Isolation and Characterization of Bacteriocin-like-Producing Companilactobacillus farciminis YLR-1 and the Inhibitory Activity of Bacteriocin Against Staphylococcus aureus
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Bacterial Isolation
2.2. Lactic Acid Bacteria Identification Using 16S rRNA Sequence Analyses
2.3. Sequencing and Sequence Assembly of the Genome
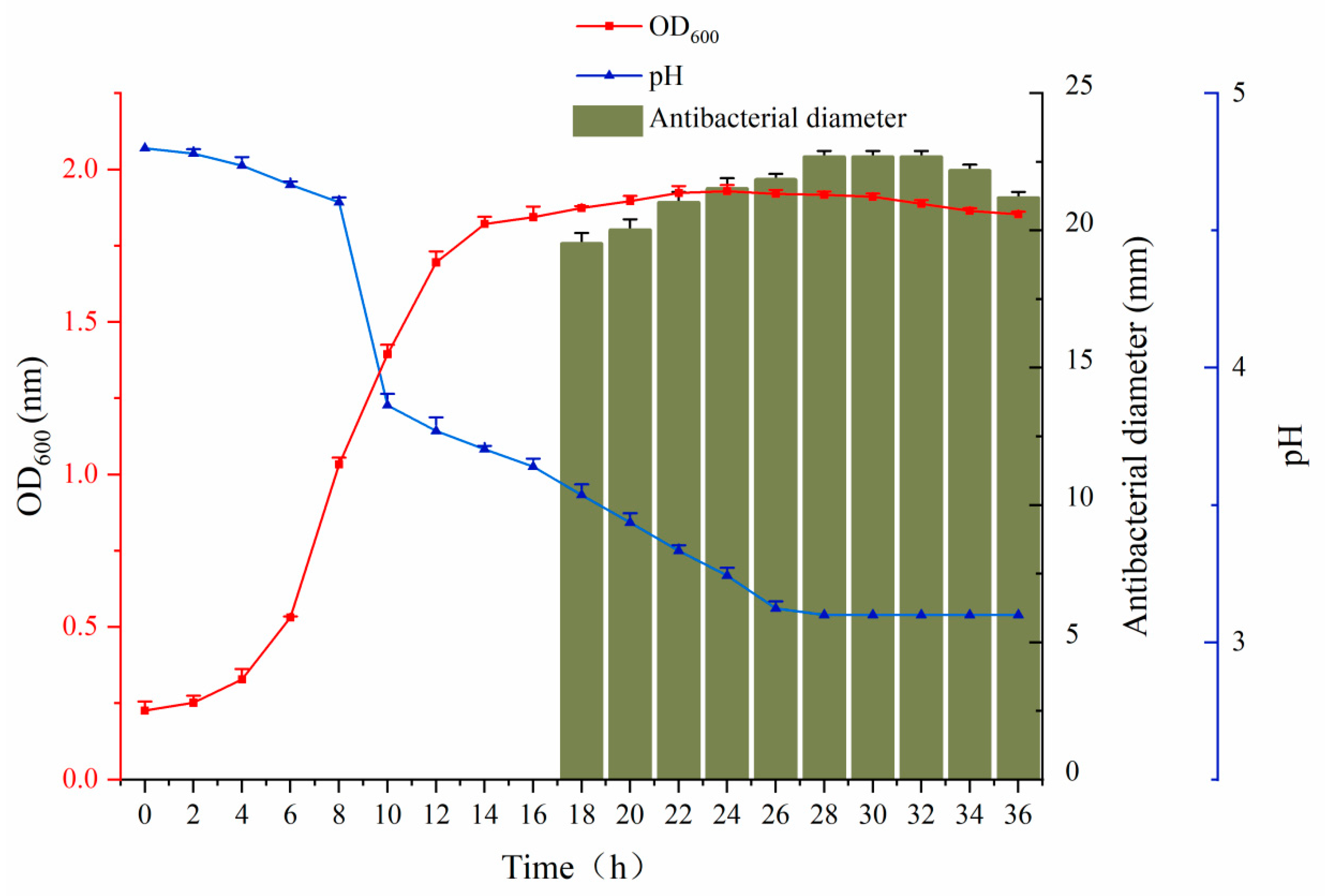
2.4. Bacterial Growth Curve
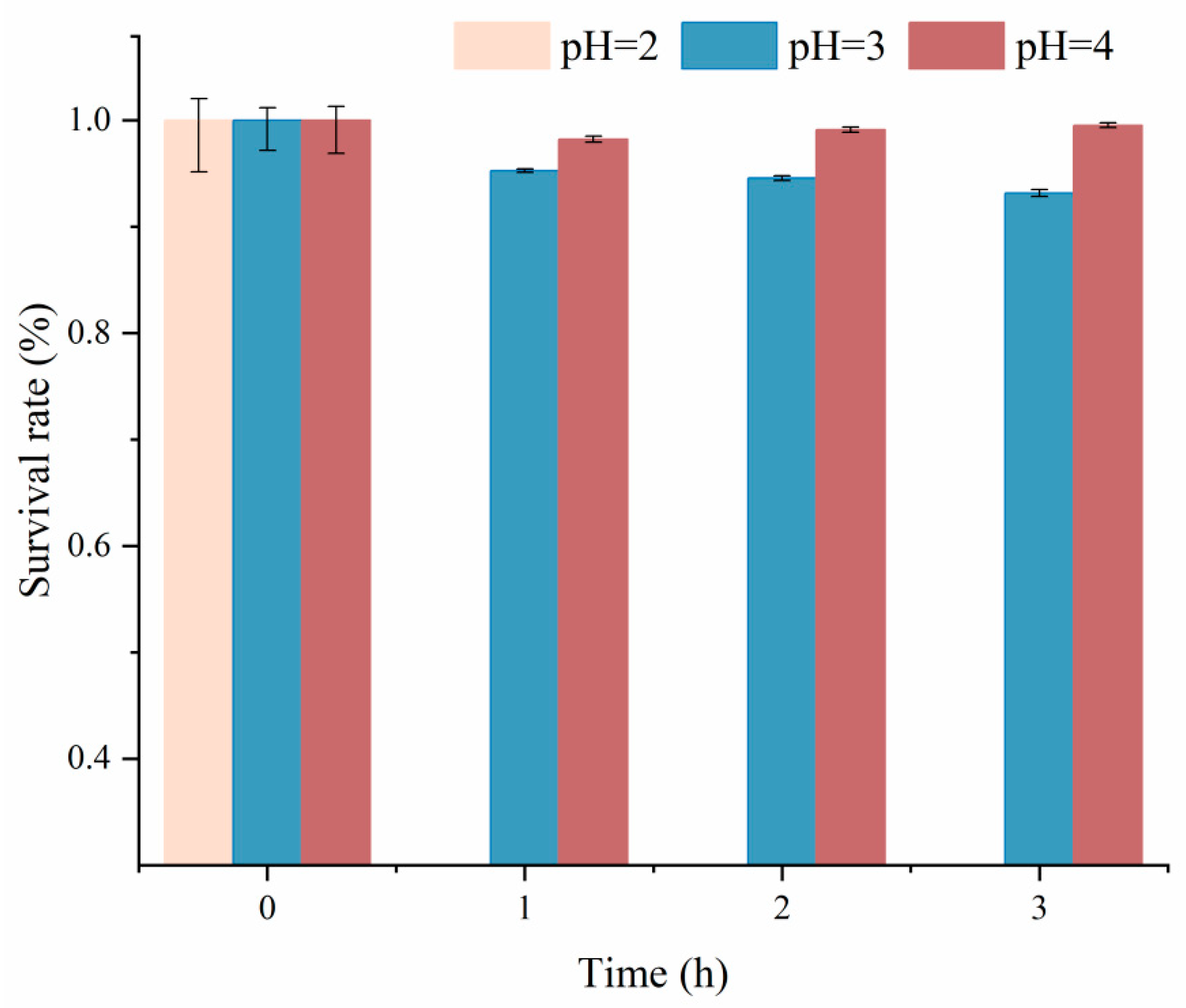
2.5. Tolerance to Low pH Conditions of C. farciminis YLR-1
2.6. Determination of Bile Tolerance of C. farciminis YLR-1
2.7. BLIS Production
2.8. Preliminary Extraction of YLR-1 BLIS
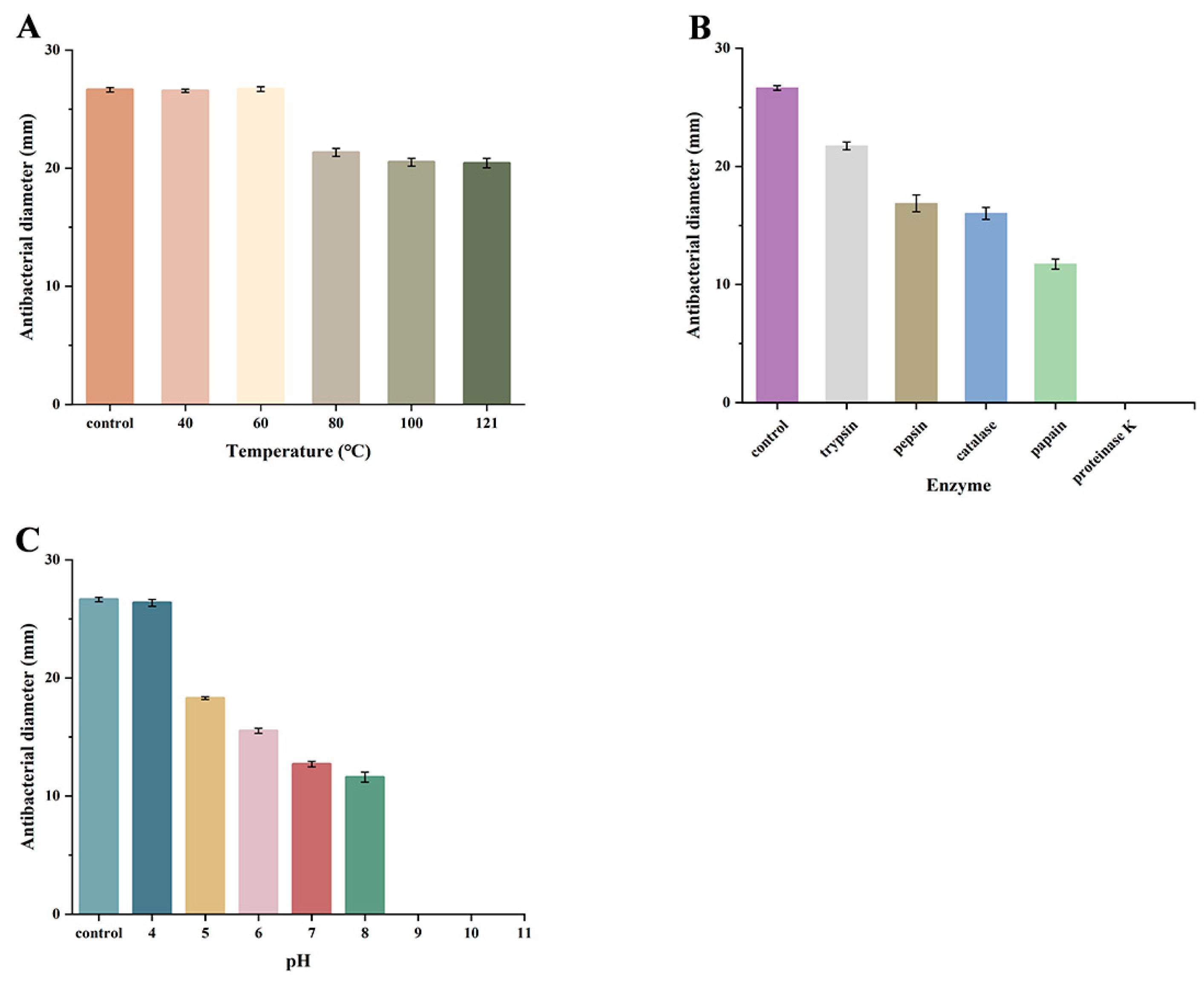
2.9. Effect of Temperature, pH, and Enzymes on Antimicrobial Activity
2.10. SDS-PAGE and In Situ Activity Assay of YLR-1 BLIS
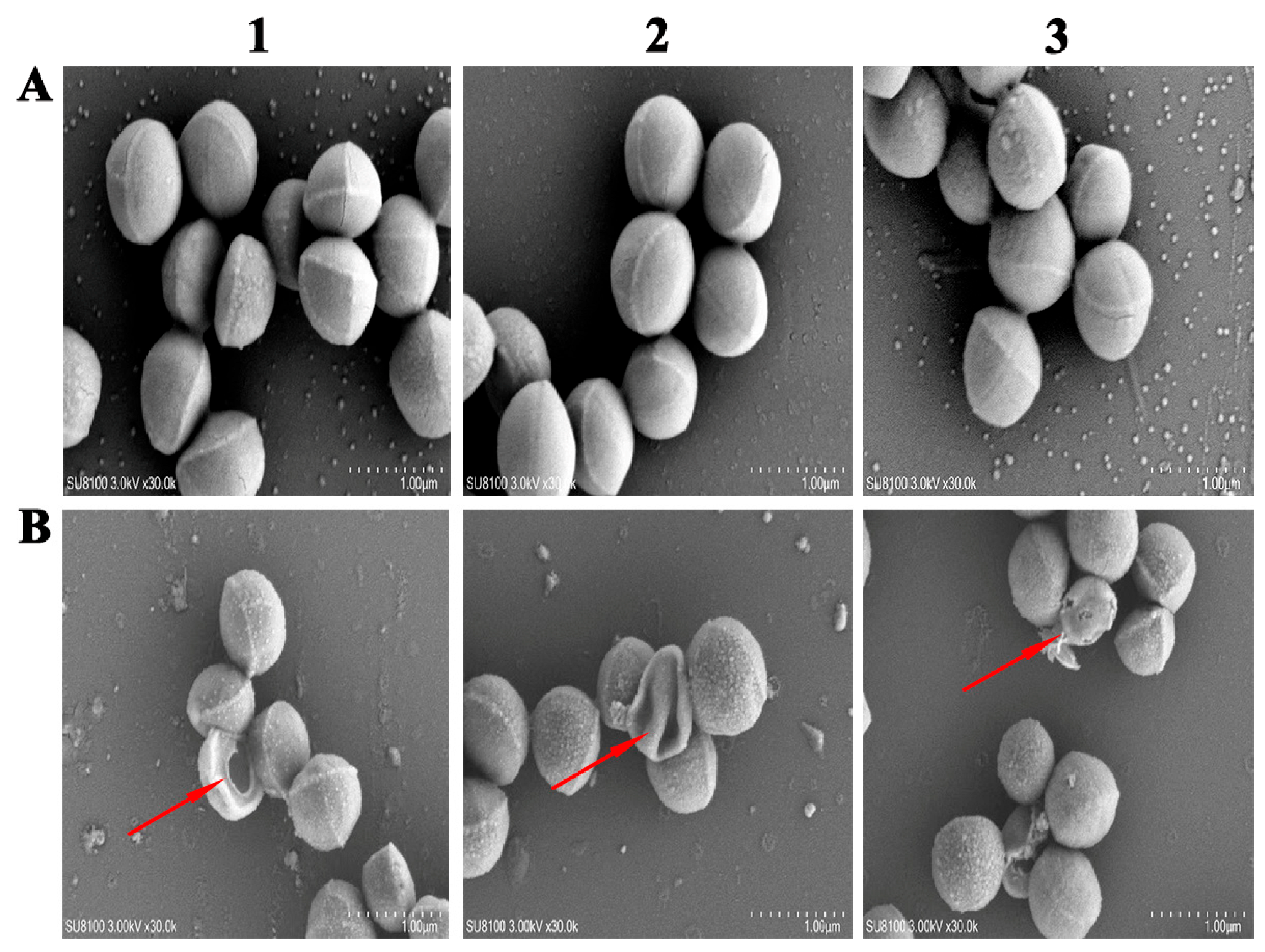
2.11. Electron Microscopy Observation
2.12. Live–Dead Staining Assay
3. Results
3.1. Identification of Bacterial Strain
3.2. Genome Assembly Results Statistics
3.3. Growth Kinetics of C. farciminis YLR-1
3.4. Tolerance to Low pH Conditions of the Isolate
3.5. Bile Salt Tolerance Test
3.6. Rough Determination of Cutoff Membrane
3.7. Heat, pH, and Enzymatic Stability of YLR-1 BLIS
3.8. SDS-PAGE Analysis of BLIS Produced by C. farciminis YLR-1
3.9. SEM Analysis
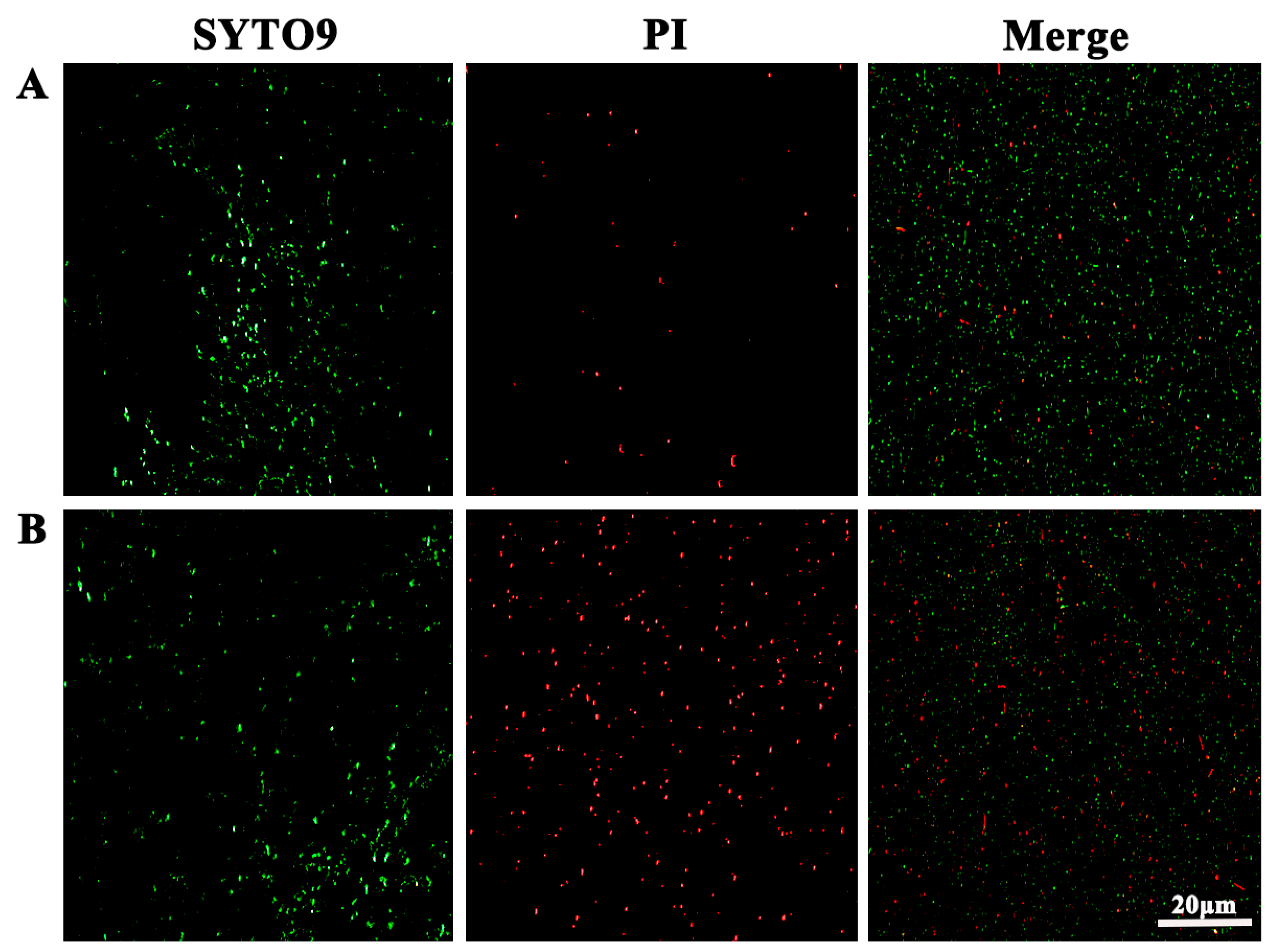
3.10. Bactericidal Effect Observed Using Fluorescence Microscope
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kadariya, J.; Smith, T.C.; Thapaliya, D. Staphylococcus aureus and Staphylococcal Food-Borne Disease: An Ongoing Challenge in Public Health. BioMed Res. Int. 2014, 1, 827965. [Google Scholar] [CrossRef]
- Argudín, M.Á.; Mendoza, M.C.; Rodicio, M.R. Food Poisoning and Staphylococcus aureus Enterotoxins. Toxins 2010, 2, 1751–1773. [Google Scholar] [CrossRef]
- Mancusi, A.; Egidio, M.; Marrone, R.; Scotti, L.; Paludi, D.; Dini, I.; Proroga, Y.T.R. The In Vitro Antibacterial Activity of Argirium SUNc against Most Common Pathogenic and Spoilage Food Bacteria. Antibiotics 2024, 13, 109. [Google Scholar] [CrossRef]
- Newell, D.G.; Koopmans, M.; Verhoef, L.; Duizer, E.; Aidara-Kane, A.; Sprong, H.; Opsteegh, M.; Langelaar, M.; Threfall, J.; Scheutz, F.; et al. Food-borne diseases—The challenges of 20years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 2010, 139, S3–S15. [Google Scholar] [CrossRef]
- Mavani, N.R.; Mohd Ali, J.; Hussain, M.A.; Rahman, N.A.; Hashim, H. Determining food safety in canned food using fuzzy logic based on sulphur dioxide, benzoic acid and sorbic acid concentration. Heliyon 2024, 10, e26273. [Google Scholar] [CrossRef]
- Settanni, L.; Corsetti, A. Application of bacteriocins in vegetable food biopreservation. Int. J. Food Microbiol. 2008, 121, 123–138. [Google Scholar] [CrossRef]
- O’sullivan, L.; Ross, R.; Hill, C.J.B. Potential of bacteriocin-producing lactic acid bacteria for improvements in food safety and quality. Biochimie 2002, 84, 593–604. [Google Scholar] [CrossRef]
- Odah, K.A.; Dong, W.-L.; Lei, L.; Atiah, L.A.; Wang, Y.-m.; Kong, L.-C.; Ma, H.-X. Isolation, Identification, and Characterization of a Novel Bacteriocin Produced by Brevibacillus laterosporus DS-3 Against Methicillin-Resistant Staphylococcus aureus (MRSA). Int. J. Pept. Res. Ther. 2019, 26, 709–715. [Google Scholar] [CrossRef]
- Ahmad, V.; Khan, M.S.; Jamal, Q.M.S.; Alzohairy, M.A.; Al Karaawi, M.A.; Siddiqui, M.U. Antimicrobial potential of bacteriocins: In therapy, agriculture and food preservation. Int. J. Antimicrob. Agents 2017, 49, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Field, D.; Ross, R.P.; Hill, C. Developing bacteriocins of lactic acid bacteria into next generation biopreservatives. Curr. Opin. Food Sci. 2018, 20, 1–6. [Google Scholar] [CrossRef]
- O’Connor, P.M.; Ross, R.P.; Hill, C.; Cotter, P.D. Antimicrobial antagonists against food pathogens: A bacteriocin perspective. Curr. Opin. Food Sci. 2015, 2, 51–57. [Google Scholar] [CrossRef]
- Mathur, H.; Field, D.; Rea, M.C.; Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocin-Antimicrobial Synergy: A Medical and Food Perspective. Front. Microbiol. 2017, 8, 1205. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhou, K.; Yin, S.; Liu, S.; Zhu, Y.; Yang, Y.; Wang, C. Purification and characterization of an exopolysaccharide produced by Lactobacillus plantarum HY isolated from home-made Sichuan Pickle. Int. J. Biol. Macromol. 2019, 134, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chenhao, Z.; Fengsong, L.; Zhengyu, J.; Xia, X. Ecological succession and functional characteristics of lactic acid bacteria in traditional fermented foods. Crit. Rev. Food Sci. Nutr. 2023, 63, 5841–5855. [Google Scholar] [CrossRef]
- Thao, T.T.P.; Thoa, L.T.K.; Ngoc, L.M.T.; Lan, T.T.P.; Phuong, T.V.; Truong, H.T.H.; Khoo, K.S.; Manickam, S.; Hoa, T.T.; Tram, N.D.Q.; et al. Characterization halotolerant lactic acid bacteria Pediococcus pentosaceus HN10 and in vivo evaluation for bacterial pathogens inhibition. Chem. Eng. Process. Process Intensif. 2021, 168, 108576. [Google Scholar] [CrossRef]
- Leite, A.M.O.; Miguel, M.A.L.; Peixoto, R.S.; Ruas-Madiedo, P.; Paschoalin, V.M.F.; Mayo, B.; Delgado, S. Probiotic potential of selected lactic acid bacteria strains isolated from Brazilian kefir grains. J. Dairy Sci. 2015, 98, 3622–3632. [Google Scholar] [CrossRef]
- Chen, C.; Chen, X.; Jiang, M.; Rui, X.; Li, W.; Dong, M. A newly discovered bacteriocin from Weissella hellenica D1501 associated with Chinese Dong fermented meat (Nanx Wudl). Food Control 2014, 42, 116–124. [Google Scholar] [CrossRef]
- Cloeckaert, A.; Miljkovic, M.; Jovanovic, S.; O’Connor, P.M.; Mirkovic, N.; Jovcic, B.; Filipic, B.; Dinic, M.; Studholme, D.J.; Fira, D.; et al. Brevibacillus laterosporus strains BGSP7, BGSP9 and BGSP11 isolated from silage produce broad spectrum multi-antimicrobials. PLoS ONE 2019, 14, e0216773. [Google Scholar] [CrossRef]
- Talib, N.; Mohamad, N.E.; Yeap, S.K.; Hussin, Y.; Aziz, M.N.M.; Masarudin, M.J.; Sharifuddin, S.A.; Hui, Y.W.; Ho, C.L.; Alitheen, N.B. Isolation and Characterization of Lactobacillus spp. from Kefir Samples in Malaysia. Molecules 2019, 24, 2606. [Google Scholar] [CrossRef]
- Lu, H.; Yang, P.; Zhong, M.; Bilal, M.; Xu, H.; Zhang, Q.; Xu, J.; Liang, N.; Liu, S.; Zhao, L.; et al. Isolation of a potential probiotic strain Bacillus amyloliquefaciens LPB-18 and identification of antimicrobial compounds responsible for inhibition of food-borne pathogens. Food Sci. Nutr. 2022, 11, 2186–2196. [Google Scholar] [CrossRef]
- Arakawa, K.; Yoshida, S.; Aikawa, H.; Hano, C.; Bolormaa, T.; Burenjargal, S.; Miyamoto, T. Production of a bacteriocin-like inhibitory substance by Leuconostoc mesenteroides subsp. dextranicum 213M0 isolated from Mongolian fermented mare milk, airag. Anim. Sci. J. 2015, 87, 449–456. [Google Scholar] [CrossRef]
- Mandal, V.; Sen, S.K.; Mandal, N.C. Optimized culture conditions for bacteriocin production by Pediococcus acidilactici LAB 5 and its characterization. Indian J. Biochem. Biophys. 2008, 45, 106–110. [Google Scholar] [PubMed]
- Janes, M.E.; Nannapaneni, R.; Proctor, A.; Johnson, M.G. Rice Hull Ash and Silicic Acid as Adsorbents for Concentration of Bacteriocins. Appl. Environ. Microbiol. 1998, 64, 4403–4409. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Haqmal, M.A.; Liang, Y.d.; Muhammad, I.; Zhao, X.O.; Elken, E.M.; Gao, Y.H.; Jia, Y.; He, C.g.; Wang, Y.M.; et al. Antibacterial activity and cytotoxicity of a novel bacteriocin isolated from Pseudomonas sp. strain 166. Microb. Biotechnol. 2022, 15, 2337–2350. [Google Scholar] [CrossRef] [PubMed]
- Polat, T.; Soyhan, İ.; Cebeci, S.; İldeniz, T.A.Ö.; Gök, Ö.; Elmas, M.A.; Mozioğlu, E.; Ünübol, N. New-generation biofilm effective antimicrobial peptides and a real-time anti-biofilm activity assay: CoMIC. Appl. Microbiol. Biotechnol. 2024, 108, 316. [Google Scholar] [CrossRef]
- Zhang, W.; An, Z.; Bai, Y.; Zhou, Y.; Chen, F.; Wang, K.-J. A novel antimicrobial peptide Scyreptin1-30 from Scylla paramamosain exhibiting potential therapy of Pseudomonas aeruginosa early infection in a mouse burn wound model. Biochem. Pharmacol. 2023, 218, 115917. [Google Scholar] [CrossRef]
- Wieneke, A.A.; Roberts, D.; Gilbert, R.J. Staphylococcal food poisoning in the United Kingdom, 1969–1990. Epidemiol. Infect. 2009, 110, 519–531. [Google Scholar] [CrossRef]
- Andersson, R.J.I.J.o.F.M. Inhibition of Staphylococcus aureus and spheroplasts of Gram-negative bacteria by an antagonistic compound produced by a strain of Lactobacillus plantarum. Int. J. Food Microbiol. 1986, 3, 149–160. [Google Scholar] [CrossRef]
- Fagheei Aghmiyuni, Z.; Saderi, H.; Owlia, P.; Saidi, N.; Laranjo, M. Evaluation of the Effect of Lactobacillus acidophilus ATCC 4356 Bacteriocin against Staphylococcus aureus. BioMed Res. Int. 2024, 2024, 4119960. [Google Scholar] [CrossRef]
- Martín, I.; Barbosa, J.; Pereira, S.I.A.; Rodríguez, A.; Córdoba, J.J.; Teixeira, P. Study of lactic acid bacteria isolated from traditional ripened foods and partial characterization of their bacteriocins. LWT 2023, 173, 114300. [Google Scholar] [CrossRef]
- Yasar, S.; Yerlikaya, S.; Sen Arslan, H.; Akgul, K.; Simsek, H. Determination of probiotic properties of lactic acid bacteria and yeasts isolated from three lacto-fermented cereals mixed with whey, citrus, and tomato pomace. J. Food Process. Preserv. 2022, 46, e17231. [Google Scholar] [CrossRef]
- Albene, D.; Lema, N.K.; Tesfaye, G.; Andeta, A.F.; Ali, K.; Guadie, A. Probiotic potential of lactic acid bacteria isolated from Ethiopian traditional fermented Cheka beverage. Ann. Microbiol. 2024, 74, 25. [Google Scholar] [CrossRef]
- Kato, K.; Serata, M.; Nakamura, M.; Ando, M.; Suzuki, T.; Okumura, T. Cell wall polysaccharide enhances Lacticaseibacillus paracasei strain Shirota growth in milk and contributes to acid and bile tolerance. Int. J. Food Microbiol. 2024, 422, 110811. [Google Scholar] [CrossRef] [PubMed]
- Balko, O.B. Low Molecular Weight Pseudomonas aeruginosa Bacteriocins. Mikrobiolohichnyi Zhurnal 2019, 81, 97–109. [Google Scholar] [CrossRef]
- Noroozi, E.; Mojgani, N.; Motevaseli, E.; Modarressi, M.H.; Tebianian, M.J.I.J.o.M. Physico-chemical and cytotoxic analysis of a novel large molecular weight bacteriocin produced by Lactobacillus casei TA0021. Iran. J. Microbiol. 2019, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, X.; Wang, Y.; Wang, J.; Kong, L.; Guo, H. Antibacterial Activity and Cytotoxicity of the Novel Bacteriocin Pkmh. Int. J. Mol. Sci. 2024, 25, 9153. [Google Scholar] [CrossRef]
- Bédard, F.; Biron, E. Recent Progress in the Chemical Synthesis of Class II and S-Glycosylated Bacteriocins. Front. Microbiol. 2018, 9, 1048. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, N.; Mao, R.; Hao, Y.; Teng, D.; Wang, J. In Vitro/Vivo Mechanisms of Antibacterial Peptide NZ2114 against Staphylococcus pseudintermedius and Its Biofilms. Antibiotics 2024, 13, 341. [Google Scholar] [CrossRef]
- Thuy, T.T.D.; Lu, H.-F.; Bregente, C.J.B.; Huang, F.-C.A.; Tu, P.-C.; Kao, C.-Y. Characterization of the broad-spectrum antibacterial activity of bacteriocin-like inhibitory substance-producing probiotics isolated from fermented foods. BMC Microbiol. 2024, 24, 85. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Su, H.; Wang, J.; Sun, S.; Liu, S.; Yin, B.; Dong, W.; Li, G. Isolation and Characterization of Bacteriocin-like-Producing Companilactobacillus farciminis YLR-1 and the Inhibitory Activity of Bacteriocin Against Staphylococcus aureus. Fermentation 2025, 11, 460. https://doi.org/10.3390/fermentation11080460
Yang L, Su H, Wang J, Sun S, Liu S, Yin B, Dong W, Li G. Isolation and Characterization of Bacteriocin-like-Producing Companilactobacillus farciminis YLR-1 and the Inhibitory Activity of Bacteriocin Against Staphylococcus aureus. Fermentation. 2025; 11(8):460. https://doi.org/10.3390/fermentation11080460
Chicago/Turabian StyleYang, Lirong, Hui Su, Jiayue Wang, Sijia Sun, Sibo Liu, Baishuang Yin, Wenlong Dong, and Guojiang Li. 2025. "Isolation and Characterization of Bacteriocin-like-Producing Companilactobacillus farciminis YLR-1 and the Inhibitory Activity of Bacteriocin Against Staphylococcus aureus" Fermentation 11, no. 8: 460. https://doi.org/10.3390/fermentation11080460
APA StyleYang, L., Su, H., Wang, J., Sun, S., Liu, S., Yin, B., Dong, W., & Li, G. (2025). Isolation and Characterization of Bacteriocin-like-Producing Companilactobacillus farciminis YLR-1 and the Inhibitory Activity of Bacteriocin Against Staphylococcus aureus. Fermentation, 11(8), 460. https://doi.org/10.3390/fermentation11080460




