1. Introduction
The geological team of the Hungarian State Geological Institute discovered the country’s first alginite deposit in November 1973 [
1]. The formation is similar to conventional oil shale. Alginite colonies are defined more specifically as forming in volcanic craters and are primarily made of algae [
1]. We currently know of four volcanic craters in Hungary that contain alginite based on research by the Hungarian State Geological Institute. All locations are in Transdanubia: Pula in Bakony, Gérce in Kemeneshát, Várkesz villages, and the southwest edge of Egyházaskesz village [
1].
Alginite mineral rock is formed through the fossilization of accumulated organic (algae) and inorganic materials such as quartz, carbonates, clay, and modified amorphous silicic acid in a watery environment. Humic acids, as a component of alginite, are organic molecules that naturally form during long-term decomposition and transformation of biomass residues [
2,
3].
Humic substances (HSs) are complex organic materials that arise from the decomposition of plant and animal matter, and they exhibit a variety of properties that are crucial for their roles in soil health, plant growth, and microbial activity. These substances can be broadly categorized into three main fractions: humic acid, fulvic acid, and humin, each with distinct characteristics and functions [
4,
5]. The numerous positive research results are not surprising since humic substances have many beneficial properties, such as redox activity, surface activity, and being a nutrient source, thanks to which they can participate in many microbiological processes [
6]. Humic substances also exhibit strong antioxidant properties, which can mitigate oxidative stress in biological systems. They contain polyphenolic components that contribute to their ability to scavenge free radicals, thus protecting cells from oxidative damage [
7]. In addition to their effects on soil and plants, humic substances play a crucial role in microbial ecology. They serve as electron donors and acceptors in various redox reactions, facilitating anaerobic respiration in microorganisms [
8,
9]. This electron transfer capability is vital for the metabolic processes of many soil bacteria and can enhance the degradation of organic matter, thus contributing to nutrient cycling within ecosystems [
8,
9]. Furthermore, humic substances can stimulate microbial growth and activity (in the cow’s rumen), increasing fermentation efficiency and producing beneficial metabolites such as short-chain fatty acids [
10]. Moreover, there was an increase in the number of LAB found in chicks that were ten days old and had an inclusion level of 0.45% HSs. According to this finding, a humic compound derived from worm compost can be utilized as a growth enhancer component in broiler feeds.
Thanks to its unique composition, alginite has many benefits and uses in agriculture because of its water adsorption capability and pH-stabilizing effect. Numerous studies on this mineral have reported that, depending on the dose, alginite can significantly boost agricultural productivity [
1,
2,
3]. Recent studies have explored innovative approaches to using alginite. For instance, it turned out that alginite is efficient in demulsification. The mineral contributed significantly to the demulsification of W/O emulsion, which was stable over two months—the emulsion was prepared from 50 wt.% crude oil (Brent type) and 50 wt.% brine. Furthermore, chemical analysis of the separated oil showed compliance with industrial standards [
11,
12].
Other researchers have found alginite to be gastroenterally beneficial with lactobacilli (LAB) in animal models [
13,
14,
15]. In 2017, Hlubeňová K. and colleagues demonstrated the immunomodulatory potential of lactobacilli and humic substances present in alginite. The administration of
L. reuteri and alginite in the group infected with
S. typhimurium significantly stimulated the cellular immune response, particularly in the mesenteric lymph nodes of mice. This was evidenced by the activation of CD4 + CD8 + lymphocytes, natural killer and natural killer T cells, as well as the activation of phagocytosis within the innate immune system component [
13]. In the same year, in a separate study, alginite was introduced into the canine diet at a dose of 1% for 14 days, along with probiotic supplementation. The combination of alginite and LAB decreased coliform and
Clostridium-like bacteria while increasing the lactic acid bacteria of the gastrointestinal system, as well as hemoglobin concentration in blood. At the same time, the treatment stimulated cellular immunity parameters and an improvement in serum mineral levels [
14]. Another research study reported comprehensive positive results comparable to those reported before regarding the combination of alginite with a probiotic strain [
15]. Their research concentrated on the impact of a probiotic strain combined with alginite on the intestinal milieu of SPF mice infected with
Salmonella typhimurium. The group supplemented with
Lactobacillus reuteri CCM 8617 and alginite exhibited a significant decrease in the growth of
S. typhimurium in mouse feces at 24 and 72 h (
p < 0.001) post-infection. The addition of additives significantly influenced nitrogen, enzymatic, hepatic, and energy metabolism in mice. The effect of
S. typhimurium infection on morphology was examined in the jejunum and ileum of the LAB group of mice. Mice livers subjected to treatment with both alginite and
Lactobacillus reuteri CCM 8617 exhibited a significant decrease in overall inflammation, hepatocyte necrosis, and the size of typhoid nodules. These promising findings bring us one step closer to making better non-dairy probiotic products. A review by Chaudhary et al. (2024) emphasizes the individual roles of site-specific microbiomes in maintaining health and preventing diseases; furthermore, the complex interactions between different microbiomes across the body are also highlighted [
16]. These interactions originate with the mouth and lungs, extending to the vagina, skin, and the central hub of the digestive system. Comprehensive research has demonstrated that the gut microbiota significantly influences the health of other microbiome sites and vice versa. Previously, the health benefits of probiotics were provided by milk/other dairy products. However, the growth of dairy probiotics is limited by lactose intolerance and health concerns related to cholesterol, allergic milk proteins, and fat content in dairy products [
17,
18,
19]. Silanikove N. et al. (2015) state that 75% of the world’s population suffers from lactose intolerance [
20]. The investigation of non-dairy probiotic products, which utilize food matrices derived from fruit, vegetables, and cereals, has received significant attention and has been widely studied due to a growing number of persons with lactose intolerance [
21]. Species such as
L. acidophilus,
L. casei,
L. plantarum,
L. rhamnosus, and
L. lactis are the most employed in the development of novel probiotic products [
22,
23,
24,
25]. Hence, we have chosen particular strains of
Lactococcus lactis,
Lactocaseibacillus rhamnosus, and
Lactobacillus acidophilus for our research.
Given the positive influence of alginite and lactic acid bacteria (LAB) on gastrointestinal function in animal models, as well as the growing need for lactose-free probiotic food products, we aimed to conduct an experimental study to examine the impact of alginite on the fermentation by potentially probiotic LAB strains. The assessment involved quantifying the fermentation process using multiple methodologies, like applying two cultivation systems (Bactrac® 4100 and fermenter), monitoring impedance levels, determining the specific colony-forming units (CFU/mL), and employing an online living cell sensor to monitor the fermentation progress.
2. Materials and Methods
2.1. Used Strains and Media
The strains were ordered from the National Collection of Agricultural and Industrial Microorganisms (NCAIM). The following microorganisms and media were used in this study: L. rhamnosus (strain: NCAIM B.02274T, i.e., ATCC 7469, i.e., DSMZ 20021) in MRS medium, L. acidophilus (strain: NCAIM B.02085, i.e., ATCC 4356, i.e., DSMZ 20079) in MRS medium, and L. lactis (strain: NCAIM B.02123T, i.e., DSMZ 20661) in M17 medium.
MRS medium: Glucose 20 g/L, peptone 10 g/L, meat extract 10 g/L, yeast extract 5.0 g/L, sodium acetate 2.0 g/L, K2HPO4 2.0 g/L, ammonium citrate 2.0 g/L, MgSO4·7∙H2O 0.2 g/L, MnSO4·H2O 0.05 g/L, Tween 80 1.08 g/L.
M17 medium: Lactose 5 g/L, disodium glycerol-β-phosphate 10 g/L, tryptone 5.0 g/L, soy peptone 5.0 g/L, beef extract 5.0 g/L, yeast extract 2.5 g/L, L-ascorbic acid 0.5 g/L, MgSO4 0.25 g/L.
Alginite-added fermentation media were supplemented with 10 g/L of alginite mineral before sterilization. Alginite’s particle size was 20–40 microns, determined via sieving after mining near Gérce, Transdanubian region, Hungary. The used alginite mineral had an average 20-micron particle size. The used alginite contained, according to Kádár et al. [
26], 4% moisture, 15% CaCO
3, and 4.6% organic matter. Total N was 0.15%, K 63 mg/kg, Al-K
2O 386 mg/kg, and Al-P
2O
5 216 mg/kg. The alginite used contained approximately 5% elemental Ca, 3.6% Al, 2.9% Fe, 1.9% Mg, 0.82% K, 0.15% P, and 0.12% S. Aqua regia soluble content was as follows: Ca 49.942 mg/kg, Al 36.026 mg/kg, Fe 28.501 mg/kg, Mg 19.188 mg/kg, K 8166 mg/kg, P 1501 mg/kg, S 1237 mg/kg, Mn 587 mg/kg, Na 454 mg/kg, Sr 419 mg/kg, Ba 281 mg/kg, Ni 75.0 mg/kg, Zn 65.8 mg/kg, Cr 63.9 mg/kg, B 26.8 mg/kg, Cu 19.2 mg/kg, Co 15.9 mg/kg, Pb 9.75 mg/kg, As 8.84 mg/kg, Sn 2.84 mg/kg, Mo 1.86 mg/kg, Se 1.02 mg/kg, Cd 0.12 mg/kg.
2.2. Fermentations
The small-scale fermentations were conducted in 10 mL reusable aerobic glass vials of the BacTrac
® 4100 (Sy-Lab, Neupurkersdorf, Austria) instrument at a temperature of 37 °C, with a total liquid volume of 10 mL. In each vial, after sterilization with distilled water in an autoclave (121 °C, 20 min), the distilled water was replaced by 10 mL of the corresponding media (including or free from alginite), followed by inoculation with 20 μL cell suspension (1 sterile loop from fresh agar plate culture in 1 mL media). The fermentations were monitored using relative impedance changes of the medium (M%) as we had previously reported [
27]. Uninoculated vials served as a reference since the relative impedance of the media may also vary. The BacMonitor Y 1.39Er program presented the results.
Specific growth rates of the static cultures were calculated from the derivative of generalized logistic equations as follows:
where
t represents fermentation time;
x represents biomass cell dry weight; and
a,
b,
c,
d, and
xmax represent model constants.
The bioreactor experiments were conducted in a 1 L benchtop bioreactor with a working volume of 0.8 L (Biostat Q fermenter, B. Braun Biotech International, Melsungen, Germany) with 5%
v/
v inoculum (having 10
9 CFU/mL). The temperature for production was set to 37 °C with an agitation speed of 300 rpm. The pH was regulated using 25% H
3PO
4 and 25% NaOH; the initial pH was set and held at 6.5 [
28]. The cell count was followed during fermentation online, and observed time courses were compared by paired
t-test with Statistica 13.5.
As a descriptive kinetic auxiliary model, the Weibull equation was used. Sigma Plot 7.0 was used for curve fitting of the Weibull equation as follows:
where
t represents fermentation time;
x represents biomass cell dry weight; and
t0,
x0,
a,
b, and
c represent model constants.
Weibull was the best-fitting model among log-logistic, gamma, log-normal, and exponential in survival analysis using the Akaike information and Bayesian information criterion [
29].
2.3. Analytic
A Waters Breeze HPLC system determined glucose and lactic acid content from samples. The instrument consisted of a Waters 717 Plus Autosampler (Wyatt Technology LLC, Santa Barbara, CA, USA), Waters 1515 Isocratic Pump (Wyatt Technology LLC), Biorad Aminex HPX 87H (300 × 7.8 mm, 9 µm) column (65 °C) (BioRad Laboratories, Hercules, CA, USA), and Waters 2414 RI detector (40 °C) (Wyatt Technology LLC). After appropriate dilution steps, the samples were filtered with a 0.2-micron mixed ester syringe filter (ViaLab Ltd., Budapest, Hungary). The mobile phase was 5 mM H2SO4 in ion-exchanged water (Simplicity, Millipore, Burlington, MA, USA), and the elution rate was 0.5 mL/min.
The colony-forming unit (CFU) determination was carried out as follows: Samples (1 mL/sample) were diluted ten-fold in a sterile Falcon tube with 9 mL of sterile saline (9.0 g/L NaCl). The ten-fold dilution was repeated six and seven times based on the expected cell count, and then 100 µL of the diluted samples was spread on MRS agar Petri dishes. The Petri dishes were then incubated at 37 °C for 24 h.
The dry matter was quantified by transferring 10 mL of fermentation broth from the BacTrac tubes into Falcon Conical Centrifuge Tubes, which were then centrifuged at 6000 rpm for 10 min using a Hermle Z200A centrifuge (Hermle, Wehingen, Germany). The supernatant was decanted, after which the cells were resuspended in distilled water and subjected to centrifugation again. The supernatant was decanted once again, and the biomass was placed into the crystallization cup with 2–3 mL of distilled water and dried expeditiously using the Sartorius MA35 moisture analyzer (Sartorius, Göttingen, Germany) until mass constancy [
28].
For
in-line living cell numbers, we used a Hamilton Incyte (Hamilton, Switzerland) viable cell density sensor. This sensor performs capacitance-based detection of living cells since it uses alternating current for polarizing biomass (dead cells cannot be polarized). By applying different frequencies, dielectric spectroscopy can be applied to determine the volume, size, and size distribution of living cells [
30]. The registered permittivity is proportional to the living cell concentration. Moreover, capacitance sensors are insensitive to micro-carriers, gas bubbles, cell debris, and other particles in suspension [
31].
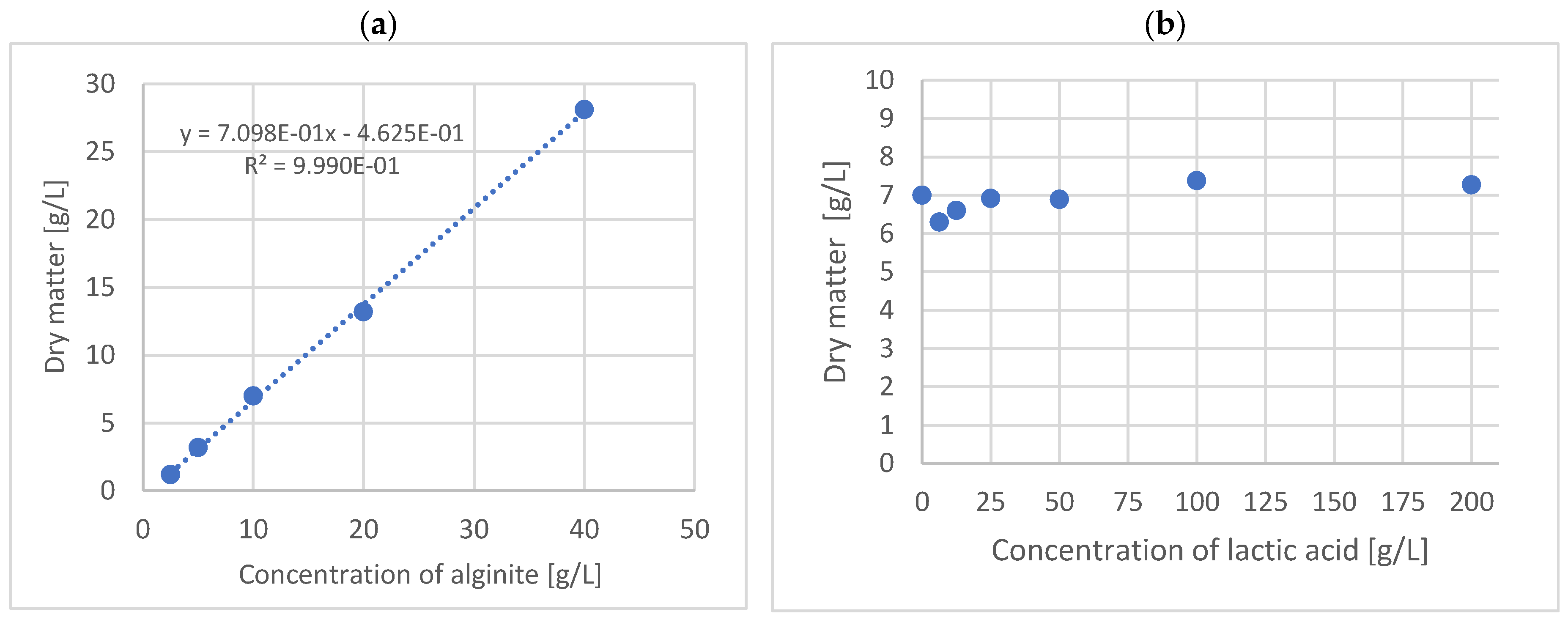
To determine what percentage of alginite mineral total mass was lost via solubilization during fermentation and via dry matter measurement, where the water content and volatile compounds could vaporize, a calibration curve was constructed using 100 g/L lactic acid solution with 2.5, 5, 10, 20, and 40 g/L alginite. Solutions were placed into BacTrac, where they could also be checked to see whether the alginite samples contained contaminants. After three days, the solutions were poured into Falcon tubes and then centrifuged, and the sediments were washed with distilled water as described above. Finally, the dry matter content of the sediment was measured and plotted versus alginite concentrations (
Figure 1a). Furthermore, another solubilization test was prepared with lactic acid solutions of different concentrations, to which 10 g/L of alginite was added uniformly, modeling alginite solubilization during the lactobacillus fermentation. The concentration of lactic acid solutions was 12.5, 25, 50, 100, and 200 g/L; the sample processing method was the same as the previous one. Finally, the dry matter content of the samples was measured and plotted versus lactic acid concentration (
Figure 1b).
3. Results
Alginite, characterized by its notable humin and humic acid content, exhibits the potential to enhance the availability of trace elements to plants [
32]. Furthermore, its buffering capacity contributes to the soil’s pH shift from acidic to neutral. These factors promote optimal plant growth and maximize crop yield [
3]. Our expectations were similar in the fermentation experiments. Firstly, we examined the processes in static cultures using BacTrac. We observed a considerable difference between the fermentations supplemented with alginite (with A.) and without it (
Figure 2). This suggests that the alginite positively affected these lactic acid-producing bacteria’s (LAB’s) cell growth because they have 2–3 times higher impedance levels than the alginite-free ones.
Maximum specific growth rates of the static cultures were determined through curve fitting, and the resulting values are presented in
Figure 3. The results were similar in the case of particular growth rates and maximal impedance values: the µmax is significantly higher in all cases where alginite was used in the fermentation medium compared to the alginite-free cases.
To validate the observations above, we conducted measurements of biomass dry matter. In order to accurately determine the dry weight of biomass without interference originating from solid alginite, we used solubilization curves (see
Figure 1). These curves revealed that 70% of the alginate remained as carbonates after reacting with lactic acid. Additionally, soluble and volatile compounds were released regardless of the lactic acid concentration at each alginite concentration tested. So, according to the solubilization curves, the remainder of the alginite was subtracted, and the final data is represented in
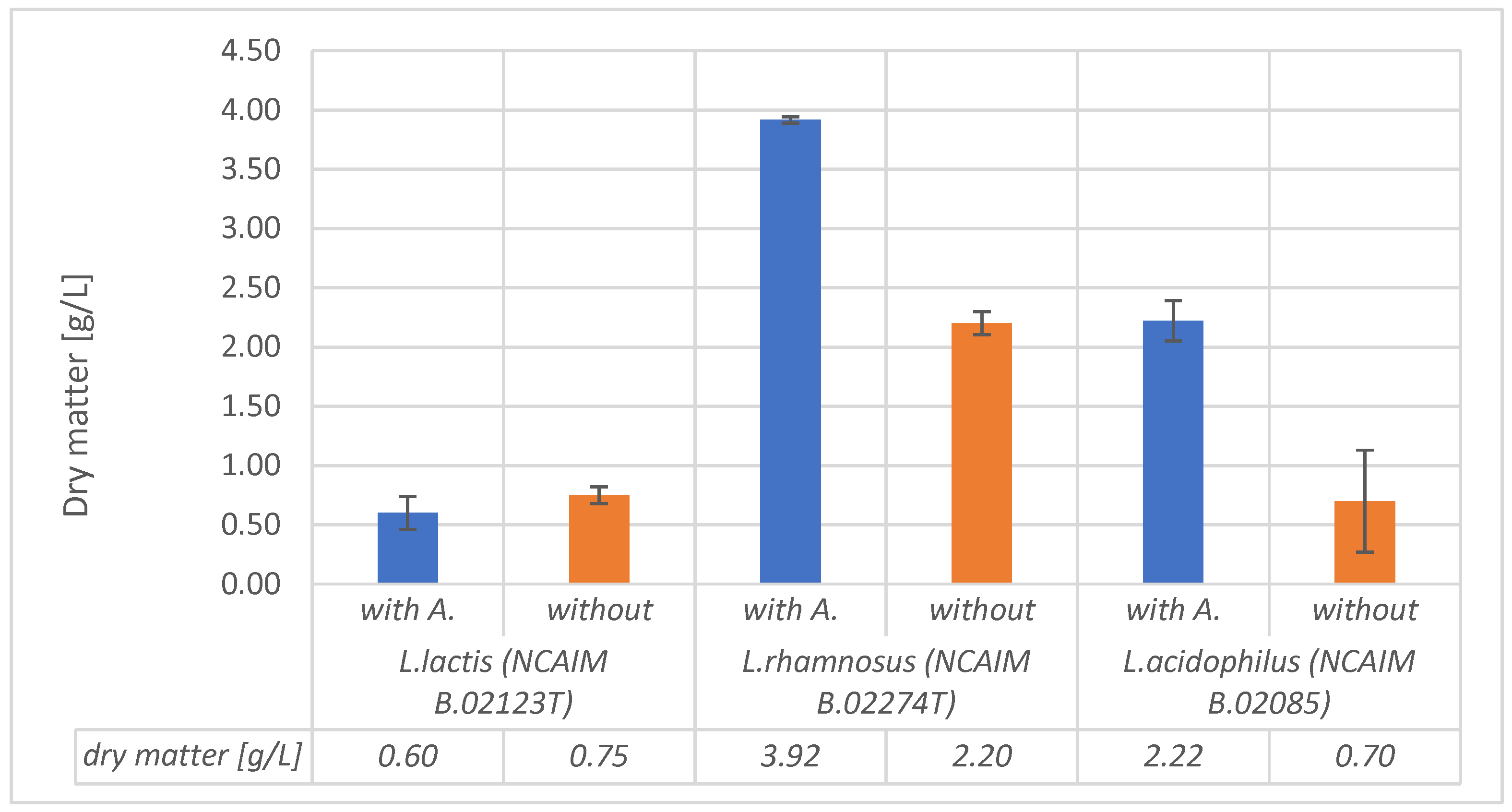
Figure 4.
The dry mass results were not significantly different in the case of alginite-containing and alginite-free runs of L. lactis. In all other cases, alginite-containing fermentations resulted in significantly higher biomass dry weight, especially in the case of L. acidophilus, where biomass dry weight was three times higher than that in the normal alginite-free fermentations, which was considered a significant enhancement since p-values were lower than 0.05.
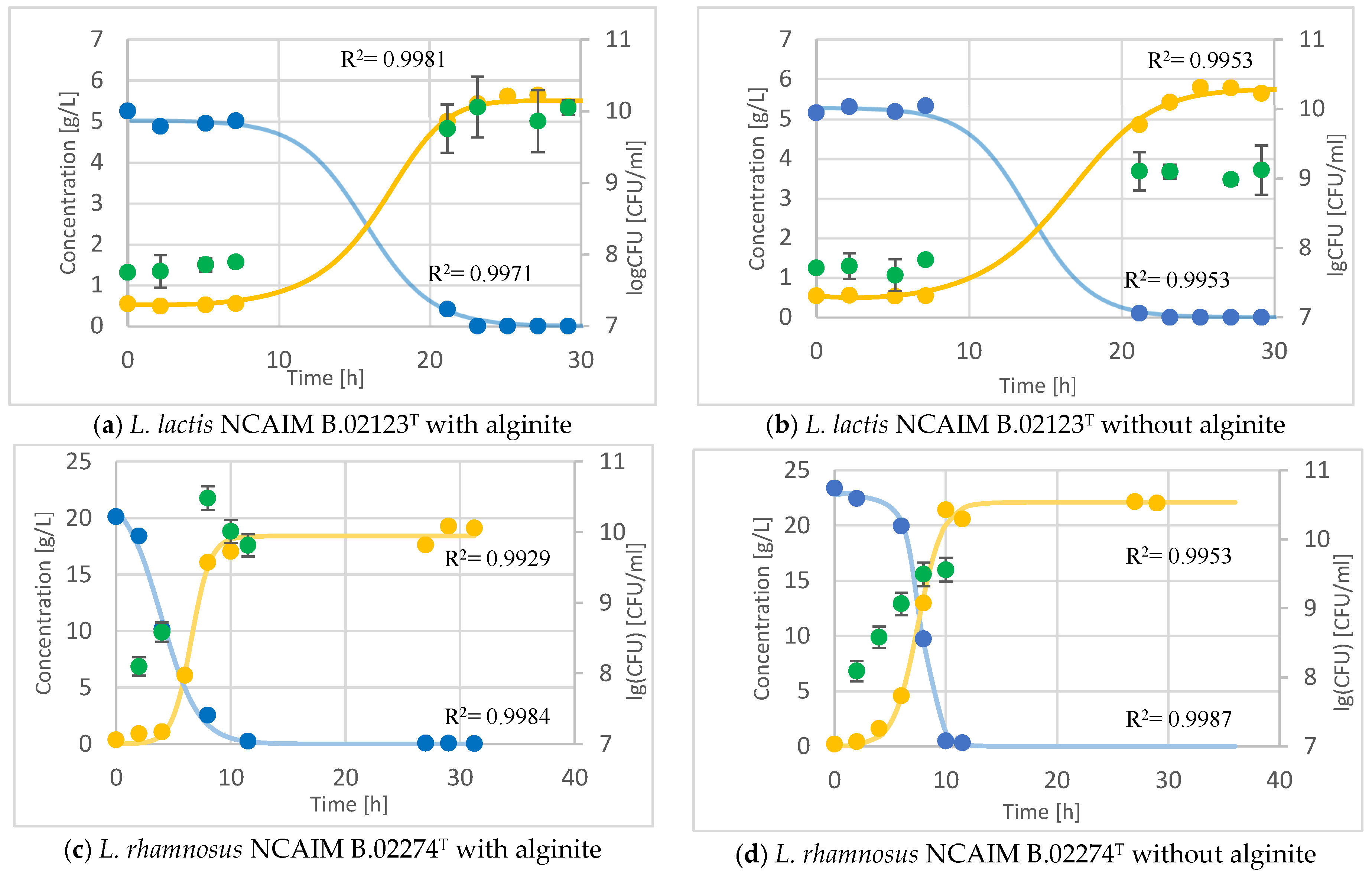
The effect of alginite addition to LAB fermentations was also compared in 1 L laboratory fermenters since probiotics are rarely produced in static cultures like BacTrac. While offline HPLC measurements monitored substrate and product concentrations, the biomass was followed by CFU determinations since solid alginite disturbed the commonly used optical density and cell dry weight measurements.
In the case of
Lactococcus lactis fermentation, the sugar (lactose) consumption and the lactic acid production are the same (
Figure 5a,b). Nevertheless, the CFU in alginite fermentation is ca. one order of magnitude higher (on a logarithmic scale) than in alginite-free fermentation.
During the fermentation of
Lactocaseibacillus rhamnosus with alginite, the CFU reached a higher value by one order of magnitude, even though, for some reason, the starting sugar amount was less than in the alginite-free fermentation (
Figure 5c,d). However, the product reached a higher concentration of 20% in the alginate-free fermentation, following the higher substrate amount consumed, and there was no more production after the 10th hour of fermentation.
We may infer that the production of lactic acid was slower from the flat curve showing product formation in the Lactobacillus acidophilus alginite-supplemented fermentation (
Figure 5e,f). Interestingly, while the lactic acid production was slower, the CFUs were actually higher in the alginite-supplemented fermentation compared to the alginite-free fermentation, indicating a greater bacterial population despite the slower acid production.
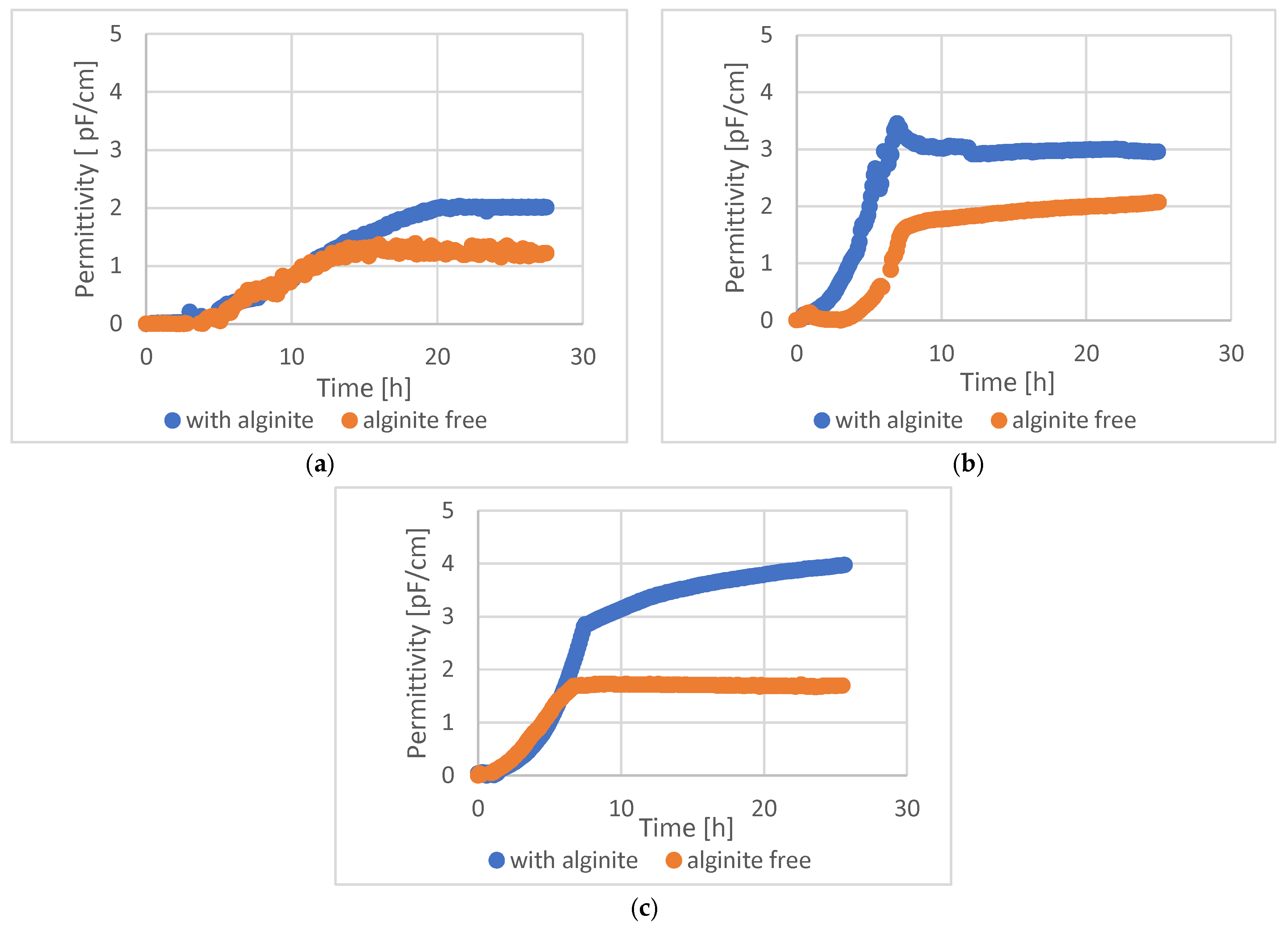
The BacTrac static cultures’ cell dry weight (CDW) and bioreactor-based dynamic cultures’ CFU results are in full accordance; despite this, a third method was used to confirm the results: an online capacitance-based living cell sensor was also used and evaluated in bioreactor experiments (showing permittivity, which correlates with cell density) (
Figure 6). This method shows only the living cells; other particles and the dead cells do not give a signal during the measurement.
In the case of all tested lactic acid bacteria, 1.5–2-fold higher cell density could be reached with alginite supplementation compared to alginite-free cultures.
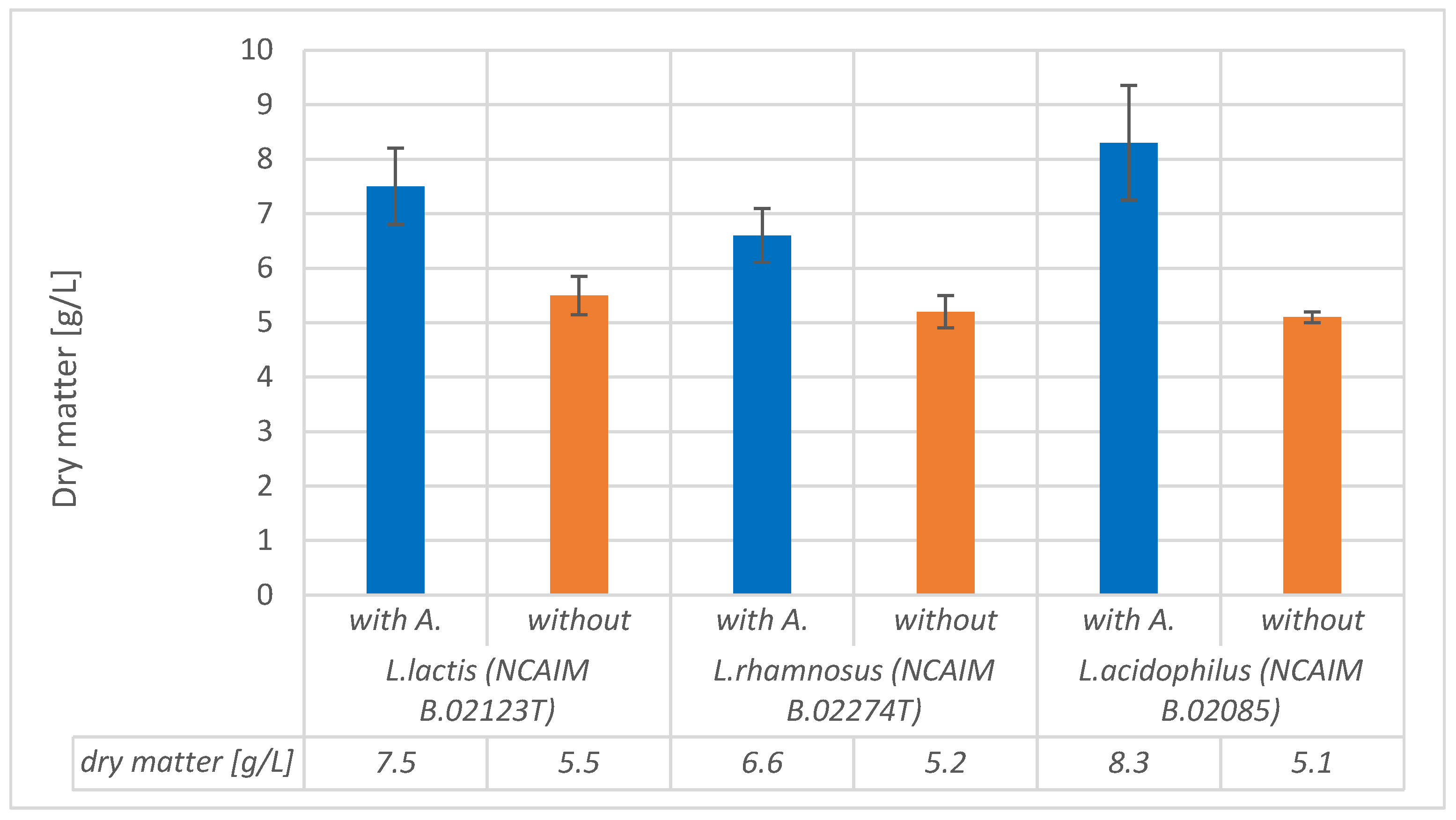
We also measured dry matter during the fermentation processes to prevent the potential measurement-disturbing effect of alginite-released ions. These measurements consistently confirmed that fermentations involving alginite resulted in higher cell biomass at the end of the process (see
Figure 7).
The results were corrected with the previously determined alginite residual amounts (
Figure 1). So, in all cases of the tested strains’ fermentations, the alginite-supplemented ones significantly reached higher biomass dry matter. These observations are based on
Figure 7 and contribute to declaring that alginite is beneficial for LAB biomass production, but based on
Figure 5, alginite does not affect the fermentation products.
4. Discussion
Facilitating microbial growth, alginite has been demonstrated to improve the overall fermentation environment because the buffering capacity of alginite helps stabilize pH levels during fermentation, hence facilitating ideal conditions for microbial activity [
15]. This stabilization can enhance fermentation rates and product yields, as pH changes can negatively impact microbial metabolism and fermentation efficiency [
15]. Following the completion of our preliminary BacTrac investigations, we obtained the same result as well, given that the experiments lacked pH control. For this exact reason, the trials were carried out in a fermenter with a capacity of one liter, with constant mixing (300 rpm) and pH (6.5) that was controlled. Because of this, the buffer capacity that alginite offers cannot be taken into consideration in these experiments. The results were very remarkable in terms of the number of living cells as well as the amount of biomass that was produced.
We assume that the presence of humic substances (HSs) in alginite plays a crucial role in enhancing the amount of LAB biomass. Among the several types of soils, alginite has the lowest amount of aromatic carbon and the highest quantity of aliphatic carbon. The compared soils include lignite, lignohumate, acadian, compost, and alginite [
33]. The source of alginite humic acids (HAs) is marine organic materials with some contribution of algae and not higher plants, as in soil or lignite. Lubica P. et al. (2015) found that alginite-derived humic acid and humic acids obtained from compost, lignohumates, and acadian sources are newly formed humic acids with a lower level of aromaticity, estimated to be around 40% [
33]. Conversely, the humic acids obtained from soils and lignite demonstrate a notable degree of aromaticity, causing enhanced stability and reduced vulnerability to oxidation. The information on alginite is significant in comprehending our findings, as previous studies have demonstrated that humic compounds, regardless of their source, can be utilized by 70–80% of bacterial strains [
34]. Initially, when Visser [
35] introduced this area of study, it was proposed that bacteria might not have easy access to humic compounds. However, further studies conducted by Tranvik and Sieburth [
36], Moran and Hodson [
37], and Donderski and Wodkowska [
38] highlighted the significant role of humic chemicals as nourishment for bacteria. The findings have resulted in diverse studies on the interactions between microorganisms and humic chemicals. Strains of
Lactococcus lactis subsp.
lactis,
Propionibacterium freudenreichii, and
Enterococcus cecorum with humic compounds were investigated, and it was found that these microorganisms were able to reduce humic substances and produce more oxidized products [
39]. Notwithstanding, we have not observed over-oxidized products (for example, pyruvic acid from lactic acid, etc.). Nevertheless, laboratory experiments resembling the ones detailed in our study have not yet been performed, and according to our information, no one has investigated the effect of alginite mineral on living cell numbers and biomass.
In summary, based on our living-cell-sensor-supported results and other reports, it is assumed that the presence of humic and fulvic acid compounds and minerals (i.e., inorganic metal ions) in alginite leads to a higher total amount of LAB biomass. In addition to humic substances, alginite also contains other useful components for microbes, such as minerals, amino acids, lipids, polyphenols, and proteins, which the microbes are able to release from the macromolecules of humic and fulvic acid in alginite [
39].
To gain information on what type of substances were consumed by the tested LAB strains, we compared initial and final samples of LAB fermentations with and without alginite by Near-Infrared Spectroscopy (see
Supplementary S1). While these are only preliminary tests, they suggested that the spectra had lower transmittance at wavelengths of 1020, 1410, and 1570 1/cm, indicating more solubility in initial alginite-supplemented fermentations compared to their final samples and alginite-free pairs.
In conclusion, it seems that the strains we are studying may be able to utilize certain sugar components found in alginite. In addition, alginite has organic content between 4.6 and 13%, which may result in a theoretical maximum increase of 1.3 g/L in organic material (10 g/L of alginite having max. 13% organic compounds). Since LAB biomass increased by more than 1.3 g/L in several cases, alginite’s organic content is not enough to reach as high a biomass concentration as we reached with alginite supplementation. Thus, alginite’s effect is rather considered as a source of inorganics (minerals), which resolves media bottleneck compounds, resulting in higher utilization of media protein. LAB convert carbohydrates almost fully into lactic acids and build their own biomass from organic N sources (like amino acids and proteins). Without alginite from MRS (having overall protein content above 25 g/L), only ca. 5 g/L of L. acidophilus NCAIM B.02085, L. rhamnosus NCAIM B02274, and L. lactis NCAIM B02123T was obtained, but with alginite supplementation, 25 g/L of protein was converted into 8, 6.5, and 7.5 g/L of LAB biomass, respectively.
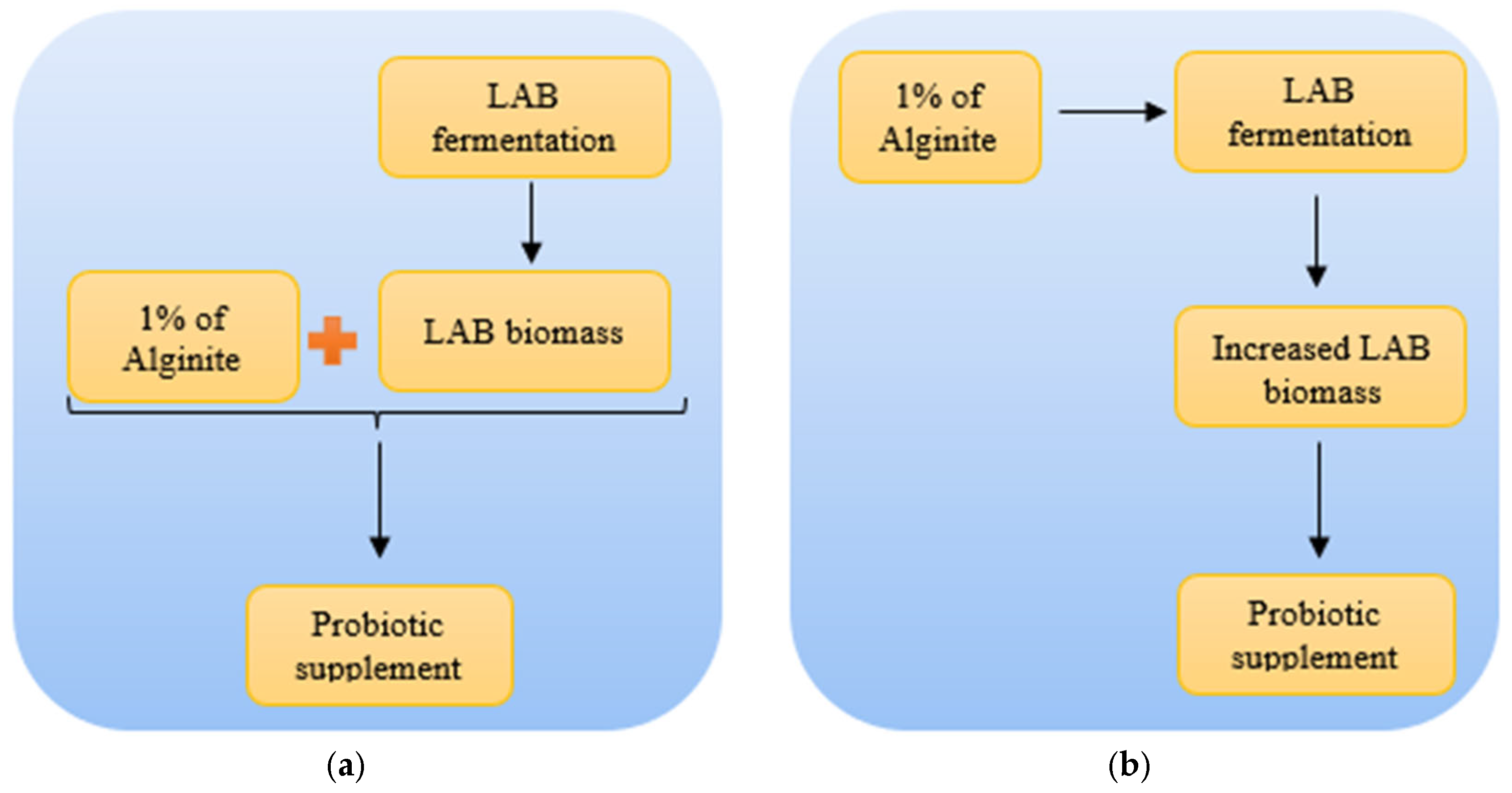
Since studies have shown that alginite and lactic acid bacteria (LAB) together have positive and synergic effects on gastrointestinal function in animal models [
13,
14,
15], our findings on the enhancing effect of alginite may result in more effective probiotic goods with higher living cell numbers. However, whether the fermentation of alginite changes its post-fermentative positive synergic effect or not is the target of further experiments and needs confirmation (
Figure 8).
If the pre- and post-fermentation positive synergic effect of alginite is later confirmed, it could lead to the development of a groundbreaking probiotic product. Prior to that time, we can only emphasize that the use of alginite in LAB fermentation has the potential to enhance biomass production yields without drastically altering lactic acid yield. This, in turn, may contribute to meeting the growing demand for lactose-free probiotics [
40].
These findings support the future development and production of potentially probiotic non-dairy-based superfoods or dietary supplements. They also add to our research on LAB-based cosmetics combined with alginite minerals.


 glucose (with L. lactis lactose),
glucose (with L. lactis lactose),  lactic acid,
lactic acid,  CFU/mL.
CFU/mL.
 glucose (with L. lactis lactose),
glucose (with L. lactis lactose),  lactic acid,
lactic acid,  CFU/mL.
CFU/mL.