Impacts of Sonication on Fermentation Process and Physicochemical, Microbiological and Sensorial Characteristics of Fermented Black Carrot Juice
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
2.2.1. Fermentation and Sonication Methods
2.2.2. Analysis Methods
pH, Soluble Solid Content, Total Titratable Acidity, Color and Turbidity Analyses
Organic Acid Profile and Ethyl Alcohol Analyses
Microbiological Analyses
Sensory Evaluation
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bermúdez-Aguirre, D.; Mobbs, T.; Barbosa-Cánovas, G.V. Ultrasound applications in food processing. In Ultrasound Technologies for Food and Bioprocessing; Feng, H., Barbosa-Cánovas, G.V., Weiss, J., Eds.; Springer: New York, NY, USA, 2011; Volume 1, pp. 65–105. [Google Scholar] [CrossRef]
- Leadley, C.E.; Williams, A. Pulsed electric field processing, power ultrasound and other emerging technologies. In Food Processing Handbook; Brennan, J.G., Ed.; Wiley-VCH: Weinheim, Germany, 2005; pp. 201–236. [Google Scholar] [CrossRef]
- Virot, M.; Tomao, V.; Ginies, C.; Chemat, F. Total lipid extraction of food using d-limonene as an alternative to n-hexane. Chromatographia 2008, 68, 311–313. [Google Scholar] [CrossRef]
- Luque-Garcıa, J.; De Castro, M.L. Ultrasound-assisted soxhlet extraction: An expeditive approach for solid sample treatment: Application to the extraction of total fat from oleaginous seeds. J. Chromatogr. A 2004, 1034, 237–242. [Google Scholar] [CrossRef]
- Suslick, K.S.; Hammerton, D.A.; Cline, R.E. Sonochemical hot spot. J. Am. Chem. Soc. 1986, 108, 5641–5642. [Google Scholar] [CrossRef]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Goula, A.M. Ultrasound-assisted extraction of pomegranate seed oil—Kinetic modeling. J. Food Eng. 2013, 117, 492–498. [Google Scholar] [CrossRef]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- Bidari, A.; Ganjali, M.R.; Norouzi, P.; Hosseini, M.R.M.; Assadi, Y. Sample preparation method for the analysis of some organophosphorus pesticides residues in tomato by ultrasound-assisted solvent extraction followed by dispersive liquid-liquid microextraction. Food Chem. 2011, 126, 1840–1844. [Google Scholar] [CrossRef]
- Sengül, M.; Erkaya, T.; Baslar, M.; Ertugay, M.F. Effect of photosonication treatment on inactivation of total and coliform bacteria in milk. Food Control 2011, 22, 1803–1806. [Google Scholar] [CrossRef]
- Kiani, H.; Zhang, Z.H.; Sun, D.W. Effect of ultrasound irradiation on ice crystal size distribution in frozen agar gel samples. Innov. Food Sci. Emerg. 2013, 18, 126–131. [Google Scholar] [CrossRef]
- Mason, T.J.; Paniwnyk, L.; Lorimer, J.P. The uses of ultrasound in food technology. Ultrason. Sonochem. 1996, 3, S253–S260. [Google Scholar] [CrossRef]
- Akdeniz, V.; Akalın, A.S. Recent advances in dual effect of power ultrasound to microorganisms in dairy industry: Activation or inactivation. Crit. Rev. Food Sci. 2022, 62, 889–904. [Google Scholar] [CrossRef]
- Avhad, D.N.; Rathod, V.K. Ultrasound assisted production of a fibrinolytic enzyme in a bioreactor. Ultrason. Sonochem. 2015, 22, 257–264. [Google Scholar] [CrossRef]
- Bochu, W.; Lanchun, S.; Jing, Z.; Yuanyuan, Y.; Yanhong, Y. The influence of Ca2+ on the proliferation of S. cerevisiae and low ultrasonic on the concentration of Ca2+ in the S. cerevisiae cells. Colloids Surf. B Biointerfaces 2003, 32, 35–42. [Google Scholar] [CrossRef]
- Sulaiman, A.Z.; Ajit, A.; Yunus, R.M.; Chisti, Y. Ultrasound-assisted fermentation enhances bioethanol productivity. Biochem. Eng. J. 2011, 54, 141–150. [Google Scholar] [CrossRef]
- Nitayavardhana, S.; Shrestha, P.; Rasmussen, M.L.; Lamsal, B.P.; van Leeuwen, J.; Khanal, S.K. Ultrasound improved ethanol fermentation from cassava chips in cassava-based ethanol plants. Bioresour. Technol. 2010, 101, 2741–2747. [Google Scholar] [CrossRef]
- Dai, C.Y.; Wang, B.C.; Duan, C.R.; Sakanishi, A. Low ultrasonic stimulates fermentation of riboflavin producing strain. Colloids Surf. B Biointerfaces 2003, 30, 37–41. [Google Scholar] [CrossRef]
- Matsuura, K.; Hirotsune, M.; Nunokawa, Y.; Satoh, M.; Honda, K. Acceleration of Cell-Growth and Ester Formation by Ultrasonic Wave Irradiation. J. Ferment. Bioeng. 1994, 77, 36–40. [Google Scholar] [CrossRef]
- Vercet, A.; Oria, R.; Marquina, P.; Crelier, S.; Lopez-Buesa, P. Rheological properties of yoghurt made with milk submitted to manothermosonication. J. Agric. Food Chem. 2002, 50, 6165–6171. [Google Scholar] [CrossRef]
- Wu, H.; Hulbert, G.J.; Mount, J.R. Effects of ultrasound on milk homogenization and fermentation with yogurt starter. Innov. Food Sci. Emerg. 2000, 1, 211–218. [Google Scholar] [CrossRef]
- Tanguler, H.; Erten, H. Chemical and microbiological characteristics of shalgam (şalgam): A traditional turkish lactic acid fermented beverage. J. Food Qual. 2012, 35, 298–306. [Google Scholar] [CrossRef]
- Akbulut, M.; Tekçe, N.; Çoklar, H. Evaluation of Grape Pomace in Production of Shalgam Juice: Effects on Some Physicochemical, Microbiological and Sensorial Properties. Braz. Arch. Biol. Technol. 2024, 67, e24230582. [Google Scholar] [CrossRef]
- Altay, F.; Karbancıoglu-Güler, F.; Daskaya-Dikmen, C.; Heperkan, D. A review on traditional Turkish fermented non-alcoholic beverages: Microbiota, fermentation process and quality characteristics. Int. J. Food Microbiol. 2013, 167, 44–56. [Google Scholar] [CrossRef]
- Ekinci, F.Y.; Baser, G.M.; Özcan, E.; Üstündağ, Ö.G.; Korachi, M.; Sofu, A.; Blumberg, J.B.; Chen, C.-Y.O. Characterization of chemical, biological, and antiproliferative properties of fermented black carrot juice, shalgam. Eur. Food Res. Technol. 2016, 242, 1355–1368. [Google Scholar] [CrossRef]
- Sengun, I.Y.; Yalcin, H.T.; Kilic, G.; Ozturk, B.; Peker, A.K.; Terzi, Y.; Atlama, K. Identification of lactic acid bacteria found in traditional Shalgam juice using 16S rRNA sequencing and evaluation of their probiotic potential in vitro. Food Biosci. 2024, 60, 104300. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Liu, Y.; Xu, M.; Zhang, T.H.; Ren, H.; Liu, W.; Li, M.Y. Accelerated aging of grape pomace vinegar by using additives combined with physical methods. J. Food Process Eng. 2020, 43, e13398. [Google Scholar] [CrossRef]
- Gao, X.L.; Liu, E.M.; Zhang, J.K.; Yang, L.X.; Huang, Q.R.; Chen, S.; Ma, H.L.; Ho, C.T.; Liao, L. Accelerating aroma formation of raw soy sauce using low intensity sonication. Food Chem. 2020, 329, 127118. [Google Scholar] [CrossRef]
- Zhang, Q.-A.; Shen, Y.; Fan, X.-H.; García Martín, J.F. Preliminary study of the effect of ultrasound on physicochemical properties of red wine. CYTA J. Food 2016, 14, 55–64. [Google Scholar] [CrossRef]
- Ma, X.; Li, T.; He, Y.; Chen, M.; Zhou, J.; Yin, L.; Ma, H. Preliminary study on ultrasonic ageing zhenjiang vinegar mechanism based on maillard simulation system. J. Food Qual. 2020, 2020, 1087863. [Google Scholar] [CrossRef]
- Çoklar, H.; Akbulut, M.; Aygun, A.; Akbulut, M.T. Valorization of Dairy By-Products, Sweet Whey, and Acid Whey, in the Production of Fermented Black Carrot Juice: A Comparative Study of the Phytochemical, Physicochemical, Microbiological, and Sensorial Aspects. Foods 2025, 14, 218. [Google Scholar] [CrossRef] [PubMed]
- Ulucan, E.; Çoklar, H.; Akbulut, M. Application of ultrasound to extend the shelf life of shalgam juice: Changes in various physicochemical, nutritional, and microbiological properties. J. Food Process. Preserv. 2022, 46, e16501. [Google Scholar] [CrossRef]
- Erol, K.F.; Kutlu, G.; Baslar, M.; Tornuk, F. Effects of heat treatment and ultraviolet radiation on physicochemical, microbiological, and bioactive properties of shalgam juice. Food Sci. Nutr. 2024, 12, 8441–8453. [Google Scholar] [CrossRef]
- Kahve, H.I.; Akbulut, M.; Coklar, H. Identification and technological characterization of endogenous yeast isolated from fermented black carrot juice, shalgam. LWT-Lebensm. Wiss. Technol. 2022, 154, 112823. [Google Scholar] [CrossRef]
- Hassan, S.S.; Ravindran, R.; Jaiswal, S.; Tiwari, B.K.; Williams, G.A.; Jaiswal, A.K. An evaluation of sonication pretreatment for enhancing saccharification of brewers’ spent grain. Waste Manag. 2020, 105, 240–247. [Google Scholar] [CrossRef]
- Cheung, Y.-C.; Siu, K.-C.; Wu, J.-Y. Kinetic models for ultrasound-assisted extraction of water-soluble components and polysaccharides from medicinal fungi. Food Bioprocess Technol. 2013, 6, 2659–2665. [Google Scholar] [CrossRef]
- Miszczak, I.; Tańska, M.; Rejmer, W.; Konopka, I.; Zielińska, M. Spontaneous Fermentation of Beetroot–Effect of Fermentation Time and Temperature and Slice Thickness on Leaven Quality. Pol. J. Food Nutr. Sci. 2024, 74, 255–267. [Google Scholar] [CrossRef]
- Tanguler, H.; Erten, H. Occurrence and growth of lactic acid bacteria species during the fermentation of shalgam (salgam), a traditional Turkish fermented beverage. LWT-Food Sci. Technol. 2012, 46, 36–41. [Google Scholar] [CrossRef]
- Yu, Z.; Su, Y.; Zhang, Y.; Zhu, P.; Mei, Z.; Zhou, X.; Yu, H. Potential use of ultrasound to promote fermentation, maturation, and properties of fermented foods: A review. Food Chem. 2021, 357, 129805. [Google Scholar] [CrossRef]
- Mulet, A.; Cárcel, J.; Benedito, J.; Sanjuan, N. Applications of low-intensity ultrasonics in the dairy industry. In Engineering and Food for the 21st Century; CRC Press: Boca Raton, FL, USA, 2002; pp. 793–814. [Google Scholar]
- Cheng, Z.; Yang, J.; Yan, R.; Wang, B.; Bai, Y.; Miao, Z.; Sun, J.; Li, H.; Wang, X.; Sun, B. Interactive mechanism-guided microbial interaction dynamics in food fermentations: Lactic acid bacteria and yeasts as a case example. Food Biosci. 2025, 68, 106453. [Google Scholar] [CrossRef]
- Tanguler, H.; Selli, S.; Sen, K.; Cabaroglu, T.; Erten, H. Aroma composition of shalgam: A traditional Turkish lactic acid fermented beverage. J. Food Sci. Technol. 2017, 54, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- Tanguler, H.; Sen, K.; Dinc, S.O. Aroma profiles of shalgam: Effect of purple carrot (Daucus carota) amount. Food Sci. Nutr. 2024, 12, 5162–5175. [Google Scholar] [CrossRef] [PubMed]
- Kırlangıç, O.; Ilgaz, C.; Kadiroğlu, P. Influence of pasteurization and storage conditions on microbiological quality and aroma profiles of shalgam. Food Biosci. 2021, 44, 101350. [Google Scholar] [CrossRef]
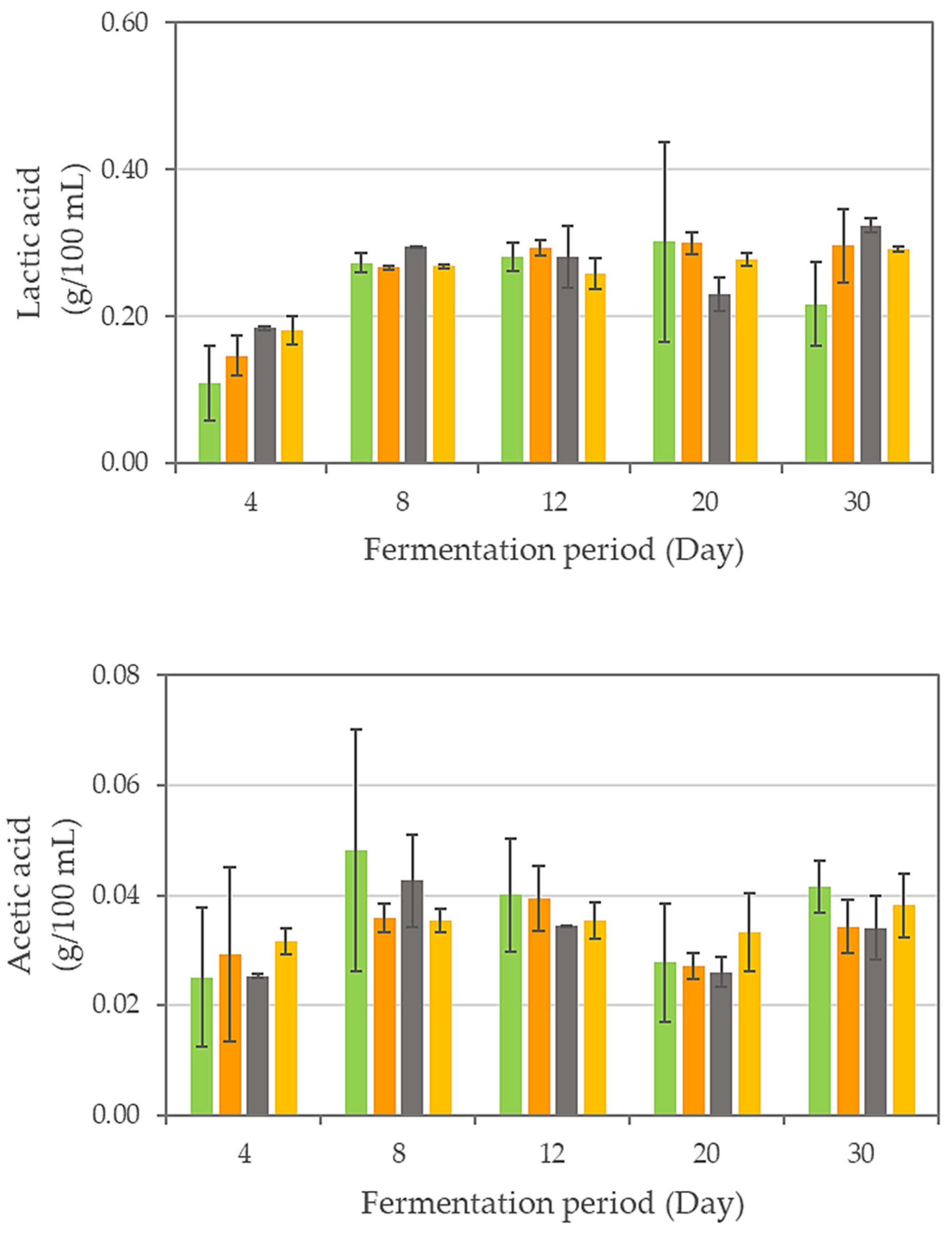
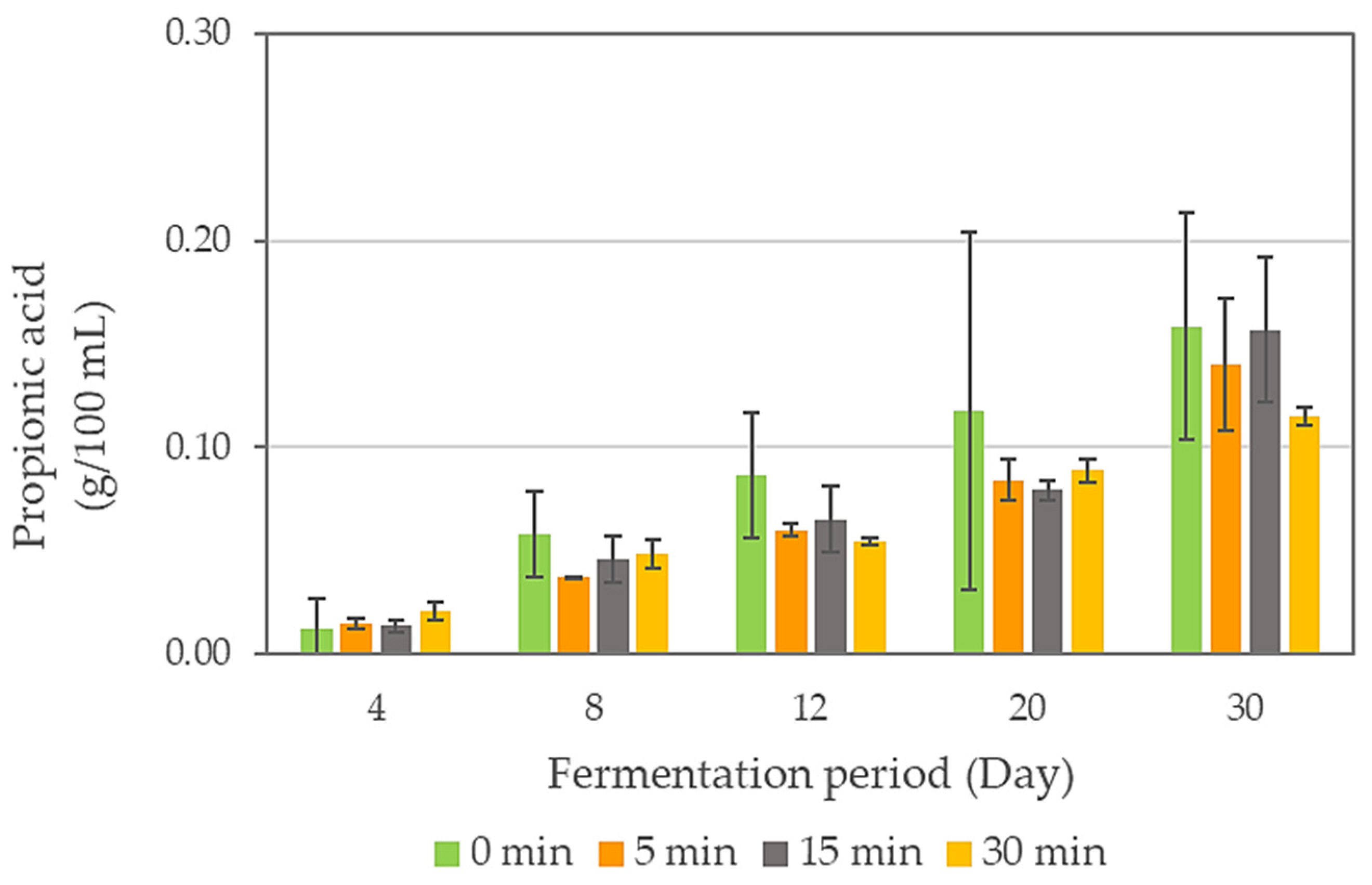
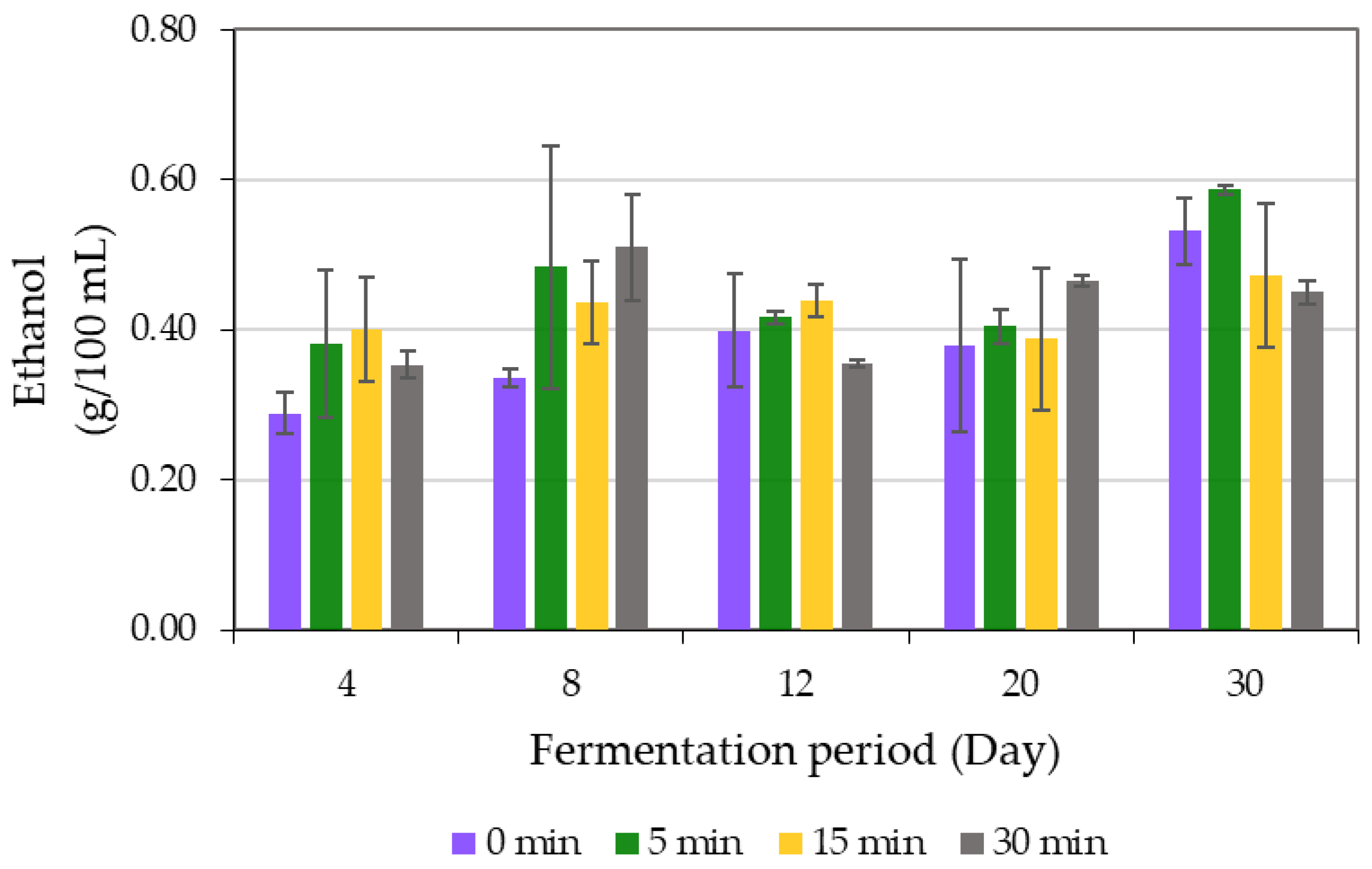
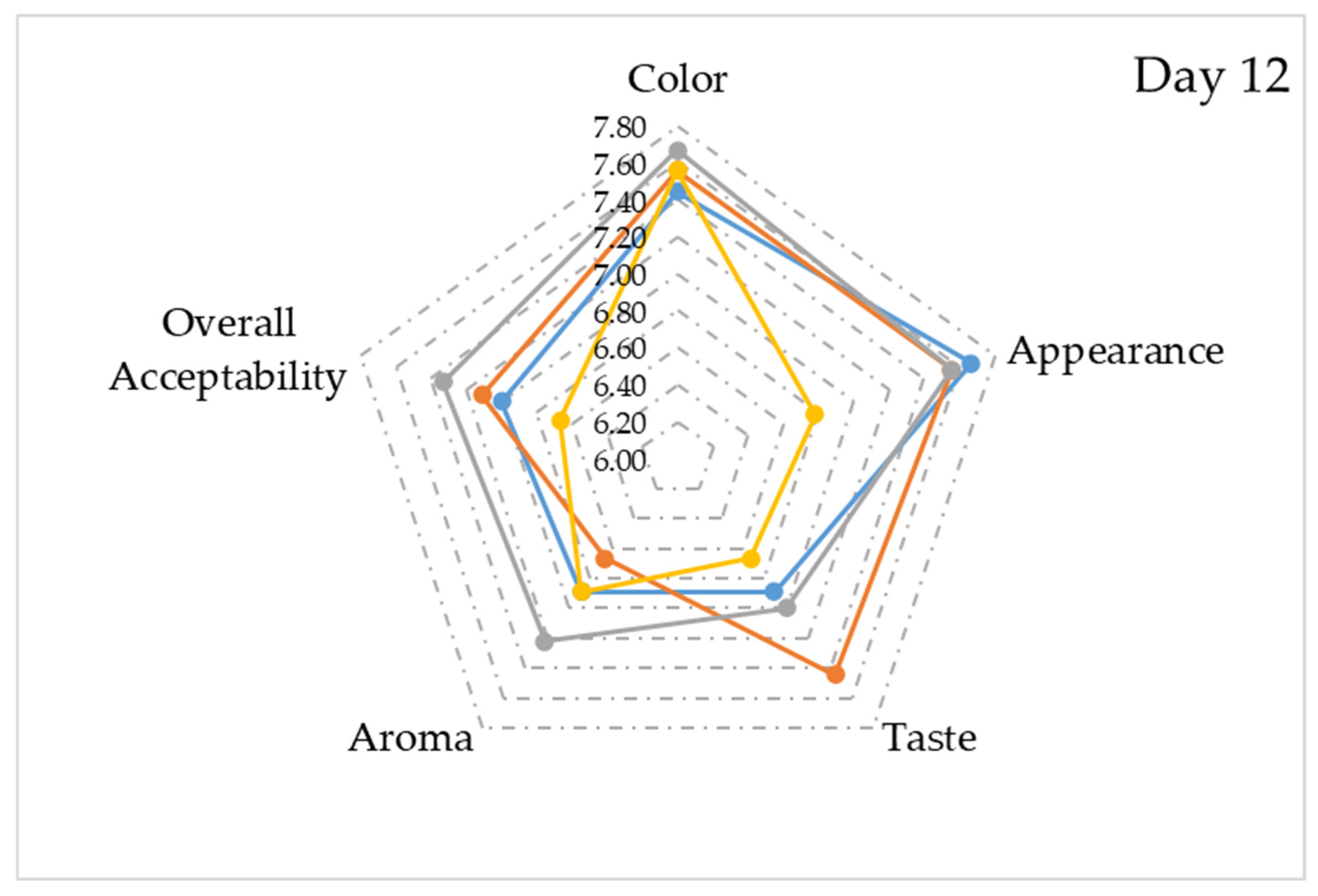
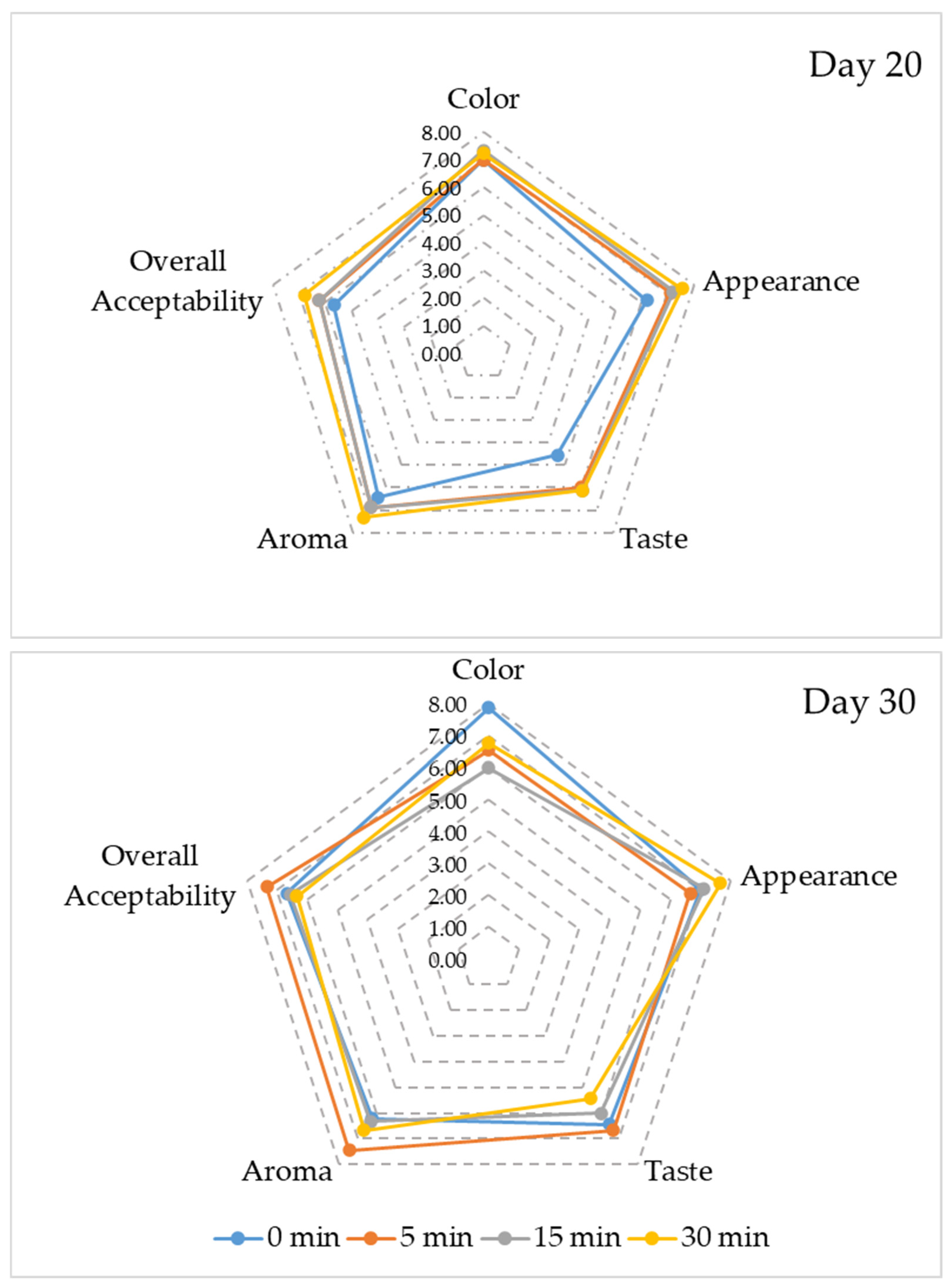
| Sonication Time (min) | Fermentation Period (Day) | pH | Total Titratable Acidity (g/L) | Soluble Solid Content (%) | Turbidity (NTU) |
|---|---|---|---|---|---|
| 0 | 0 | 5.85 ± 0.09 | 0.011 ± 0.000 | 1.40 ± 0.28 | 163.00 ± 12.73 |
| 4 | 3.72 ± 0.05 | 0.207 ± 0.003 | 2.00 ± 0.00 | 255.00 ± 0.00 | |
| 8 | 3.44 ± 0.02 | 0.377 ± 0.018 | 2.40 ± 0.28 | 208.50 ± 31.80 | |
| 12 | 3.52 ± 0.04 | 0.301 ± 0.018 | 1.80 ± 0.00 | 121.30 ± 74.60 | |
| 20 | 3.36 ± 0.09 | 0.378 ± 0.0912 | 2.15 ± 0.21 | 223.50 ± 77.10 | |
| 30 | 3.53 ± 0.00 | 0.359 ± 0.018 | 2.25 ± 0.21 | 162.50 ± 0.71 | |
| 5 | 0 | 6.17 ± 0.45 | 0.008 ± 0.005 | 1.40 ± 0.00 | 181.00 ± 17.00 |
| 4 | 3.61 ± 0.10 | 0.205 ± 0.087 | 2.00 ± 0.00 | 271.00 ± 45.30 | |
| 8 | 3.49 ± 0.02 | 0.268 ± 0.054 | 1.80 ± 0.28 | 183.50 ± 2.12 | |
| 12 | 3.48 ± 0.01 | 0.292 ± 0.066 | 1.70 ± 0.42 | 167.50 ± 37.50 | |
| 20 | 3.38 ± 0.01 | 0.407 ± 0.026 | 2.20 ± 0.00 | 217.00 ± 7.07 | |
| 30 | 3.50 ± 0.05 | 0.353 ± 0.061 | 2.00 ± 0.57 | 190.00 ± 80.60 | |
| 15 | 0 | 6.05 ± 0.20 | 0.006 ± 0.000 | 1.70 ± 0.14 | 221.50 ± 7.78 |
| 4 | 3.62 ± 0.02 | 0.211 ± 0.048 | 2.05 ± 0.07 | 271.00 ± 58.00 | |
| 8 | 3.44 ± 0.02 | 0.364 ± 0.026 | 2.40 ± 0.00 | 157.50 ± 40.30 | |
| 12 | 3.45 ± 0.00 | 0.288 ± 0.036 | 1.65 ± 0.21 | 208.50 ± 26.20 | |
| 20 | 3.49 ± 0.06 | 0.299 ± 0.051 | 1.85 ± 0.50 | 219.00 ± 65.10 | |
| 30 | 3.43 ± 0.01 | 0.420 ± 0.059 | 1.75 ± 0.35 | 148.00 ± 35.40 | |
| 30 | 0 | 5.83 ± 0.34 | 0.009 ± 0.005 | 1.75 ± 0.07 | 175.00 ± 31.10 |
| 4 | 3.56 ± 0.06 | 0.265 ± 0.003 | 2.20 ± 0.28 | 253.50 ± 31.80 | |
| 8 | 3.46 ± 0.01 | 0.314 ± 0.041 | 2.20 ± 0.28 | 228.50 ± 26.20 | |
| 12 | 3.56 ± 0.06 | 0.276 ± 0.110 | 1.40 ± 0.00 | 203.50 ± 26.20 | |
| 20 | 3.46 ± 0.00 | 0.332 ± 0.015 | 1.80 ± 0.28 | 208.00 ± 4.24 | |
| 30 | 3.53 ± 0.01 | 0.317 ± 0.005 | 1.70 ± 0.14 | 208.00 ± 62.20 |
| Ultrasound Time (min) | Fermentation Period (Day) | TMAB (log cfu/mL) | LAB (log cfu/mL) | Yeast (log cfu/mL) |
|---|---|---|---|---|
| 0 | 0 | 9.19 ± 0.06 abc | 7.81 ± 0.39 ab | 7.17 ± 0.01 ab |
| 8 | 9.15 ± 0.01 abc | 8.37 ± 0.06 a | 6.03 ± 0.16 bcd | |
| 12 | 8.10 ± 0.23 de | 7.23 ± 0.26 bc | 4.23 ± 0.10 e | |
| 5 | 0 | 9.88 ± 0.06 a | 6.03 ± 0.12 d | 7.92 ± 0.05 a |
| 8 | 9.10 ± 0.00 abc | 7.80 ± 0.22 ab | 5.60 ± 0.05 cd | |
| 12 | 7.15 ± 0.50 f | 7.05 ± 0.361 bcd | 4.83 ± 0.38 de | |
| 15 | 0 | 9.76 ± 0.09 ab | 6.39 ± 0.05 cd | 8.14 ± 0.11 a |
| 8 | 8.98 ± 0.07 bc | 8.50 ± 0.28 a | 6.53 ± 0.01 bc | |
| 12 | 7.41 ± 0.28 ef | 7.75 ± 0.52 ab | 5.05 ± 0.52 de | |
| 30 | 0 | 9.62 ± 0.16 ab | 7.61 ± 0.15 ab | 8.15 ± 0.12 a |
| 8 | 8.42 ± 0.19 cd | 8.32 ± 0.22 a | 6.27 ± 0.28 bc | |
| 12 | 7.70 ± 0.22 def | 8.02 ± 0.13 ab | 5.84 ± 0.56 cd |
| Ultrasound Time (min) | Fermentation Period (Day) | L* | a* | b* | C* | h |
|---|---|---|---|---|---|---|
| 0 | 0 | 70.83 ± 1.94 | 0.78 ± 0.14 | 5.02 ± 0.62 | 5.08 ± 0.63 | 81.16 ± 0.49 |
| 4 | 27.59 ± 3.21 | 60.36 ± 2.37 | 45.41 ± 4.26 | 75.55 ± 4.45 | 36.92 ± 1.51 | |
| 8 | 22.72 ± 0.30 | 56.06 ± 0.24 | 38.15 ± 0.424 | 67.81 ± 0.44 | 34.24 ± 0.18 | |
| 12 | 23.42 ± 0.00 | 56.46 ± 0.27 | 39.34 ± 0.042 | 68.82 ± 0.21 | 34.87 ± 0.16 | |
| 20 | 24.74 ± 3.90 | 57.75 ± 3.85 | 41.56 ± 6.63 | 71.19 ± 6.98 | 35.62 ± 2.53 | |
| 30 | 20.10 ± 2.62 | 52.48 ± 3.02 | 33.68 ± 4.43 | 62.38 ± 4.94 | 32.61 ± 1.93 | |
| 5 | 0 | 68.30 ± 1.77 | 0.70 ± 0.15 | 4.11 ± 0.33 | 4.170 ± 0.35 | 80.47 ± 1.31 |
| 4 | 27.63 ± 3.16 | 60.73 ± 2.43 | 45.69 ± 4.26 | 76.02 ± 4.50 | 36.91 ± 1.47 | |
| 8 | 25.01 ± 2.63 | 58.28 ± 2.37 | 41.98 ± 4.36 | 71.85 ± 4.47 | 35.71 ± 1.73 | |
| 12 | 24.13 ± 3.63 | 57.16 ± 3.22 | 40.56 ± 6.17 | 70.13 ± 6.19 | 35.24 ± 2.60 | |
| 20 | 21.71 ± 0.75 | 55.04 ± 0.85 | 36.37 ± 1.31 | 65.97 ± 1.43 | 33.45 ± 0.54 | |
| 30 | 22.13 ± 4.33 | 54.78 ± 4.60 | 37.15 ± 7.42 | 66.24 ± 7.98 | 33.95 ± 3.10 | |
| 15 | 0 | 68.77 ± 0.11 | 0.83 ± 0.04 | 4.905 ± 0.516 | 4.98 ± 0.52 | 80.38 ± 0.53 |
| 4 | 26.27 ± 2.92 | 59.41 ± 2.50 | 43.94 ± 4.67 | 73.91 ± 4.79 | 36.42 ± 1.76 | |
| 8 | 20.24 ± 0.85 | 53.58 ± 0.90 | 33.87 ± 1.36 | 63.39 ± 1.48 | 32.30 ± 0.60 | |
| 12 | 25.52 ± 0.19 | 58.85 ± 0.13 | 42.95 ± 0.24 | 72.85 ± 0.26 | 36.13 ± 0.09 | |
| 20 | 22.32 ± 5.90 | 54.98 ± 6.55 | 37.38 ± 10.15 | 66.56 ± 11.12 | 33.87 ± 4.10 | |
| 30 | 20.69 ± 6.17 | 53.16 ± 7.14 | 34.63 ± 10.68 | 63.55 ± 11.80 | 32.64 ± 4.64 | |
| 30 | 0 | 68.25 ± 3.76 | 0.91 ± 0.23 | 5.56 ± 0.72 | 5.64 ± 0.76 | 80.83 ± 1.22 |
| 4 | 24.91 ± 1.85 | 58.21 ± 1.77 | 41.88 ± 3.11 | 71.72 ± 3.25 | 35.71 ± 1.20 | |
| 8 | 23.23 ± 1.90 | 56.62 ± 1.98 | 38.99 ± 3.21 | 68.75 ± 3.45 | 34.52 ± 1.27 | |
| 12 | 25.01 ± 5.47 | 57.91 ± 5.01 | 41.70 ± 8.88 | 71.42 ± 9.25 | 35.53 ± 3.46 | |
| 20 | 23.27 ± 1.92 | 56.44 ± 1.92 | 39.05 ± 3.38 | 68.65 ± 3.50 | 34.65 ± 1.41 | |
| 30 | 24.41 ± 3.06 | 56.83 ± 2.86 | 40.86 ± 5.09 | 69.51 ± 4.58 | 35.64 ± 2.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ercan, M.; Akbulut, M.; Çoklar, H.; Demirci, T. Impacts of Sonication on Fermentation Process and Physicochemical, Microbiological and Sensorial Characteristics of Fermented Black Carrot Juice. Fermentation 2025, 11, 475. https://doi.org/10.3390/fermentation11080475
Ercan M, Akbulut M, Çoklar H, Demirci T. Impacts of Sonication on Fermentation Process and Physicochemical, Microbiological and Sensorial Characteristics of Fermented Black Carrot Juice. Fermentation. 2025; 11(8):475. https://doi.org/10.3390/fermentation11080475
Chicago/Turabian StyleErcan, Muhammet, Mehmet Akbulut, Hacer Çoklar, and Talha Demirci. 2025. "Impacts of Sonication on Fermentation Process and Physicochemical, Microbiological and Sensorial Characteristics of Fermented Black Carrot Juice" Fermentation 11, no. 8: 475. https://doi.org/10.3390/fermentation11080475
APA StyleErcan, M., Akbulut, M., Çoklar, H., & Demirci, T. (2025). Impacts of Sonication on Fermentation Process and Physicochemical, Microbiological and Sensorial Characteristics of Fermented Black Carrot Juice. Fermentation, 11(8), 475. https://doi.org/10.3390/fermentation11080475






