Abstract
Fermentation has been a crucial process in the preparation of foods and beverages for consumption, especially for the purpose of adding value to nutrients and bioactive compounds; however, conventional approaches have certain drawbacks such as not being able to fulfill the requirements of the ever-increasing global population as well as the sustainability goals. This review aims to evaluate how the application of advanced fermentation techniques can transform the food production system to be more effective, nutritious, and environmentally friendly. The techniques discussed include metabolic engineering, synthetic biology, AI-driven fermentation, quorum sensing regulation, and high-pressure processing, with an emphasis on their ability to enhance microbial activity with a view to enhancing product output. Authentic, wide-coverage scientific research search engines were used such as Google Scholar, Research Gate, Science Direct, PubMed, and Frontiers. The literature search was carried out for reports, articles, as well as papers in peer-reviewed journals from 2010 to 2024. A statistical analysis with a graphical representation of publication trends on the main topics was conducted using PubMed data from 2010 to 2024. In this present review, 112 references were used to investigate novel fermentation technologies that fortify the end food products with nutritional and functional value. Images that illustrate the processes involved in novel fermentation technologies were designed using Adobe Photoshop. The findings indicate that, although there are issues regarding costs, the scalability of the process, and the acceptability of the products by the consumers, the technologies provide a way of developing healthy foods and products produced using sustainable systems. This paper thus calls for more research and development as well as for the establishment of a legal frameworks to allow for the integration of these technologies into the food production system and make the food industry future-proof.
1. Introduction
Fermentation represents one of the most ancient biotechnological methods in food production; its relevance has continued into modern times because this process can substantially improve the preservation and nutritional values of food. In line with the Sustainable Development Goals (SDG) of Good Health and Wellbeing, coupled with Industry, Innovation, and Infrastructure, recent advances in fermentation technologies place health benefits at the forefront of activity rather than its conventional roles of shelf-life extension and sensory enhancement [1,2]. This shift has been a reflection of the global trend towards healthier, more functional foods. Recent studies have shifted a focus toward the health benefits that fermented products, especially those for gut microbiome support, can offer; fermentation is considered an economic and energy-efficient method to enhance the digestibility, nutrient bioavailability, and organoleptic properties of foods [3,4].
Traditionally, fermentation depended on the wild microorganisms present around it, which always resulted in anomalies, since food products were developed based on regional environmental factors as shown in Table 1. However, consumer demand and an awareness of safety standards urge standardization processes. This has also necessitated industry measures, which include the use of starter cultures with controlled and specified fermentation methods [5]. Fermentation science began with the discovery of Aspergillus oryzae by Korschelt in 1878 for koji production, which was one of the first formal recognitions of microbial interventions in fermentation. Further acquisition of knowledge in molecular biology, next-generation sequencing, multi-omics, and bioinformatics have transformed fermentation science, adding greater depth to microbial diversity, metabolic pathways, and fermentation outcomes [6,7].
2. Traditional Fermentation Processes
2.1. Alcoholic Fermentation
The application of Saccharomyces cerevisiae in alcoholic fermentation is one of the oldest uses of biotechnology for the production of beverages that result from the conversion of sugars to ethanol and carbon dioxide [8]. This metabolic process plays a fundamental role in the production of beer, wine, and spirits, where yeast selection is fundamental for the optimization not only of alcohol yields but also of the sensory profiles. Indeed, this reaction mainly concerns sugars such as fructose and glucose. The metabolism by yeast improves both the alcohol content and the flavor of the final product [9].
2.2. Lactic Acid Fermentation
Lactic acid fermentation, mediated through a microbial group with glucose metabolism, is applied widely in food and non-food industries. According to [10], the uses of lactic acid are said to be multifunctional in foods, cosmetics, and the chemical industry. These include food preservation, maintaining pH, skin conditioning, and even anti-aging. Lactic acid has therefore proven to be multifunctional in the light of fermentation. It has been used as a complexing agent, mosquito repellent, and also as an ingredient in cosmetics for rejuvenation of the skin and skin moisturizing [11].
2.3. Acetic Acid Fermentation
Acetic acid is an organic acid that is widely needed owing to its strong preservative capability and its characteristic sensory properties; its synthesis is often carried out synthetically or via microbial fermentation. The common microbial producers are Acetobacter, Gluconacetobacter, and Gluconobacter. Acetic acid is generally used in food applications, including vinegar and pickled vegetables [12]. Acetic acid can also be produced from petroleum-derived substrates such as butane and ethylene. Microbial fermentation is again an attractive sustainable option, in particular for food-grade vinegar production [13].
3. Novel Food Fermentation Technologies
3.1. Precision Fermentation
Precision fermentation is an up-and-coming trend that leverages the advances of synthetic biology, which programs microbial organisms to synthesize specific and complex organic compounds as seen in Table 2. Motivated by the need to make food production sustainable, the technology provides alternative ways of producing key components of food—carbohydrates, lipids, and proteins—from conventional agriculture [14,15]. Precision fermentation, using engineered microbial strains as cell factories, can provide ways to produce high-value ingredients for food and pharmaceuticals, thereby expanding the possibilities for producing nutrient-rich food at scale [16].
Figure 1 depicts an increasing trend in the number of publications on precision fermentation from 2010 to 2024. It can be observed that from 2010 to 2018, there was a steady increase in publications; thereafter, the trend showed a more sudden growth starting in 2019, with a sharp increase in 2023 and 2024. Clearly, from the trend, there has been a growing interest in, and research on, the field of precision fermentation over the last decade. Figure 2 describes the step-by-step operation of precision fermentation in the food industry for novel functional products like animal-free milk. The process shows how specific microbial processes may be tapped for sustainable and creative food solutions.
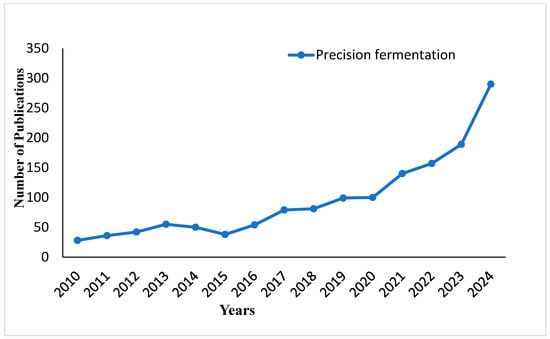
Figure 1.
Publication trends on precision fermentation from 2010 to 2024.
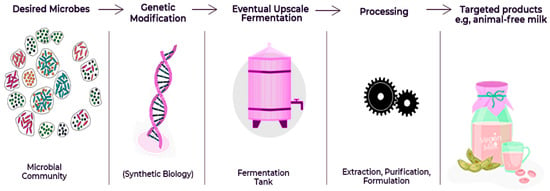
Figure 2.
Application of precision fermentation in food processing.
The most commonly used microorganisms are filamentous fungi, especially species of Trichoderma, particularly T. reesei, Saccharomyces cerevisiae, Pichia pastoris Komagaetella phaffii, and some Bacillus species. Most of these microorganisms are generally considered GRAS, and their GRAS status is further enhanced by synthetic biology and genetic engineering in order to optimize target production and yield in substrate conversion [17]. Furthermore, recent advances in synthetic biology and multi-omics tools have unraveled vast microbial biodiversity, enabling precision control over fermentation processes to precisely tailor products to specific quality standards [18].
Precision fermentation enables animal-free proteins, such as Perfect Day’s dairy proteins and Every Company’s egg proteins, to be produced [19,20]. These represent a movement toward an environmentally sustainable source of food that mimics, in taste and nutritional properties, animal-derived products but with less effect on land and marine ecosystems [21,22].
Nevertheless, the logistics and economics of transporting agricultural by-products or food waste to fermentation facilities pose a big challenge. Co-location of fermentation facilities with food production locations may go some way toward overcoming this problem by using available processing by-products [23]. In addition, if precision fermentation is to scale up to commercially viable levels, it must overcome a series of challenges in bioreactor adaptation (adapting bioreactors to accommodate the unique requirements of specific microorganisms, such as nutrient availability, temperature, and pH levels, while maintaining high yields), and molecule isolation (isolating desired molecules from microorganisms efficiently and cost-effectively). Its current limitations in understanding microorganism behavior and environmental adaptation within bioreactor systems impacts scalability [24].
3.2. Microbial Consortia
Microbial consortia are promising, collaborative approaches to fermentation that exploit the synergy of several microbial species, complementing each other’s metabolic activities. In contrast to the traditional monoculture, where a single strain performs all functions, microbial consortia divide labor among different strains optimized for some metabolic or biosynthetic tasks. This division of labor significantly increases process efficiency, reduces the metabolic burden on individual strains, and thus potentially yields more robust production capabilities [25,26].
The trend in the number of publications on microbial consortia from 2010 to 2024 is shown in Figure 3. From 2010 to 2016, there is a steep increase in research activity, with the number of publications reaching a peak of about 900 in 2016. After this peak, there is a sharp decline between 2017 and 2020, with the number of publications falling to approximately 400 by 2020. From 2021 onward, the field sees a period of relative stability, followed by a modest resurgence in 2024, where the publication count rises slightly above 500. The trend indicates fluctuating but sustained interest in microbial consortia research, with renewed attention in recent years. Figure 4 illustrates how important microbial consortia are for the production of fermented foods; it indicates their role in generating flavor and improving the quality of food through the activities of umami amino acids and peptides. Umami, often referred to as a “delicious savory taste,” is part of the basic flavor profile experienced in many food items. The figure highlights how microbial consortia increase the content of functional components in fermented foods, for example, the antioxidant tetramethylpyrazine (TMP) and rutin. Additionally, the figure presents the ability of microbial consortia to modulate and decrease the production of undesirable compounds, such as furfural and geosmin, during fermentation.
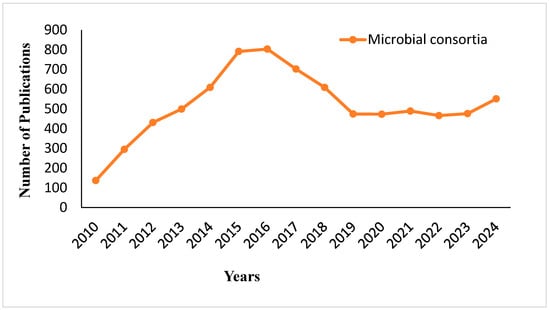
Figure 3.
Publication trends on microbial consortia in food fermentation from 2010 to 2024.
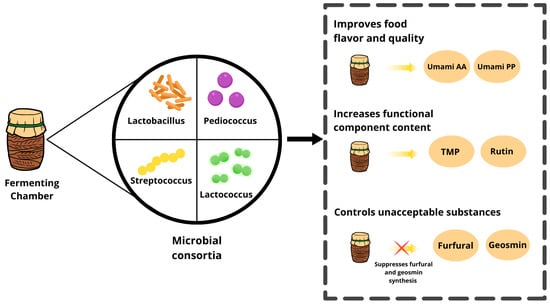
Figure 4.
Benefits of microbial consortia in production of fermented foods.
Synthetic biology plays a vital role in the engineering of microbial consortia for targeted applications. The distribution of genetic circuits across different strains enables complicated functions such as biofilm formation, logic gate construction, and the production of multiple compounds in one bioreactor setup [27]. Scientists might also engineer the populations of microbes to communicate and synchronize activities through quorum sensing or chemical signaling so that there are stable interactions and a reliable output. For example, Bacillus subtilis communities have been engineered to produce biofilms that improve the output of metabolic pathways for higher levels of targeted metabolites and distributed protein production [28].
However, there are still critical barriers to the full realization of the potential of microbial consortia for industrial-scale applications. The artificially constructed microbial communities are inherently unstable due to possible competition, mutations, and environmental variability. Control over those interactions will require continued advances in genetic stability, orthogonal communication pathways, and more precise mechanisms of population control, to prevent overgrowth and mutations from driving declines in production efficiency [29]. Continued research in the optimization of such consortia for stability and productivity is a prerequisite to exploring their full potential.
3.3. Gene Engineering
Gene engineering in microbial fermentation tries to develop the best strains with the desired characteristics of industrial enzyme production. Compared with plant or animal sources of enzymes, microbial enzymes have intrinsic advantages in terms of scalability, yield, and specificity. Therefore, microbial sources, especially genetically modified organisms (GMOs), are a major source for producing food enzymes, since genetic modifications can be relatively easily conducted to enhance production efficiency, enzyme stability, and reduce the production of undesired by-products [30].
Gene engineering has played a significant role in the enhancement of the fermentation process through the manipulation of microbial genomes. Using CRISPR-Cas9 and other genome editing tools, it is possible to instruct the microorganisms to ferment almost any substrate, optimize their stress response, and produce higher levels of the desired metabolites. For instance, yeast strains have been genetically modified for the production of ethanol and lactic acid in industrial fermentation processes. These modifications not only enhance the quantity of the products but also the ability of the process to work under a wide range of fermentation conditions, thereby reducing the cost of production [31].
The trend of publications on gene engineering from 2010 to 2024 is presented in Figure 5. The data demonstrate a constantly increasing trend of research activity over the years, with a gradual rise observed from 2010 to 2014. There is a marked increase from 2015 onwards; by 2020, the number of publications crosses the 1000 mark. Even after 2020, although there are minor ups and downs, research in the field continues to remain at a high activity level and reaches around 1100 publications by 2024. It reflects the consistent global interest in developments in gene engineering. Figure 6 shows the genetic engineering of enzymes obtained from microorganisms for a variety of uses in food and chemical industries, showing how genetic changes can tune enzyme function, enabling their applications across different industrial manufacturing processes.
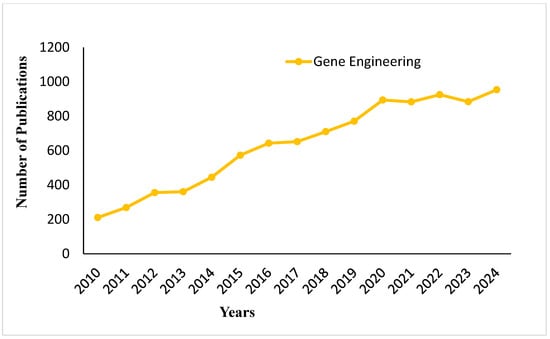
Figure 5.
Publication trends on gene engineering in food fermentation from 2010 to 2024.
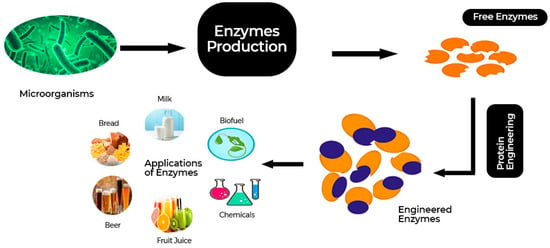
Figure 6.
Application of gene engineering for enzyme production.
The design of genetically modified microorganisms (GMMs) usually begins with the selection of a host organism, chosen based on the intended industrial application and considering criteria such as the amount of the desired enzyme to be produced, the cost of production, or simplicity in cultivation. Having identified a suitable host, scientists concentrate on adding specific genetic elements, such as expression vectors, selection markers, and promoter sequences, all designed to yield optimal levels of the enzyme in question within the host cell. The choice of transformation method is also critical and should be tailored to ensure high integration efficiency and genetic stability through generations [32].
Safety assessments of the microbial strains applied in food manufacturing are statutory requirements, particularly where genetic modification is concerned. Applicants for GMMs should provide a detailed taxonomic identification, a history of safe use, and qualifications under standards such as the Qualified Presumption of Safety (QPS) status. Technical and toxicological assessment should further ensure that the GMM does not produce harmful substances such as toxins, allergens, or other unwanted by-products [33]. While rigorous, these analyses are important for the assurance of public safety and consumer acceptance of GMM-produced enzymes in increasingly large-scale food processing.


Table 1.
Comparison of traditional and novel fermentation technologies.
Table 1.
Comparison of traditional and novel fermentation technologies.
| Parameter | Traditional Fermentation | Novel Fermentation | References |
| Process Control | Relies on natural conditions, leading to varied outcomes and potential off-flavors | controlled environments ensure consistency. | [34,35,36,37] |
| Example: Acetic acid fermentation for vinegar, allowing for manual regulation of oxygen levels. | Example: Advanced AI and methods, like quorum sensing in precision brewing, improve microbial signal control and genetic engineering. | ||
| Sustainability | Focuses on local resources but can demand significant water and energy, generating waste. | Sustainability goals aim for resource-efficient practices and waste reduction in production. | [38,39] |
| Example: Alcoholic fermentation in wine exemplifies high water and energy demands | Example: Precision fermentation minimizes high water and energy demands. | ||
| Production Consistency | Variability in flavor and texture results from reliance on natural fermentation and external factors, affecting product quality. | The fermentation processes tend to be better controlled by strain selection to reach higher reliability in product quality. | [40,41] |
| Example: Swiss cheese from propionic acid fermentation tends to vary in texture and flavors. | Example: Precision fermentation standardizes dairy proteins across plant-based products. | ||
| Fermentation Time | Can take longer (days to weeks). | The time of fermentation is shortened due to the improvement of the processes and active growth of the engineered strains. | [42] |
| Example: The fermentation of cabbage into sauerkraut takes about 1 to 4 weeks through lactic acid fermentation. | Example: Precision fermentation optimizes production time for dairy proteins. | ||
| Product Diversity | Conventional techniques produce diverse products influenced by microbial diversity, reflecting cultural heritage. | Novel technologies enable specific product qualities that traditional methods cannot achieve such as cultured meat proteins. | [42,43] |
| Example: Saccharomyces cerevisiae in alcoholic beverages and various fermented foods like ‘fufu’ and ‘dosa’. | Example: Innovative methods, like synthetic biology and metabolic engineering, create diverse products, including lab-grown proteins and bioactive compounds. |
3.4. Synthetic Biology and Metabolic Engineering
Metabolic engineering and synthetic biology are two closely interrelated fields that together advance the frontiers of bioengineering to enable desired metabolite production in microorganisms, plants, and other biological systems. Synthetic biology primarily involves the construction of genetic circuits, devices, and organisms through integration of genetic parts, such as promoters, coding sequences, transcriptional factors, and binding sequences, while simultaneously bringing quantitative data together to enable predictive models of biology [44]. The data-driven approach becomes one of the founding stones of metabolic engineering, which is the manipulation of cellular functions in specific organisms to produce the desired products from low-cost substrates to increase yield and decrease undesirable byproducts [45].
Synthetic biology aids in fermentation by developing new strains of microorganisms with the help of new metabolic pathways. These engineered microbes are capable of synthesizing high-value products, such as vitamins, amino acids and bioactive peptides, during the fermentation process [46]. The control of microbial activity in fermentation by synthetic circuits guarantees the reproducibility of the process and its effectiveness, even when the environmental conditions change. For instance, it has been possible through synthetic biology to produce compounds, such as recombinant proteins and enzymes, through the fermentation of microorganisms and without the need of extraction [47].
Figure 7 shows the trend in publications on synthetic biology and metabolic engineering for the same period. A growth in publications begins in this area from about 2014, with a sharp rise between 2015 and 2018. From 2019 onward, the publications stabilize at 500 and upward to 2023. Note the upward trend in 2024, reaching roughly 700 publications. The upward trend may reflect an increased interest concerning metabolic and synthetic biology discoveries with industrial applications. Figure 8 illustrates how metabolic engineering and synthetic biology can be used to develop microorganisms with improved functional properties. Such engineered microorganisms confer other beneficial characteristics to commonly known fermented foods, improving their nutritional and functional profiles.
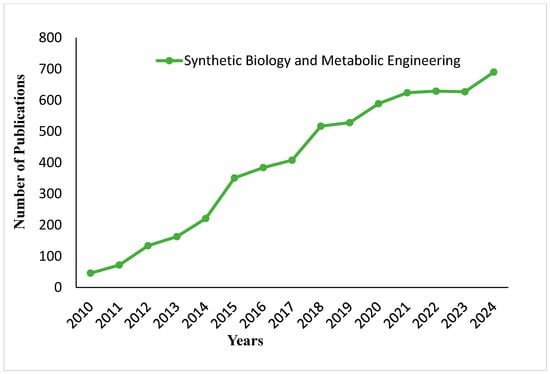
Figure 7.
Publication trends on synthetic biology and metabolic engineering in food fermentation from 2010 to 2024.
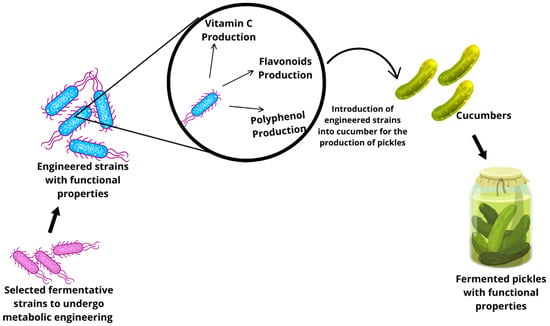
Figure 8.
Application of metabolic engineering and synthetic biology.
Metabolic engineering is used to alter the metabolic pathways of microorganisms in a way that will increase fermentation yield. Through the control of metabolic flow, it is possible to increase the production of bioactive metabolites such as probiotics, organic acids, and peptides. Furthermore, this approach also reduces the formation of side products and improves the substrate conversion [48]. For instance, the metabolic engineering of Lactobacillus strains has led to the development of probiotics with enhanced potency and bioavailability, leading them to play a more significant role in the growth of the functional food market [49].
Some of the feats achieved through metabolic engineering have included nutritionally enhanced crops. For example, synthetic biology has been used to engineer rice to produce beta-carotene, a precursor of vitamin A, creating what has been dubbed “Golden Rice.” This innovation offers a solution to vitamin A deficiencies in regions reliant on rice as a staple and thus improves diets in at-risk populations [50]. One of the most promising metabolic engineering approaches is metabolic flux manipulation, which seeks to enhance efficiency in metabolic pathways so as to obtain the maximum yield of a product by controlling cellular resources and inhibitors.
However, engineering the introduction of synthetic pathways into living organisms often faces challenges. For instance, the addition of exogenous genetic elements may interfere with the native functions of a host organism by affecting protein stability, gene expression, and growth rates [51]. The enhancement of target metabolite production must be balanced with an organism’s native functions, as sometimes the side effects can result in reduced viability or metabolic dysregulation. These challenges demand the thoughtful design of metabolic pathways and advanced genetic tools to ensure compatibility and minimize perturbations, which clearly indicates the further development of pathway integration strategies.
3.5. Quorum Sensing Regulation
Quorum sensing (QS) is a complex communication system among bacteria that allows for coordinated responses to environmental conditions through the detection of changes in cell-population density. The process regulates microbial behaviors in relation to food safety and quality, including biofilm formation, virulence, and stress adaptation [52]. In the context of food preservation, quorum sensing inhibition (QSI) holds great promise for reductions in bacterial spoilage, as it can intervene with biofilm formation—an important factor contributing to bacterial persistence and resistance [53]. Some foods have been indicated to exhibit quorum quenching properties in a natural way. These are mostly linked to defensive compounds in plant and animal tissues or microbial adaptations that neutralize QS activity coming from nearby microbes. Among probiotics, Lactobacillus strains show quorum quenching potential, with L. plantarum and L. curvatus able to suppress the motility and biofilm formation of pathogens [54]. Through QS inhibition, these probiotics reduce the virulence of pathogens, paving new ways to food preservation and biofilm control.
Figure 9 shows the trend of the number of publications on quorum sensing regulation from 2010 to 2024. Most of the data points indicate a constant rise, with an initial rapid increase between 2010 and 2011, followed by a gradual but upward trend from 2012 to 2020. This steadily increases after a slight plateau around 2021 and 2022, thus showing that the field has attracted continued interest over the last decade. Figure 10 is a short explanation of the quorum sensing and quorum sensing inhibition process. Quorum sensing is a form of communication between bacterial cells, where signaling molecules, such as acyl-homoserine lactones (AHLs) and autoinducing peptides (AIPs), produced by bacteria in a community, are recognized by specific receptors on other bacteria belonging to the same species. In contrast, quorum sensing inhibition prevents this communication with the introduction of AHL and AIP analogs. These analogues, by inhibiting the binding of signaling molecules to receptor sites, interfere with bacterial communication; therefore, they inhibit the production of biofilms that are antibiotic-resistant.
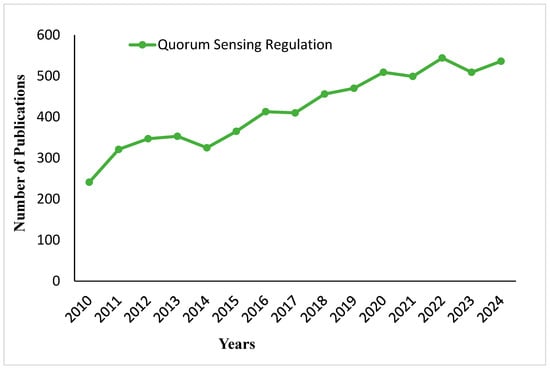
Figure 9.
Publication trends on quorum sensing regulation from 2010 to 2024.
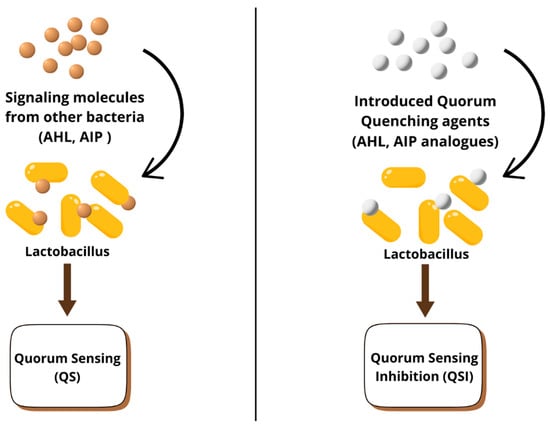
Figure 10.
Quorum sensing inhibition.
While QS and quorum quenching (QQ) present promising avenues for research, the translation of in vitro findings to industrial applications is still restrained. Although much of the research showing the effectiveness of QQ has been conducted in laboratory settings, there is a great need for large-scale studies in real food systems [55]. The stability and effectiveness of QQ require further validation in different environments such as various food matrices and under variable storage conditions. This constraint demands more research to stabilize QQ molecules and study their effects in dynamic industrial settings to fully realize the potential of their beneficial impacts on food safety.
3.6. High-Pressure Processing (HPP)
High-pressure processing (HPP) has emerged as a significant advancement in sustainable food processing, responding to growing consumer concerns related to food safety, nutritional quality, and environmental impact. Unlike traditional thermal methods, HPP employs pressures of up to 600 MPa to eradicate pathogenic and spoilage microorganisms, reduce antibiotics and pesticides in foodstuffs, and maintain the nutritional and sensory attributes of food products as seen in Table 2 [56,57]. This technology has shown promise in applications from dairy products to seafood, fruits, vegetables, honey and ready-to-eat meals, by possibly aiding in the preservation of bioactive compounds and the extension of shelf life, offering clean-label food products devoid of synthetic preservatives [58,59]. Continuous high-pressure processing (CHPP) is a novel method of food preservation, operating on a continuous flow system in contrast to the traditional batch HPP. It is ideal for liquid and semi-liquid products, such as juices, soups, and sauces, in order to provide extended shelf life, safety, and nutrient retention without chemical preservatives [60].
Figure 11 illustrates the publication number of high-pressure processing studies from 2010 to 2024. From the chart, it can be seen that publications have grown constantly over the years; a steady upward trend in growth is seen from 2010 to 2018. Starting from 2019, the rising trajectory continues, with slight ups and downs between 2021 and 2023, reaching the highest recorded value in 2024. This indicates that the research interest in high-pressure processing technologies remains constant and tends to increase. Figure 12 shows how desired microorganisms can be preserved during high-pressure processing, a technique known for destroying most microorganisms except spores. The figure shows the encapsulation of desired microorganisms in cocoa butter, which protects them from the low temperatures of freeze-drying and the destructive forces of the HPP machine.
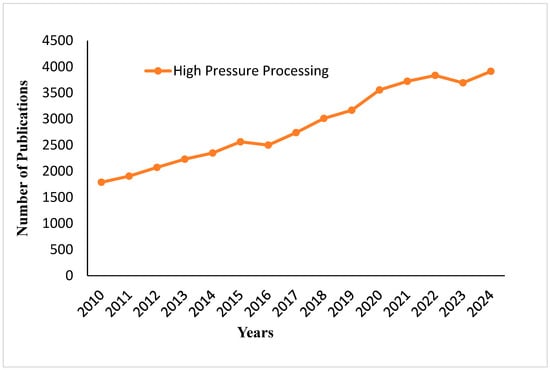
Figure 11.
Publication trends on high-pressure processing from 2010 to 2024.
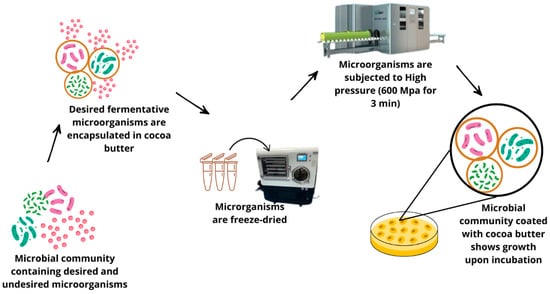
Figure 12.
Application of high-pressure processing (HPP).
High-pressure processing (HPP) has been found to be effective in enhancing fermentation processes through the control of microbial activity and substrate utilization. HPP can activate stress responses in microorganisms which results in increased enzyme activity and improved metabolic activity which leads to increased fermentation rates [61]. Furthermore, HPP is used as a pre-treatment for raw materials where the cell structures are disrupted in order to release fermentable substrates for the microorganisms. It also enhances the formation of bioactive compounds, such as antioxidants and peptides, through the induction of secondary metabolite biosynthesis in response to stress. Furthermore, HPP can also help to eliminate other undesirable microorganisms in the system, thereby enabling the starter cultures to grow and dominate, guaranteeing proper and quality fermentation [62]. A study carried out by [63] showed a solution for a common problem with high-pressure processing: preserving the beneficial microbes necessary for fermentation or bio-preservation. The HPP process usually inactivates nearly all microorganisms, often including those that may be purposely added to foods. Researchers have shown that the encapsulation of microorganisms under a thin layer of cocoa butter protected the beneficial microorganisms during freeze-drying and subsequent pressure treatment. The encapsulated cultures survived under HPP conditions at 600 MPa at 5 °C for 3 min, eventually showing some growth during further incubation (Figure 12), whereas non-encapsulated ones were almost fully inactivated.
Pressure-tolerant microorganisms, existing ones or the newly developed ones, work well with HPP, which opens new possibilities for fermentation and product creation. HPP also enables the control of fermentation profiles as the pressure applied alters the activity of the microorganisms responsible for fermentation to set the desired flavors, textures, and nutrient profiles [64]. Not only does HPP increase efficiency, it also enhances the safety of food by inhibiting the growth of spoilage microorganisms. Its application in fermentation is a way of enhancing product quality, nutritional content, and production yields; therefore, it can be seen as a vital technology for the future of fermentation processing [65].
HPP has some challenges that restrict its wider industrial application in the food industry, with the first being the high cost due to energy-intensive processing. The pressure levels—usually between 300 and 600 MPa—necessary to effectively run the process can only be realized using machinery able to sustain very intense conditions, which is usually up to seven times more energy-intensive than that needed for other common processing technologies such as heat processing [66]. These costs are further compounded by the expensive nature of HPP equipment—both its acquisition and maintenance—which can make the technology economically unfeasible for smaller-scale producers.
Another critical limitation is that HPP is not efficient in the inactivation of bacterial spores. Due to their highly resistant nature regarding high pressures, additional heat treatments need to be combined with HPP in order to make food safe. However, this dual approach can affect the sensory and nutritional qualities of food and reduces the energy efficiency of the process, which contradicts the aim of sustainability. One area that still needs innovation for the wider application of HPP in the food industry is spore inactivation [67].
Scalability and equipment design also prove to be a challenge. Most HPP systems, being batch in nature, are limited in throughput and less efficient compared to methods using continuous processing. The development of cost-effective, continuous-flow HPP systems is important but is still at early stages, hence restrictive for industrial-scale implementation [68].
Furthermore, HPP is not effective in all food matrices; its efficacy may vary with food composition, pH, and water activity, which requires a significant amount of optimization for each product. This complicates process standardization and increases development costs. Lastly, greater consumer awareness of HPP is an indirect challenge in the sense that, although sold as a technology that really preserves freshness and nutritional quality in food, consumer skepticism, or a lack of familiarity, could restrain market acceptance, thus requiring investment in education and marketing [69].
These challenges will require focused research in the area of spore inactivation, equipment cost reduction, and the development of continuous processing systems. Collaboration among researchers, equipment manufacturers, and the food industry will be important in overcoming these barriers, which will finally unleash the full potential of HPP [70].
3.7. Enzymatic Fermentation
The use of microbial enzymes in fermentation is an age-old practice, founded on the manipulation of the ability of microorganisms to produce stable and multi-functional enzymes critical to the food industry. Many of these microorganisms, such as Aspergillus niger, Saccharomyces cerevisiae, and Bacillus subtilis, are sources of enzymes critical to the baking, brewing, and the dairy industries, as they provide consistency in the products and ensure maximal productivity in operations [71]. Amylolytic enzymes, in particular, hold great importance in the production of glucose and fructose syrups, and play fundamental roles in other industries such as confectionery and processed food production [72]. Industrially produced microbially derived enzymes are known for their high activity and scalability. Some of the widely produced enzymes include protease, α-amylase, and glucose isomerase. The discovery of glucose isomerase has greatly influenced the sweetener industry in its production of high-fructose corn syrup [73].
Figure 13 shows the number of publications on enzymatic fermentation from 2010 to 2024. The data show a progressive increase in research activity from 2010 to 2016, with a peak in 2017. Between 2018 and 2020, the number of publications leveled off; slight fluctuations have occurred from 2021 until 2024. This is indicative of persistent scientific interest in enzymatic fermentation, with minor ups and downs over the past few years. Figure 14 shows the production process of enzymes in several important steps, including enzyme production, solid–liquid separation, concentration and stabilization, germ filtration, enzyme formulation, and packaging. All the steps are interlinked to ensure the quality and efficacy of the final enzyme product.
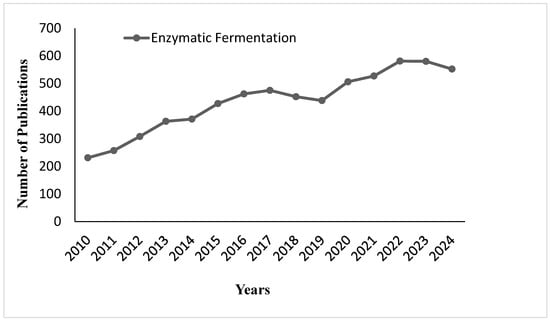
Figure 13.
Publication trends on enzymatic fermentation from 2010 to 2024.
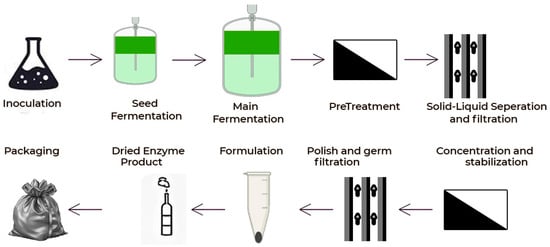
Figure 14.
Processes involved in enzymatic fermentation.
This, however, becomes a challenge at scale-up. Most scaled-up microbial production is expensive and complex, generally requiring sophisticated bioreactors and the tailoring of conditions for growth. It is also at risk of having high production costs and resource requirements, despite active research into cheaper solid-state fermentation (SSF) techniques and lower-cost substrates. These challenges must be met in order for enzymatic fermentation to maintain its importance in the current industrial field and to expand beyond its present limitations [73].
3.8. Artificial Intelligence (AI)-Driven Fermentation Optimization
Artificial intelligence has been one of the most important tools in the optimization of fermentation processes, offering new data-driven approaches with the potential to revolutionize bioprocessing by raising conversion efficiency and reducing costs. Advanced algorithms, such as genetic algorithms and neural networks, can be used to develop flexible models of fermentation processes that closely mimic the actual processes. Consequently, these models make the online prediction of variables, such as substrate concentration and alcohol production, possible, enabling the accurate control of fermentation conditions in order to increase both yields and operational efficiency [74,75].
AI-driven fermentation optimization revolutionizes the way functional foods are developed. It makes possible the most precise control over fermentation parameters and maximizes nutritious value in the products. Figure 15 shows the number of publications on artificial intelligence (AI)-driven fermentation optimization from 2010 to 2024. The chart shows very minimal research activity between 2010 and 2018, with a gradual increase starting in 2019. A big surge in publications is seen from 2021 onwards, with an exponential rise reaching the highest value in 2024. This trend shows the rapidly growing interest and advancement in integrating AI technologies into fermentation optimization over the past few years.
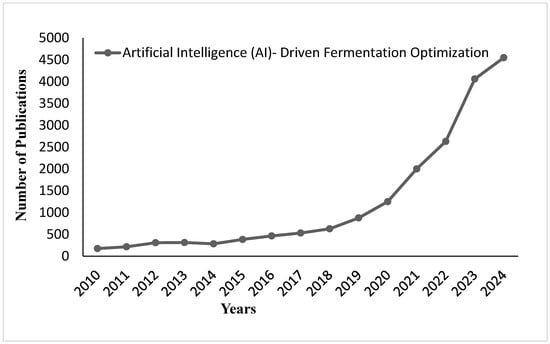
Figure 15.
Publication trends on AI-driven fermentation optimization from 2010 to 2024.
Figure 16 emphasizes the artificial intelligence (AI)-driven fermentation processes. AI has been found to be of utmost importance in the prediction of outcomes in fermentations, depending on the input data, hence allowing for optimization and increasing efficiency in the fermentation process.
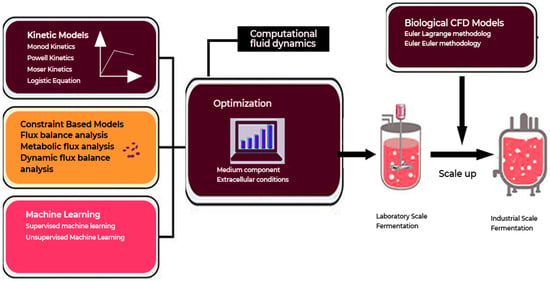
Figure 16.
An AI-driven fermentation optimization process.
AI algorithms analyze a huge amount of data originating from fermentation processes, including microbial behavior, the production of metabolites, and the environmental conditions for process optimization towards desired outcomes [76]. It ensures consistent quality, reduces the wastage of nutrients, and optimizes synthesis to their maximal potential for bioactive compounds. Similarly, AI supports the real-time monitoring and predictive modeling of adjustments in fermentation parameters for maintaining microbial balance and increasing the production of probiotics, antioxidants, and other functional components [77].
Machine learning models can also predict microbial interactions with each other, optimizing coculture systems that produce synergistic effects on functionality. The models also support strain selection and engineering based on the identification of microbes with specific metabolic pathways to produce targeted functional compounds [78]. The integration of AI in the fermentation process enables the producer to tailor functional food profiles, including enhanced gut health benefits, improved bioavailability of nutrients, and designer sensory attributes. Equally, the potential to significantly reduce waste and smooth out production flows means that AI-driven fermentation is even more in tune with nature, and is, therefore, a game-changer in modern food science.
Using advanced feed control methodology and genetic algorithms, artificial intelligence adjusts parameters by building mathematical models that optimize process regulation and predict configurations for further improvement, with reduced reliance on manual adjustments [79]. Moreover, the AI-driven sensor, or virtual analysis, can enable autonomous monitoring to have direct insight into the fermentation process, even where it is impossible to use physical sensors in certain scenarios. This will then allow for adaptive adjustments in these complex systems, increasing the quality of production while allowing for the data collection required for continuous optimization [80].
In the area of enzyme engineering, AI has been of great benefit in saving computation time and speeding up the discovery of optimized enzymes for food processing (Figure 16). For instance, AlphaFold, an AI-driven tool, has predicted the structures of more than 200 million proteins, ushering in a new era of structural biology and enzyme design [81]. This capability allows for the rapid and inexpensive discovery of enzymes that can drive innovation in food biotechnology while simultaneously tackling experimental roadblocks and enhancing production efficiencies [82,83].
While the promise of artificial intelligence-assisted fermentation optimization holds great potential, there are also significant barriers to the migration of data-driven frameworks from experimental settings to practical industrial applications. Laboratory investigations typically involve controlled parameters and comprehensive genetic information; however, the adaptability of these models to high-throughput processes requires further modification to adjust for natural variability among industrial substrates and conditions [75]. Consequently, though it provides groundbreaking insight, the practical scalability and integration of artificial intelligence into commercial systems—for which integration is necessary to ensure reliable industrial applications—are still under investigation [80].
3.9. Immobilized Enzymes Fermentation
The use of enzymes in food production is quite old; however, specific disadvantages of these biocatalysts—instability and sensitivity toward processing conditions—have been major constraints on industrial applications. Immobilized enzyme technology overcomes these drawbacks, since enzymes are anchored to carriers, often inert polymers or inorganic supports, which enhances their reusability and stability as seen in Table 2. This technique not only stabilizes the enzymes during varying process conditions but also makes industrial applications more economically viable by reducing product contamination and allowing for the recycling of enzymes through multiple cycles [84].
Discovery of the right carriers, with features including porosity, stability, and scalability, will assist in immobilization efficiency. Suitable carriers enable the availability of active sites and contribute to the physicochemical stability of enzymes. Such features enhance industrial applications in various fields such as food and pharmaceuticals. Figure 17 shows the trend in the number of publications on immobilized enzyme fermentation from 2010 to 2024. From the graph, one can see that there was a progressive increase in the number of research studies from 2010 to 2013, and a steep rise, reaching its peak in 2014. In general, after 2015, the trend is fluctuating; publication numbers have stabilized at around 40 publications per year after 2017, although slight drops are observed in 2021 and 2023. This indicates consistent interest in the research topic of immobilized enzyme fermentation over the past ten years, with variations in publication output. Figure 18 shows enzyme immobilization methods such as adsorption, covalent bonding, entrapment or encapsulation, and crosslinking. These methods confer the advantage of increased stability, reusability, and efficiency of the enzymes in several industrial applications.
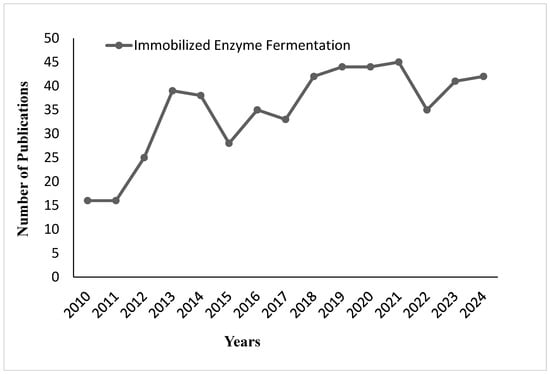
Figure 17.
Publication trends on immobilized enzyme fermentation from 2010 to 2024.
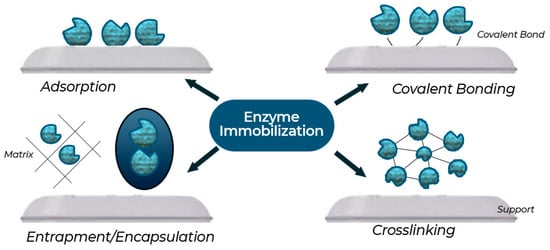
Figure 18.
Enzyme immobilization methods.
Advanced carrier technology has presently enabled enzymes, such as lipases and proteases, to catalyze a number of transformations, including hydrolysis, esterification, and aminolysis. This versatility has widened their roles, especially in food processing applications, where they participate in flavor development, dairy product stabilization, and lipid modification in products such as cheese and yogurt [85,86].
However, immobilized enzymes present some limitations, such as higher sensitivity toward severe changes in temperature and pH, which may deteriorate their catalytic activity [87,88]. While many traditional heterogeneous chemical catalysts work well in a variety of solvents, enzymes tend to exhibit optimal activity in water, which complicates the non-aqueous processes for which the binding to the solid carrier becomes ineffective. Moreover, the application of immobilized enzyme systems remains costly and complex when scaled up, revealing a requirement for the further development of more affordable, sustainable carriers and improved tolerance to fluctuations in processing conditions [89].


Table 2.
Key innovations in fermentation technology and their benefits.
Table 2.
Key innovations in fermentation technology and their benefits.
| Innovation | Description | Nutritional/Functional Benefits | References |
| Precision fermentation | Using microorganisms, like yeast, fungi, or bacteria, at the molecular level enables the production of proteins, enzymes, fats, and other complex compounds. | Benefits include controlling allergens and reducing cholesterol and saturated fats. | [90] |
| Microbial consortia | This involves a structured microbial community (bacteria, fungi, algae, archaea) that coexists and interacts in a shared environment. | For producing probiotics and improving protein quality. | [91] |
| Synthetic Biology and Metabolic engineering | Applying biological modifications for uses in healthcare, industry, agriculture, and environmental protection. | Enhanced production of essential nutrients, including vitamins, amino acids, and omega-3s. | [92] |
| Genetic engineering | Genetically modifying microorganisms for targeted compound production. | Creating functional foods while enhancing flavor and extending shelf life. | [93] |
| Quorum sensing regulation | It serves as a powerful tool for fine-tuning fermentation processes. | Enhanced bioavailability, community stability, and resistance mechanism. | [94] |
| High-pressure processing (HPP) | A high-pressure method that preserves food by inactivating harmful microbes and enzymes without heat. | Maintains vitamins, bioactive compounds, flavor, aroma, and reduces enzyme activity, extending shelf life. | [95] |
| Enzymatic fermentation | It is a biological process where enzymes (protein catalysts) convert organic compounds into simpler substances. | Enhances nutrient bioavailability, increases levels of vitamins, and reduces allergens and toxins. | [96] |
| Artificial Intelligence (AI)-Driven Fermentation Optimization | AI technologies, like machine learning and data analytics, enhance fermentation in food and biotechnology. | Improved nutrient retention, higher probiotic content, consistent quality, and shorter fermentation times. | [97] |
| Immobilized Enzymes Fermentation | Enzymes are immobilized on a solid support rather than suspended freely in the medium. | Enzyme reusability, cost savings, improved stability and activity, and better control over fermentation, resulting in increased yield and purity. | [98] |
4. Safety Issues and Risk Assessment of New Fermentation Technologies
Factors that determine the growth of pathogenic microorganisms in fermented food products include the quality of raw materials, the processing environment, and hygiene practices. Inferior ingredients are exemplified by Vietnamese nem chua, a traditional raw sausage that often contains contaminants, including Staphylococcus aureus and Escherichia coli, especially when good manufacturing practices (GMPs) are not implemented [99,100]. The quality of water used in fermentation will also directly impact food safety, especially in rural areas with limited access to treated water, increasing the risks of contamination with pathogens such as Salmonella spp. and E. coli [101].
In many cases, fermented products in low-resource areas may use reused, non-sterile containers to package products, further increasing contamination risks [102]. Moreover, antibiotic residues in foods from the treatment of livestock may introduce antimicrobial resistance genes to consumers and might promote multidrug-resistant bacterial strains. The transfer of resistance genes between foodborne pathogens through horizontal gene transfer demonstrates the need for close monitoring in terms of antibiotic use and the presence of these genes in starter cultures and the final products [103].
One of the significant secondary products detected in some fermented food products, especially those rich in free amino acids, is the presence of biogenic amines (BAs). Higher levels of BAs, deriving from decarboxylation or transamination reactions, may signal spoilage and potential health hazards [104]. Contamination of pathogens in the fermentation stage may mean an increased risk in exposing consumers to fermented products, especially in the case of dry or semi-dry fermented sausages; therefore, more stringent safety controls and microbial surveillance throughout the production chain must be guaranteed [105]. Despite being linked to various health advantages, the safety of fermented products presents a complex issue stemming from traditional and spontaneous fermentation processes that incorporate diverse control mechanisms and quality evaluations.
5. Market Trends and Consumer Acceptance of Fermented Foods
The fermented foods market has grown considerably worldwide as the importance of gut health and nutrient assimilation becomes more recognized by consumers. Foods that are fermented, such as yogurt, kimchi, and kombucha, provide good environments for beneficial microorganisms, especially probiotics, which can improve digestion and contribute to overall well-being [106]. The demand for products containing probiotics has seen a rising trend, as reflected in the valuation of the fermented foods market, which was estimated to be around USD 578.1 billion in 2023, driven by consumer trends in functional food choices [107]. Species of Lactobacillus, for example, hold great industrial applications owing to their genetic expression profiles, enabling them to tolerate environmental stressors. In the UK alone, more than one billion euros in sales of probiotic yogurt were recorded in 2021, pointing out the increasing market for fermented dairy [108].
Fermented food matrices, such as yogurt, kefir, and fermented milk, are good probiotic delivery vehicles. This matrix may convey viable fermentation microorganisms to the consumer, anticipating current health trends and the popularity of products such as kefir—which is about to see global growth of more than USD 500 million by the year 2032 [109]. Despite the increased consumer interest, some fermented products may present microbial safety issues or inconsistent probiotic content; there is an industrial need for standardization in order to maintain consumer confidence and assure product effectiveness.
6. Future Directions and Opportunities in Novel Fermentation Technologies
Innovations in fermentation depend on greater knowledge of microbial genetics and how they function metabolically. Knowledge of gene–environment interactions can help to improve lactic acid production by choosing strains based on genetic markers that identify favorable metabolic traits. The introduction of high-throughput sequencing (HTS) has allowed for an in-depth examination of the microbial communities of fermented foods, thus creating a basis for tailored strain selection and higher production efficiency [110].
Moreover, genomics has clarified the mechanisms in Lactobacillus that play a role in proteolysis—crucial for the development of flavor and texture—by transforming amino acids into particular flavor compounds [111]. Although fermentation has long been used as a means to preserve food, traditional or spontaneous approaches to fermentation are always at risk of contamination by pathogenic microorganisms. This fact underlines the need for genomic research to enable the detection of such harmful microorganisms at an early stage. Synthetic biology and genomics carry huge potential in terms of engineering strains to convert non-food biomass into nutritious, safe, and consumer-friendly products through fermentation [112].
7. Conclusions
Rapid fermentation technologies have opened up new food production avenues in line with consumer preferences for health-benefiting products characterized by better nutritional profiles. Challenges still persist with respect to microbial safety, antibiotic resistance, and quality control. The integration of an AI-assisted fermentation approach with genomics and immobilized enzyme systems is opening up possibilities for enhanced efficiency, safety, and scalability in the production of fermented foods. Future innovations based on genomic insights and synthetic biology could further transform fermentation processes and unlock new potential for safer, nutritionally enhanced, and consumer-accepted products within the global food market.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Ağagündüz, D.; Yılmaz, B.; Koçak, T.; Altıntaş Başar, H.B.; Rocha, J.M.; Özoğul, F. Novel Candidate Microorganisms for Fermentation Technology: From Potential Benefits to Safety Issues. Foods 2022, 11, 3074. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, C.E.; Olaniran, A.F.; Adeyi, O.; Adeyi, A.J.; Ojediran, J.O.; Adewumi, A.D.; Iranloye, Y.M.; Erinle, O.C. Drying characteristics of fermented-cooked cassava chips used in the production of complementary food: Mathematical and Gaussian process regression modeling approaches. J. Food Process Eng. 2021, 44, e13715. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Puniya, A.K.; Dhewa, T. Enhancing Micronutrients Bioavailability through Fermentation of Plant-Based Foods: A Concise Review. Fermentation 2021, 7, 63. [Google Scholar] [CrossRef]
- Olojede, A.O.; Sanni, A.I.; Banwo, K.; Michael, T. Improvement of Texture, Nutritional Qualities, and Consumers’ Perceptions of Sorghum-Based Sourdough Bread Made with Pediococcus pentosaceus and Weissella confusa Strains. Fermentation 2022, 8, 32. [Google Scholar] [CrossRef]
- Galimberti, A.; Bruno, A.; Agostinetto, G.; Casiraghi, M.; Guzzetti, L.; Labra, M. Fermented food products in the era of globalization: Tradition meets biotechnology innovations. Curr. Opin. Biotechnol. 2021, 70, 36–41. [Google Scholar] [CrossRef]
- Mukherjee, A.; Gómez-Sala, B.; O’Connor, E.M.; Kenny, J.G.; Cotter, P.D. Global Regulatory Frameworks for Fermented Foods: A Review. Front. Nutr. 2022, 9, 902642. [Google Scholar] [CrossRef]
- Malomo, A.A.; Olawoye, B.; Olaniran, A.F.; Olaniyi, O.I.; Adedayo, A.; Adeniran, H.A.; Abiose, S.H. Evaluation of sugar and free amino acid during fermentation of ogi from maize, acha and sorghum. Food Sci. Appl. Biotechnol. 2021, 4, 111–118. [Google Scholar] [CrossRef]
- Perez, D.; Assof, M.; Bolcato, E.; Sari, S.; Fanzone, M. Combined effect of temperature and ammonium addition on fermentation profile and volatile aroma composition of Torrontes Riojano wines. LWT 2018, 87, 488–497. [Google Scholar] [CrossRef]
- Schwarz, M.; Rodríguez-Dodero, M.C.; Jurado, M.S.; Puertas, B.; Barroso, C.G.; Guillén, D.A. Analytical Characterization and Sensory Analysis of Distillates of Different Varieties of Grapes Aged by an Accelerated Method. Foods 2020, 9, 277. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, G.; Tian, X.; He, L.; Li, C.; Zeng, X.; Wang, X. Effect of Fermentation Parameters on Natto and Its Thrombolytic Property. Foods 2021, 10, 2547. [Google Scholar] [CrossRef]
- Kłosowski, G.; Mikulski, D.; Pielech-Przybylska, K. Pyrazines Biosynthesis by Bacillus Strains Isolated from Natto Fermented Soybean. Biomolecules 2021, 11, 1736. [Google Scholar] [CrossRef]
- Handa, C.L.; Lima, F.S.; Guelfi, M.F.G.; Fernandes, M.S.; Georgetti, S.R.; Ida, E.I. Parameters of the fermentation of soybean flour by Monascus purpureus or Aspergillus oryzae on the production of bioactive compounds and antioxidant activity. Food Chem. 2019, 271, 274. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, J.; Zhang, A.; Yang, S.T. Propionic acid fermentation. In Bioprocessing Technologies in Biorefinery for Sustainable Production of Fuels, Chemicals, and Polymers; Wiley: Hoboken, NJ, USA, 2013; pp. 331–350. [Google Scholar]
- Tubb, C.; Seba, T. Rethinking food and agriculture 2020–2030: The second domestication of plants and animals, the disruption of the cow, and the collapse of industrial livestockfarming. Ind. Biotechnol. 2021, 17, 57–72. [Google Scholar] [CrossRef]
- Hassoun, A.A.; El-Din Bekhit, A.R.; Jambrak, J.M.; Regenstein, F.; Chemat, J.D.; Morton, M.; Gudjónsdóttir, M.; Carpena, M.A.; Prieto, P. The fourth industrial revolution in the foodindustry—Part II: Emerging food trends. Crit. Rev. Food Sci. Nutr. 2022, 64, 407–437. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.V. Chapter 19 Medical biotechnology: Techniques and applications. In Omics Technologies and Bio-Engineering; Barh, D., Azevedo, V., Eds.; Academic Press: San Diego, CA, USA; Cambridge, MA, USA, 2018; Volume 1, pp. 449–469. [Google Scholar] [CrossRef]
- Gomes, A.M.V.; Carmo, T.S.; Carvalho, L.S.; Bahia, F.M.; Parachin, N.S. Comparison of yeasts as hosts for recombinant protein production. Microorganisms 2018, 6, 38. [Google Scholar] [CrossRef]
- Teng, T.S.; Chin, Y.L.; Chai, K.F.; Chen, W.N. Fermentation for future food systems. EMBO Rep. 2021, 22, e52680. [Google Scholar] [CrossRef]
- Nestlé Joins Forces with Perfect Day on Animal-Free Dairy Project. Available online: https://www.just-food.com/news/nestle-joins-forces-with-perfect-day-on-animal-free-dairy-project/ (accessed on 27 September 2024).
- Cullen, L. 5 Precision Fermentation Startups that Have Closed Major Funding Rounds. 2022. Available online: https://provegincubator.com/5-precision-fermentation-startups-that-have-closed-major-funding-rounds/ (accessed on 26 August 2025).
- Terefe, N.S. Recent Developments in Fermentation Technology: Toward the Next Revolution in Food Production; Academic Press: Cambridge, MA, USA, 2022; Chapter 5. [Google Scholar]
- Olojede, A.O.; Oahimire, I.O.; Gbande, J.I.; Osondu-Igbokwe, A.D.; Thomas, R.M.; Olojede, D.S.; Banwo, K. Evaluation of acha flour in the production of gluten-free sourdough cookies. Int. J. Food Sci. 2023, 58, 3244–3251. [Google Scholar] [CrossRef]
- Verni, M.; Minisci, A.; Convertino, S.; Nionelli, L.; Rizzello, C.G. Wasted bread as substrate for the cultivation of starters for the food industry. Front. Microbiol. 2020, 11, 293. [Google Scholar] [CrossRef]
- Nishi, T.; Ito, Y.; Nakamura, Y.; Yamaji, T.; Hashiba, N.; Tamai, M.; Yasohara, Y.; Ishii, J.; Kondo, A. One-step in vivo assembly of multiple DNA fragments and genomic integration in Komagataella phaffii. ACS Synth. Biol. 2022, 11, 644–654. [Google Scholar] [CrossRef]
- Du, P.; Huiwei, Z.; Haoqian, Z.; Ruisha, W.; Jianyi, H.; Ye, T.; Chunbo, L. De novo design of an intercellular signaling toolbox for multi-channel cell–cell communication and biological computation. Nat. Commun. 2020, 11, 4226. [Google Scholar] [CrossRef]
- Duncker, K.E.; Holmes, Z.A.; You, L. Engineered microbial consortia: Strategies and applications. Microb. Cell Fact. 2021, 20, 211. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Laneri, S. Spices, condiments, extra virgin olive oil and aromas as not only flavorings, but precious allies for our wellbeing. Antioxidants 2021, 10, 868. [Google Scholar] [CrossRef] [PubMed]
- Johnston, T.G. Compartmentalized microbes and co-cultures in hydrogels for on-demand bioproduction and preservation. Nat. Commun. 2020, 11, 563. [Google Scholar] [CrossRef] [PubMed]
- Lino, F.; Bajic, D.; Vila, J.; Sánchez, A.; Sommer, M. Complex yeast–bacteria interactions affect the yield of industrial ethanol fermentation. Nat. Commun. 2021, 12, 1498. [Google Scholar] [CrossRef]
- Salazar-Cerezo, S.; Kun, R.S.; de Vries, R.P.; Garrigues, S. CRISPR/Cas9 technology enables the development of the filamentous ascomycete fungus Penicillium subrubescens as a new industrial enzyme producer. Enzym. Microb. Technol. 2020, 133, 109463. [Google Scholar] [CrossRef]
- Ku, S.; Yang, S.; Lee, H.H.; Choe, D.; Johnston, T.; Ji, G.E. Biosafety assessment of Bifidobacterium animalis subsp. lactis AD011 used for human consumption as a probiotic microorganism. Food Control 2020, 117, 106985. [Google Scholar] [CrossRef]
- Herman, L.; Chemaly, M.; Cocconcelli, P.S.; Fernandez, P.; Klein, G.; Peixe, L.; Prieto, M.; Querol, A.; Suarez, J.E.; Sundh, I. The qualified presumption of safety assessment and its role in EFSA risk evaluations: 15 years past. FEMS Microbiol. Lett. 2019, 366, fny260. [Google Scholar] [CrossRef]
- Fraiture, M.A.; Deckers, M.; Papazova, N.; Roosens, N.H.C. Detection strategy targeting a chloramphenicol resistance gene from genetically modified bacteria in food and feed products. Food Control 2020, 108, 106873. [Google Scholar] [CrossRef]
- Elhalis, H.; Cox, J.; Zhao, J. Coffee fermentation: Expedition from traditional to controlled process and perspectives for industrialization. Appl. Food Res. 2023, 3, 100253. [Google Scholar] [CrossRef]
- Mota, J.; Vilela, A. Aged to Perfection: The Scientific Symphony behind Port Wine, Vinegar, and Acetic Acid Bacteria. Fermentation 2024, 10, 200. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Xin, X.; Liu, D.; Zhang, W. Comparative analysis of traditional and modern fermentation for Xuecai and correlations between volatile flavor compounds and bacterial community. Front. Microbiol. 2021, 12, 631054. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F.; Hassoun, A.; Zouari, A.; Tülbek, M.Ç.; Mefleh, M.; Aït-Kaddour, A.; Castellari, M. Fermentation for designing novel plant-based meat and dairy alternatives. Foods 2023, 12, 1005. [Google Scholar] [CrossRef] [PubMed]
- Aworh, O.C. African traditional foods and sustainable food security. Food Control 2023, 145, 109393. [Google Scholar] [CrossRef]
- Michel, M.; Eldridge, A.L.; Hartmann, C.; Klassen, P.; Ingram, J.; Meijer, G.W. Benefits and challenges of food processing in the context of food systems, value chains and sustainable development goals. Trends Food Sci. Technol. 2024, 104, 703. [Google Scholar] [CrossRef]
- Antone, U.; Ciprovica, I.; Zolovs, M.; Scerbaka, R.; Liepins, J. Propionic acid fermentation—Study of substrates, strains, and antimicrobial properties. Fermentation 2022, 9, 26. [Google Scholar] [CrossRef]
- Boukid, F.; Ganeshan, S.; Wang, Y.; Tülbek, M.Ç.; Nickerson, M.T. Bioengineered enzymes and precision fermentation in the food industry. Int. J. Mol. Sci. 2023, 24, 10156. [Google Scholar] [CrossRef]
- Sionek, B.; Szydłowska, A.; Küçükgöz, K.; Kołożyn-Krajewska, D. Traditional and new microorganisms in lactic acid fermentation of food. Fermentation 2023, 9, 1019. [Google Scholar] [CrossRef]
- Bhalla, T.C.; Savitri. Yeasts and traditional fermented foods and beverages. Yeast Divers. Hum. Welf. 2017, 53, 82. [Google Scholar]
- Cameron, D.E.; Bashor, C.J.; Collins, J.J. A brief history of synthetic biology. Nat. Rev. Microbiol. 2016, 12, 381. [Google Scholar] [CrossRef]
- Stephanopoulos, G. Synthetic biology and metabolic engineering. ACS Synth. Biol. 2018, 1, 514–525. [Google Scholar] [CrossRef]
- Park, H.S.; Jun, S.C.; Han, K.H.; Hong, S.B.; Yu, J.H. Diversity, application, and synthetic biology of industrially important Aspergillus fungi. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 161–202. [Google Scholar] [CrossRef]
- Kim, G.B.; Kim, W.J.; Kim, H.U.; Lee, S.Y. Machine learning applications in systems metabolic engineering. Curr. Opin. Biotechnol. 2020, 64, 1–9. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, P.; Li, N.; Li, J.; Chen, J.; Zhou, J. Production of 2-keto-L-gulonic acid by metabolically engineered Escherichia coli. Bioresour. Technol. 2020, 318, 124069. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Mishra, S.; Zhao, H. Recent advances in metabolic engineering of Saccharomyces cerevisiae: New tools and their applications. Metab. Eng. 2018, 50, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Pyne, M.E.; Narcross, L.; Martin, V.J.J. Engineering plant secondary metabolism in microbial systems. Plant Physiol. 2019, 179, 844–861. [Google Scholar] [CrossRef] [PubMed]
- Roell, G.W.; Zha, J.; Carr, R.R.; Koffas, M.A.; Fong, S.S.; Tang, Y.J. Engineering microbial consortia by division of labor. Microb. Cell Factor. 2019, 18, 35. [Google Scholar] [CrossRef]
- Sikdar, R.; Elias, M. Quorum quenching enzymes and their effects on virulence, biofilm, and microbiomes: A review of recent advances. Expert Rev. Anti-Infect. Ther. 2020, 18, 1221–1233. [Google Scholar] [CrossRef]
- Shivaprasad, D.P.; Taneja, N.K.; Lakra, A.; Sachdev, D. In vitro and in situ abrogation of biofilm formation in E. coli by vitamin C through ROS generation, disruption of quorum sensing, and exopolysaccharide production. Food Chem. 2021, 341, 128171. [Google Scholar] [CrossRef]
- Hossain, I.; Mizan, F.R.; Roy, P.K.; Nahar, S.; Toushik, S.H.; Ashrafudoulla, M.; Jahid, I.K.; Lee, J.; Ha, S.D. Listeria monocytogenes biofilm inhibition on food contact surfaces by application of postbiotics from Lactobacillus curvatus B.67 and Lactobacillus plantarum M.2. Food Res. Int. 2021, 148, 110–595. [Google Scholar] [CrossRef]
- Wei, Q.; Bhasme, P.; Wang, Z.; Wang, L.; Wang, S.; Zeng, Y.; Wang, Y.; Ma, L.Z.; Li, Y. Chinese medicinal herb extract inhibits PQS-mediated quorum sensing system in Pseudomonas aeruginosa. J. Ethnopharmacol. 2020, 248, 112272. [Google Scholar] [CrossRef]
- Fidalgo, L.G.; Moreira, S.A.; Ormando, P.; Pinto, C.A.; Queirós, R.P.; Saraiva, J.A. Chapter 6—High-pressure processing associated with other technologies to change enzyme activity. In Foundations and Frontiers in Enzymology: Effect of High-Pressure Technologies on Enzymes; de Castro Leite Júnior, B.R., Tribst, A.A.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 141–168. [Google Scholar] [CrossRef]
- Sidirokastritis, N.D.; Tsiantoulas, I.; Tananaki, C.; Vareltzis, P. The effect of high hydrostatic pressure on tetracycline hydrochloride and sulfathiazole residues in various food matrices—comparison with ultrasound and heat treatment. Food Addit. Contam. Part A 2022, 39, 687–698. [Google Scholar] [CrossRef]
- Ademosun, O.T.; Ajanaku, K.O.; Adebayo, A.H.; Oloyede, M.O.; Okere, D.U.; Akinsiku, A.A.; Ajayi, S.O.; Ajanaku, C.O.; Dokunmu, T.M.; Owolabi, A.O. Physico-chemical, microbial and organoleptic properties of yoghurt fortified with tomato juice. J. Food Nutr. Res. 2019, 7, 810–814. [Google Scholar] [CrossRef]
- Thakur, M.; Modi, V.K. Emerging Technologies in Food Science. Available online: https://www.springerprofessional.de/en/emerging-technologies-in-food-science/18042750 (accessed on 4 January 2025).
- Sevenich, R.; Mathys, A. Continuous versus discontinuous ultra-high-pressure systems for food sterilization with focus on ultra-high-pressure homogenization and high-pressure thermal sterilization: A review. Compreh. Rev. Food Sci. Food Saf. 2018, 17, 646–662. [Google Scholar] [CrossRef] [PubMed]
- Raghubeer, E.V.; Phan, B.N.; Onuoha, E.; Diggins, S.; Aguilar, V.; Swanson, S.; Lee, A. The use of high-pressure processing (HPP) to improve the safety and quality of raw coconut (Cocos nucifera L.) water. Int. J. Food Microbiol. 2020, 331, 108697. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, O.P. Non-Thermal Processing of Foods; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- McGillin, M.R.; deRiancho, D.L.; DeMarsh, T.A.; Hsu, E.D.; Alcaine, S.D. Selective survival of protective cultures during high-pressure processing by leveraging freeze-drying and encapsulation. Foods 2022, 11, 2465. [Google Scholar] [CrossRef] [PubMed]
- Asaithambi, N.; Singh, S.K.; Singha, P. Current status of non-thermal processing of probiotic foods: A review. J. Food Eng. 2021, 303, 110567. [Google Scholar] [CrossRef]
- Balamurugan, S.; Gemmell, C.; Tsun Yin Lau, A.; Arvaj, L.; Strange, P.; Gao, A. High pressure processing during the drying of fermented sausages can enhance safety and reduce time required to produce a dry fermented product. Food Control 2020, 113, 107224. [Google Scholar] [CrossRef]
- Huang, Y.L.; Tsai, Y.H. Extraction of chitosan from squid pen waste by high hydrostatic pressure: Effects on physicochemical properties and antioxidant activities of chitosan. Int. J. Biol. Macromol. 2020, 160, 677–687. [Google Scholar] [CrossRef]
- Gavahian, M.; Pallares, N.; Al Khawli, F.; Ferrer, E.; Barba, F.J. Recent advances in the application of innovative food processing technologies for mycotoxins and pesticide reduction in foods. Trends Food Sci. Technol. 2020, 106, 209–2018. [Google Scholar] [CrossRef]
- Vieira, P.; Ribeiro, C.; Pinto, C.A.; Saraiva, J.A.; Barba, F.J. Application of HPP in food fermentation processes. In Present and Future of High Pressure Processing; Elsevier: Amsterdam, The Netherlands, 2020; pp. 329–351. [Google Scholar] [CrossRef]
- Koubaa, M.; Barba, F.J.; Roohinejad, S.; Saraiva, J.; Lorenzo, J.M. New challenges and opportunities of food fermentation processes: Application of conventional and innovative techniques. Food Res. Int. 2018, 114, 88–100. [Google Scholar] [CrossRef]
- Duffuler, P.; Giarratano, M.; Naderi, N.; Suwal, S.; Marciniak, A.; Perreault, V.; Pouliot, Y. High hydrostatic pressure induced extraction and selective transfer of β-phosvitin from the egg yolk granule to plasma fractions. Food Chem. 2020, 321, 126696. [Google Scholar] [CrossRef]
- Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB). Enzyme Nomenclature. Available online: https://iubmb.qmul.ac.uk/enzyme/ (accessed on 10 October 2024).
- Fadel, M.; AbdEl-Halim, S.; Sharada, H.; Yehia, A.; Ammar, M. Production of glucoamylase, α-amylase and cellulase by Aspergillus oryzae F-923 cultivated on wheat bran under solid state fermentation. J. Adv. Biol. Biotechnol. 2020, 23, 8–22. [Google Scholar] [CrossRef]
- Ganeshan, S.; Kim, S.H.; Vujanovic, V. Scaling-up production of plant endophytes in bioreactors: Concepts, challenges and perspectives. Bioresour. Bioprocess. 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Pistikopoulos, E.N.; Barbosa-Povoa, A.; Lee, J.H.; Misener, R.; Mitsos, A.; Reklaitis, G.V.; Venkatasubramanian, V.; You, F.; Gani, R. Process systems engineering—The generation next? Comput. Chem. Eng. 2021, 147, 107252. [Google Scholar] [CrossRef]
- Brindhadevi, K.; Shanmuganathan, R.; Pugazhendhi, A.; Gunasekar, P.; Manigandan, S. Biohydrogen production using horizontal and vertical continuous stirred tank reactor—A numerical optimization. Int. J. Hydrogen Energy 2021, 46, 11305–11312. [Google Scholar] [CrossRef]
- Urtubia, A.; León, R.; Vargas, M. Identification of chemical markers to detect abnormal wine fermentation using support vector machines. Comput. Chem. Eng. 2021, 145, 107158. [Google Scholar] [CrossRef]
- Sipos, A.; Florea, A.; Arsin, M.; Fiore, U. Using neural networks to obtain indirect information about the state variables in an alcoholic fermentation process. Processes 2021, 9, 74. [Google Scholar] [CrossRef]
- Guadalupe-Daqui, M.; Chen, M.; Thompson-Witrick, K.; MacIntosh, A. Yeast morphology assessment through automated image analysis during fermentation. Fermentation 2021, 7, 44. [Google Scholar] [CrossRef]
- Chu, R.Y.; Li, S.X.; Zhu, L.D.; Yin, Z.H.; Hu, D.; Liu, C.C.; Mo, F. A review on co-cultivation of microalgae with filamentous fungi: Efficient harvesting, wastewater treatment, and biofuel production. Renew. Sust. Energy Rev. 2021, 139, 110689. [Google Scholar] [CrossRef]
- Sawant, A.M.; Vamkudoth, K.R. Biosynthetic process and strain improvement approaches for industrial penicillin production. Biotechnol. Lett. 2022, 44, 179–192. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Protzel, A.; Green, T.; Figurnov, M.; Ronnebrger, O. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Wang, J.; Yang, P.; Zhao, B.; Liu, S. AI-assisted food enzymes design and engineering: A critical review. Syst. Microb. BiOmanuf. 2023, 3, 75–87. [Google Scholar] [CrossRef]
- Mavani, N.R.; Ali, J.M.; Othman, S.; Hussain, M.A.; Hashim, H.; Rahman, N.A. Application of artificial intelligence in the food industry. Food Eng. Rev. 2022, 14, 134–175. [Google Scholar] [CrossRef]
- Kurzbaum, E.; Raizner, Y.; Kuc, M.E.; Kulikov, A.; Hakimi, B.; Kruh, L.I.; Menashe, O. Phenol biodegradation by bacterial cultures encapsulated in 3D microfiltration-membrane capsules. Environ. Technol. 2020, 41, 2875–2883. [Google Scholar] [CrossRef]
- Razzaghi, M.; Homaei, A.; Vianello, F.; Azad, T.; Sharma, T.; Nadda, A.K.; Stevanato, R.; Bilal, M.; Iqbal, H. Industrial applications of immobilized nano-biocatalysts. Bioprocess Biosyst. Eng. 2022, 45, 237–256. [Google Scholar] [CrossRef] [PubMed]
- Tarafdar, A.; Sirohi, R.; Gaur, V.; Kumar, S.; Sharma, P.; Varjani, S.; Pandey, H.; Sindhu, R.; Madhavan, A.; Rajasekharan, R.; et al. Engineering interventions in enzyme production: Lab to industrial scale. Bioresour. Technol. 2021, 326, 124771. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, U.; Sohail, M.; Ghanemi, A. Cellulases: From bioactivity to a variety of industrial applications. Biomimetics 2021, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Jafari, S.M.; Mahoonak, A.S.; Ghorbani, M. Liposomal/nanoliposomal encapsulation of food-relevant enzymes and their application in the food industry. Food Bioproc. Technol. 2021, 14, 23–38. [Google Scholar] [CrossRef]
- Bahri, S.; Homaei, A.; Mosaddegh, E. Zinc sulfide-chitosan hybrid nanoparticles as a robust surface for immobilization of Sillago sihama α-amylase. Colloids Surf. B Biointerfaces 2022, 218, 112754. [Google Scholar] [CrossRef]
- Hilgendorf, K.; Wang, Y.; Miller, M.J.; Jin, Y. Precision fermentation for improving the quality, flavor, safety, and sustainability of foods. Curr. Opin. Biotechnol. 2024, 86, 103084. [Google Scholar] [CrossRef]
- Muhammad, I.S.; Riaz, A.; Sajjad, H.; Arshad, M.; Azeem, K. Role of bacterial consortia in promoting plant growth and nutrients bioavailability. Pak. J. Agric. Res. 2020, 57, 211–1220. [Google Scholar] [CrossRef]
- Aboobacker, P.A.; Ragunathan, L.; Sanjeevi, T. Synthetic Biology’s Latest Trends in Antimicrobial Resistance and Biofilm. J. Pure Appl. Microbiol. 2023, 17, 23–34. [Google Scholar] [CrossRef]
- US Food and Drug Administration Website. Agricultural Biotechnology. Feed Your Mind. Available online: https://www.fda.gov/food/consumers/agricultural-biotechnology (accessed on 26 August 2025).
- Arvin, N.; Eric, D. Adaptive Significance of Quorum Sensing-Dependent Regulation of Rhamnolipids by Integration of Growth Rate in Burkholderia glumae: A Trade-Off between Survival and Efficiency. Front. Microbiol. 2016, 7, 1215. [Google Scholar] [CrossRef]
- Chandrakala, R.; Lakshmi, E.J.; Anjineyulu, K.; Pandiselvam, R.; Balasubramaniam, V.M. Influence of high-pressure pasteurization on nutritional, functional, and rheological characteristics of fruit and vegetable juices and purees—An updated review. Food Control 2023, 146, 109516. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef] [PubMed]
- Sak, J.; Suchodolska, M. Artificial Intelligence in Nutrients Science Research: A Review. Nutrients 2021, 13, 322. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.H.; Reyhaneh, S.; Fabio, V.; Roberto, S. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Adebo, O.A. African Sorghum-Based Fermented Foods: Past, Current and Future Prospects. Nutrients 2020, 12, 1111. [Google Scholar] [CrossRef]
- Mannaa, M.; Han, G.; Seo, Y.S.; Park, I. Evolution of food fermentation processes and the use of multi-omics in deciphering the roles of the microbiota. Foods 2021, 10, 2861. [Google Scholar] [CrossRef]
- Anyogu, A.; Olukorede, A.; Anumudu, C.K.; Onyeaka, H.; Areo, E.; Adewale, O.; Nwaiwu, O. Microorganisms and food safety risks associated with indigenous fermented foods from Africa. Food Control 2021, 129, 108227. [Google Scholar] [CrossRef]
- Bousquet, J.; Anto, J.M.; Czarlewski, W.; Haahtela, T.; Fonseca, S.C.; Iaccarino, G.; ARIA group. Cabbage and fermented vegetables: From death rate heterogeneity in countries to candidates for mitigation strategies of severe COVID-19. Allergy 2021, 76, 735–750. [Google Scholar] [CrossRef]
- Kariyawasam, K.M.G.M.M.; Lee, N.K.; Paik, H.D. Fermented dairy products as delivery vehicles of novel probiotic strains isolated from traditional fermented Asian foods. J. Food Sci. Technol. 2021, 58, 2467–2478. [Google Scholar] [CrossRef]
- Gonzalez-Fandos, E.; Vazquez de Castro, M.; Martinez-Laorden, A. Behaviour of Listeria monocytogenes and natural microflora during the manufacture of Riojano chorizo (Spanish dry cured sausage). Microorganisms 2021, 9, 1963. [Google Scholar] [CrossRef]
- Boxman, I.L.A.; Jansen, C.C.C.; Zwartkruis-Nahuis, A.J.T.; Hägele, G.; Sosef, N.P.; Dirks, R.A.M. Detection and quantification of hepatitis E virus RNA in ready-to-eat raw pork sausages in The Netherlands. Int. J. Food Microbiol. 2020, 333, 108791. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; El Sheikha, A.F.; Hammami, R.; Kumar, W. Traditionally fermented pickles: How the microbial diversity associated with their nutritional and health benefits? J. Funct. Foods 2020, 70, 103971. [Google Scholar] [CrossRef]
- Capozzi, V.; Fragasso, M.; Russo, P. Microbiological safety and the management of microbial resources in artisanal foods and beverages: The need for a transdisciplinary assessment to conciliate actual trends and risks avoidance. Microorganisms 2020, 8, 306. [Google Scholar] [CrossRef]
- Campisi, V. What Are the Growth Prospects for Fermented Foods? The Food Institute. 2022. Available online: https://foodinstitute.com/focus/what-are-the-growth-prospects-for-fermented-foods/ (accessed on 26 August 2025).
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented foods, health and the gut microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef]
- Chai, K.F.; Chang, L.S.; Adzahan, N.M.; Karim, R.; Rukayadi, Y.; Ghazali, H.M. Physicochemical properties and toxicity of cocoa powder-like product from roasted seeds of fermented rambutan (Nephelium lappaceum L.) fruit. Food Chem. 2019, 271, 298–308. [Google Scholar] [CrossRef]
- Tan, Y.X.; Mok, W.K.; Lee, J.; Kim, J.; Chen, W.N. Solid state fermentation of brewers’ spent grains for improved nutritional profile using Bacillus subtilis WX-17. Fermentation 2019, 5, 52. [Google Scholar] [CrossRef]
- Linder, T. Making the case for edible microorganisms as an integral part of a more sustainable and resilient food production system. Food Secur. 2019, 11, 265–278. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).