Abstract
To explore an industrial fermentation approach for traditional mung bean sour pulp, this study isolated core microorganisms including lactic acid bacteria and yeasts from naturally fermented samples and constructed a synthetic microbial community. The optimized community consisted of Lactiplantibacillus pentosus, Lactococcus garvieae, and Cyberlindnera jadinii at a ratio of 7:3:0.1 and was used to ferment cooked mung bean pulp with a material-to-water ratio of 1:8 and 1% sucrose addition. Under these conditions, the final product exhibited significantly higher levels of protein (4.55 mg/mL), flavonoids (0.10 mg/mL), polyphenols (0.11 mg/mL), and vitamin C (7.75 μg/mL) than traditionally fermented mung bean sour pulp, along with enhanced antioxidant activity. The analysis of organic acids, free amino acids, and volatile compounds showed that lactic acid was the main acid component, the bitter amino acid content was reduced, the volatile flavor compounds were more abundant, and the level of harmful compound dimethyl sulfide was significantly decreased. These results indicate that fermentation using a synthetic microbial community effectively improved the nutritional quality, flavor, and safety of mung bean sour pulp.
1. Introduction
Mung bean sour noodles, made from fermented mung bean sour pulp (MBSP), have long been a popular traditional snack in China. Known for their distinctive sour and aromatic flavor, these noodles are easy to digest and offer a rich nutritional profile. They are considered a signature dish of Luoyang, an ancient capital city of China, and are particularly beloved by local residents. Mung beans, the main ingredient in the noodles, are known for their high protein content and also provide dietary fiber [1], vitamins [2], and minerals [3]. Additionally, mung beans are a rich source of bioactive compounds such as polyphenols and flavonoids, which have various health benefits [4]. These nutrients, combined with the fermentation process, contribute to the noodles’ nutritional value and overall health-promoting properties.
Legumes represent a critical, underutilized substrate for functional fermentation due to their high protein, fiber, and phytochemical content. Mung beans undergo fermentation, during which their nutrients are preserved, starch and protein degrade, and the resulting components become easily absorbable by the human body, contributing to a rich and unique flavor profile. Currently, the traditional fermentation process of mung bean sour pulp (MBSP) is typically carried out through spontaneous fermentation in family-run workshops. Several factors influence the quality of MBSP, with microbial composition playing a crucial role [5]. The microbial community in traditionally fermented MBSP is complex and poorly understood, and the fermentation process can be prone to contamination by harmful microorganisms. This often leads to inconsistencies in taste and flavor. Therefore, researchers have conducted extensive studies on the impact of microbial communities on the flavor and nutritional quality of fermented foods. For example, Li [6] analyzed the relationships among microbial communities, physicochemical properties, and volatile compounds in traditional MBSL. They identified the key bacterial and fungal genera involved in fermentation and flavor formation and detected 40 volatile compounds, mainly alcohols, acids, and phenols. Protein, acid content, and pH significantly influence microbial composition, offering insights into MBSL flavor development. Wang [7] investigated the changes in volatile compounds and free amino acids during the fermentation of Luoyang mung bean sour (LMBS) using GC-MS and an automatic amino acid analyzer. They found that beany flavor compounds significantly decreased, while the concentrations of esters and acids increased. Moreover, Liang [8] used Lactococcus lactis RQ1066 to ferment mung bean milk, which improved the protein hydrolysis, antioxidant capacity, and sensory quality while reducing anti-nutritional factors. This demonstrated its potential for enhancing the nutritional and functional value of mung bean products. Minna [9] found that fermentation with lactic acid and propionic acid bacteria significantly reduced galacto-oligosaccharides in legumes, concurrently degrading vicine and convicine in faba beans, while the impact on tannins in faba beans and lentil was moderate. However, the construction of synthetic microbial starters in MBSP fermentation had not been previously reported. Thus, screening key fermentation strains from traditionally fermented MBSP was essential for developing high-quality MBSP suitable for industrial production.
In this study, two strains of lactic acid bacteria and one strain of yeast, isolated from traditional Luoyang MBSP, were used to ferment mung bean sour pulp. Through single-factor experiments and response surface optimization, synthetic microbial communities were constructed for the fermentation of MBSP, and their nutritional components and flavor substances were compared with those of traditionally fermented MBSP. This research provides a foundation for the future standardization and industrialization of traditional MBSP production.
2. Materials and Methods
2.1. Media and Microbial Cultivation
Mung beans, traditionally fermented MBSP, and sucrose were purchased from a local agricultural market in Luoyang City, Henan Province, China. For the isolation and cultivation of lactic acid bacteria, we used MRS media (DeMan, Rogosa and Sharpe Medium) (Aobox, Beijing, China); for the isolation and cultivation of yeast, we used Rose Bengal medium (Aobox, Beijing, China) and YPD media (Yeast Extract Peptone Dextrose Medium) (peptone, 20 g/L, glucose, 20 g/L, yeast extract, 10 g/L, H2O 1000 mL), respectively.
2.2. Screening and Identification of Dominant Microbia in Traditionally Fermented MBSP
The traditionally fermented MBSP samples were purchased from two agricultural markets in Luoyang and then subjected to gradient dilution. Rose Bengal agar plates containing Chloramphenicol to inhibit bacterial growth were spread with 10−1, 10−2, and 10−3 dilutions to screen for yeast. For the screening of lactic acid bacteria, MRS plates were spread with 10−3, 10−4, and 10−5 dilutions and combined with a relative diameter measurement of calcium dissolution zones formed by CaCO3. Acid-producing microorganisms were screened using the calcium dissolution zone method, while yeast colonies were identified based on their moist and viscous surface and large, thick colony morphology.
The gradient-diluted samples were spread onto MRS agar plates and incubated at 28 °C for 72 h, and then they were spread onto Rose Bengal agar plates and incubated at 30 °C for 48 h, respectively. Each gradient was repeated 3 times.
The isolated strains were purified and sent to Sangon Biotech (Shanghai, China) Co., Ltd. for sequencing. The 16S rDNA sequences of lactic acid bacteria and ITS (Internal Transcribed Spacer) sequences of yeast were compared with those in the NCBI database using BLAST, and a phylogenetic tree was constructed using MEGA 7.0 software.
2.3. Construction of Synthetic Microbial Communities for MBSP’s Preparation
The traditional MBSP fermentation process was modified to simulate industrial conditions. In total, 1 Kg of mung beans was soaked in 4 L of purified water for 12 h, after which the water was drained. The beans were sterilized at 100 °C for 3 min. Water was then added to achieve a bean-to-water ratio of 1:8 (m/v), followed by the addition of varying amounts of sucrose according to the experimental design. The cell walls were disrupted, and the mixture was blended thoroughly. The resulting slurry was stirred and boiled for 20 min and then cooled to room temperature. Different ratios of microbial strains were inoculated, and fermentation was carried out at 28 °C for 24 h.
The culture conditions of synthetic microbial communities for MBSP were optimized using a single-factor design. Factors such as the inoculation ratio of the lactic acid bacteria (3.5 × 108 CFU/mL) complex, the yeast (2.0 × 108 CFU/mL) inoculation amount, and the sucrose addition level were optimized based on acidity and sensory evaluation during mung bean sour pulp fermentation. Based on the results of the single-factor experiments (Figure S2), the highest value of either total acidity (TA) or the sensory evaluation score was selected as the central point (0 level) for the Box–Behnken design (BBD) experiment. The BBD experiment was applied to optimize the MBSP fermentation process. Using sensory evaluation scores as the response variable, response surface methodology was employed to optimize the composite inoculation ratio of lactic acid bacteria (with lactic acid bacteria total inoculation level of 10%), the yeast inoculation amount, and the sucrose addition level.
2.4. Sensory Evaluation
Twenty trained undergraduate students majoring in Food Science and Technology conducted sensory evaluations of the MBSP samples, which were coded and presented in a randomized order. The evaluators assessed the samples based on ‘taste’, ‘flavor’, and ‘viscosity’ according to the criteria outlined in Table 1. The total sensory score was calculated by summing the scores for these three attributes.

Table 1.
Scoring criteria for sensory evaluation of MBSP.
2.5. Test of Nutrition Components
The MBSP sample was diluted with 60% ethanol and precipitated overnight at 4 °C. The samples were then centrifuged at 9600× g for 15 min at 4 °C, and the supernatant was collected to determine the contents of titratable acidity (TA), vitamin C (Vc), polyphenols, flavonoids, free amino acids (FAAs), and organic acids (OAs). TA was analyzed using a titration method [10]. The Vc content was determined by the 2,6-dichlorophenol indophenol titration method [11]. The polyphenol content was measured following the method of Yang et al. [12] and calculated using a gallic acid equivalent (GAE) standard curve. The flavonoid content was determined using the method of Huang et al. [13] and calculated using a rutin equivalent (RE) standard curve. FAAs were analyzed using an amino acid analyzer (A300, Membrapure GmbH, Frankfurt, Germany) according to the method of Ai [14]. OAs were measured by HPLC (1260 Infinity, Agilent Technologies, Palo Alto, CA, USA) following the method of Wang [15]. For protein analysis, 60% ethanol was replaced with distilled water, and the protein content was determined using the method of Zhai [16], with quantification based on a bovine serum albumin standard curve.
2.6. Determining the In Vitro Antioxidant Capacity
The antioxidant capacity was evaluated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging assays. The DPPH radical scavenging activity of MBSP was determined according to the method described by Su [17]. The ABTS+ scavenging activity was assessed following the procedure outlined by Re [18].
2.7. Analysis Volatile Flavor Compounds
The headspace solid-phase microextraction (HS-SPME) technique was used to extract volatile compounds. A total of 10 mL of the MBSP sample, 2 g of NaCl, and 10 μL of 2-octanol (100 mg/L, used as the internal standard) were added to a 20 mL headspace sampling vial. The mixture was equilibrated in a water bath at 60 °C for 20 min. Then, a microextraction fiber (50/30 μm DVB/CAR/PDMS, Supelco, Bellefonte, PA, USA) was exposed to the sample headspace for 40 min at 60 °C. Volatile flavor compounds were analyzed using a gas chromatograph–triple quadrupole tandem mass spectrometer (TSQ9000, Thermo Fisher Scientific, Waltham, MA, USA) equipped with a TG-WAXMS capillary column (30 m × 0.25 mm × 0.25 μm, Thermo Fisher Scientific, Waltham, MA, USA). Helium was used as the carrier gas at a flow rate of 1 mL/min. The injection port temperature was set at 250 °C. The GC oven temperature was initially set at 35 °C for 5 min, it was increased to 170 °C at a rate of 3 °C/min, and then it was held for 2 min. Mass spectra were obtained by electron ionization (EI) scanning from 30 to 350 m/z at 70 eV. Qualitative analysis of the compounds was performed by comparison with the Replib and Mainlib databases. The content of each flavor compound was calculated as a percentage of its peak area relative to the total peak area [15].
2.8. Statistical Analysis
Origin 2021 was used for graph generation, and Design Expert was employed to perform independent sample t-tests and Pearson’s correlation analyses. A significance level of p < 0.05 was considered statistically significant, while p < 0.01 indicated a highly significant difference. The results were expressed as mean ± standard deviation.
3. Results and Discussion
3.1. Strain Isolation and Identification
Traditionally fermented foods underwent mixed-strain fermentation during natural fermentation processes, which often resulted in unstable product characteristics and the potential presence of pathogenic or harmful microorganisms [19]. To improve the food safety of MBSP, elucidate its fermentation mechanism, and enable industrial-scale production, dominant microorganisms were first isolated from MBSP. Based on the cultivation characteristics, microscopic morphology, and phylogenetic analysis, three representative strains were identified. LYSJ-01 was preliminarily classified as a Gram-positive bacterium. Phylogenetic analysis showed that it clustered with Lactococcus garvieae strains, exhibiting a sequence similarity of 99%, suggesting that it belonged to the genus Lactococcus (Figure 1A). Similarly, LYSJ-02 was identified as a Gram-positive bacterium, with gene sequences showing 98–99% similarity to Lactiplantibacillus pentosus and Lactiplantibacillus paraplantarum, indicating that it was likely a functional strain within the family Lactobacillaceae (Figure 1B). LYSJ-03 exhibited typical yeast morphology, and its phylogenetic profile showed the closest relationship to Cyberlindnera jadinii, with a sequence similarity of 99–100%, identifying it as a yeast-like fungus (Figure 1C). These three strains were subsequently used for the fermentation of MBSP to reduce the accumulation of harmful microorganisms and enhance the stability of the MBSP.
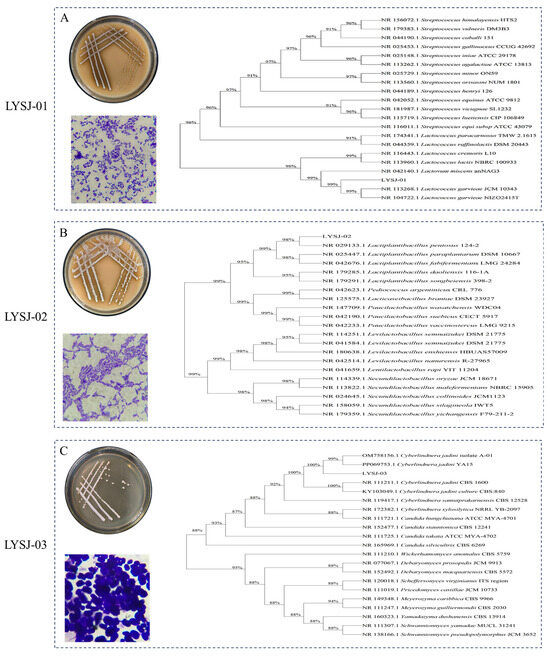
Figure 1.
Comparative morphological characterization and phylogenetic analysis of LYSJ-01 (A), LYSJ-02 (B), and LYSJ-03 (C) strains.
3.2. Response Surface Methodology (RSM) Optimizes Artificial Constructed Strain Fermented MBSP
The Design Expert software was used in multivariate quadratic regression fitting on the experimental result data. A multiple quadratic regression equation was obtained as follows:
Y = 22.0 − 0.875A + 0.375B + 0.75C − 0.25AB − 1.5AC − 0.5BC − 5.63A2 − 3.63B2 − 2.87C2
The results of the analysis of variance (ANOVA) for the regression model are presented in Table 2. As shown, the F-value of the regression equation was 278.73, with a corresponding p-value of <0.0001, indicating that the model was highly significant. The correlation coefficient R2 was 0.9972, indicating that the model fits the experiment well. The lack-of-fit term was not significant, indicating that the model had high predictive reliability. As shown in Table 2, factors A (inoculation ratio of the two lactic acid bacteria), B (inoculation amount of Cyberlindnera jadinii LYSJ-03), and C (amount of sucrose added) had significant effects on the sensory evaluation of mung bean sour paste. Among the interaction terms, AC and BC were significant, whereas AB was not. Based on the regression equation, the order of importance of the factors influencing the sensory score was A > C > B. Response surface plots and contour maps were generated accordingly (Figure S3). As shown in Figure S2, the sensory evaluation scores increased and then decreased with rising levels of each factor. The contour plots for AC and BC displayed elliptical shapes, indicating significant interaction effects on the sensory evaluation, whereas the contour plot for AB was nearly circular, suggesting a relatively minor interaction effect. These findings were consistent with the ANOVA results.

Table 2.
Analysis of variance (ANOVA) results for the regression model.
Based on the regression model, the optimal fermentation conditions were determined as follows: a lactic acid bacteria (total inoculation 10%, v/v) ratio of 7:3 (Lactiplantibacillus pentosus: Lactococcus garvieae, v/v), an inoculation amount of Cyberlindnera jadinii at 0.1% (v/v), and a sucrose addition of 1% (m/v). Under these conditions, the model predicted a sensory evaluation score of 20.510. To validate the model’s accuracy, three replicate fermentation experiments were conducted. The average sensory score of the three replicates was 20.000, which closely matched the predicted value, confirming that the model was both accurate and reliable.
3.3. Changes in Nutritional Components and In Vitro Antioxidant Capacity
Mung beans were considered highly nutritious due to their rich protein content [20], and they exhibited antioxidant properties attributed to active components such as vitamins, flavonoids, and polyphenols. The levels of protein, vitamin C, flavonoids, and polyphenols were measured in traditionally fermented commercial MBSP (sample A) and MBSP fermented with an artificially constructed microbial consortium (sample B). Table 3 shows that sample B contained 4.55 mg/mL of protein, 0.10 mg/mL of flavonoids, 0.11 mg/mL of polyphenols, and 7.75 μg/mL of vitamin C. The protein content in sample B accounted for 93.81% of the total protein in both samples A and B, which correlated with the higher proportion of Lactiplantibacillus in sample B fermentation and was consistent with the findings of Li [6]. Vitamin C, one of the most sensitive vitamins in food [21], was better preserved in sample B due to the low pH environment generated by lactic acid bacteria, accounting for 66.7% of the total in both samples. The flavonoid and polyphenol contents were also higher in sample B, representing 64.71% and 57.49% of the total, respectively, likely due to the fermentation of cooked mung bean pulp. The heat treatment of soybeans, as reported by Cui [22], reduced the anti-nutritional factors while increasing the polyphenol levels. Additionally, lactic acid bacteria produced enzymes such as phenolic acid decarboxylases and esterases, promoting the release and transformation of phenolic compounds. The organic acids generated during fermentation further inhibited polyphenol degradation [23].

Table 3.
Changes in the nutritional composition and in vitro antioxidant capacity of samples A and B. Sample A represented MBSP fermented using traditional methods, and sample B represented MBSP fermented using an artificially constructed microbial consortium.
The antioxidant activity of fermented foods delayed aging, reduced the risk of chronic diseases, enhanced immune function, and extended shelf life, thereby contributing to an improvement in health [24,25]. To evaluate the antioxidant activity of MBSP, DPPH and ABTS assays were performed to assess the in vitro antioxidant capacity of both samples. As shown in Table 3, sample B exhibited DPPH and ABTS radical scavenging activities of 49.07% and 37.45% (vitamin C equivalent), accounting for 67.12% and 66.73% of the total values from both samples, respectively. Moreover, the changes in the proportions of vitamin C, flavonoids, and polyphenols between the two samples showed a consistent trend, suggesting that MBSP fermented by the artificially constructed microbial community contained higher levels of antioxidant-active compounds and demonstrated enhanced in vitro antioxidant activity.
3.4. Changes in Free Amino Acids Content
The free amino acids (FAAs) in mung bean sour pulp not only possess a substantial nutritional value but also significantly contribute to the formation of its flavor profile. The content of FAAs in commercially available traditionally fermented mung bean sour pulp (sample A) and artificially constructed microbial flora fermented mung bean sour pulp (sample B) was tested. Figure 2a shows that the total amount of free amino acids (FAAs) in sample A was 311.83 mg/100 mL, which was significantly higher than that in sample B (99.24 mg/100 mL). The decrease in FAAs in sample B may have been due to the use of cooked mung bean pulp for fermentation. Given the relatively poor thermal stability of amino acids, the high temperatures during mung bean pulping likely led to the degradation of certain amino acids and their binding with saccharides [26]. It was also possible that amino acids were metabolized and utilized by microorganisms present in the mung bean sour pulp [27]. Moreover, the ratios of essential amino acids to total amino acid content in the two samples are 17.67% (A) and 16.87% (B), respectively.
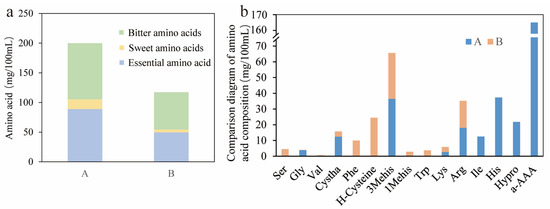
Figure 2.
(a) Comparison of different categories of amino acids between samples A and B. (b) Comparison of amino acid contents between samples A and B. Sample A represented MBSP fermented using traditional methods, and sample B represented MBSP fermented using an artificially constructed microbial consortium. Ser: serine; Gly: glycine; Val: valine; Cystha: L-cystathionine; phe: phenylalanine; H-Cysteine: cysteine; 3Mehis: 3-methylhistidine; 1Mehis: 1-methyl-L-histidine; Trp: tryptophan; Lys: lysine; Arg: arginine; Ile: isoleucine; His: histidine; Hypro: hydroxyproline; a-AAA: α-aminoadipic acid.
Different FAAs exhibited distinct flavors, and their types and concentrations directly affected the taste of foods [28,29]. As shown in Figure 2a, the proportions of sweet-tasting, bitter-tasting, and essential amino acids in total amino acids were generally consistent between the two samples. Figure 2b shows that nine free amino acids were detected in the traditionally fermented commercial MBSP, while eleven were identified in the MBSP fermented by the artificially constructed microbial consortium. Different amino acids contributed to the complex flavor of MBSP. For example, Ser and Gly provided sweetness, while Val, Phe, Lys, Arg, Ile, and His imparted bitterness [30]. Thr, Val, Phe, Cys, and Trp were uniquely detected in sample B, with concentrations of 4.57, 0.65, 9.98, 24.48, and 3.74 mg/100 mL, respectively. Thr contributed sweetness, and Cys enhanced umami. Although Val, Phe, and Trp were bitter, they acted as flavor precursors and helped shape the unique taste of sour pulp. As essential amino acids, they also improved nutritional value. MBSP fermented by the artificial microbial consortium was considered more palatable, as confirmed by the sensory evaluation results.
3.5. Changes in Organic Acid Content
Organic acids contributed to the development of the sour taste and organoleptic properties of MBSP and, similar to vinegar, played a key role in shaping its overall flavor [31]. The organic acid (OA) contents in traditionally fermented commercial mung bean sour pulp (sample A) and in MBSP fermented by an artificially constructed microbial consortium (sample B) were analyzed, and the results are shown in Table 4. As shown in Table 4, sample B contained four types of organic acids—lactic acid, pyruvic acid, acetic acid, and oxalic acid—with a total concentration of 21.13 ± 0.95 mg/mL, of which lactic acid accounted for 92.24%. In contrast, sample A contained seven types of organic acids with a total concentration of 15.97 ± 0.76 mg/mL; lactic acid and malic acid together accounted for 83.28%, and lactic acid alone accounted for 44.04%. Notably, tartaric acid, malic acid, and α-ketoglutaric acid were uniquely detected in sample A. These organic acids contributed to the development of the characteristic sour flavor observed in sample A.

Table 4.
Comparison of organic acid contents between samples A and B.
The types and contents of organic acids influenced the sensory characteristics of MBSP [32]. Microorganisms produced malic acid, tartaric acid, pyruvic acid, lactic acid, α-ketoglutaric acid, and acetic acid during the fermentation process. Malic acid possessed a distinctive flavor and a long-lasting aroma; even small amounts imparted a fresh sourness to traditionally fermented MBSP. Tartaric acid, which was slightly astringent with a strong sour taste, was also detected [33]. Acetic acid, a volatile compound, exhibited a sharp vinegar-like aroma. Yu [34] found that high concentrations of acetic acid could mask the aroma of fermented products, reducing flavor harmony and making the product less mellow. Lactic acid, a highly acidic compound, provided a mild, tangy sourness that was smoother and less sharp compared to citric or acetic acids. Its smooth and refreshing acidity made it a preferred component in many fermented foods and beverages. In this study, lactic acid was identified as the primary source of acidity in MBSP. Sample B contained significantly higher lactic acid levels than sample A, resulting in a softer texture and a cleaner, more rounded sour flavor. This feature directly influenced the sensory quality and uniqueness of the product. Therefore, compared to traditionally fermented MBSP, the version produced by the synthetic microbial community exhibited a smoother texture and a purer sour taste, consistent with the sensory evaluation results.
3.6. Changes in Volatile Flavor Compounds
The volatile flavor compounds in samples A and B were analyzed using SPME-HS-GC-MS. Sample A contained 50 compounds with a total concentration of 70.86 mg/mL, including 6 acids, 14 esters, 6 aldehydes and ketones, 21 alcohols, 3 phenols, and 3 others. Sample B had 71 compounds with a total concentration of 58.64 mg/mL, including 6 acids, 16 esters, 9 aldehydes and ketones, 22 alcohols, 4 phenols, and 14 others (Figure S2a,b, Figure 3a–f). As shown in Figure S2b, the acid content in sample B reached 6.68 mg/100 mL, which was nearly four times higher than that in sample A (1.72 mg/100 mL). The concentration of acetic acid in sample B was 4.95 mg/100 mL, significantly higher than the 1.07 mg/100 mL observed in sample A. Additionally, the ethanol levels in sample B reached 8.63 mg/100 mL, compared to 5.20 mg/100 mL in sample A. The relatively higher ethanol content in sample B may have helped mask the sharpness of acetic acid, resulting in a more balanced aroma and a smoother, mellower taste [35].
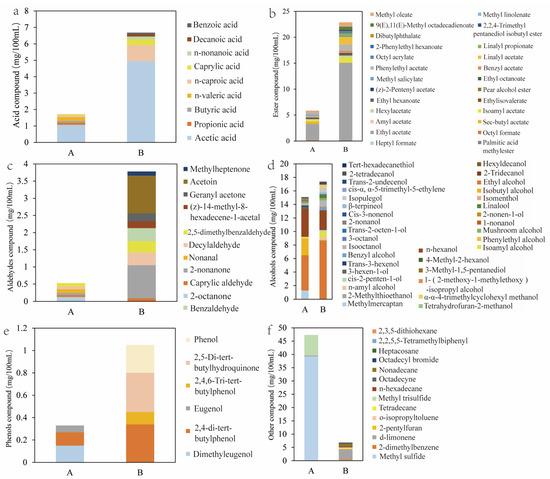
Figure 3.
(a) Comparison of acid compound contents between samples A and B. (b) Comparison of ester compound contents between samples A and B. (c) Comparison of aldehyde compound contents between samples A and B. (d) Comparison of alcohol compound contents between samples A and B. (e) Comparison of phenol compound contents between samples A and B. (f) Comparison of other compound contents between samples A and B. Sample A represented MBSP fermented using traditional commercial methods, and sample B represented MBSP fermented using an artificially constructed microbial consortium.
As shown in Figure 3b, the ester content in sample B reached 22.86 mg/100 mL, accounting for 39.1% of total volatiles, which was significantly higher than that in sample A (5.85 mg/100 mL, 8.26%). Ethyl acetate, a major contributor to fruity and pleasant aromas, was present at 15.09 mg/100 mL in sample B, nearly four times the amount in sample A (3.05 mg/100 mL). It was mainly produced by yeast and lactic acid bacteria during fermentation. Isoamyl acetate, responsible for banana and pear-like aromas, reached 1.09 mg/100 mL in sample B, also four times higher than in sample A (0.27 mg/100 mL). Additionally, floral and fruity compounds such as linalyl acetate, 2-phenylethyl hexanoate, and dibutyl phthalate were detected only in sample B. Aldehydes and ketones, key aromatic compounds in fermented products, were mainly associated with fruity and creamy notes and have been shown to positively influence product quality [35]. As shown in Figure 3c, their total content in sample B reached 3.78 mg/100 mL, which was 6.13 times higher than that in sample A (0.53 mg/100 mL). Ketones are known for their pleasant aromatic characteristics. Geranylacetone (0.12 mg/mL) and 3-hydroxy-2-butanone (1.10 mg/100 mL), both detected only in sample B, contributed magnolia-like floral notes [5] and creamy, buttery, slightly sweet aromas, respectively [36]. Both samples contained decanal and nonanal; the former has a fruity aroma, while the latter provides strong rose and sweet orange notes, enhancing the overall fruity and floral profile of MBSP [37].
As shown in Figure 3d, the alcohol content in sample B was 17.36 mg/100 mL, slightly higher than the 15.12 mg/100 mL in sample A. Linalool (0.34 mg/100 mL) and methanethiol (1.29 mg/100 mL), which were detected only in sample A, contribute a fresh green leaf fragrance [38] and a rotten cabbage odor [39], respectively. Methanethiol is one of the key compounds responsible for the unpleasant flavor in commercially available MBSP. The content of 1-octen-3-ol in sample B was 0.35 mg/100 mL, higher than the 0.15 mg/100 mL in sample A. This compound is the main contributor to the beany flavor in soybeans [40]. However, the higher ester content in sample B helped mask the beany odor of 1-octen-3-ol, resulting in a more desirable aroma in sample B. Moreover, 2,5-di-tert-butylhydroquinone (0.35 mg/100 mL), detected only in sample B (Figure 3e), is known for its strong antioxidant properties [41], contributing to the enhanced antioxidant activity in sample B. As shown in Figure 3f, d-limonene, which has a light floral aroma [42], is an approved food flavoring by national standards, and its content in sample B was 11 times higher than in sample A. In sample A, the dimethyl sulfide content was the highest, reaching 39.30 mg/100 mL, while the methanethiol content was 1.29 mg/100 mL. Volatile organic sulfur compounds are known for their unpleasant odor and very low odor threshold [43]. However, the dimethyl sulfide content in sample B was reduced to 0.31 mg/100 mL, and methanethiol was not detected, leading to an improved aroma and greater overall healthiness in sample B.
The results confirmed that the artificially constructed microbial community fermentation was effective in preserving the key flavor characteristics of MBSP. The construction of synthetic microbial communities contributed to a higher proportion of esters, alcohols, acids, aldehydes, and ketones, which played a significant role in the formation of the overall desirable flavor of MBSP. Therefore, synthetic microbial communities for MBSP exhibited a better aroma compared to traditionally fermented products, which was consistent with the sensory evaluation results.
4. Conclusions
In this study, three primary fermentation strains were isolated from traditionally fermented mung bean sour pulp and identified as Lactococcus garvieae, Lactiplantibacillus pentosus, and Cyberlindnera jadinii through 16S rDNA sequencing. The constructed synthetic microbial community fermented cooked mung bean pulp with optimal conditions: a material-to-water ratio of 1:8, 10% (v/v) lactic acid bacteria inoculation, 0.1% (v/v) yeast inoculation, and 1% (m/v) sucrose addition. The microbial community, consisting of Lactiplantibacillus pentosus/Lactococcus garvieae/Cyberlindnera jadinii in a 7:3:0.1 ratio, resulted in higher protein (4.55 mg/mL), flavonoid (0.10 mg/mL), polyphenol (0.11 mg/mL), and vitamin C (7.75 μg/mL) contents compared to traditionally fermented mung bean sour pulp, consistent with the in vitro antioxidant activity results. While retaining the flavor profile of traditionally fermented MBSP, synthetic microbial communities for MBSP exhibited more defined sourness, enhanced nutritional richness, a significant reduction in harmful flavor components, and a more abundant taste profile. This study demonstrates a process for enhancing the nutritional value and safety of fermented mung bean sour pulp, though further research is needed to explore strain interactions and the mechanisms of metabolic component changes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation11080443/s1, Figure S1: Characteristics of strains LYSJ-01, LYSJ-02 and LYSJ-03. (A) Growth of strains LYSJ-01, LYSJ-02 and LYSJ-03. (B) The acidity and pH value of the fermentation broth during the growth of strains LYSJ-01 and LYSJ-02. (C) The OD600 values of strains LYSJ-01 and LYSJ-02 cultured at different pH for 72 h. (D) The OD600 values of strains LYSJ-03 cultured at 19 different pH for 72 h; Figure S2: Result of single Factor effect to the MBSP-SMC fermented. (A)Inoculation ration of LYSJ-01. (B) Inoculate amount of LYSJ-03. (C) Sucrose add amount; Figure S3: RSM analysis of sensory ratings as influenced by Lactic acid bacteria ratio, Yeast inoculum amount, and sucrose addition amount in fermentation optimization. A represented the compound ratio of lactic acid bacteria; B represented Yeast inoculation amount; C represented sucrose addition amount; Figure S4: The proportion of volatile flavor compound categories in samples A (a) and B (b); Table S1: Factor and levels of BBD; Table S2: Experiment Design and Results of RSM.
Author Contributions
Y.Z.: writing—review and editing, supervision, project administration; L.C.: writing—original draft, methodology, data curation; P.L.: software, formal analysis; H.Y.: visualization, data curation; D.W.: supervision, methodology, investigation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Postgraduate Education Reform and Quality Improvement Project of Henan Province, China, grant number: YJS2024JD51 and the Joint Fund Project of Science and Technology Research and Development Plan of Henan Province, China, grant number: 235101610045.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors confirm that they have no conflicts of financial interests or personal relationships that could have appeared to influence the work described in this manuscript.
References
- Chandrasiri, S.D.; Liyanage, R.; Vidanarachchi, J.K.; Weththasinghe, P.; Jayawardana, B.C. Does processing have a considerable effect on the nutritional and functional properties of mung bean (Vigna radiata)? Procedia Food Sci. 2016, 6, 352–355. [Google Scholar] [CrossRef]
- Li, J.Q.; Lu, Y.Y.; Chen, H.L.; Wang, L.X.; Wang, S.H.; Guo, X.B.; Cheng, X.Z. Effect of photoperiod on vitamin E and carotenoid biosynthesis in mung bean (Vigna radiata) sprouts. Food Chem. 2021, 358, 129915. [Google Scholar] [CrossRef]
- Jahan, S.; Bisrat, F.; Omar, M.; Faruque Md Jannatul, F.; Shompa, S.K.; Tasnim, F. Formulation of nutrient enriched germinated wheat and mung-bean based weaning food compare to locally available similar products in Bangladesh. Heliyon 2021, 7, e06974. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Shen, X.; Shen, H.; Shen, H.F.; Zhou, Y.; Yao, X.M. Effect of optimized germination technology on polyphenol content and hypoglycemic activity of mung bean. Front. Nutr. 2023, 10, 1138739. [Google Scholar] [CrossRef]
- Li, C.; Tang, C.; Zeng, X.Y.; Zhang, Y.; He, L.P.; Yan, Y. Exploration of carbonyl compounds in red-fleshed kiwifruit wine and perceptual interactions among non-volatile organic acids. Food Chem. 2024, 448, 139118. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Shu, L.Y.; Zhao, L.N.; Cao, L.; Li, X.; Tie, S.S.; Tian, P.P.; Gu, S.B. Unravelling the correlations among the microbial community, physicochemical properties, and volatile compounds of traditional mung bean sour liquid. LWT Food Sci. Technol. 2024, 198, 115971. [Google Scholar] [CrossRef]
- Wang, X.D.; Wang, X.Y.; Lou, H.W.; Li, Y.; Reham k Zhao, R.Y. Understanding the correlation between formation of flavor compounds and dominant bacteria during Luoyang mung bean sour fermentation. Food Biosci. 2024, 60, 104374. [Google Scholar] [CrossRef]
- Liang, Z.Q.; Sun, J.W.; Yang, S.; Wen, R.; Liu, L.B.; Du, P.; Li, C.; Zhang, G.F. Fermentation of mung bean milk by Lactococcus lactis: Focus on the physicochemical properties, antioxidant capacities and sensory evaluation. Food Biosci. 2022, 48, 101798. [Google Scholar] [CrossRef]
- Kahala, M.; Blasco, L.; Bragge, R.; Porcellato, D.; Østlie, H.M.; Rundberget, T.; Baz-Lomba, J.A.; Pihlava, J.-M.; Hellström, J.; Jørgensen, E.G.; et al. Lactic and propionic acid bacteria starter cultures for improved nutritional properties of pea, faba bean and lentil. LWT Food Sci. Technol. 2024, 208, 116691. [Google Scholar] [CrossRef]
- Li, Y.; Nguyen, T.T.H.; Jin, J.; Lim, J.; Lee, J.; Piao, M.; Mok, I.-K.; Kim, D. Brewing of glucuronic acid-enriched apple cider with enhanced antioxidant activities through the co-fermentation of yeast (Saccharomyces cerevisiae and Pichia kudriavzevii) and bacteria (Lactobacillus plantarum). Food Sci. Biotechnol. 2021, 30, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.P.; Mahajan, M.; Jain, P. Photometric methods for the determination of vitamin C: Reviews. Anal. Sci. 2005, 14, 889–895. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Ning, Y.; Wang, X.; Zhang, X.; Weng, P.; Wu, Z.F. Characterization of an intracellular alkaline serine protease from Bacillus velezensis SW5 with fibrinolytic activity. Curr. Microbiol. 2020, 77, 1610–1621. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Bian, Y.Y.; Liu, T.Z.; Xu, Z.; Song, Z.Q.; Wang, F.; Li, T.P.; Li, S.H. Antioxidant potential and in vitro inhibition of starch digestion of flavonoids from Crataegus pinnatifida. Heliyon 2022, 8, e11058. [Google Scholar] [CrossRef]
- Ai, M.; Qiu, X.; Huang, J.; Wu, C.; Jin, Y.; Zhou, R. Characterizing the microbial diversity and major metabolites of Sichuan bran vinegar augmented by Monascus purpureus. Int. J. Food Microbiol. 2019, 292, 83–90. [Google Scholar] [CrossRef]
- Wang, D.H.; Wang, M.Y.; Cao, L.W.; Wang, X.T.; Sun, J.R.; Yuan, J.F.; Gu, S.B. Changes and correlation of microorganism and flavor substances during persimmon vinegar fermentation. Food Biosci. 2022, 46, 101565. [Google Scholar] [CrossRef]
- Zhai, C.K.; Lu, C.M.; Zhang, X.Q.; Sun, G.J.; Lorenz, K.J. Comparative study on nutritional value of Chinese and north American wild rice. J. Food Compos. Anal. 2001, 14, 371–382. [Google Scholar] [CrossRef]
- Su, M.S.; Silva Juan, L. Antioxidant activity, anthocyanins, and phenolics of rabbiteye blueberry (Vaccinium ashei) by-products as affected by fermentation. Food Chem. 2005, 97, 447–451. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolourization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Jimenez, M.E.; O’Donovan, C.M.; Ullivarri, M.F.; Cotter, P.D. Microorganisms present in artisanal fermented food from South America. Front. Microbiol. 2022, 13, 941866. [Google Scholar] [CrossRef]
- Huang, Z.L.; Li, Y.; Fan, M.C.; Qian, H.F.; Wang, L. Recent advances in mung bean protein: From structure, function to application. Int. J. Biol. Macromol. 2024, 273 Pt 2, 133210. [Google Scholar] [CrossRef]
- Raffaella, D.C.; Rosalinda, F.S.; Annalisa, P.; Maria, D.A.; Jean-Christophe, S.; Solange, B.; Laura, D.G.; Marco, G. Effect of autochthonous lactic acid bacteria starters on health-promoting and sensory properties of tomato juices. Int. J. Food Microbiol. 2009, 128, 473–483. [Google Scholar] [CrossRef]
- Cui, R.B.; Yoo Michelle, J.Y.; Zhu, F. Comparison of microwave and conventional heating on physicochemical properties and phenolic profiles of purple sweetpotato and wheat flours. Food Biosci. 2022, 46, 101602. [Google Scholar] [CrossRef]
- Tkacz, K.; Chmielewska, J.; Turkiewicz, I.P.; Nowicka, P.; Wojdyło, A. Dynamics of changes in organic acids, sugars and phenolic compounds and antioxidant activity of sea buckthorn and sea buckthorn-apple juices during malolactic fermentation. Food Chem. 2020, 332, 127382. [Google Scholar] [CrossRef]
- Sarıtaş, S.; Portocarrero, A.C.M.; Miranda, L.J.M.; Lombardo, M.; Koch, W.; Raposo, A.; Hesham, R.E.S.; Alves, J.L.D.B.; Esatbeyoglu, T.; Karav, S.; et al. The impact of fermentation on the antioxidant activity of food products. Molecules 2024, 29, 3941. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Yan, J.; Qi, X.R.; Wang, Y.H.; Zheng, Z.T.; Liang, J.Q.; Ling, J.T.; Chen, Y.X.; Tang, X.Y.; et al. Application of fermented Chinese herbal medicines in food and medicine field: From an antioxidant perspective. Trends Food Sci. Technol. 2024, 148, 104410. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Luan, D. Thermal degradation characteristics of amino acids in rainbow trout fillets during traditional high temperature short time processing and microwave processing. J. Food Meas. Charact. 2023, 17, 1940–1952. [Google Scholar] [CrossRef]
- Neis, E.; Dejong, C.; Rensen, S. The Role of Microbial Amino Acid Metabolism in Host Metabolism. Nutrients 2015, 7(4), 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Curiel, J.A.; Coda, R.; Centomani, I.; Summo, C.; Gobbetti, M.; Rizzello, C.G. Exploitation of the nutritional and functional characteristics of traditional Italian legumes: The potential of sourdough fermentation. Int. J. Food Microbiol. 2015, 196, 51–61. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, P.C.; Liu, P.P.; Song, X.W.; Guo, F.; Li, Y.Y.; Ni, D.J.; Jiang, C.J. Novel insight into the role of withering process in characteristic flavor formation of teas using transcriptome analysis and metabolite profiling. Food Chem. 2019, 272, 313–322. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Zhu, Y.W.; Wang, W.L.; Zhou, X.R.; Chen, G.L.; Liu, Y. Seven novel umami peptides from Takifugu rubripes and their taste characteristics. Food Chem. 2020, 330, 127204. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, S.; Kong, X.; Ji, Z.; Han, X.; Wu, J.; Mao, J. Elucidation of the aroma compositions of Zhenjiang aromatic vinegar using comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry and gas chromatography- olfactometry. J. Chromatogr. A 2017, 1487, 218–226. [Google Scholar] [CrossRef]
- Wu, Z.; Tu, M.; Yang, X.; Xu, J.; Yu, Z. Effect of cutting and storage temperature on sucrose and organic acids metabolism in post-harvest melon fruit. Postharvest Biol. Technol. 2020, 161, 111081. [Google Scholar] [CrossRef]
- Liu, N.; Pan, J.H.; Miao, S.; Qin, L.K. Microbial community in Chinese traditional fermented acid rice soup (rice-acid) and its correlations with key organic acids and volatile compounds. Food Res. Int. 2020, 137, 109672. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.J.; Lu, Z.M.; Yu, N.H.; Xu, W.; Li, G.Q.; Shi, J.S.; Xu, Z.H. HS-SPME/GC-MS and chemometrics for volatile composition of Chinese traditional aromatic vinegar in the Zhenjiang region. J. Inst. Brew. 2012, 118, 133–141. [Google Scholar] [CrossRef]
- Xu, S.; Ma, Z.; Chen, Y.; Li, J.; Jiang, H.; Qu, T.; Zhang, W.; Li, C.; Liu, S. Characterization of the flavor and nutritional value of coconut water vinegar based on metabolomics. Food Chem. 2022, 369, 130872. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.X.; Ye, S.L.; Huang, X.L.; Li, C.G.; Feng, Y.Q.; Min, X.; Yu, Q.; Zhang, X.L.; Wang, Q. Fermentation with a multi-strain to enhance the flavor of HongJun Tofu, a Chinese fermented okara food. LWT Food Sci. Technol. 2023, 189, 115495. [Google Scholar] [CrossRef]
- Kim, K.N.; Chun, I.J.; Suh, J.H.; Sung, J.H. Relationships between sensory properties and metabolomic profiles of different apple cultivars. Food Chem. X 2023, 18, 100641. [Google Scholar] [CrossRef]
- Ishita, P.; Pratap, B.S.B.; Adinpunya, M. Seasonal and diel variations in scent composition of ephemeral Murraya paniculata (Linn.) Jack flowers are contributed by separate volatile components. Biochem. Syst. Ecol. 2020, 89, 104004. [Google Scholar] [CrossRef]
- Wu, J.H.; Li, Y.; Zhao, H.; Huang, M.Q.; Sun, Y.; Zhang, J.L.; Sun, B.J. Recent advances in the understanding of off-flavors in alcoholic beverages: Generation, regulation, and challenges. J. Food Compos. Anal. 2021, 103, 104117. [Google Scholar] [CrossRef]
- Lu, Y.H.; Chi, Y.L.; Lv, Y.P.; Yang, G.H.; He, Q. Evolution of the volatile flavor compounds of Chinese horse bean-chili-paste. LWT Food Sci. Technol. 2019, 102, 131–135. [Google Scholar] [CrossRef]
- Chen, Y.W.; Jiang, J.Q.; Li, Y.K.; Xie, Y.; Cui, M.; Hu, Y.; Yin, R.; Ma, X.M.; Niu, J.M.; Cheng, W.D.; et al. Enhancing physicochemical properties, organic acids, antioxidant capacity, amino acids and volatile compounds for ‘Summer Black’ grape juice by lactic acid bacteria fermentation. LWT Food Sci. Technol. 2024, 209, 116791. [Google Scholar] [CrossRef]
- Akkad, R.; Buchko, A.; Johnston, S.P.; Han, J.; House, J.D.; Curtis, J.M. Sprouting improves the flavour quality of faba bean flours. Food Chem. 2021, 364, 130355. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ma, X.R.; Wang, W.P.; Xu, D.D.; Zhang, X.; Zhang, J.; Sun, Y. Characterization of flavor fingerprinting of red sufu during fermentation and the comparison of volatiles of typical products. Food Sci. Hum. Wellness 2019, 8, 375–384. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).