Abstract
Bio-hydrogen and bio-methane co-production was a promising way to enhance the energy conversion efficiency, and enzyme loading and pH are key factors influencing anaerobic fermentation processes. Therefore, in this study, the co-production process of bio-hydrogen and bio-methane was evaluated based on the effect of enzyme loading (20%, 30%, and 40%) combined with initial pH (6.0, 7.0, 8.0, and 9.0). The results indicated that, compared with other conditions, 30% enzyme loading with an initial pH of 8.0 was more feasible for bio-hydrogen and bio-methane co-production from duckweed, achieving a bio-hydrogen yield of 114.56 mL/g total solid (TS) and a bio-methane yield of 260.32 mL/g TS. Under optimum condition, the energy conversion efficiency was 71.4%, which was 6-fold and 4.8-fold higher than that of the single bio-hydrogen production stage (pH 8, 40% and 10.2%) and single methane production stage (control group with 12.30%), respectively.
1. Introduction
Developing clean and renewable energy became an imperative due to finite supplies and pollution, an issue of the utilization of fossil fuels [1,2]. Biological hydrogen combined with methane production from biomass provides a solution to overcome the disadvantages [3]. Photo-fermentative hydrogen production standouts out from different biological hydrogen production methods because of its capacities to use various sources of feedstocks and high substrate conversion efficiency [4]. However, in order to maximize the bioenergy conversion potential of biomass, bio-menthane production could follow after bio-hydrogen production, which uses the end-products of the first stage, bio-hydrogen production.
Duckweed, a floating plant of family Lemnaceae, is famous for its high crude protein content, productivity, and adaptive capabilities, which has been considered as a promising bioenergy feedstock [5,6]. As a kind of lignocellulosic biomass, duckweed is consists of cellulose, hemicellulose, and lignin, making prior treatment necessary before bioenergy conversion [7]. However, during the bioenergy conversion process (such as bio-hydrogen production and bio-ethanol production) of lignocellulosic biomass, enzymatic hydrolysis becomes a decisive step in harvesting higher digestibility [8]. Moreover, the efficiency of the digestibility of lignocellulosic biomass was closely related with enzyme loadings, which also affect the enzyme activity in the system [9]. Nahar, et al. [10] pointed out that the hydrolysis rate of corn stover was increased with enzyme loadings, while the optimal enzyme loading also depended on the enzyme costs, bioenergy price, and process temperature. During the process of photo-fermentative hydrogen production with lignocellulosic biomass, the macromolecular cellulose needs to be degraded by cellulase into small-molecule sugars before the metabolic actives of photosynthetic bacteria for hydrogen generation [11]. Enzymes are crucial for enhancing the conversion efficiency of feedstocks to hydrogen, but their high cost also increases the operational expenses of bio-hydrogen production to some extent. Hence, researchers have wanted the enzymatic hydrolysis step to be more efficient so that a higher energy conversion efficiency during the process of photo-fermentative hydrogen production with duckweed can be obtained; therefore, an appropriate amount of enzyme loading became necessary.
pH is an important parameter for biological hydrogen production because it affects enzymatic activities and biochemical reactions [12,13]. In addition, the pH also influences the metabolic by-products (such as volatile fatty acids (VFAs)), which would further affect the subsequent reuse of these waste liquids [14,15]. Zhang et al. [7] achieved a maximum hydrogen yield of 85.6 mL/g TS from a mixed feedstock of duckweed and corn stover via photo-fermentation at an initial pH of 8.0, which also accompanied a maximum concentration of total VFA of 2.23 g/L. Liu et al. [14] found that the subsequent anaerobic digestion had a similar methane yield of 313.6–318.7 mL CH4/g VS when the initial pHs of the first stage of the dark fermentative, bio-hydrogen production were in a range of 5.5–10.0, and the higher initial pH was more preferable for methane evolution. However, to the best of authors’ acknowledge, the co-production of bio-hydrogen and bio-methane production using end-products of hydrogen production was concentrated on dark fermentation and anerobic digestion; photo-fermentation combined with an anerobic digestion has not been carried out with different enzyme loadings and pH values.
The objectives of this study were to (1) evaluate the effect of enzyme loading combined with the initial pH on the bio-hydrogen and bio-methane co-production of duckweed and (2) investigate the energy conversion efficiency enhancement during the bio-hydrogen and bio-methane co-production process of duckweed. In order to achieve these objectives, batch experiments were performed under different enzyme loadings with different initial pH values, and the sugar concentration changes were detected during the photo-fermentative hydrogen production process, and the pH values and VFAs variations during the bio-hydrogen and bio-methane co-production processes were also measured. In addition, the energy conversion efficiencies of bio-hydrogen production, bio-methane production, and bio-hydrogen and bio-methane co-production were compared.
2. Materials and Methods
2.1. Properties of Substrate, Photosynthetic Bacteria, and Seeding Sludge
The duckweed used in this experiment was purchased from Jinantang Traditional Chinese Medicine Co., Ltd. (Bozhou, China). After it was air-dried, the duckweed was ground into powder and then passed through a 60-mesh sieve (<250 μm). Duckweed powder was stored in a sealed plastic and kept at room temperature before experimentation. The total solid (TS), volatile solid (VS), and ash content of feedstock were 94.01 ± 0.00%, 81.55 ± 0.04%, and 16.77 ± 0.02%, respectively. Cellulose, hemicellulose, and lignin contents of duckweed were 12.0 ± 0.3%, 9.6 ± 0.4%, and 9.5 ± 0.4%, respectively. The elemental composition of the duckweed was as follows (% of TS): carbon, 36.26 ± 0.29; hydrogen, 4.97 ± 0.10; nitrogen, 3.33 ± 0.12; and sulfur, 0.63 ± 0.18. The energy content was calculated based on the determined weight percentages of C/H/S/O using the modified Dulong formula as 15.03 kJ/g TS.
The inoculum used in photo-fermentation was obtained from sludge collected from the anaerobic area of Wulongkou Water Company in Zhengzhou, China. Photosynthetic bacterial consortium was enriched via an iterative batch cultivation in enrichment-growth medium under continuous illumination (3000 lx). The dominant genera of the photosynthetic bacteria were identified as Rhodobacter and Proteiniphilum acetatigenes through 16S rDNA sequencing. The enrichment-growth medium for photosynthetic bacteria had the following composition (all in g/L): NH4Cl, 0.5; KH2PO4, 0.1; MgSO4, 0.1; NaCl, 1; sodium acetate, 2; yeast extract, 0.5; and NaHCO3, 1. Detailed cultivation protocols for the photosynthetic bacteria were performed according to the methodology described in the previous study [15]. The dry cell weight (DCW) of the photosynthetic bacteria cultivated for 72 h, used for the photo-fermentative bio-hydrogen production system, was 4.37 ± 0.06 mg TS/mL.
The seed inoculum used for anaerobic digestion was obtained from the liquid digestate of a digester reactor feeding corn straw in Zhengzhou, China. The seed inoculum was kept at another anaerobic reactor to degas before experimentation until no gas was produced. WCHB1-02, SC-I-84, and Bacteroidetes_vadinHA17 were the dominant genera of the inoculum detected by 16S rDNA. The fermentation broth was filtered through a 50-mesh sieve, and the TS of seed inoculum before inoculation was 9.37 ± 0.02%.
2.2. Experimental Setup
In this study, the experimental was comprised of two operational phases: photo-fermentative bio-hydrogen production (Phase I) and anaerobic digestion bio-methane production (Phase II).
Phase I included the following: A photo-fermentative bio-hydrogen production batch experiment was conducted in a 250 mL Erlenmeyer flask containing a 150 mL working volume. Each flask was successively loaded with the following: 4.5 g (based on TS) of duckweed powder, cellulase enzyme at specified loading rate (Solarbio Science & Technology Co., Ltd., Beijing, China), 100 mL mixture containing a citrate-sodium citrate buffer solution (0.05 M at a pH of 4.8), and a bio-hydrogen production medium (K2HPO4 0.5, NH4Cl 0.4, sodium glutamate 3.56, MgCl2 0.2, yeast extract 0.1, and NaCl 2, all in g/L). The initial pH was adjusted to the target setpoint using 5 M NaOH or HCl solutions. Enzyme loading rates and initial pH predetermined rates value were set as 20%, 30%, and 40% (w/w, enzyme/substrate) and 6.0, 7.0, 8.0, and 9.0 ± 0.1. Therefore, the experimental conditions were designated as 20-6, 20-7, 20-8, and 20-9; 30-6, 30-7, 30-8, and 30-9; and 40-6, 40-7, 40-8 and 40-9 (i.e., enzyme loading rate-initial pH). After inoculation with 50 mL of an exponential-phase photosynthetic bacteria, the conical flasks were sealed with rubber stoppers fitted with two ports: one for periodic sampling of fermentation broth and another connected to a 500 mL gas collection bag. After that, all bioreactors were incubated in a temperature-controlled incubator (30.0 ± 0.5 °C) under continuous illumination (3000 lx and 60 W of incandescent light) for 72 h. Gas and fermentation broth samples were collected every 12 h. All experiments were performed in triplicate, and the mean of the experimental data was used for the results.
Phase II included the following: After photo-fermentative bio-hydrogen production, a part of the effluents was sampled for further analyses, and the residual effluents were neutralized to a pH of 7.0 ± 0.1 using 5 M NaOH and then inoculated with the degassed seed sludge for the second-stage anaerobic digestion bio-methane production. The working volume in each flask was calculated as 200 mL based on a 3:7 volume ratio of the inoculum to effluent. Besides, two control groups were established: (1) deionized water (140 mL) + duckweed (4.5 g TS) + seed inoculum (60 mL), named as the control group, and (2) 0.05 M citrate-sodium citrate buffer (pH 4.8, 140 mL) + duckweed (4.5 g TS) + seed inoculum (60 mL), named as the buffer group. The initial pH of both control groups was adjusted to 7.0 ± 0.1 (as in prior procedures). Subsequently, each flask was sealed with a rubber stopper and placed in a constant-temperature incubator at 35 °C for 18 days. Sampling was performed every 2 days to measure methane content and pH value. All experiments were conducted in triplicate, and the average values of experimental results were calculated.
2.3. Analysis Methods
TS and VS of duckweed were determined according to Standard Methods [7]. The contents of cellulose, hemicellulose, and lignocellulose in duckweed were determined using the Van Soest method. The carbon, hydrogen, nitrogen, and sulfur of duckweed was quantified using an elemental analyzer (Vario EL/cube, elemental, Frankfurt, Germany). A TES-1332A digital illuminometer (TES Electronic Industry Co., Ltd., Taiwan, China) was employed to monitor the light intensity. Dynamic pH changes in the fermentation broth were recorded using a PHS-3C pH meter (Shanghai Yidian Scientific Instrument Co., Ltd., Shanghai, China). Total reducing sugar concentration was determined by using the 3,5-dinitrosalicylic acid (DNS) colorimetric method (glucose as the standard).
The volume of gas produced in the experiment was measured using a 60 mL gastight syringe. A biogas component analysis was performed via a GC 9790II gas chromatograph (Fuli Analytical Instruments Co., Ltd., Wenling, China) equipped with a thermal conductivity detector (TCD). Nitrogen served as the carrier gas at 45 mL/min. For bio-hydrogen detection, the temperatures of the injection port, TCD, and column oven were maintained at 120 °C, 150 °C, and 100 °C, respectively, and the gas retention time was 2 min. For bio-methane analysis, the injector port temperature was set to 100 °C with a retention time of 4 min, while all other operating parameters remained consistent with those for bio-hydrogen detection conditions. Soluble metabolic products (SMPs) in the fermentation broth were performed using an Agilent HP 7890B gas chromatograph (Agilent, Palo Alto, CA, USA) with a flame ionization detector (FID) and an HP-5ms ultra-inert column (30 m × 0.25 mm) with 5 mL/min of nitrogen as the carrier gas (0.4 Mpa inlet pressure). The detector, column, and FID temperatures were constant at 210 °C, 120 °C, and 250 °C, respectively. Prior to SMPs analysis, samples were centrifuged at 10,000 rpm for 20 min, and the supernatant filtered through 0.45 μm nylon syringe filters.
2.4. Kinetic Parameter Analysis
The cumulative production of bio-hydrogen and bio-methane were simulated by a modified Gompertz equation ((Equation (1) [16], and the kinetic parameters (Hm, Rm, and λ) were estimated via OriginPro Learning Edition 2021 (Origin Lab Corporation, Northampton, MA, USA). The bio-hydrogen production rate (HPR) throughout the photo-fermentation process was derived via mathematical differentiation (Equation (2)) [17].
where H(t) is bio-hydrogen yield cumulated from the photo-fermentation system, using mL/g TS. Hm denotes the bio-hydrogen production potential (mL/g TS); Rm is the maximum bio-hydrogen production rate, using mL/g substrate/h; λ is lag time of photo-fermentation system, h; t is photo-fermentative time; and e denotes exponential constant.
2.5. Energy Conversion Efficiency
The energy conversion efficiency (ECE, %) for the conversion of duckweed to bio-hydrogen and bio-methane was described by Equations (3)–(5) [18].
where ECE is the energy conversion efficiency, %, V is gas volume, , and signifies the heat values of hydrogen and methane, which are 12.86 and 35.82 J/mL, respectively. Qduckweed refers to the heat value of duckweed, 15.03 kJ/g TS. m is the weight of duckweed, 1 g TS.
3. Results and Discussion
3.1. Effect of Different Enzyme Loadings Combined with Initial pH on Bio-Hydrogen–Bio-Methane Co-Production
Effect of different enzyme loadings combined with initial pH on the bio-hydrogen and bio-methane co-production of duckweed is present in Figure 1. The kinetic parameters for bio-hydrogen production and bio-methane production are showed in Table 1 and Table 2. The R2 at different conditions were higher than 0.99, indicating good fitting between the experimental data and model (Table 1 and Table 2). In cases of 20% enzyme loading, the maximum bio-hydrogen yield of 97.06 mL/g TS was obtained at an initial pH of 8.0, and the maximum bio-methane yield of 195.85 mL/g TS, during the subsequent anerobic digestion process feeding the relative end-products of bio-hydrogen production, was also obtained at an initial pH of 8.0 (Figure 1a,b, Table 1 and Table 2). Similar results were observed at cases of 30% enzyme loading with different pH values (Figure 1c,d, Table 1 and Table 2); however, different results were present in cases of 40% enzyme loading with different pH values, with a maximum bio-hydrogen yield of 119.27 mL/g TS achieved at an initial pH of 8.0, while the maximum bio-methane yield was achieved at an initial pH of 7.0 (Figure 1e,f, Table 1 and Table 2). The results indicated that a slight alkaline environment is more suitable for photosynthetic bacteria for bio-hydrogen production and subsequent bacteria for bio-methane production.
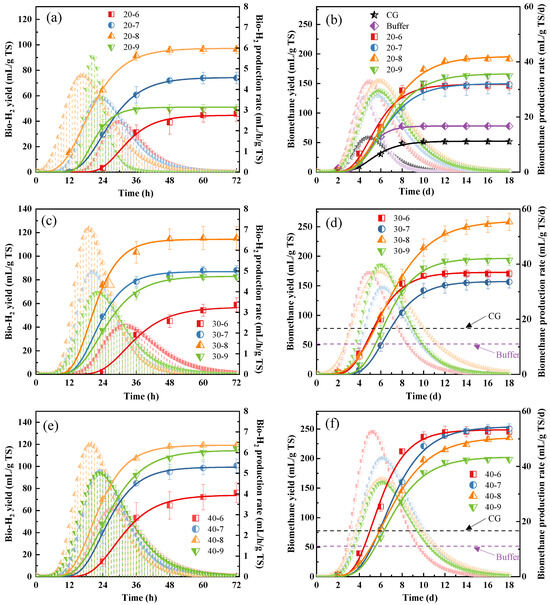
Figure 1.
Effect of different enzyme loadings combined with initial pH on bio-hydrogen and bio-methane co-production. Bio-H2 yield and bio-H2 production rate under 20% enzyme loading (a), 30% enzyme loading (c), and 40% enzyme loading (e) combined with different pHs. Bio-CH4 yield and bio-CH4 production rate under 20% enzyme loading (b), 30% enzyme loading (d), and 40% enzyme loading (f) combined with different pHs.

Table 1.
Kinetic parameters for bio-hydrogen production via photo-fermentation from duckweed.

Table 2.
Kinetic parameters for the bio-methane production process feeding the end-products of photo-fermentative bio-hydrogen production.
In cases of initial pH of 6.0, the bio-hydrogen yield was increased with the increasing of enzyme loading, and the subsequent anerobic digestion process feeding the relative end-products of bio-hydrogen production, was also increased with the increasing of enzyme loading (Figure 1, Table 1 and Table 2). Similar tendencies were also found in cases with an initial pH of 7.0, 8.0, and 9.0 in bio-hydrogen production and bio-methane production processes, except for bio-methane at the initial pH 8.0 with 30% enzyme loading; indeed, the bio-methane yield was 10.2% higher than those with 40% enzyme loading (Figure 1, Table 1 and Table 2). Considering the cost of the enzyme, 30% enzyme loading combined with an initial pH of 8.0 was more feasible for bio-hydrogen and bio-methane co-production, giving a bio-hydrogen yield of 114.56 mL/g TS and bio-methane yield of 260.32 mL/g TS (Figure 1c,d, Table 1 and Table 2).
In general, it was found that the maximum bio-hydrogen production rates appeared with the maximum bio-hydrogen yield with same enzyme loadings, meaning the cases with an initial pH of 8.0 shared the most of the maximum bio-hydrogen production rate, except for the case with an initial pH of 9.0 with 20% enzyme loading, whose maximum bio-hydrogen yield was 19.7% higher over that of an initial pH of 8.0 with the same enzyme loading (Figure 1, Table 1 and Table 2). At same initial pH values, the maximum bio-hydrogen production rates were not liner correlated with enzyme loadings, which means that increasing enzyme loading cannot improve the maximum bio-hydrogen production rate (Figure 1, Table 1 and Table 2). During the second stage of the bio-methane production process feeding the relative end-products of bio-hydrogen production, the linger correlation between the maximum bio-methane production rate and initial pH values was observed in the case of 40% enzyme loading, which shared a negative relationship (Figure 1, Table 1 and Table 2). However, for the same initial pH values of 6.0 and 7.0, there was positive correlative between the maximum bio-methane production rate and enzyme loadings (Figure 1, Table 1 and Table 2).
The lag time of bio-hydrogen and bio-methane production means the response of bacteria to the sudden change of environment [14]. During the bio-hydrogen production process, the shortest lag time of 9.17 h was obtained from the case of 20% enzyme loading with an initial pH of 8.0 (Table 1). Moreover, the pH of 6.0 gave the longest lag time whatever the enzyme loadings, which suggested that lower pH values made the bacteria take a longer time to adapt to the environment [19]. However, during the bio-methane production process feeding the relative end-products of bio-hydrogen production, there was no significant difference of lag time was found, and the lag time under different conditions were in a range of 3.20–4.22 h (Table 2).
3.2. Effect of Different Enzyme Loadings Combined with Initial pH on Time-Change Profile of Sugar and pH of Broth During Bio-Hydrogen–Bio-Methane Co-Production Process
Effect of different enzyme loadings combined with an initial pH on the time-change profile of sugar concentration during the bio-hydrogen production process with duckweed is present in Figure 2. In general, the sugar concentration decreased over the time, and the maximum sugar concentrations at different conditions were found at 12 h, since the bio-hydrogen production reaction through photo-fermentation is still in the preparatory stage during the period of 0–12 h, and the sugars were used by the photosynthetic bacteria for cell growth and small-molecular-acid production, leading to lower consumption rate of reducing sugar (Figure 2). The rapid progress of the bio-hydrogen production reaction was accompanied by the rapid consumption of the substrate and the continuous accumulation of acidic metabolites, which led to insufficient energy supply in the reaction system, deterioration of the growth environment for the microorganism, and consequently a significant decrease in the hydrogen production rate as the reaction proceeded [20,21]. The sugar concentrations with 40% enzyme loading at 12 h were higher over those with 20% and 30% enzyme loadings at 12 h, and the maximum sugar concentration of 6.8 g/L was achieved at 40% enzyme loading with an initial pH of 6.0 (Figure 2c). In addition, the pH has a more significant impact on sugar concentrations with 40% enzyme loading, indicating that a higher sugar concentration appeared with lower pH values (Figure 2c). The results might be explained in that the lower pH inhibited the metabolic activities of photosynthetic bacteria, leading to more sugar maintained in the broth [22]. However, after 24 h, it was found that the sugar concentrations under different conditions were all kept in a lower stable range until the end of the photo-fermentation, meaning the majority of sugars realized by the enzyme hydrolysis of duckweed had been used for bio-hydrogen production (Figure 2).
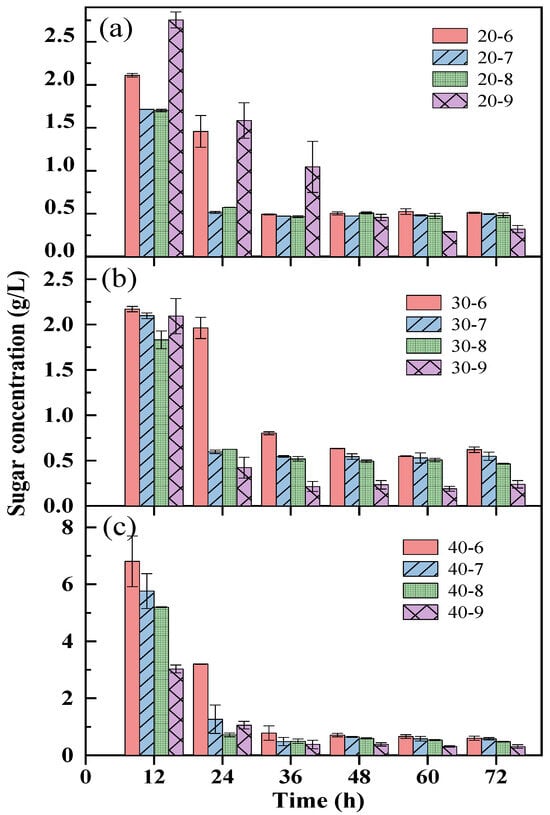
Figure 2.
Effect of different enzyme loadings combined with initial pH on time-change profile of sugar concentration during bio-H2 production process. 20% enzyme loading (a), 30% enzyme loading (b), and 40% enzyme loading (c) combined with different pHs.
The pH value has a significant impact on multiple key factors in microbial processes, including the type of metabolic by-products, substrate utilization efficiency, protein synthesis, and material storage processes [19]. As shown in Figure 3a,c,d, a dramatic pH decrease was found in 24 h during the bio-hydrogen production process, as the consumed sugars were converted into small molecular acids. In addition, the Δ pH between 0 h and 72 h had a liner correlation with the initial pH and the enzyme loadings during the bio-hydrogen production process, showing that a lower initial pH would lead to a relative stable pH of the medium, since a lower pH is more preferable for cell growth, acid accumulation, and polyhydroxyfatty acid ester (PHA) synthesis rather than H2 generation [23,24]. Moreover, the maximum Δ pH of 3.3 was obtained from 40% enzyme loading with an initial pH of 9.0, indicating a higher enzyme loading might increase the sugar concentration while also increasing the conversion of sugar to acids, resulting in a lower pH (Figure 3e) [25].
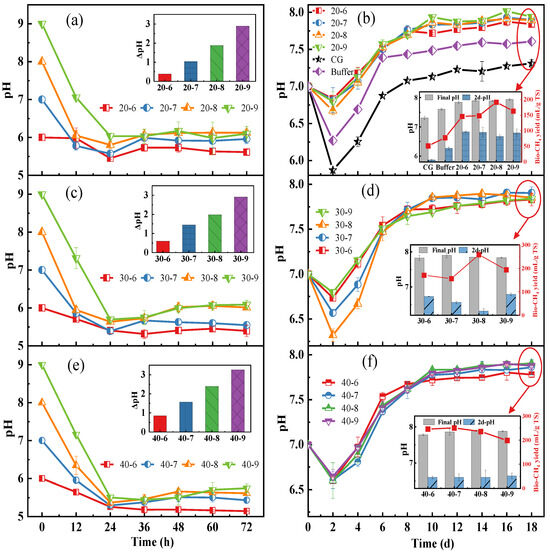
Figure 3.
Effect of different enzyme loadings combined with initial pH on time-change profile of pH. pH changes of broth during bio-H2 production process: 20% enzyme loading (a), 30% enzyme loading (c), and 40% enzyme loading (e) combined with different pHs. pH changes of broth during bio-CH4 production process: 20% enzyme loading (b), 30% enzyme loading (d), and 40% enzyme loading (f) combined with different pHs.
The effect of different enzyme loadings combined with a pH on the time-change profile of pH during the bio-methane production process is present in Figure 3b,d,f. Since the anaerobic digestion was fed with the end-products of the photo-fermentative bio-hydrogen production, the accumulated VFAs would cause a drop of pH at the beginning (the 2nd day in this study) of the bio-methane production process [26]. The maximum pH drops of 0.68 was observed at 30% enzyme loading with the initial pH of 8.0 (Figure 3d). Additionally, at the initial stage of anaerobic digestion, the pH values of the CG and buffer groups dropped rapidly. This might be due to the fact that the buffering capacity of deionized water is almost zero, and acidic substances directly release H+ in the unbuffered system, causing the pH to fall freely. After an 18 days anaerobic digestion, the pH increased to a narrow range of 7.31~7.90, suggesting a small number of acids were left in the digestate, which was further verified by Figure 4b.
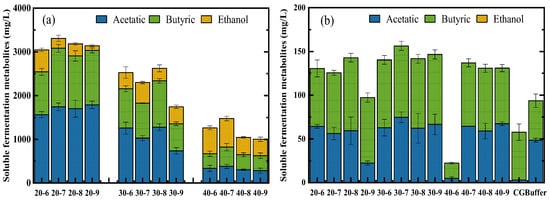
Figure 4.
Effect of different enzyme loadings combined with initial pH on soluble fermentation metabolites during bio-hydrogen and bio-methane co-production process. Soluble fermentation metabolites at the end of bio-hydrogen production (a) and bio-methane production (b).
3.3. Effect of Different Enzyme Loadings Combined with Initial pH on Soluble Fermentation Metabolites During Bio-Hydrogen–Bio-Methane Co-Production Process
As showed in Figure 4, the acetic acid, butyric acid, and ethanol were detected in the end-products of bio-hydrogen production via photo-fermentation, while no ethanol was detected in the end-products of bio-methane production via anaerobic digestion. For bio-hydrogen production, all of the concentrations of acetic acid and butyric acid were declined with the increasing of enzyme loadings (Figure 4a). The reason might be explained in that a higher bio-hydrogen yield consumed more substrates leaving less acids in the end-products, since the acids converted from sugars would be further utilized for the metabolic actives of bacteria [27]. No liner correlation was found between these two soluble fermentation metabolites with pH, and the maximum of acetic acid and butyric acid were achieved at the pH of 9.0 and 7.0 with 20% enzyme loading, respectively (Figure 4a). However, the opposite trend in concentration of ethanol was found, which increased with the increasing of enzyme loadings (except for 30% enzyme loading with an initial pH of 6.0), as a higher sugar concentration owning to higher enzyme loading would result in more ethanol accumulation based on the pathway ethanol generation () [28] (Figure 4a). Similarly, the ethanol concentration did not show a liner correlation with pH (Figure 4a).
For bio-methane production, trace amounts of acetate acid and butyric acid were detected at the end of anaerobic digestion (Figure 4b). In general, the concentrations of butyric acid under different conditions were higher than those of acetic acid, except for cases of feeding the buffer and effluent of bio-hydrogen production from 40% enzyme loading with an initial pH of 9.0 (Figure 4b). In addition, the highest concentrations of the acetic acid of 74.6 mg/L and butyric acid of 83.5 mg/L were found in cases feeding the effluent of bio-hydrogen production with a 30% enzyme loading with an initial pH of 7.0 and 20% enzyme loading with an initial pH of 8.0, respectively (Figure 4b).
3.4. Effect of Enzyme Loading Combined with pH on Energy Conversion Efficiency During Bio-Hydrogen–Bio-Methane Co-Production Process
The effect of different enzyme loadings combined with the initial pH on the energy conversion efficiency is shown in Figure 5. It was found that the initial pH of 8.0 achieved the highest energy conversion efficiency over the other initial pH values no matter the enzyme loadings during the bio-hydrogen production process, and the maximum energy conversion efficiency of 10.2% was obtained from the 40% enzyme loading (Figure 5). However, a different tendency was observed during the bio-methane production process; the groups feeding the end-products that had an initial pH of 8.0 still achieved the highest energy conversion efficiency when the enzyme loadings were 20% and 30%, while the groups feeding the end-products that had an initial pH of 7.0, when the enzyme loadings were 40%, gave the maximum energy conversion efficiency of 59.7%, which was 30.6% higher and 3.0% lower than the highest levels of enzyme loadings of 20% and 30% (Figure 5). Moreover, the maximum of the energy conversion efficiency of the bio-H2-CH4 co-production, 71.4%, was observed from an enzyme loading of 30% with an initial pH of 8.0, which was 32.5% and 4.5% higher over that of the highest level of 20% and 40% enzyme loadings, respectively (Figure 5). Under the optimal conditions, the energy conversion efficiency of the combined hydrogen and methane production was 6-fold and 4.8-fold higher than that of single bio-hydrogen production (pH 8–40% and 10.2% (maximum)) and the single bio-methane production stage (control group with 12.30%), respectively. As a conclusion, increasing the enzyme loading might enhance the energy conversion efficiency during the bio-H2-CH4 co-production process.
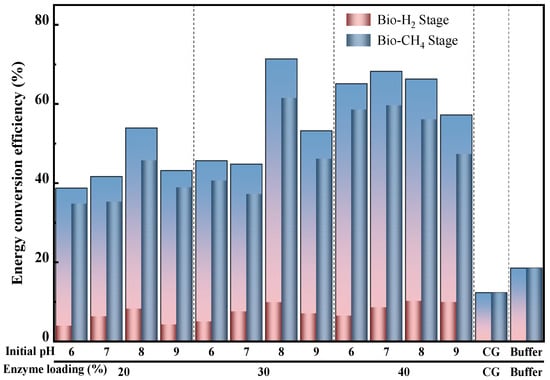
Figure 5.
Effect of different enzyme loadings combined with initial pH on energy conversion efficiency during bio-hydrogen and bio-methane co-production process with duckweed.
4. Conclusions
Duckweed was utilized for co-production of bio-hydrogen and bio-methane via a two-stage integrated process comprising photo-fermentation and anaerobic digestion. In general, the enzyme loadings showed a positive relationship with the bio-hydrogen yield and subsequent bio-methane yield. The highest bio-hydrogen yield in the bio-hydrogen production stage (Phase I) was 119.27 mL/g TS, achieved under the conditions of an initial pH of 8 and an enzyme loading of 40%. Furthermore, the bio-hydrogen effluent at an enzyme loading of 30% and an initial pH of 8.0 was more feasible in obtaining the highest bio-methane yield of 260.32 mL/g TS. The highest energy conversion efficiency of 71.4% was obtained combining photo-fermentation at the pH of 8, an enzyme loading of 30%, and an AD at a pH of 7 at 35 °C. These research results will provide an efficient way towards higher energy conversion efficiency harvesting from duckweed.
Author Contributions
Conceptualization, X.Z. and D.J.; methodology, X.Z.; software, C.X.; validation, X.Z., Z.S. and C.X.; formal analysis, Z.L.; investigation, D.J.; resources, Q.Z.; data curation, X.Z.; writing—original draft preparation, X.Z.; writing—review and editing, Z.S.; visualization, D.J.; supervision, W.L.; project administration, Q.Z.; funding acquisition, Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Postdoctoral Science Foundation of China (2024T170240 and 2024M750807), Key Special Project for International Scientific and Technological Innovation Cooperation between Governments under the National Key R & D Program (2023YFE0106000), Natural Science Foundation of Henan, China (242300421251), Training Plan for Young Backbone Teachers in Undergraduate Universities of Henan, China (2023GGJS027).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hosseini, S.E.; Wahid, M.A. Hydrogen Production from Renewable and Sustainable Energy Resources: Promising Green Energy Carrier for Clean Development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Mishra, P.K.; Kausar, M.A.; Saeed, M.; Gupta, V.K.; Singh, R.; Ramteke, P.W. Advances in Nanomaterials Induced Biohydrogen Production Using Waste Biomass. Bioresour. Technol. 2020, 307, 123094. [Google Scholar] [CrossRef]
- Xia, A.; Cheng, J.; Ding, L.; Lin, R.; Song, W.; Zhou, J.; Cen, K. Enhancement of Energy Production Efficiency from Mixed Biomass of Chlorella pyrenoidosa and Cassava Starch Through Combined Hydrogen Fermentation and Methanogenesis. Appl. Energy 2014, 120, 23–30. [Google Scholar] [CrossRef]
- Pachaiappan, R.; Cornejo-Ponce, L.; Sagade, A.A.; Mani, M.; Aroulmoji, V.; Femilaa Rajan, V.; Manavalan, K. A Concise Review of Recent Biohydrogen Production Technologies. Sustain. Energy Technol. Assess. 2024, 62, 103606. [Google Scholar] [CrossRef]
- Baek, G.Y.; Saeed, M.; Choi, H.K. Duckweeds: Their Utilization, Metabolites and Cultivation. Appl. Biol. Chem. 2021, 64, 73. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, N.E.; Walsh, É.; Bolger, P.; Burnell, G.; O’Leary, N.; O’Mahoney, M.; Paolacci, S.; Wall, D.; Jansen, M.A.K. Duckweed Bioreactors: Challenges and Opportunities for Large-Scale Indoor Cultivation of Lemnaceae. J. Clean. Prod. 2022, 336, 130285. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, D.; Zhang, H.; Wang, Y.; Zhang, Z.; Lu, C.; Zhang, Q. Enhancement of the Biohydrogen Production Performance from Mixed Substrate by Photo-Fermentation: Effects of Initial PH and Inoculation Volume Ratio. Bioresour. Technol. 2021, 319, 124153. [Google Scholar] [CrossRef]
- Venturin, B.; Frumi Camargo, A.; Scapini, T.; Mulinari, J.; Bonatto, C.; Bazoti, S.; Pereira Siqueira, D.; Maria Colla, L.; Alves, S.L.; Paulo Bender, J.; et al. Effect of Pretreatments on Corn Stalk Chemical Properties for Biogas Production Purposes. Bioresour. Technol. 2018, 266, 116–124. [Google Scholar] [CrossRef]
- Abellanas-Perez, P.; Carballares, D.; Rocha-Martin, J.; Fernandez-Lafuente, R. The Effects of Buffer Nature on Immobilized Lipase Stability Depend on Enzyme Support Loading. Catalysts 2024, 14, 105. [Google Scholar] [CrossRef]
- Nahar, N.; Ripplinger, D.; Pryor, S.W. Process Yield and Economic Trade-Offs for Enzymatic Hydrolysis of Alkaline Pretreated Corn Stover. Biomass Bioenergy 2017, 99, 97–105. [Google Scholar] [CrossRef]
- Dong, L.; Cao, G.; Zhao, L.; Liu, B.; Ren, N. Alkali/Urea Pretreatment of Rice Straw at Low Temperature for Enhanced Biological Hydrogen Production. Bioresour. Technol. 2018, 267, 71–76. [Google Scholar] [CrossRef]
- Lazaro, C.Z.; Varesche, M.B.A.; Silva, E.L. Effect of Inoculum Concentration, PH, Light Intensity and Lighting Regime on Hydrogen Production by Phototrophic Microbial Consortium. Renew. Energy 2015, 75, 1–7. [Google Scholar] [CrossRef]
- Qyyum, M.A.; Ismail, S.; Ni, S.Q.; Ihsanullah, I.; Ahmad, R.; Khan, A.; Tawfik, A.; Nizami, A.S.; Lee, M. Harvesting Biohydrogen from Industrial Wastewater: Production Potential, Pilot-Scale Bioreactors, Commercialization Status, Techno-Economics, and Policy Analysis. J. Clean. Prod. 2022, 340, 130809. [Google Scholar] [CrossRef]
- Liu, X.; Li, R.; Ji, M.; Wu, N.; Yang, F. Effects of Initial PH on Two-Stage Biogas Production and Hydrolysis in a Co-Digestion Process of Food Waste and Waste Activated Sludge. Environ. Eng. Sci. 2021, 38, 266–276. [Google Scholar] [CrossRef]
- Jiang, D.; Ge, X.; Zhang, T.; Liu, H.; Zhang, Q. Photo-Fermentative Hydrogen Production from Enzymatic Hydrolysate of Corn Stalk Pith with a Photosynthetic Consortium. Int. J. Hydrogen Energy 2016, 41, 16778–16785. [Google Scholar] [CrossRef]
- Jiang, D.; Zhang, L.; Pei, W.; Song, Z.; Zhang, T.; Jing, Y.; Chen, Z.; Li, W.; Zhang, Q.; Lu, C. Correlation Between Ethanol Addition and Photo-Fermentative Biohydrogen Production of Arundo donax L. with a Consortium. Renew. Energy 2025, 248, 123077. [Google Scholar] [CrossRef]
- Li, Y.; Fan, X.; Zhang, H.; Ai, F.; Jiao, Y.; Zhang, Q.; Zhang, Z. Pretreatment of Corn Stover by Torrefaction for Improving Reducing Sugar and Biohydrogen Production. Bioresour. Technol. 2022, 351, 126905. [Google Scholar] [CrossRef]
- Xia, C.; Jiang, D.; Zhang, X.; Xie, N.; Lu, C.; Zhang, Q. A Performance Comparison of Three Amino Acid Additives in the Process of Photo-Fermentative Biohydrogen Production with Corn Straw. Fermentation 2025, 11, 108. [Google Scholar] [CrossRef]
- Ananthi, V.; Ramesh, U.; Balaji, P.; Kumar, P.; Govarthanan, M.; Arun, A. A Review on the Impact of Various Factors on Biohydrogen Production. Int. J. Hydrogen Energy 2024, 52, 33–45. [Google Scholar] [CrossRef]
- Santos, G.A.; Bortoli, L.D.; Santos, D.A.; Lobato, F.S.; Cardoso, V.L.; Xavier Batista, F.R. Insights of Light Spectra on Biohydrogen Production by Photo-Fermentation. Int. J. Hydrogen Energy 2025, 149, 150088. [Google Scholar] [CrossRef]
- Anzola-Rojas, M.D.P.; Fuess, L.T.; Zaiat, M. Specific Organic Loading Rate Control for Improving Fermentative Hydrogen Production. Fermentation 2024, 10, 213. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Kumar, G.; Mudhoo, A.; Rene, E.R.; Saratale, G.D.; Kobayashi, T.; Xu, K.; Kim, S.H.; Kim, D.H. Fermentative Hydrogen Production Using Lignocellulose Biomass: An Overview of Pre-Treatment Methods, Inhibitor Effects and Detoxification Experiences. Renew. Sustain. Energy Rev. 2017, 77, 28–42. [Google Scholar] [CrossRef]
- Amulya, K.; Venkateswar Reddy, M.; Venkata Mohan, S. Acidogenic Spent Wash Valorization through Polyhydroxyalkanoate (PHA) Synthesis Coupled with Fermentative Biohydrogen Production. Bioresour. Technol. 2014, 158, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Venkateswar Reddy, M.; Venkata Mohan, S. Influence of Aerobic and Anoxic Microenvironments on Polyhydroxyalkanoates (PHA) Production from Food Waste and Acidogenic Effluents Using Aerobic Consortia. Bioresour. Technol. 2012, 103, 313–321. [Google Scholar] [CrossRef]
- Shao, Y.; Song, J.; Sun, Y. Advances in Biochar’s Effect on Anaerobic Fermentation Systems. Pol. J. Environ. Stud. 2024, 33, 3009–3018. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, L.; Su, H. The Effect of a Buffer Function on the Semi-Continuous Anaerobic Digestion. Bioresour. Technol. 2013, 139, 43–49. [Google Scholar] [CrossRef]
- Komolwanich, T.; Tatijarern, P.; Prasertwasu, S.; Khumsupan, D.; Chaisuwan, T.; Luengnaruemitchai, A.; Wongkasemjit, S. Comparative Potentiality of Kans Grass (Saccharum spontaneum) and Giant Reed (Arundo donax) as Lignocellulosic Feedstocks for the Release of Monomeric Sugars by Microwave/Chemical Pretreatment. Cellulose 2014, 21, 1327–1340. [Google Scholar] [CrossRef]
- De Amorim, E.L.C.; Sader, L.T.; Silva, E.L. Effect of Substrate Concentration on Dark Fermentation Hydrogen Production Using an Anaerobic Fluidized Bed Reactor. Appl. Biochem. Biotechnol. 2012, 166, 1248–1263. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).