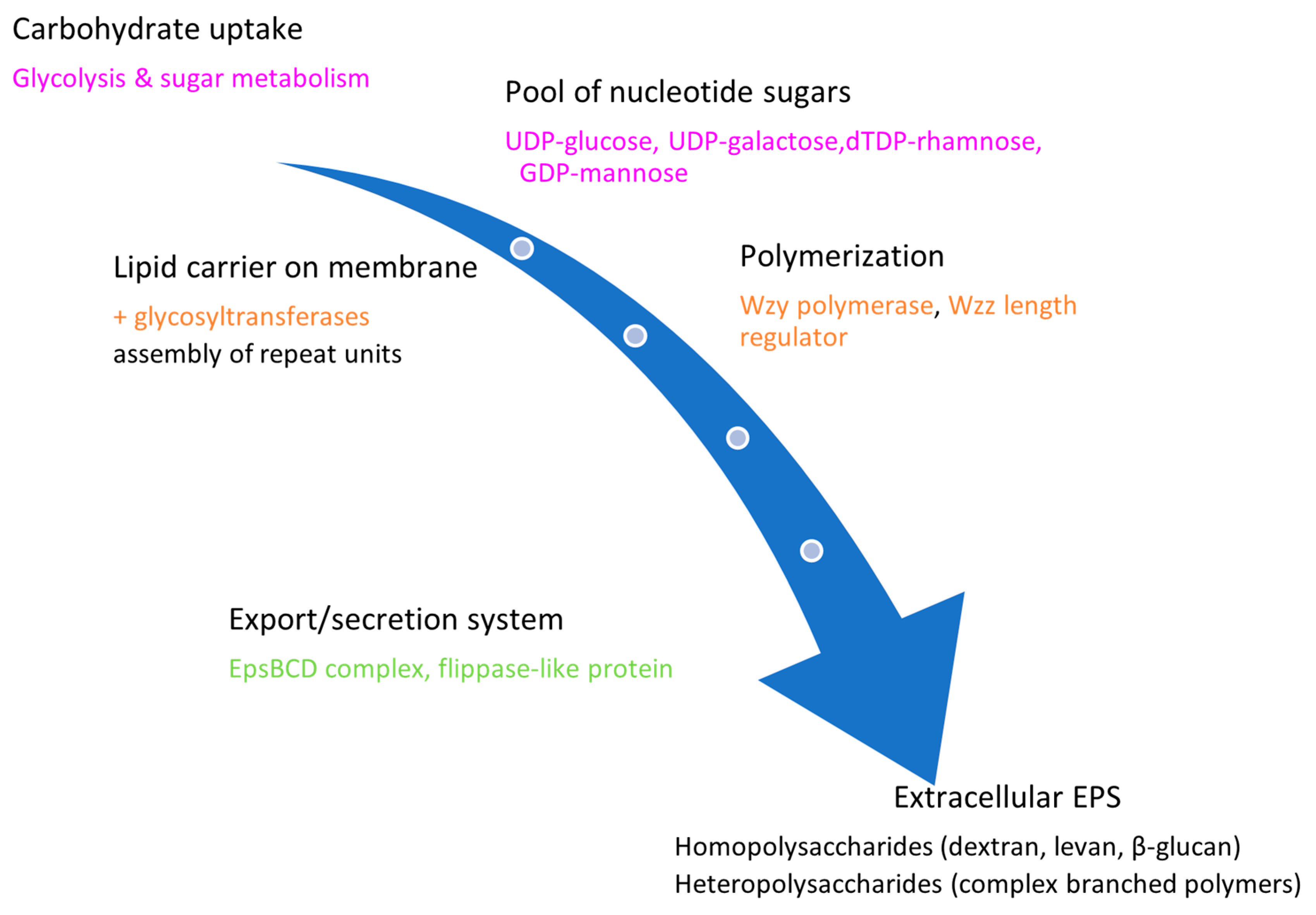
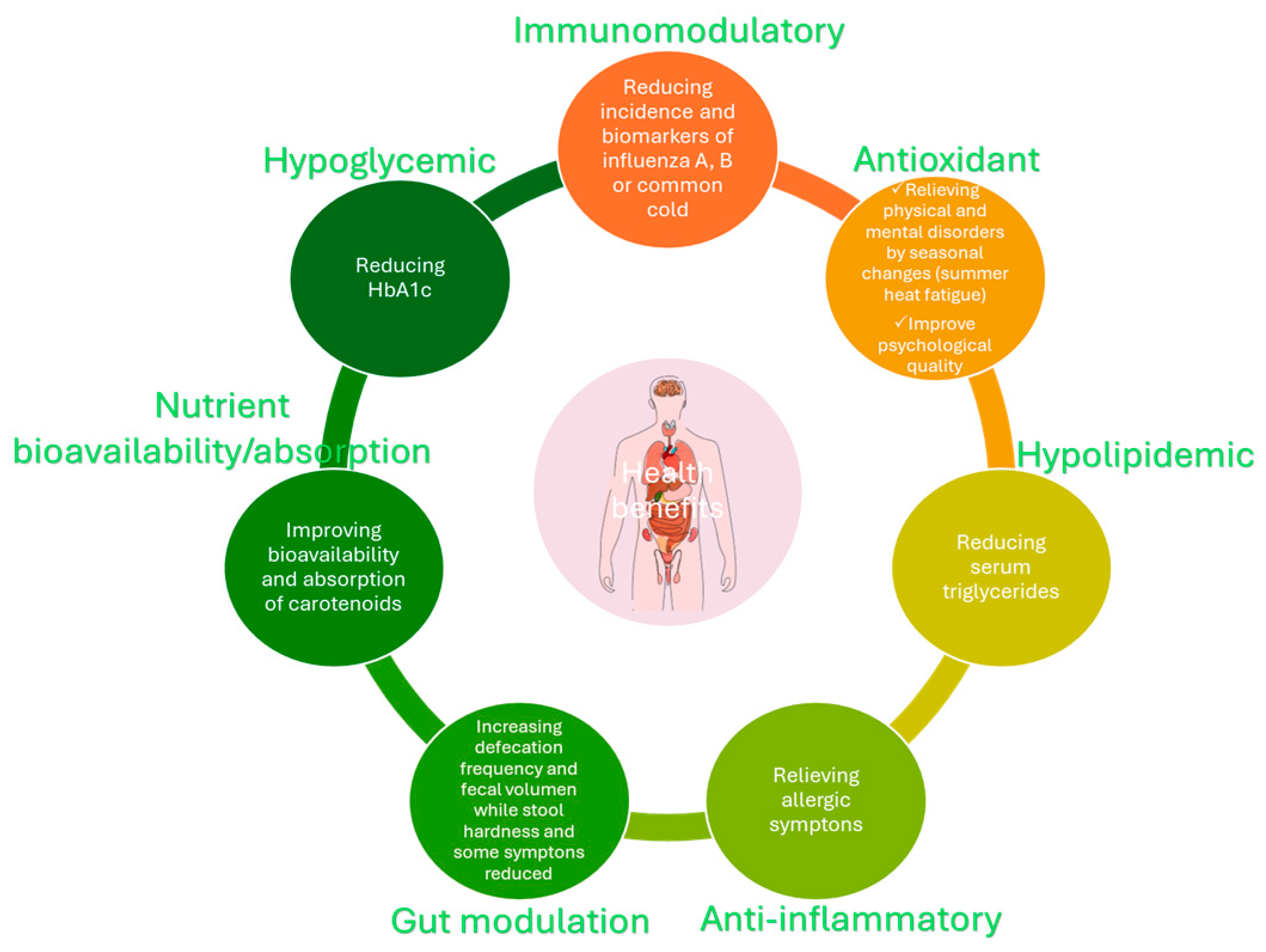

| Omics Discipline | Primary Focus | Contribution to EPS Research | Limitations |
|---|---|---|---|
| Genomics | Identification of EPS gene clusters (eps operons) | Enables strain screening and rational pathway targeting | Does not capture regulation or dynamic behavior |
| Transcriptomics | Expression profiling under specific conditions | Reveals regulatory cues and fermentation triggers | Snapshot view: affected by culture conditions |
| Proteomics | Detection of biosynthetic enzymes and transporters | Connects genotype to active biosynthetic machinery | Challenges in analyzing membrane-bound proteins |
| Metabolomics | Monitoring of sugar fluxes and fermentation by-products | Identifies metabolic bottlenecks and EPS precursors | Requires careful interpretation; complex sample handling |
| Glycomics | EPS structure mapping (e.g., linkages, branching) | Elucidates functional motifs influencing techno- and bioactivity | Technically complex; limited standardization |
| Functional Role of EPS | Mechanism/Benefit | Target Population/Use Case |
|---|---|---|
| Prebiotic activity | Selective stimulation of beneficial gut microbiota | Individuals with dysbiosis, the elderly |
| Immunomodulation | Modulation of immune signaling pathways | Metabolic syndrome, Inflammatory Bowel Disease, and aging |
| Protective matrix for probiotics | Enhances viability and stability during digestion | Symbiotic formulations in functional foods |
| Synergistic formulations | Combination with fibers/polyphenols for precision effects | Personalized nutrition interventions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Figueroa, R.H.; López-Malo, A.; Mani-López, E. Lactic Acid Bacteria-Derived Exopolysaccharides: Dual Roles as Functional Ingredients and Fermentation Agents in Food Applications. Fermentation 2025, 11, 538. https://doi.org/10.3390/fermentation11090538
Hernández-Figueroa RH, López-Malo A, Mani-López E. Lactic Acid Bacteria-Derived Exopolysaccharides: Dual Roles as Functional Ingredients and Fermentation Agents in Food Applications. Fermentation. 2025; 11(9):538. https://doi.org/10.3390/fermentation11090538
Chicago/Turabian StyleHernández-Figueroa, Ricardo H., Aurelio López-Malo, and Emma Mani-López. 2025. "Lactic Acid Bacteria-Derived Exopolysaccharides: Dual Roles as Functional Ingredients and Fermentation Agents in Food Applications" Fermentation 11, no. 9: 538. https://doi.org/10.3390/fermentation11090538
APA StyleHernández-Figueroa, R. H., López-Malo, A., & Mani-López, E. (2025). Lactic Acid Bacteria-Derived Exopolysaccharides: Dual Roles as Functional Ingredients and Fermentation Agents in Food Applications. Fermentation, 11(9), 538. https://doi.org/10.3390/fermentation11090538






