Neuroprotective Effect of Lactobacillus gasseri MG4247 and Lacticaseibacillus rhamnosus MG4644 Against Oxidative Damage via NF-κB Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Probiotic Samples
2.2. Acetylcholinesterase (AChE) Inhibitory Activity
2.3. Cell Culture
2.4. Measurement of Cell Viability and Damage
2.5. Intracellular ROS Levels
2.6. Protein Extraction and Western Blotting
2.7. Genomic DNA (gDNA) Isolation
2.8. WGS and Genome Annotation
2.9. Morphological Analysis
2.10. Antibiotic Susceptibility
2.11. Statistical Analysis
3. Results
3.1. Screening of Strains Based on AChE Inhibition and Neuroprotective Potential
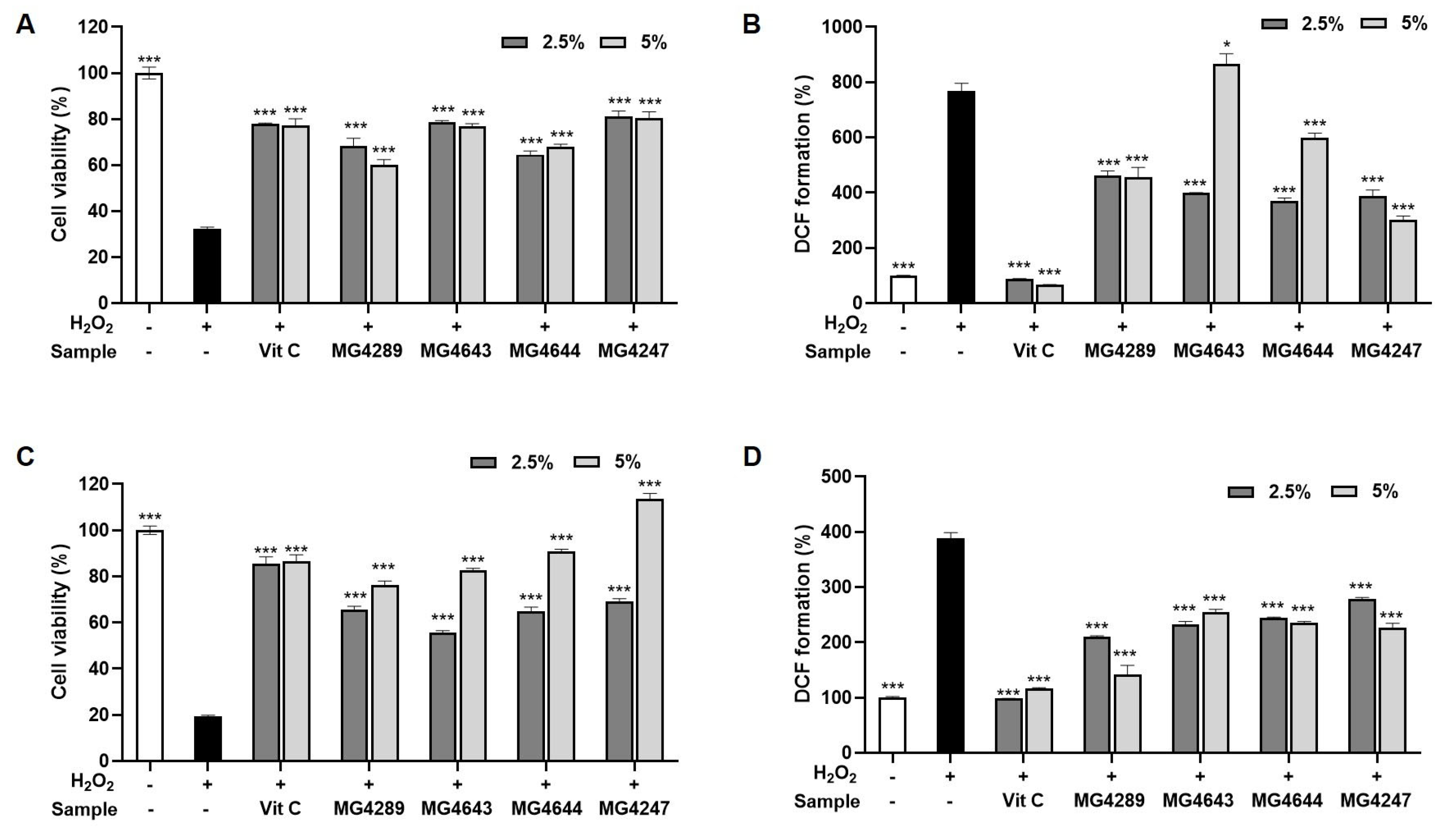
3.2. CFS Improves Cell Viability and Reduces ROS in H2O2-Treated HT-22 and SK-N-MC Cells
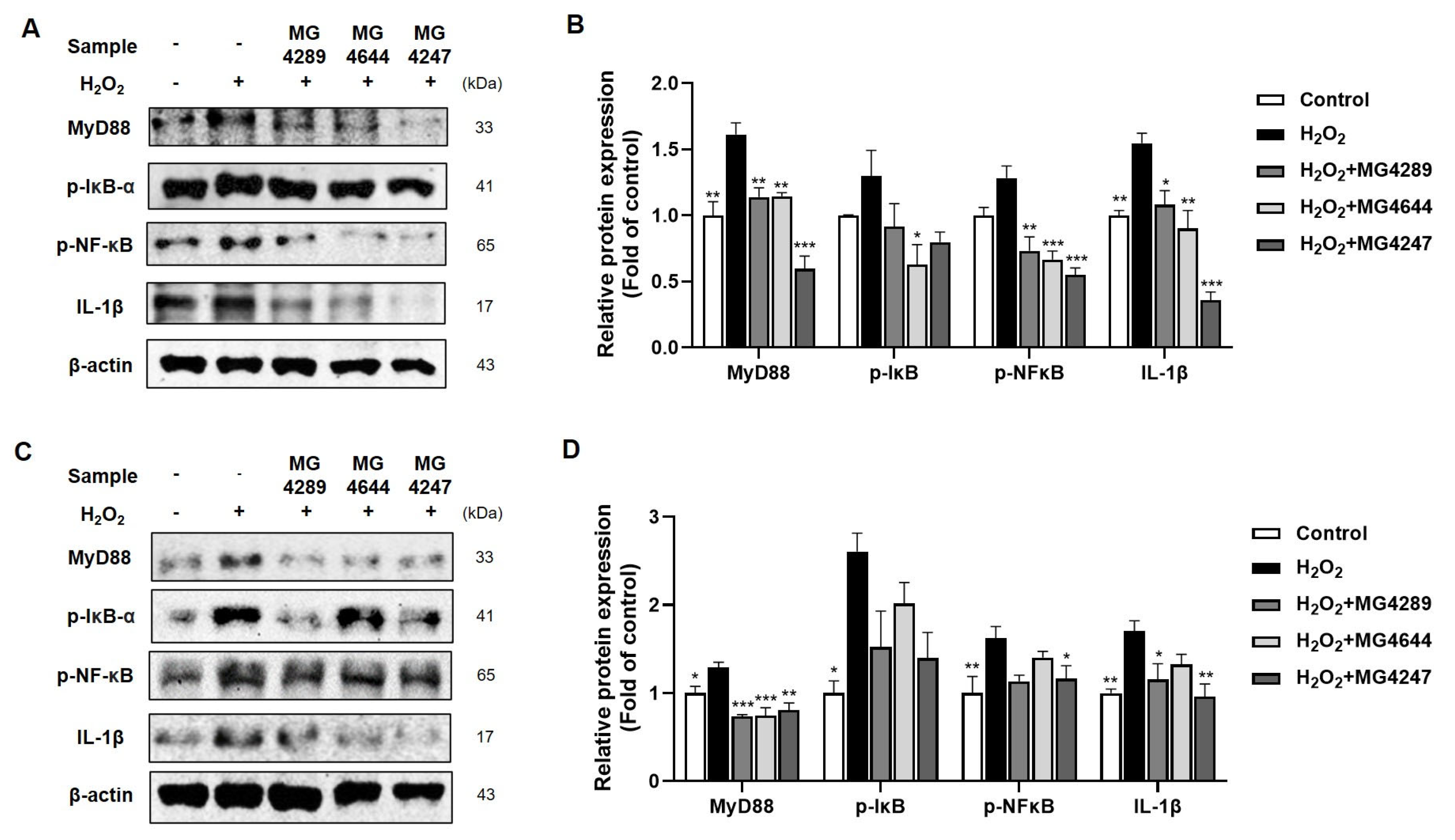
3.3. Anti-Inflammatory Effects of Selected Strains in H2O2-Treated HT-22 and SK-N-MC Cells
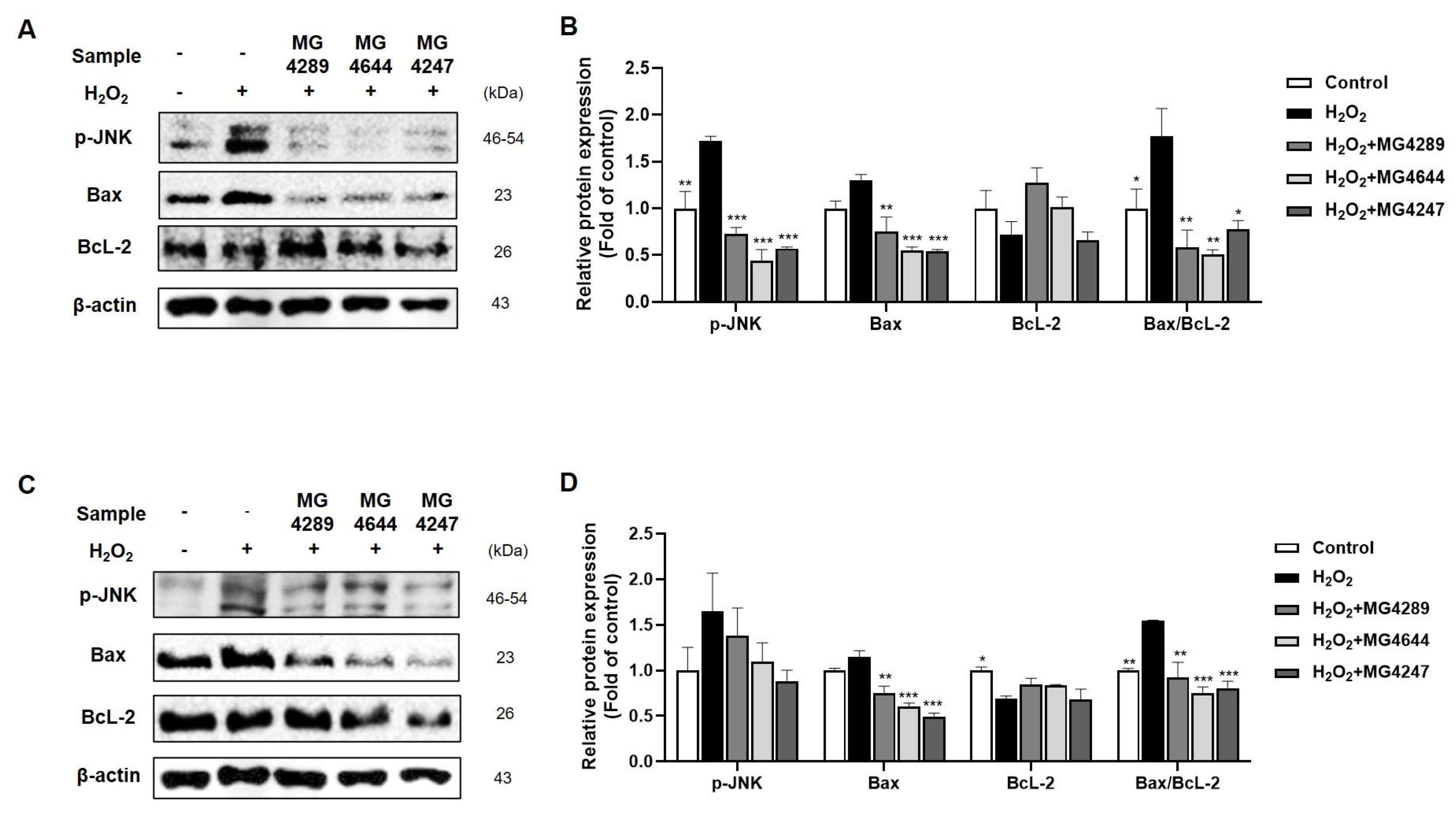
3.4. Modulation of Apoptotic Pathways by Selected Strainsin H2O2-Treated HT-22 and SK-N-MC Cells
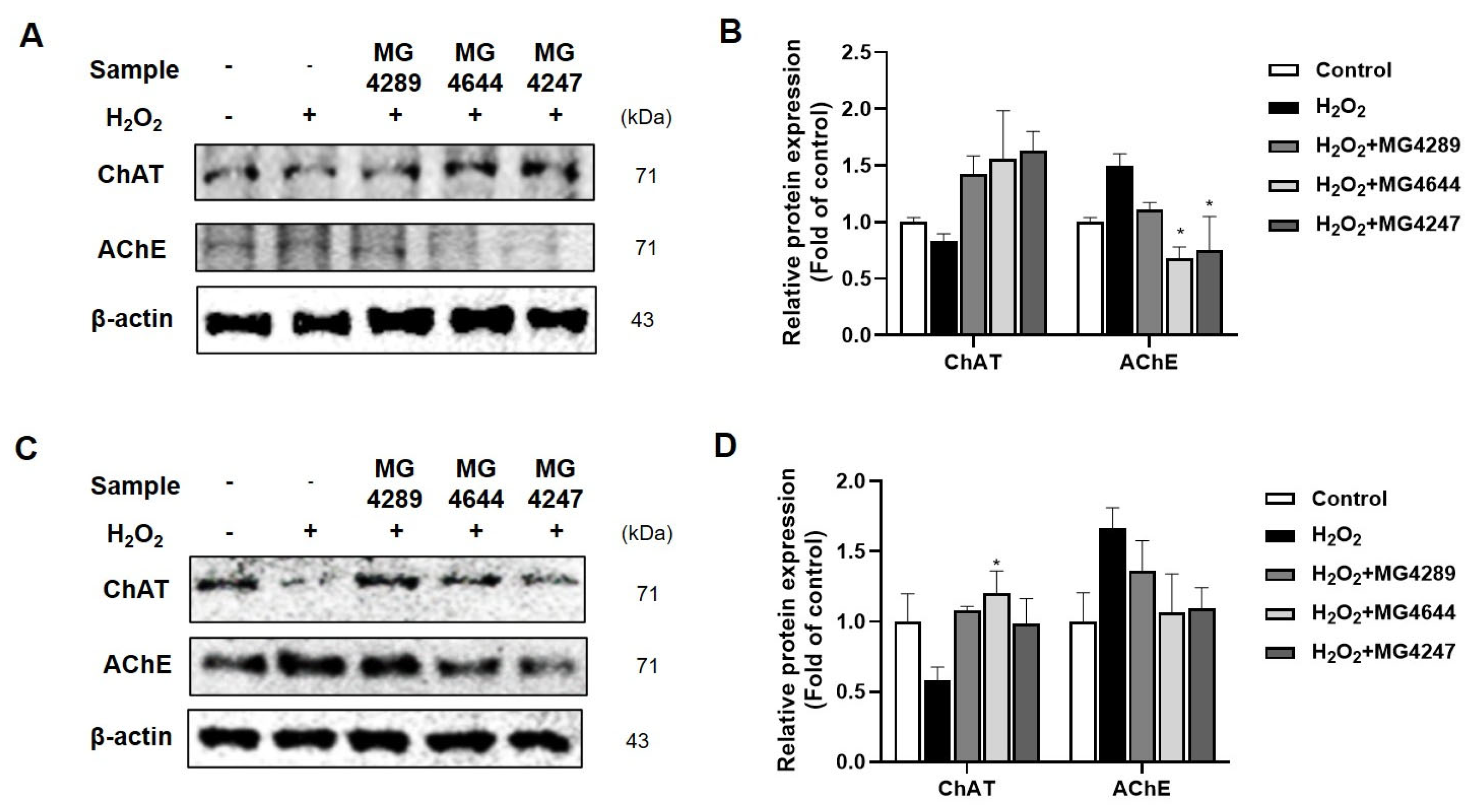
3.5. Regulation of Cholinergic System by Probiotic Strains in H2O2-Treated HT-22 and SK-N-MC Cells
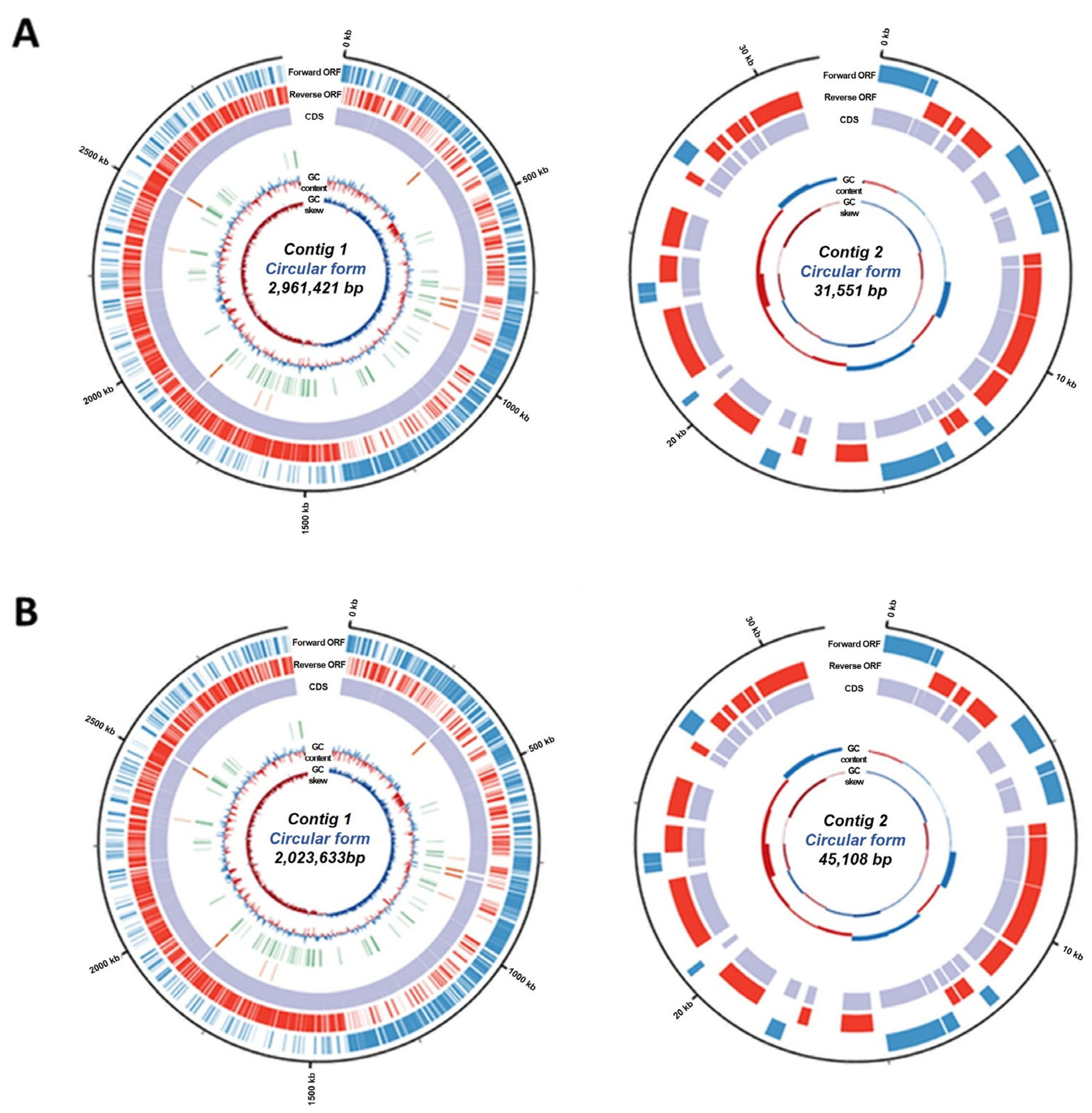
3.6. General Features of L. rhamnosus MG4644 and L. gasseri MG4247
3.7. Antibiotic Susceptibility of L. rhamnosus MG4644 and L. gasseri MG4247
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species-sources, functions, oxidative damage. Pol Merkur Lekarski. 2020, 48, 124–127. [Google Scholar]
- Beckhauser, T.F.; Francis-Oliveira, J.; De Pasquale, R. Reactive oxygen species: Physiological and physiopathological effects on synaptic plasticity: Supplementary issue: Brain plasticity and repair. J. Exp. Neurosci. 2016, 10, S39887. [Google Scholar] [CrossRef] [PubMed]
- Massaad, C.A.; Klann, E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid. Redox Signal. 2011, 14, 2013–2054. [Google Scholar] [CrossRef]
- Hsieh, H.-L.; Yang, C.-M. Role of redox signaling in neuroinflammation and neurodegenerative diseases. BioMed Res. Int. 2013, 2013, 484613. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, C.M.; da Silva Oliveira, G.L.; do Nascimento Cavalcante, A.; Morais Chaves, S.K.; Rai, M. Determination of parameters of oxidative stress in vitro models of neurodegenerative diseases-A review. Curr. Clin. Pharmacol. 2018, 13, 100–109. [Google Scholar] [CrossRef]
- Aksenova, M.V.; Aksenov, M.Y.; Mactutus, C.F.; Booze, R.M. Cell culture models of oxidative stress and injury in the central nervous system. Curr. Neurovasc. Res. 2005, 2, 73–89. [Google Scholar] [CrossRef]
- Bermúdez-Humarán, L.G.; Salinas, E.; Ortiz, G.G.; Ramirez-Jirano, L.J.; Morales, J.A.; Bitzer-Quintero, O.K. From probiotics to psychobiotics: Live beneficial bacteria which act on the brain-gut axis. Nutrients 2019, 11, 890. [Google Scholar] [CrossRef]
- Toledo, A.R.L.; Monroy, G.R.; Salazar, F.E.; Lee, J.-Y.; Jain, S.; Yadav, H.; Borlongan, C.V. Gut–brain axis as a pathological and therapeutic target for neurodegenerative disorders. Int. J. Mol. Sci. 2022, 23, 1184. [Google Scholar] [CrossRef]
- Bi, M.; Liu, C.; Wang, Y.; Liu, S.-J. Therapeutic Prospect of New Probiotics in Neurodegenerative Diseases. Microorganisms 2023, 11, 1527. [Google Scholar] [CrossRef]
- Xiang, S.; Ji, J.-L.; Li, S.; Cao, X.-P.; Xu, W.; Tan, L.; Tan, C.-C. Efficacy and safety of probiotics for the treatment of Alzheimer’s disease, mild cognitive impairment, and Parkinson’s Disease: A systematic review and meta-analysis. Front. Aging neurosci. 2022, 14, 730036. [Google Scholar] [CrossRef]
- Aghamohammad, S.; Hafezi, A.; Rohani, M. Probiotics as functional foods: How probiotics can alleviate the symptoms of neurological disabilities. Biomed. Pharmacother. 2023, 163, 114816. [Google Scholar] [CrossRef] [PubMed]
- Salek, F.; Mirzaei, H.; Khandaghi, J.; Javadi, A.; Nami, Y. Apoptosis induction in cancer cell lines and anti-inflammatory and anti-pathogenic properties of proteinaceous metabolites secreted from potential probiotic Enterococcus faecalis KUMS-T48. Sci. Rep. 2023, 13, 7813. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the MTT assay. Cold Spring Harb. Protoc. 2018, 2018, pdb. prot095505. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the lactate dehydrogenase assay. Cold Spring Harb. Protoc. 2018, 2018, pdb. prot095497. [Google Scholar] [CrossRef]
- Heo, S.-J.; Cha, S.-H.; Kim, K.-N.; Lee, S.-H.; Ahn, G.; Kang, D.-H.; Oh, C.; Choi, Y.-U.; Affan, A.; Kim, D. Neuroprotective effect of phlorotannin isolated from Ishige okamurae against H2O2-induced oxidative stress in murine hippocampal neuronal cells, HT22. Appl. Biochem. Biotechnol. 2012, 166, 1520–1532. [Google Scholar] [CrossRef]
- Stergiou, O.S.; Tegopoulos, K.; Kiousi, D.E.; Tsifintaris, M.; Papageorgiou, A.C.; Tassou, C.C.; Chorianopoulos, N.; Kolovos, P.; Galanis, A. Whole-genome sequencing, phylogenetic and genomic analysis of Lactiplantibacillus pentosus L33, a potential probiotic strain isolated from fermented sausages. Front. Microbiol. 2021, 12, 746659. [Google Scholar] [CrossRef]
- Bae, W.-Y.; Lee, Y.J.; Jung, W.-H.; Shin, S.L.; Kim, T.-R.; Sohn, M. Draft genome sequence and probiotic functional property analysis of Lactobacillus gasseri LM1065 for food industry applications. Sci. Rep. 2023, 13, 12212. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, J.Y.; Kim, Y.; Kang, C.-H. Limosilactobacillus fermentum MG5091 and Lactococcus lactis MG4668 and MG5474 Suppress Muscle Atrophy by Regulating Apoptosis in C2C12 Cells. Fermentation 2023, 9, 659. [Google Scholar] [CrossRef]
- Zielińska, D.; Sionek, B.; Kołożyn-Krajewska, D. Safety of probiotics. In Diet, Microbiome and Health; Elsevier: Amsterdam, The Netherlands, 2018; pp. 131–161. [Google Scholar]
- Liu, D.-M.; Xu, B.; Dong, C. Recent advances in colorimetric strategies for acetylcholinesterase assay and their applications. Trends Anal. Chem. 2021, 142, 116320. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, S.; Cai, Q.; Miao, J.; Li, J. Antioxidant Capacity and Protective Effects on H2O2-Induced Oxidative Damage in PC12 Cells of the Active Fraction of Brassica rapa L. Foods 2023, 12, 2075. [Google Scholar] [CrossRef] [PubMed]
- Oswald, M.C.; Garnham, N.; Sweeney, S.T.; Landgraf, M. Regulation of neuronal development and function by ROS. FEBS Lett. 2018, 592, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Stottmeier, B.; Dick, T.P. Redox sensitivity of the MyD88 immune signaling adapter. Free Radic. Biol. Med. 2016, 101, 93–101. [Google Scholar] [CrossRef]
- Mendiola, A.S.; Cardona, A.E. The IL-1β phenomena in neuroinflammatory diseases. J. Neural Transm. 2018, 125, 781–795. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. BBA Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Bekdash, R.A. The cholinergic system, the adrenergic system and the neuropathology of Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 1273. [Google Scholar] [CrossRef]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef]
- Hafiz, Z.Z.; Amin, M.A.M.; Johari James, R.M.; Teh, L.K.; Salleh, M.Z.; Adenan, M.I. Inhibitory effects of raw-extract Centella asiatica (RECA) on acetylcholinesterase, inflammations, and oxidative stress activities via in vitro and in vivo. Molecules 2020, 25, 892. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Q.; Lu, B.; Shen, H.; Liu, S.; Shi, Y.; Leptihn, S.; Li, H.; Wei, J.; Liu, C. Whole-genome analysis of probiotic product isolates reveals the presence of genes related to antimicrobial resistance, virulence factors, and toxic metabolites, posing potential health risks. BMC Genom. 2021, 22, 210. [Google Scholar] [CrossRef]
- Kim, M.-H.; Kim, D.; Sung, J.H. A Gut-Brain Axis-on-a-Chip for studying transport across epithelial and endothelial barriers. J. Ind. Eng. Chem. 2021, 101, 126–134. [Google Scholar] [CrossRef]
- Karapetkov, N.; Georgieva, R.; Rumyan, N.; Karaivanova, E. Antibiotic susceptibility of different lactic acid bacteria strains. Benef. Microbes 2011, 2, 335–339. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012, 10, 2740. [Google Scholar]
- Lee, J.Y.; Park, Y.; Jeong, Y.; Kang, H. Anti-Inflammatory Response in TNFα/IFNγ-Induced HaCaT Keratinocytes and Probiotic Properties of Lacticaseibacillus rhamnosus MG4644, Lacticaseibacillus paracasei MG4693, and Lactococcus lactis MG5474. J. Microbiol. Biotechn. 2023, 33, 1039. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO Work Group. Guidelines for the Evaluation of Probiotics in Food; FAO/WHO Work. Group: Rome, Italy, 2002; pp. 1–11. [Google Scholar]
| Species | Strain | Accession Number | Origin |
|---|---|---|---|
| Lacticaseibacillus rhamnosus | MG4289 | MW947158.1 | Human (vagina) |
| MG4643 | ON668169.1 | Human (feces) | |
| MG4644 | ON668170.1 | Human (feces) | |
| Lacticaseibacillus paracasei | MG4267 | OP102572.1 | Human (vagina) |
| MG4272 | MW947164. | Human (vagina) | |
| MG4693 | OP077096.1 | Human (oral) | |
| Lactiplantibacillus plantarum | MG5106 | OP102458.1 | Food (fish) |
| MG4604 | OP102458.1 | Human (feces) | |
| Lactobacillus gasseri | MG4247 | MN069036.1 | Human (vagina) |
| Species | Strain | AChE Inhibition (%) | Cell Viability (%) | LDH Releases (%) |
|---|---|---|---|---|
| Control 1 | 0.00 ± 1.46 | 100.00 ± 1.33 | 100.00± 3.41 | |
| L. rhamnosus | MG4289 | 51.83 ± 0.12 | 177.67 ± 1.05 *** | 21.46 ± 0.86 *** |
| MG4643 | 52.00 ± 1.07 | 118.19 ± 3.38 ** | 16.67 ± 1.00 *** | |
| MG4644 | 60.08 ± 1.39 | 121.85 ± 1.28 *** | 14.86 ± 0.27 *** | |
| L. paracasei | MG4267 | 54.35 ± 1.90 | 82.48 ± 2.36 ** | 93.56 ± 2.42 |
| MG4272 | 60.57 ± 1.53 | 82.09 ± 3.79 * | 76.08 ± 4.44 * | |
| MG4693 | 63.85 ± 2.01 | 88.53 ± 2.57 * | 81.64 ± 7.18 | |
| L. plantarum | MG5106 | 63.02 ± 0.69 | 48.06 ± 1.00 ** | 68.35 ± 1.55 ** |
| MG4604 | 52.80 ± 1.72 | 59.64 ± 8.19 ** | 63.84 ± 2.98 ** | |
| L. gasseri | MG4247 | 67.04 ± 0.28 | 131.12 ± 5.13 ** | 14.21 ± 0.79 *** |
| Antimicrobials | L. gasseri MG4247 | L. rhamnosus MG4644 | ||
|---|---|---|---|---|
| MIC (μg/mL) | S/R 1 | MIC (μg/mL) | S/R | |
| Ampicillin | 0.125 | S | 0.5 | S |
| Gentamicin | 3 | S | 1.5 | S |
| Kanamycin | 48 | R | 24 | R |
| Streptomycin | 6 | S | 8 | S |
| Tetracycline | 0.75 | S | 0.75 | S |
| Chloramphenicol | 6 | R | 6 | R |
| Erythromycin | 0.047 | S | 0.09 | S |
| Vancomycin | 0.5 | S | n.r. 2 | |
| Clindamycin | 0.5 | S | 0.25 | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.Y.; Kim, J.H.; Park, J.-Y.; Kim, B.-K.; Heo, H.J.; Choi, S.-I. Neuroprotective Effect of Lactobacillus gasseri MG4247 and Lacticaseibacillus rhamnosus MG4644 Against Oxidative Damage via NF-κB Signaling Pathway. Fermentation 2025, 11, 385. https://doi.org/10.3390/fermentation11070385
Lee JY, Kim JH, Park J-Y, Kim B-K, Heo HJ, Choi S-I. Neuroprotective Effect of Lactobacillus gasseri MG4247 and Lacticaseibacillus rhamnosus MG4644 Against Oxidative Damage via NF-κB Signaling Pathway. Fermentation. 2025; 11(7):385. https://doi.org/10.3390/fermentation11070385
Chicago/Turabian StyleLee, Ji Yeon, Ju Hui Kim, Jeong-Yong Park, Byoung-Kook Kim, Ho Jin Heo, and Soo-Im Choi. 2025. "Neuroprotective Effect of Lactobacillus gasseri MG4247 and Lacticaseibacillus rhamnosus MG4644 Against Oxidative Damage via NF-κB Signaling Pathway" Fermentation 11, no. 7: 385. https://doi.org/10.3390/fermentation11070385
APA StyleLee, J. Y., Kim, J. H., Park, J.-Y., Kim, B.-K., Heo, H. J., & Choi, S.-I. (2025). Neuroprotective Effect of Lactobacillus gasseri MG4247 and Lacticaseibacillus rhamnosus MG4644 Against Oxidative Damage via NF-κB Signaling Pathway. Fermentation, 11(7), 385. https://doi.org/10.3390/fermentation11070385






