Phenolic Evolution During Industrial Red Wine Fermentations with Different Sequential Air Injection Regimes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
- Intensity low (IL): Approximately 40 s of air injection per cycle.
- Intensity high (IH): Approximately 100 s of air injection per cycle.
- Frequency low (FL): 4 cycles of air injection per day.
- Frequency high (FH): 8 cycles of air injection per day.
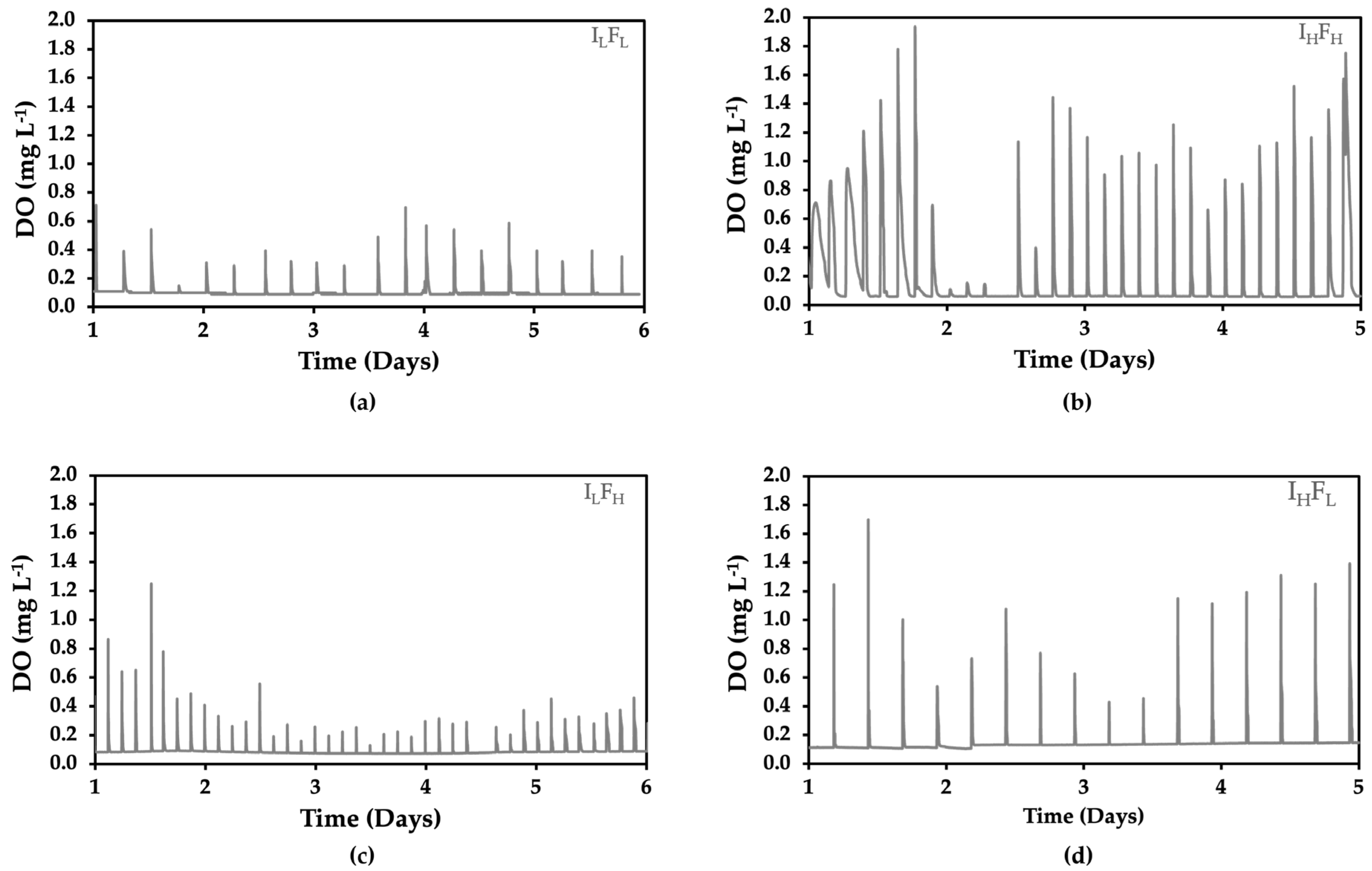
2.2. Dissolved Oxygen During Fermentation
2.3. Chemical Composition
2.3.1. General Physicochemical Measurements
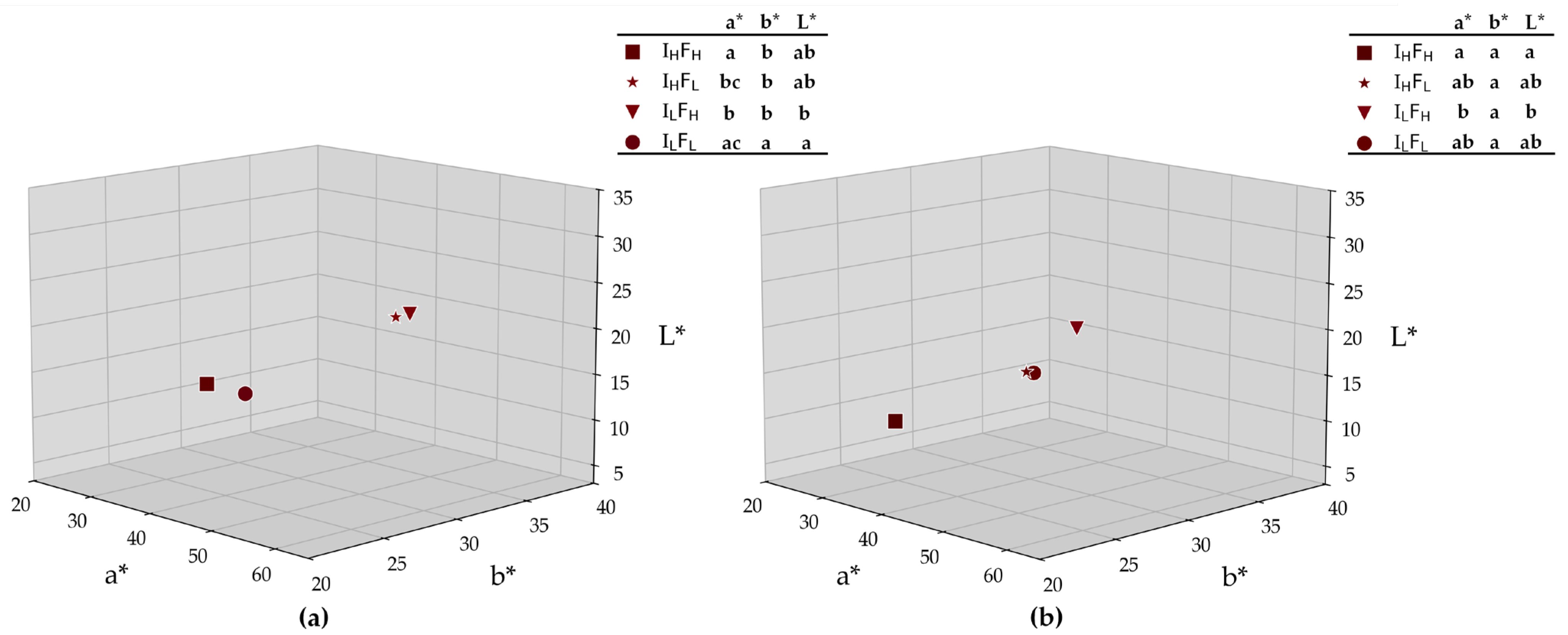
2.3.2. Phenolic Composition and Wine Color
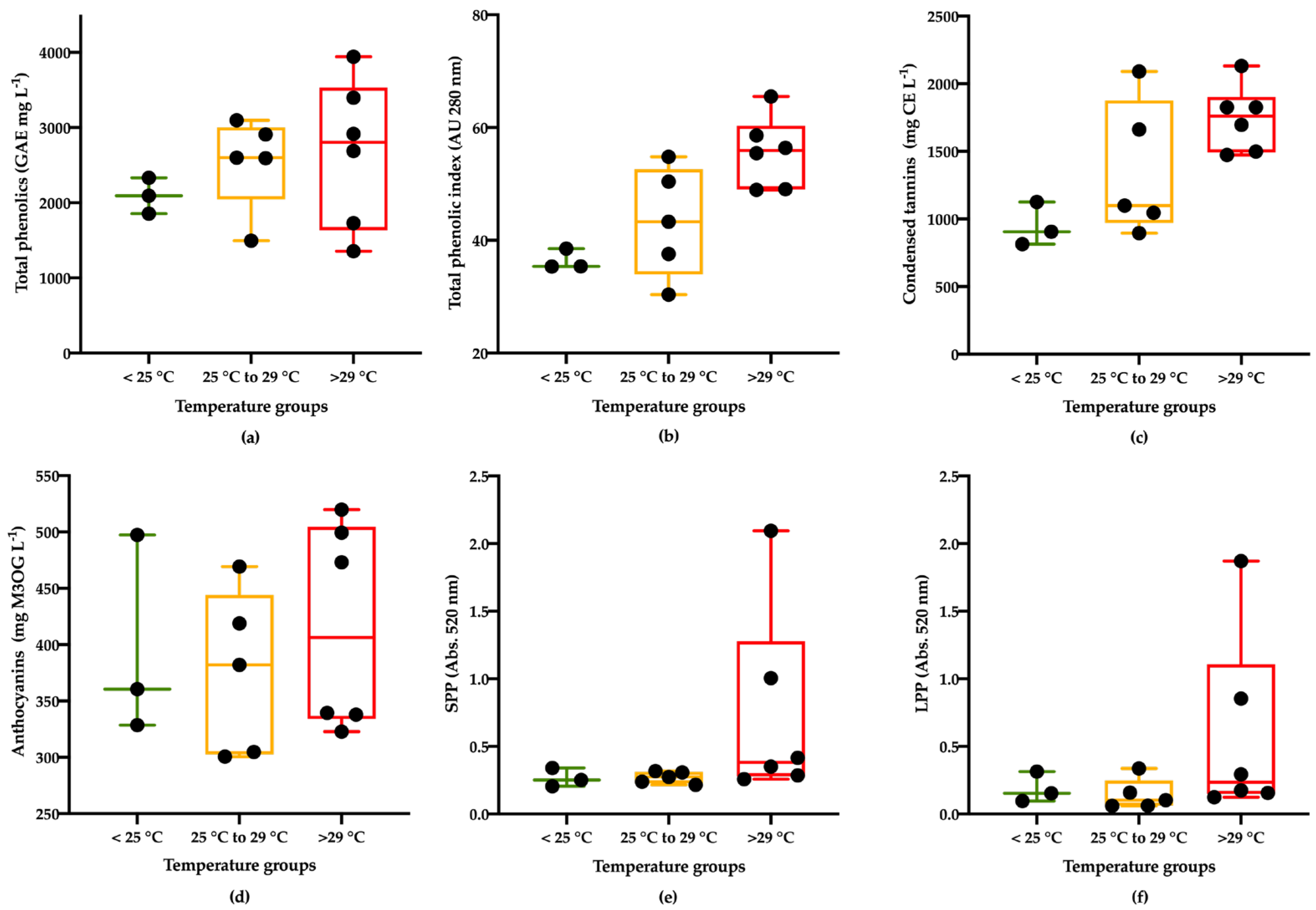
Total Phenolics
Condensed Tannins
Anthocyanins and Polymeric Pigments
Low Molecular Weight Polyphenols
2.4. Fermentation Progress Monitoring
2.5. Statistical Analyses
3. Results
3.1. Dissolved Oxygen During Fermentation
3.2. Chemical Composition
3.2.1. General Physicochemical Measurements
3.2.2. Phenolic Composition and Wine Color
Total Phenolics
Condensed Tannins
Anthocyanins and Polymeric Pigments
Low-Molecular-Weight Phenolics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carew, A.L.; Kerslake, F.L.; Bindon, K.A.; Smith, P.A.; Close, D.C.; Dambergs, R.G. Viticultural and Controlled Phenolic Release Treatments Affect Phenolic Concentration and Tannin Composition in Pinot Noir Wine. Am. J. Enol. Vitic. 2020, 71, 256–265. [Google Scholar] [CrossRef]
- Allegro, G.; Pastore, C.; Valentini, G.; Filippetti, I. The Evolution of Phenolic Compounds in Vitis vinifera L. Red Berries during Ripening: Analysis and Role on Wine Sensory—A Review. Agronomy 2021, 11, 999. [Google Scholar] [CrossRef]
- Day, M.P.; Espinase Nandorfy, D.; Bekker, M.Z.; Bindon, K.A.; Solomon, M.; Smith, P.A.; Schmidt, S.A. Aeration of Vitis Vinifera Shiraz Fermentation and Its Effect on Wine Chemical Composition and Sensory Attributes. Aust. J. Grape Wine Res. 2021, 27, 360–377. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Dipalmo, T.; Rizzello, C.G.; Corbo, F.; Crupi, P. Emerging Technology to Develop Novel Red Winemaking Practices: An Overview. Innov. Food Sci. Emerg. Technol. 2016, 38, 41–56. [Google Scholar] [CrossRef]
- Casassa, L.F.; Harbertson, J.F. Extraction, Evolution, and Sensory Impact of Phenolic Compounds during Red Wine Maceration. Annu. Rev. Food Sci. Technol. 2014, 5, 83–109. [Google Scholar] [CrossRef]
- Setford, P.C.; Jeffery, D.W.; Grbin, P.R.; Muhlack, R.A. Factors Affecting Extraction and Evolution of Phenolic Compounds during Red Wine Maceration and the Role of Process Modelling. Trends Food Sci. Technol. 2017, 69, 106–117. [Google Scholar] [CrossRef]
- Harrison, R. Practical Interventions That Influence the Sensory Attributes of Red Wines Related to the Phenolic Composition of Grapes: A Review. Int. J. Food Sci. Technol. 2018, 53, 3–18. [Google Scholar] [CrossRef]
- Lerno, L.; Reichwage, M.; Ponangi, R.; Hearne, L.; Block, D.E.; Oberholster, A. Effects of Cap and Overall Fermentation Temperature on Phenolic Extraction in Cabernet Sauvignon Fermentations. Am. J. Enol. Vitic. 2015, 66, 444–453. [Google Scholar] [CrossRef]
- Canals, R.; Llaudy, M.C.; Valls, J.; Canals, J.M.; Zamora, F. Influence of Ethanol Concentration on the Extraction of Color and Phenolic Compounds from the Skin and Seeds of Tempranillo Grapes at Different Stages of Ripening. J. Agric. Food Chem. 2005, 53, 4019–4025. [Google Scholar] [CrossRef]
- Andrich, G.; Zinnai, A.; Venturi, F.; Fiorentini, R. A Tentative Mathematical Model to Describe the Evolution of Phenolic Compounds during the Maceration of Sangiovese and Merlot Grapes. Ital. J. Food Sci. 2005, 17, 45–58. [Google Scholar]
- Sun, B.S.; Pinto, T.; Leandro, M.C.; Ricardo-Da-Silva, J.M.; Spranger, M.I. Transfer of Catechins and Proanthocyanidins from Solid Parts of the Grape Cluster into Wine. Am. J. Enol. Vitic. 1999, 50, 179–184. [Google Scholar] [CrossRef]
- Bindon, K.A.; Kassara, S.; Cynkar, W.U.; Robinson, E.M.C.; Scrimgeour, N.; Smith, P.A. Comparison of Extraction Protocols to Determine Differences in Wine-Extractable Tannin and Anthocyanin in Vitis vinifera L. Cv. Shiraz and Cabernet Sauvignon Grapes. J. Agric. Food Chem. 2014, 62, 4558–4570. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Mireles, M.S.; Harwood, E.D.; Weller, K.M.; Ross, C.F. Chemical and Sensory Effects of Saignée, Water Addition, and Extended Maceration on High Brix Must. Am. J. Enol. Vitic. 2009, 60, 450–460. [Google Scholar] [CrossRef]
- Smith, P.A.; Mcrae, J.M.; Bindon, K.A. Impact of Winemaking Practices on the Concentration and Composition of Tannins in Red Wine. Aust. J. Grape Wine Res. 2015, 21, 601–614. [Google Scholar] [CrossRef]
- Del Llaudy, M.C.; Canals, R.; Canals, J.M.; Zamora, F. Influence of Ripening Stage and Maceration Length on the Contribution of Grape Skins, Seeds and Stems to Phenolic Composition and Astringency in Wine-Simulated Macerations. Eur. Food Res. Technol. 2008, 226, 337–344. [Google Scholar] [CrossRef]
- Bindon, K.A.; Smith, P.A.; Holt, H.; Kennedy, J.A. Interaction between Grape-Derived Proanthocyanidins and Cell Wall Material. 2. Implications for Vinification. J. Agric. Food Chem. 2010, 58, 10736–10746. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Trousdale, E.K. Anthocyanin-Tannin Interactions Explaining Differences in Polymeric Phenols Between White and Red Wines. Am. J. Enol. Vitic. 1992, 43, 63–70. [Google Scholar] [CrossRef]
- Springer, L.F.; Sherwood, R.W.; Sacks, G.L. Pathogenesis-Related Proteins Limit the Retention of Condensed Tannin Additions to Red Wines. J. Agric. Food Chem. 2016, 64, 1309–1317. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Busse-Valverde, N.; Fernández-Fernández, J.I.; Gómez-Plaza, E.; Gil-Muñoz, R. The Extraction Kinetics of Anthocyanins and Proanthocyanidins from Grape to Wine in Three Different Varieties. OENO One 2016, 50, 91–100. [Google Scholar] [CrossRef]
- Gil, M.; Kontoudakis, N.; González, E.; Esteruelas, M.; Fort, F.; Canals, J.M.; Zamora, F. Influence of Grape Maturity and Maceration Length on Color, Polyphenolic Composition, and Polysaccharide Content of Cabernet Sauvignon and Tempranillo Wines. J. Agric. Food Chem. 2012, 60, 7988–8001. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; Wiley: Hoboken, NJ, USA, 2016; ISBN 9781118627808. [Google Scholar]
- Lerno, L.A.; Panprivech, S.; Ponangi, R.; Hearne, L.; Blair, T.; Oberholster, A.; Block, D.E. Effect of Pump-over Conditions on the Extraction of Phenolic Compounds during Cabernet Sauvignon Fermentation. Am. J. Enol. Vitic. 2018, 69, 295–301. [Google Scholar] [CrossRef]
- McRae, J.M.; Day, M.P.; Bindon, K.A.; Kassara, S.; Schmidt, S.A.; Schulkin, A.; Kolouchova, R.; Smith, P.A. Effect of Early Oxygen Exposure on Red Wine Colour and Tannins. Tetrahedron 2015, 71, 3131–3137. [Google Scholar] [CrossRef]
- Frost, S.C.; Blackman, J.W.; Ebeler, S.E.; Heymann, H. Analysis of Temporal Dominance of Sensation Data Using Correspondence Analysis on Merlot Wine with Differing Maceration and Cap Management Regimes. Food Qual. Prefer. 2018, 64, 245–252. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Mateus, N.; de Freitas, V. Sensorial Properties of Red Wine Polyphenols: Astringency and Bitterness. Food Sci. Nutr. 2017, 57, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xie, J.; Lin, J.; Xie, X.; Fan, S.; Han, X.; Zhang, D.; Han, L. The Material Basis of Astringency and the Deastringent Effect of Polysaccharides: A Review. Food Chem. 2023, 405, 134946. [Google Scholar] [CrossRef]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking; Springer: New York, NY, USA, 1999; ISBN 978-1-4419-5190-8. [Google Scholar]
- Pettinelli, S.; Pardini, L.; De Angeli, G.; Bianchi, A.; Najar, B.; Cerreta, R.; Bellincontro, A.; Floridia, G.; Mencarelli, F. Innovative “Soft” Maceration Techniques in Red Grape Fermentation. Beverages 2022, 8, 62. [Google Scholar] [CrossRef]
- Guerrini, S.; Barbato, D.; Mangani, S.; Mari, E.; Buscioni, G.; Ganucci, D.; Galli, V.; Granchi, L. Utilization of the AIRMIXING M.I.TM System in Producing Red Wine without Added Sulphites. Fermentation 2023, 9, 812. [Google Scholar] [CrossRef]
- Pettinelli, S.; Alfieri, G.; Bianchi, A.; Uliva, A.; Floridia, G.; Bellincontro, A.; Mencarelli, F. Use of AirMixing M.I.® Resonance Waves to Produce Aroma Richer Sangiovese Wine without Sulphites Addition in a Sustainable Way. LWT 2024, 198, 116075. [Google Scholar] [CrossRef]
- Lerno, L.; Reichwage, M.; Panprivech, S.; Ponangi, R.; Hearne, L.; Oberholster, A.; Block, D.E. Chemical Gradients in Pilot-Scale Cabernet Sauvignon Fermentations and Their Effect on Phenolic Extraction. Am. J. Enol. Vitic. 2017, 68, 401–411. [Google Scholar] [CrossRef]
- Casassa, L.F.; Kuster, S.T.; Gannet, P.; Watrelot, A.A. Temperature and Cap Management Effects on the Chemical, Phenolic, and Chromatic Composition of Pinot Noir Wines from the Central Coast of California. Am. J. Enol. Vitic. 2023, 74, 0740031. [Google Scholar] [CrossRef]
- LAFFORT TANIN VR SUPRA. Available online: https://laffort.com/en/products/tanin-vr-supra/ (accessed on 14 July 2025).
- Moenne, M.I.; Saa, P.; Laurie, V.F.; Pérez-Correa, J.R.; Agosin, E. Oxygen Incorporation and Dissolution During Industrial-Scale Red Wine Fermentations. Food Bioproc. Technol. 2014, 7, 2627–2636. [Google Scholar] [CrossRef]
- Del Alamo-Sanza, M.; Laurie, F.; Nevares, I. Wine Evolution and Spatial Distribution of Oxygen during Storage in High-Density Polyethylene Tanks. J. Sci. Food Agric. 2015, 95, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Calderón, J.F.; del Alamo-Sanza, M.; Nevares, I.; Laurie, V.F. The Influence of Selected Winemaking Equipment and Operations on the Concentration of Dissolved Oxygen in Wines. Cienc. Investig. Agrar. 2014, 41, 27–28. [Google Scholar] [CrossRef]
- Laurie, V.F.; Salazar, S.; Campos, M.I.; Cáceres-Mella, A.; Peña-Neira, Á. Periodic Aeration of Red Wine Compared to Microoxygenation at Production Scale. Am. J. Enol. Vitic. 2014, 65, 254–260. [Google Scholar] [CrossRef]
- Pérez-Caballero, V.; Ayala, F.; Echávarri, J.F.; Negueruela, A.I. Proposal for a New Standard OIV Method for Determination of Chromatic Characteristics of Wine. Am. J. Enol. Vitic. 2003, 54, 59–62. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of Total Phenolics. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Mercurio, M.D.; Dambergs, R.G.; Herderich, M.J.; Smith, P.A. High Throughput Analysis of Red Wine and Grape PhenolicsAdaptation and Validation of Methyl Cellulose Precipitable Tannin Assay and Modified Somers Color Assay to a Rapid 96 Well Plate Format. J. Agric. Food Chem. 2007, 55, 4651–4657. [Google Scholar] [CrossRef]
- Sarneckis, C.J.; Dambergs, R.G.; Jones, P.; Mercurio, M.; Herderich, M.J.; Smith, P.A. Quantification of Condensed Tannins by Precipitation with Methyl Cellulose: Development and Validation of an Optimised Tool for Grape and Wine Analysis. Aust. J. Grape Wine Res. 2006, 12, 39–49. [Google Scholar] [CrossRef]
- Dambergs, R.G.; Mercurio, M.D.; Kassara, S.; Cozzolino, D.; Smith, P.A. Rapid Measurement of Methyl Cellulose Precipitable Tannins Using Ultraviolet Spectroscopy with Chemometrics: Application to Red Wine and Inter-Laboratory Calibration Transfer. Appl. Spectrosc. 2012, 66, 656–664. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Picciotto, E.A.; Adams, D.O. Measurement of Polymeric Pigments in Grape Berry Extracts and Wines Using a Protein Precipitation Assay Combined with Bisulfite Bleaching. Am. J. Enol. Vitic 2003, 54, 301–306. [Google Scholar] [CrossRef]
- Heredia, T.M.; Adams, D.O.; Fields, K.C.; Held, P.G.; Harbertson, J.F. Evaluation of a Comprehensive Red Wine Phenolics Assay Using a Microplate Reader. Am. J. Enol. Vitic. 2006, 57, 497–502. [Google Scholar] [CrossRef]
- Peña-Neira, A.; Cáceres, A.; Pastenes, C. Low Molecular Weight Phenolic and Anthocyanin Composition of Grape Skins from Cv. Syrah (Vitis vinifera L.) in the Maipo Valley (Chile): Effect of Clusters Thinning and Vineyard Yield. Food Sci. Technol. Int. 2007, 13, 153–158. [Google Scholar] [CrossRef]
- Simmonds, C. Alcohol: Its Production, Properties, Chemistry, and Industrial Applications. With Chapters on Methyl Alcohol, Fusel Oil, and Spirituous Beverages. Nature 1920, 104, 431–432. [Google Scholar] [CrossRef]
- Devatine, A.; Chiciuc, I.; Poupot, C.; Mietton-Peuchot, M. Micro-Oxygenation of Wine in Presence of Dissolved Carbon Dioxide. Chem. Eng. Sci. 2007, 62, 4579–4588. [Google Scholar] [CrossRef]
- Day, M.P.; Schmidt, S.A.; Smith, P.A.; Wilkes, E.N. Use and Impact of Oxygen during Winemaking. Aust. J. Grape Wine Res. 2015, 21, 693–704. [Google Scholar] [CrossRef]
- Rosenfeld, E.; Beauvoit, B.; Blondin, B.; Salmon, J.-M. Oxygen Consumption by Anaerobic Saccharomyces Cerevisiae under Enological Conditions: Effect on Fermentation Kinetics. Appl. Environ. Microbiol. 2003, 69, 113–121. [Google Scholar] [CrossRef]
- Asenstorfer, R.E.; Markides, A.J.; Iland, P.G.; Jones, G.P. Formation of Vitisin A during Red Wine Fermentation and Maturation. Aust. J. Grape Wine Res. 2003, 9, 40–46. [Google Scholar] [CrossRef]
- Schneider, V.; Müller, J.; Schmidt, D. Oxygen Consumption by Postfermentation Wine Yeast Lees: Factors Affecting Its Rate and Extent under Oenological Conditions. Food Technol. Biotechnol. 2016, 54, 395–402. [Google Scholar] [CrossRef]
- Sablayrolles, J.-M.; Dubois, C.; Manginot, C.; Roustan, J.-L.; Barre, P. Effectiveness of Combined Ammoniacal Nitrogen and Oxygen Additions for Completion of Sluggish and Stuck Wine Fermentations. J. Ferment. Bioeng. 1996, 82, 377–381. [Google Scholar] [CrossRef]
- Volpe, M.G.; La Cara, F.; Volpe, F.; De Mattia, A.; Serino, V.; Petitto, F.; Zavalloni, C.; Limone, F.; Pellecchia, R.; De Prisco, P.P.; et al. Heavy Metal Uptake in the Enological Food Chain. Food Chem. 2009, 117, 553–560. [Google Scholar] [CrossRef]
- del Mar Castiñeira, M.; Brandt, R.; Jakubowski, N.; Andersson, J.T. Changes of the Metal Composition in German White Wines through the Winemaking Process. A Study of 63 Elements by Inductively Coupled Plasma−Mass Spectrometry. J. Agric. Food Chem. 2004, 52, 2953–2961. [Google Scholar] [CrossRef]
- Du, G.; Zhan, J.; Li, J.; You, Y.; Zhao, Y.; Huang, W. Effect of Fermentation Temperature and Culture Medium on Glycerol and Ethanol during Wine Fermentation. Am. J. Enol. Vitic. 2012, 63, 132–138. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Wen, X.; Song, X.; Meng, D.; Li, B.; Wang, M.; Tao, Y.; Zhao, H.; Guan, W.; et al. The Glycerol and Ethanol Production Kinetics in Low-temperature Wine Fermentation Using Saccharomyces Cerevisiae Yeast Strains. Int. J. Food Sci. Technol. 2019, 54, 102–110. [Google Scholar] [CrossRef]
- Zoecklein, B.W.; Fugelsang, K.C.; Gump, B.H.; Nury, F.S. Wine Analysis and Production; Springer: Boston, MA, USA, 1999; ISBN 978-1-4757-6969-2. [Google Scholar]
- Pérez-Navarro, J.; García Romero, E.; Gómez-Alonso, S.; Izquierdo Cañas, P.M. Comparison between the Phenolic Composition of Petit Verdot Wines Elaborated at Different Maceration/Fermentation Temperatures. Int. J. Food Prop. 2018, 21, 996–1007. [Google Scholar] [CrossRef]
- Casassa, L.F.; LoMonaco, I.; Velasco, M.; Papageorgas, D.D. Effect of Cap Management Frequency on the Phenolic, Chromatic, and Sensory Composition of Cabernet Sauvignon Wines from the Central Coast of California over Two Vintages. Molecules 2024, 29, 2509. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Girard, B.; Mazza, G.; Reynolds, A.G. Changes in Anthocyanins and Color Characteristics of Pinot Noir Wines during Different Vinification Processes. J. Agric. Food Chem. 1997, 45, 2003–2008. [Google Scholar] [CrossRef]
- Girard, B.; Yuksel, D.; Cliff, M.; Delaquis, P.; Reynolds, A. Vinifcation Efects on the Sensory, Colour and GC Profles of Pinot Noir Wines from British Columbia. Food Res. Int. 2001, 34, 483–499. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G. Influence of Temperature during Pre-Fermentative Maceration and Alcoholic Fermentation on the Phenolic Composition of ‘Cabernet Sauvignon’ Wines. Foods 2021, 10, 1053. [Google Scholar] [CrossRef]
- Cacace, J.E.; Mazza, G. Optimization of Extraction of Anthocyanins from Black Currants with Aqueous Ethanol. J. Food Sci. 2003, 68, 240–248. [Google Scholar] [CrossRef]
- Koyama, K.; Goto-Yamamoto, N.; Hashizume, K. Influence of Maceration Temperature in Red Wine Vinification on Extraction of Phenolics from Berry Skins and Seeds of Grape (Vitis vinifera). Biosci. Biotechnol. Biochem. 2007, 71, 958–965. [Google Scholar] [CrossRef]
- Coleman, M.C.; Fish, R.; Block, D.E. Temperature-Dependent Kinetic Model for Nitrogen-Limited Wine Fermentations. Appl. Environ. Microbiol. 2007, 73, 5875–5884. [Google Scholar] [CrossRef]
- Gomez-Plaza, E.; Gil, R.; Cutillas Adrian, M.; Ignacio Fernández, J. Phenolic Compounds and Color Stability of Red Wines: Effect of Skin Maceration Time. Am. J. Enol. Vitic. 2001, 52, 266–270. [Google Scholar] [CrossRef]
- Fourie, E.; Aleixandre-Tudo, J.L.; Mihnea, M.; du Toit, W. Partial Least Squares Calibrations and Batch Statistical Process Control to Monitor Phenolic Extraction in Red Wine Fermentations under Different Maceration Conditions. Food Control 2020, 115, 107303. [Google Scholar] [CrossRef]
- Picariello, L.; Gambuti, A.; Picariello, B.; Moio, L. Evolution of Pigments, Tannins and Acetaldehyde during Forced Oxidation of Red Wine: Effect of Tannins Addition. LWT Food Sci. Technol. 2017, 77, 370–375. [Google Scholar] [CrossRef]
- Sparrow, A.M.; Dambergs, R.G.; Bindon, K.A.; Smith, P.A.; Close, D.C. Interactions of Grape Skin, Seed, and Pulp on Tannin and Anthocyanin Extraction in Pinot Noir Wines. Am. J. Enol. Vitic. 2015, 66, 472–481. [Google Scholar] [CrossRef]
- Bindon, K.A.; Kassara, S.; Smith, P.A. Towards a Model of Grape Tannin Extraction under Wine-like Conditions: The Role of Suspended Mesocarp Material and Anthocyanin Concentration. Aust. J. Grape Wine Res. 2017, 23, 22–32. [Google Scholar] [CrossRef]
- Pavez, C.; González-Muñoz, B.; O’Brien, J.A.; Laurie, V.F.; Osorio, F.; Núñez, E.; Vega, R.E.; Bordeu, E.; Brossard, N. Red Wine Astringency: Correlations between Chemical and Sensory Features. LWT 2022, 154, 112656. [Google Scholar] [CrossRef]
- Laurie, V.F.; Law, R.; Joslin, W.S.; Waterhouse, A.L. In Situ Measurements of Dissolved Oxygen during Low-Level Oxygenation in Red Wines. Am. J. Enol. Vitic. 2008, 59, 215–219. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Nevares, I.; Martínez-Gil, A.; del Alamo-Sanza, M. Oxygen Consumption by Red Wines under Different Micro-Oxygenation Strategies and Q. Pyrenaica Chips. Effects on Color and Phenolic Characteristics. Beverages 2018, 4, 69. [Google Scholar] [CrossRef]
- Singleton, V.L. Oxygen with Phenols and Related Reactions in Musts, Wines, and Model Systems: Observations and Practical Implications. Am. J. Enol. Vitic. 1987, 38, 69–77. [Google Scholar] [CrossRef]
- Flanzy, C. Oenologie: Fondements Scientifiques et Technologiques; Tec Et Doc: Hobart, Australia, 1998; Volume 6, ISBN 2743002433. [Google Scholar]
- Cano-López, M.; Pardo-Mínguez, F.; Schmauch, G.; Saucier, C.; Teissedre, P.-L.; López-Roca, J.M.; Gómez-Plaza, E. Effect of Micro-Oxygenation on Color and Anthocyanin-Related Compounds of Wines with Different Phenolic Contents. J. Agric. Food Chem. 2008, 56, 5932–5941. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Laurie, V.F. Oxidation of Wine Phenolics: A Critical Evaluation and Hypotheses. Am. J. Enol. Vitic. 2006, 57, 306–313. [Google Scholar] [CrossRef]
- Castellari, M.; Matricardi, L.; Arfelli, G.; Galassi, S.; Amati, A. Level of Single Bioactive Phenolics in Red Wine as a Function of the Oxygen Supplied during Storage. Food Chem. 2000, 69, 61–67. [Google Scholar] [CrossRef]
- Schmid, F.; Schadt, J.; Jiranek, V.; Block, D.E. Formation of Temperature Gradients in Large- and Small-Scale Red Wine Fermentations during Cap Management. Aust. J. Grape Wine Res. 2009, 15, 249–255. [Google Scholar] [CrossRef]

| Treatment/Time | Bx | G_F | pH | Iron | Glycerol | Ethanol | Color Intensity | Color Tonality/Hue |
|---|---|---|---|---|---|---|---|---|
| D0 | ||||||||
| IHFH | 22.22 ± 1.14 a | 201.21 ± 11.35 a | 3.93 ± 0.36 a | 0.44 ± 0.17 a | 0.51 ± 0.32 a | 0.37 ± 0.68 ab | 2.26 ± 0.53 a | 0.89 ± 0.17 a |
| IHFL | 22.61 ± 2.01 a | 211.67 ± 15.35 a | 3.95 ± 0.23 a | 0.73 ± 0.05 b | 0.81 ± 0.44 a | NF | 4.32 ± 1.38 b | 0.87 ± 0.17 a |
| ILFH | 21.90 ± 1.75 a | 199.12 ± 15.08 a | 3.83 ± 0.07 a | 0.50 ± 0.15 a | 0.78 ± 0.18 a | NF | 1.99 ± 0.77 a | 0.75 ± 0.22 a |
| ILFL | 22.45 ± 0.86 a | 207.14 ± 13.27 a | 3.75 ± 0.14 a | 0.57 ± 0.16 ab | 0.75 ± 0.50 a | 0.62 ± 0.57 a | 2.57 ± 0.54 a | 0.7 ± 0.25 a |
| D2 | ||||||||
| IHFH | 18.23 ± 1.72 b | 131.66 ± 23.70 ab | 3.65 ± 0.23 a | 0.90 ± 0.33 ab | 3.06 ± 1.66 a | 4.81 ± 3.25 a | 4.83 ± 2.15 ab | 0.99 ± 0.49 a |
| IHFL | 17.13 ± 0.91 b | 129.26 ± 30.89 ab | 3.58 ± 0.18 a | 1.15 ± 0.39 b | 4.84 ± 0.30 b | 5.67 ± 2.46 a | 5.54 ± 1.87 ab | 0.64 ± 0.15 a |
| ILFH | 14.72 ± 1.90 a | 105.2 ± 32.84 a | 3.58 ± 0.14 a | 0.92 ± 0.08 ab | 5.68 ± 0.49 b | 5.00 ± 1.98 a | 4.65 ± 0.91 ab | 0.53 ± 0.09 a |
| ILFL | 17.88 ± 0.81 b | 156.36 ± 7.49 b | 3.56 ± 0.17 a | 0.80 ± 0.20 a | 3.32 ± 0.40 a | 3.52 ± 3.37 a | 3.59 ± 0.46 a | 0.90 ± 1.04 a |
| D4 | ||||||||
| IHFH | 9.11 ± 1.66 ab | 28.23 ± 19.07 a | 3.59 ± 0.14 a | 1.12 ± 0.57 a | 6.05 ± 0.79 a | 10.06 ± 1.28 a | 8.64 ± 2.67 ab | 0.48 ± 0.03 a |
| IHFL | 11.84 ± 1.02 c | 70.87 ± 14.38 b | 3.60 ± 0.16 a | 1.25 ± 0.12 a | 6.36 ± 0.59 a | 9.33 ± 1.75 a | 7.18 ± 1.87 a | 0.59 ± 0.07 b |
| ILFH | 8.37 ± 0.58 a | 23.31 ± 10.43 ab | 3.58 ± 0.11 a | 1.46 ± 0.26 ab | 6.95 ± 0.77 a | 10.67 ± 1.09 a | 7.03 ± 0.47 a | 0.49 ± 0.04 a |
| ILFL | 10.57 ± 1.87 bc | 48.73 ± 23.84 a | 3.63 ± 0.16 a | 1.45 ± 0.23 b | 6.34 ± 1.15 a | 9.44 ± 0.28 a | 9.29 ± 1.28 b | 0.48 ± 0.04 a |
| D6 | ||||||||
| IHFH | 7.73 ± 1.00 b | 4.11 ± 6.38 a | 3.64 ± 0.14 a | 1.3 ± 0.35 a | 6.13 ± 0.49 a | 11.56 ± 0.79 a | 10.15 ± 2.26 b | 0.47 ± 0.04 a |
| IHFL | 7.61 ± 0.96 ab | 8.10 ± 8.03 a | 3.65 ± 0.11 a | 1.85 ± 0.55 bc | 7.07 ± 1.19 a | 11.67 ± 0.50 a | 8.44 ± 1.25 ab | 0.5 ± 0.03 ab |
| ILFH | 6.76 ± 0.21 a | 1.87 ± 1.14 a | 3.69 ± 0.16 a | 1.51 ± 0.54 ab | 7.08 ± 0.98 a | 11.33 ± 0.66 a | 7.03 ± 1.43 a | 0.49 ± 0.02 ab |
| ILFL | 7.52 ± 0.54 ab | 4.21 ± 5.97 a | 3.74 ± 0.27 a | 2.14 ± 0.48 c | 6.37 ± 0.76 a | 11.50 ± 0.64 a | 9.03 ± 1.68 b | 0.53 ± 0.04 b |
| IHFH | IHFL | ILFH | ILFL | ||
|---|---|---|---|---|---|
| C | D4 | 129.95 ± 39.2 a | 105.91 ± 117.84 a | 137.55 ± 17.75 a | 198.36 ± 204.96 a |
| D6 | 243.02 ± 84.39 a | 234.38 ± 110.78 a | 115.38 ± 32.11 a | 238.33 ± 116.78 a | |
| EC | D4 | 63.51 ± 19.31 a | 46.11 ± 40.77 a | 70.32 ± 13.22 a | 111.97 ± 127.41 a |
| D6 | 52.7 ± 22.38 a | 113.98 ± 74.27 a | 65.68 ± 20.67 a | 128.44 ± 60.48 a | |
| PB3 | D4 | 14.3 ± 2.23 a | 81.21 ± 36.58 a | 95.94 ± 27.29 a | 82.83 ± 63.32 a |
| D6 | 74.45 ± 24.18 a | 128.37 ± 89.97 a | 79.45 ± 18.4 a | 178.12 ± 85.94 a | |
| GA | D4 | 41.77 ± 11.35 a | 39.46 ± 28.57 a | 51.3 ± 0.58 a | 48.75 ± 30.8 a |
| D6 | 76.88 ± 7.44 a | 81.77 ± 27.28 a | 55.26 ± 10.77 a | 55.26 ± 30.97 a | |
| PCA | D4 | 6.25 ± 1.14 a | 7.92 ± 7.14 a | 9.06 ± 3.25 a | 7.68 ± 3.62 a |
| D6 | 10.33 ± 2.51 a | 10.16 ± 1.87 a | 6.7 ± 0.34 a | 12.74 ± 5.16 a | |
| CfA | D4 | 2.08 ± 0.43 a | 7.44 ± 2.67 a | 7.06 ± 3.91 a | 5.27 ± 1.85 a |
| D6 | 4.03 ± 1.34 a | 8.03 ± 1.34 ab | 3.87 ± 0.9 a | 10.08 ± 4.11 b | |
| p-cuA | D4 | 3.63 ± 0.97 a | 5.49 ± 3.78 a | 5.13 ± 0.86 a | 4.75 ± 2.94 a |
| D6 | 11.34 ± 2.16 a | 11.02 ± 5.28 a | 3.09 ± 1.27 a | 9.3 ± 2.96 a | |
| VllA | D4 | 23.52 ± 15.57 a | 34.25 ± 32.92 a | 9.93 ± 2.75 a | 8.23 ± 1.55 a |
| D6 | 35.87 ± 1.63 b | 23.13 ± 6.54 ab | 6.56 ± 1.17 a | 20.27 ± 13.33 b | |
| CAc | D4 | 7.08 ± 0.06 a | 9.21 ± 7.76 a | 9 ± 2.35 a | 8.72 ± 4.02 a |
| D6 | 11.88 ± 1.14 a | 9.39 ± 3.19 a | 6.55 ± 2.39 a | 11.71 ± 3.53 a | |
| CAt | D4 | 4.81 ± 2.49 a | 8.7 ± 9.28 a | 12.7 ± 4.81 a | 17.59 ± 15.19 a |
| D6 | 6.42 ± 4.68 a | 7.27 ± 3.24 a | 9.79 ± 4.02 a | 18.12 ± 6.51 a | |
| CaftA | D4 | 3.91 ± 1.55 a | 2.24 ± 2.76 a | 2.69 ± 1.28 a | 2.15 ± 1.54 a |
| D6 | 4.1 ± 2.92 a | 2.35 ± 0.31 a | 13.19 ± 21.45 a | 2.32 ± 1.5 a | |
| Rsv | D4 | 8.48 ± 4.99 a | 10.83 ± 10.37 a | 3.8 ± 0.68 a | 4.22 ± 2.43 a |
| D6 | 15.3 ± 4.45 b | 7.92 ± 4.33 a | 2.84 ± 0.24 a | 3.93 ± 0.86 a | |
| Q | D4 | 17.9 ± 4.08 a | 15.51 ± 6.67 a | 26.67 ± 19.04 a | 17.13 ± 2.38 a |
| D6 | 15.27 ± 3.51 a | 19.5 ± 3.53 a | 36.55 ± 23.94 a | 31.27 ± 5.44 a | |
| Tr | D4 | 96.27 ± 14.16 a | 165.06 ± 136.48 a | 128.12 ± 8.86 a | 130.44 ± 50.44 a |
| D6 | 202.05 ± 65.89 a | 180.13 ± 30.69 a | 101.33 ± 14.84 a | 165.47 ± 63.93 a | |
| Mr3Glu | D4 | 17 ± 4.26 a | 16.85 ± 7.23 a | 18.2 ± 5.52 a | 15.84 ± 5.45 a |
| D6 | 20.89 ± 15.31 a | 11.83 ± 8.16 a | 13.67 ± 2.12 a | 19.36 ± 5.1 a | |
| Q3Gal | D4 | 33.19 ± 4.54 a | 15.04 ± 7.69 a | 25.09 ± 11.89 a | 29.08 ± 9.38 a |
| D6 | 32.19 ± 7.82 b | 18.72 ± 2.46 ab | 13.93 ± 6.48 a | 20.93 ± 0.01 ab | |
| PG1 | D4 | 9.68 ± 0.16 a | 10.36 ± 2.4 a | 10.17 ± 0.57 a | 9.45 ± 1.2 a |
| D6 | 10.62 ± 0.46 a | 11.91 ± 0.92 a | 10.18 ± 0.21 a | 10.82 ± 0.97 a | |
| PG2 | D4 | 17.68 ± 2.13 a | 20.65 ± 9.96 a | 22.32 ± 1.79 a | 19.41 ± 7.1 a |
| D6 | 14.41 ± 4.36 a | 31.66 ± 4.7 b | 22.03 ± 3.41 ab | 29.92 ± 7.83 b | |
| PG3 | D4 | 13.08 ± 0.0 b | 9.73 ± 1.68 a | 12.27 ± 0.58 ab | 10.18 ± 1.75 ab |
| D6 | 31.11 ± 19.67 a | 21.81 ± 3.28 a | 14.33 ± 2.26 a | 18.51 ± 4.34 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-Martínez, P.A.; Peña-Neira, A.; Laurie, V.F. Phenolic Evolution During Industrial Red Wine Fermentations with Different Sequential Air Injection Regimes. Fermentation 2025, 11, 446. https://doi.org/10.3390/fermentation11080446
Peña-Martínez PA, Peña-Neira A, Laurie VF. Phenolic Evolution During Industrial Red Wine Fermentations with Different Sequential Air Injection Regimes. Fermentation. 2025; 11(8):446. https://doi.org/10.3390/fermentation11080446
Chicago/Turabian StylePeña-Martínez, Paula A., Alvaro Peña-Neira, and V. Felipe Laurie. 2025. "Phenolic Evolution During Industrial Red Wine Fermentations with Different Sequential Air Injection Regimes" Fermentation 11, no. 8: 446. https://doi.org/10.3390/fermentation11080446
APA StylePeña-Martínez, P. A., Peña-Neira, A., & Laurie, V. F. (2025). Phenolic Evolution During Industrial Red Wine Fermentations with Different Sequential Air Injection Regimes. Fermentation, 11(8), 446. https://doi.org/10.3390/fermentation11080446







