Comparison of Lactic Acid Production from Different Agro-Industrial Waste Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Pretreatment
2.2. Microorganisms and Culture Conditions
2.3. Waste Substrate Fermentation
2.4. Analysis
2.5. Mathematical Modeling
3. Results and Discussion
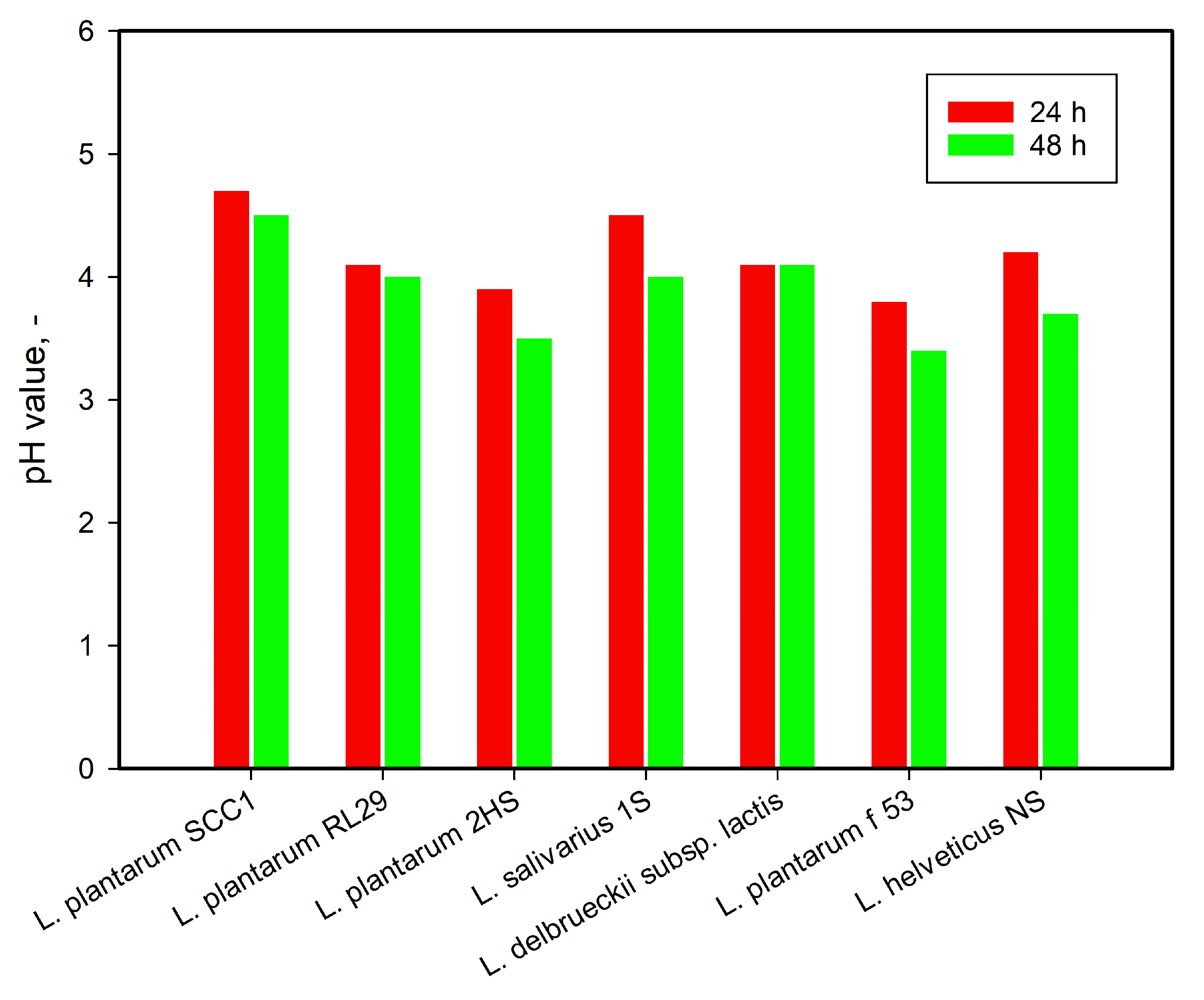
3.1. Strain Selection and Identification
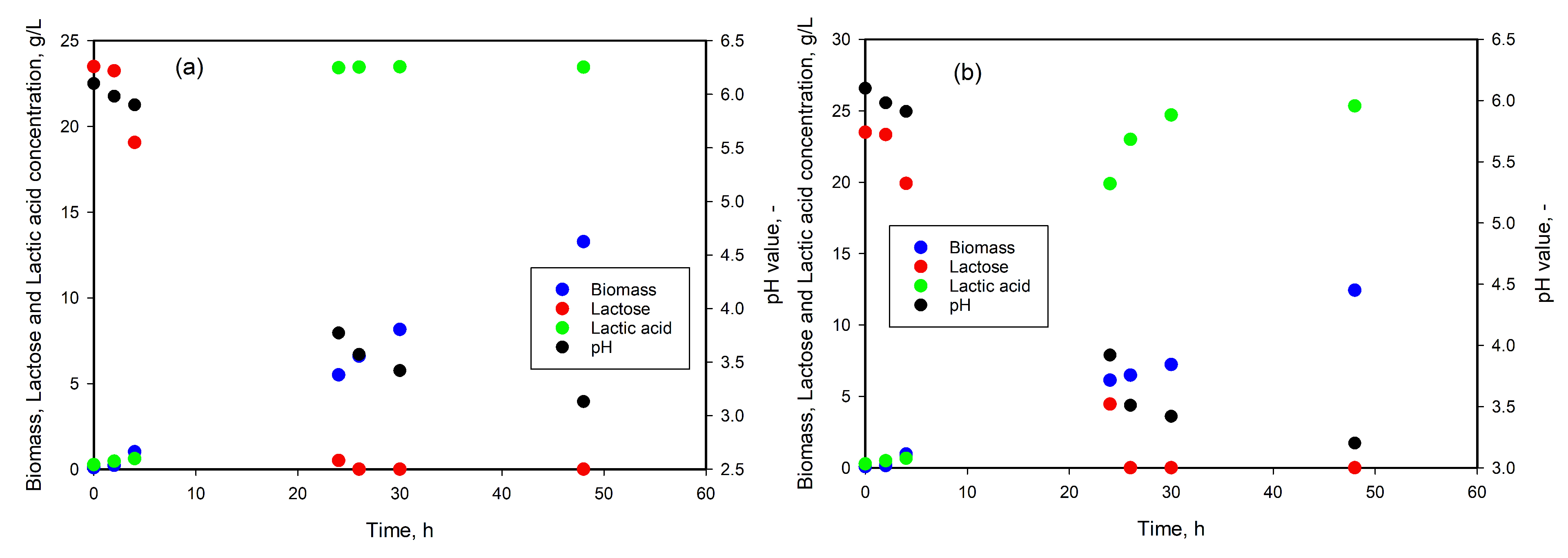
3.2. LA Production from Lactose
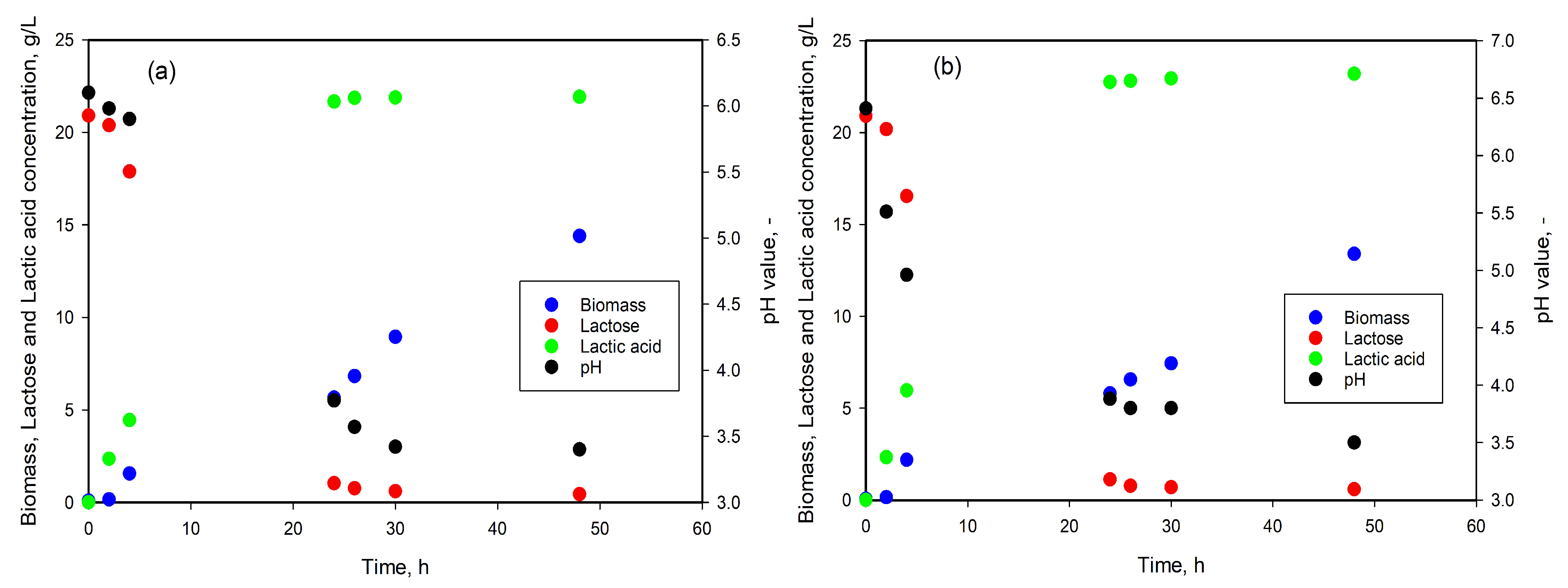
3.3. LA Production from DDGS Hydrolysates
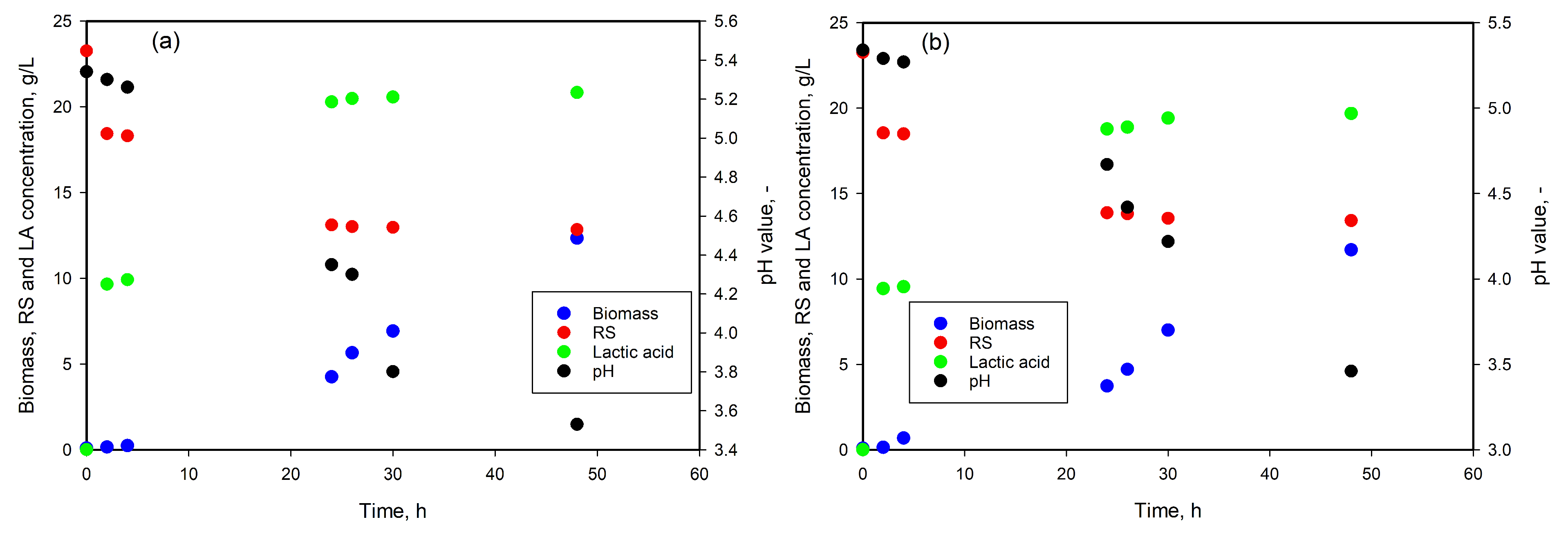
3.4. LA Production from SCG Hydrolysates
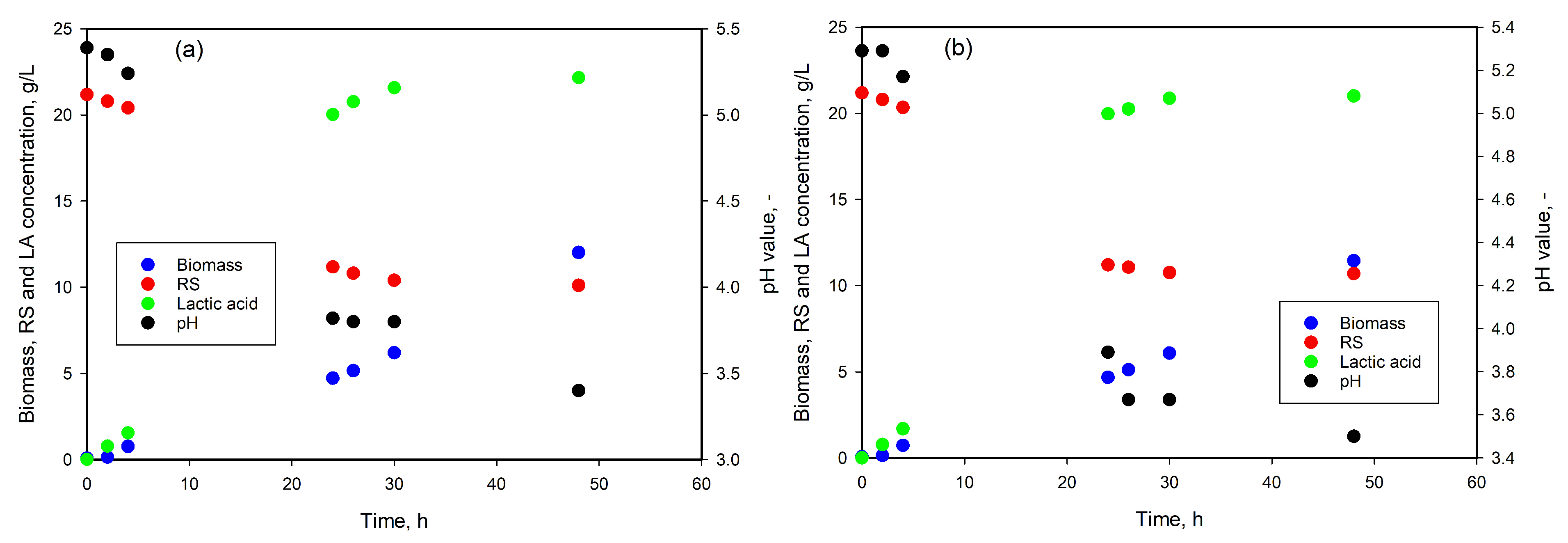
3.5. LA Production from Whey
3.6. LA Production from Wood Hydrolysate
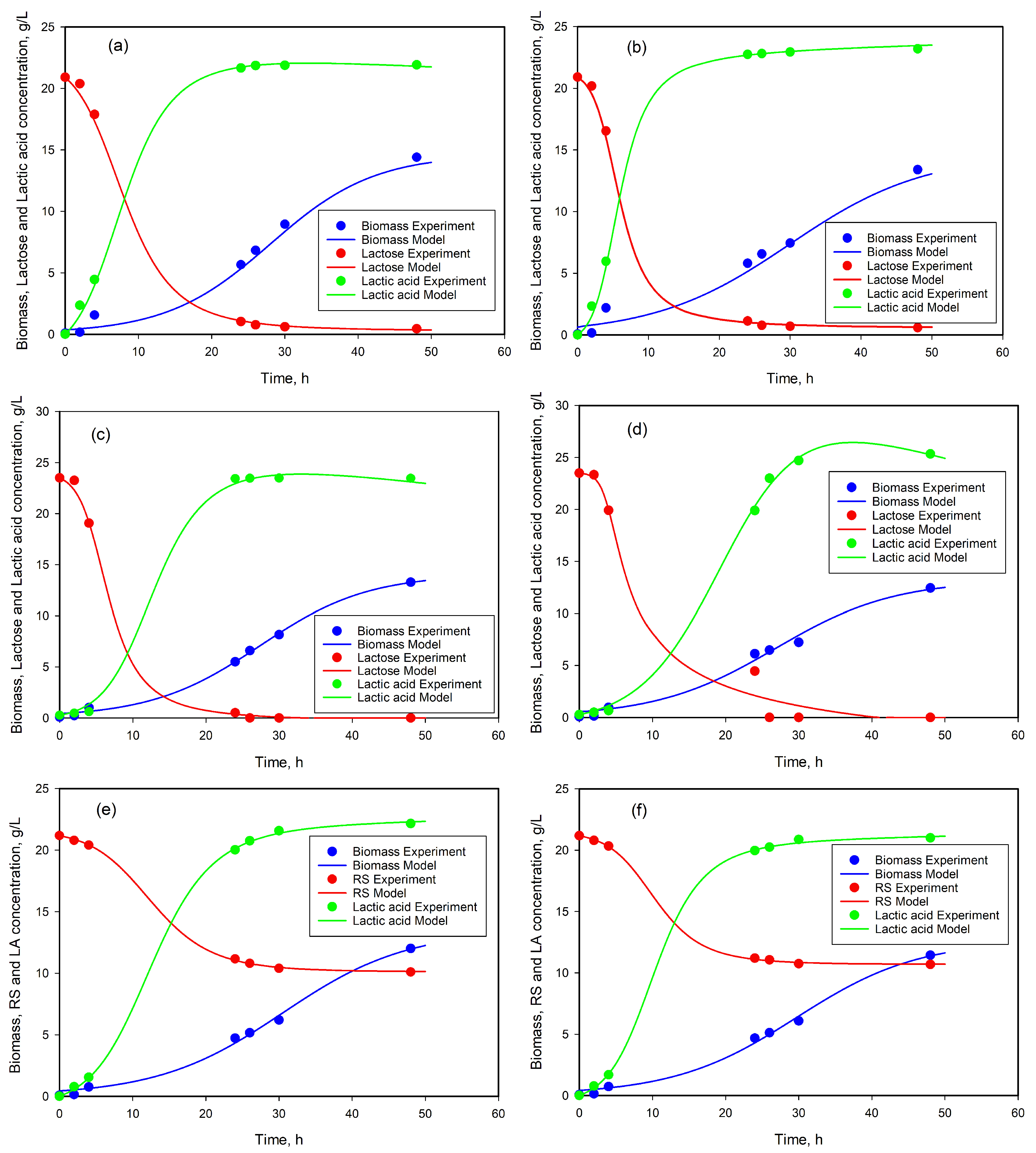
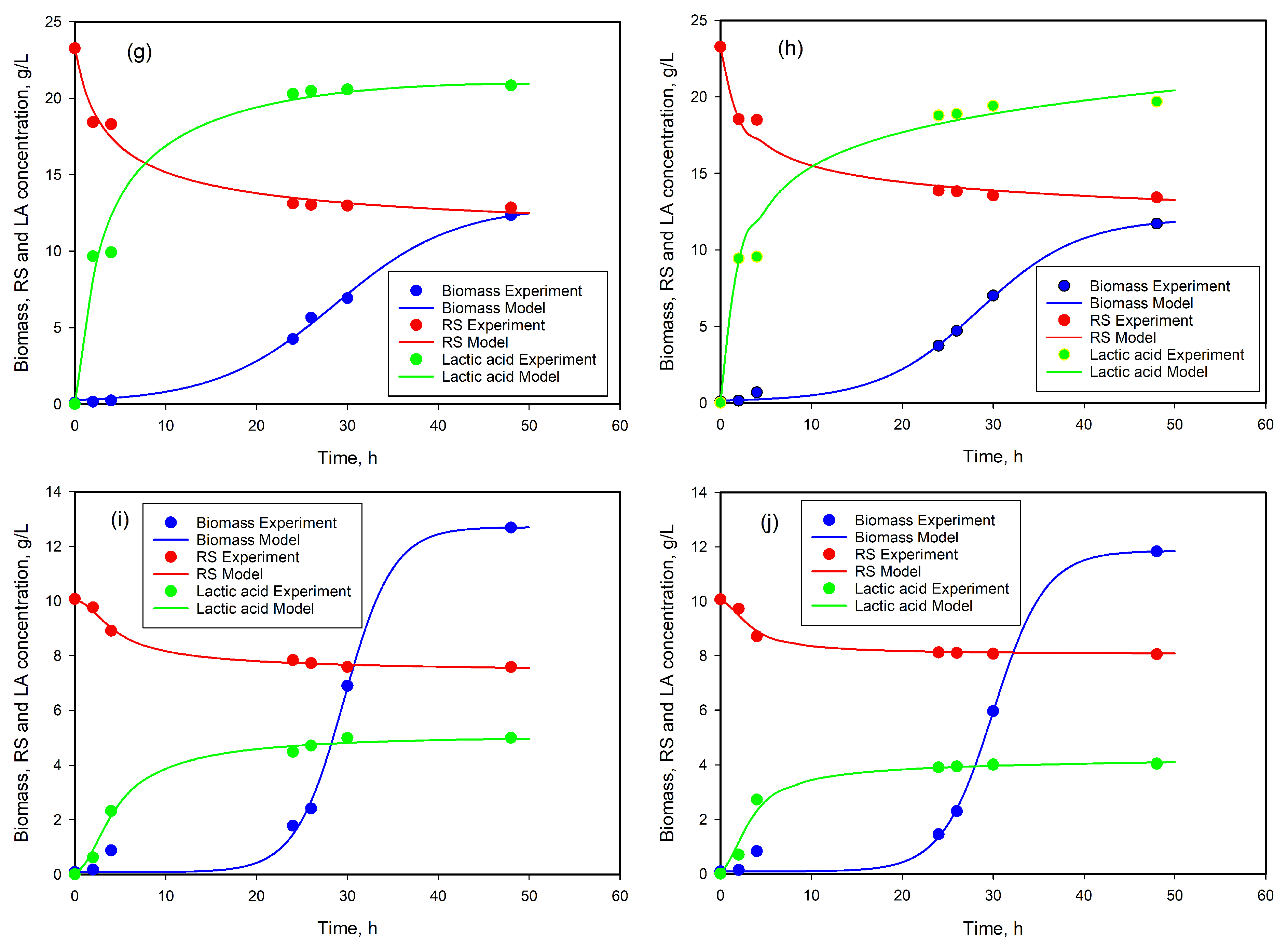
3.7. Modeling of Cell Growth, Substrate Consumption, and Product Accumulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swetha, T.A.; Ananthi, V.; Bora, A.; Sengottuvelan, N.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A Review on Biodegradable Polylactic Acid (PLA) Production from Fermentative Food Waste-Its Applications and Degradation. Int. J. Biol. Macromol. 2023, 234, 123703. [Google Scholar] [CrossRef]
- Naser, A.Z.; Deiab, I.; Darras, B.M. Poly(Lactic Acid) (PLA) and Polyhydroxyalkanoates (PHAs), Green Alternatives to Petroleum-Based Plastics: A Review. RSC Adv. 2021, 11, 17151–17196. [Google Scholar] [CrossRef]
- Adsul, M.G.; Varma, A.J.; Gokhale, D.V. Lactic Acid Production from Waste Sugarcane Bagasse Derived Cellulose. Green Chem. 2007, 9, 58–62. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Shang, N.; Li, P. Microbial Fermentation Processes of Lactic Acid: Challenges, Solutions, and Future Prospects. Foods 2023, 12, 2311. [Google Scholar] [CrossRef]
- Nwamba, M.C.; Song, G.; Sun, F.; Mukasekuru, M.R.; Ren, H.; Zhang, Q.; Cao, T.; Wang, H.; Sun, H.; Hong, J. Efficiency Enhancement of a New Cellulase Cocktail at Low Enzyme Loading for High Solid Digestion of Alkali Catalyzed Atmospheric Glycerol Organosolvent Pre-Treated Sugarcane Bagasse. Bioresour. Technol. 2021, 338, 125505. [Google Scholar] [CrossRef]
- Nwamba, M.C.; Sun, F.; Mukasekuru, M.R.; Song, G.; Harindintwali, J.D.; Boyi, S.A.; Sun, H. Trends and Hassles in the Microbial Production of Lactic Acid from Lignocellulosic Biomass. Environ. Technol. Innov. 2021, 21, 101337. [Google Scholar] [CrossRef]
- Rombouts, J.L.; Kranendonk, E.M.M.; Regueira, A.; Weissbrodt, D.G.; Kleerebezem, R.; Van Loosdrecht, M.C.M. Selecting for Lactic Acid Producing and Utilising Bacteria in Anaerobic Enrichment Cultures. Biotechnol. Bioeng. 2020, 117, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Long, S.; Tang, X.; Sun, Y.; Luque, R.; Zeng, X.; Lin, L. Earth-Abundant 3d-Transition-Metal Catalysts for Lignocellulosic Biomass Conversion. Chem. Soc. Rev. 2021, 50, 6042–6093. [Google Scholar] [CrossRef]
- 2025 Ethanol Industry Outlook. Available online: https://ethanolrfa.org/file/2912/2025%20Outlook.pdf (accessed on 1 April 2025).
- Naydenova, G.; Popova-Krumova, P.; Danova, S.; Yankov, D. Lactic Acid Production from Distiller’s Dried Grains Dilute Acid Hydrolysates. Fermentation 2024, 10, 581. [Google Scholar] [CrossRef]
- Iram, A.; Cekmecelioglu, D.; Demirci, A. Distillers’ Dried Grains with Solubles (DDGS) and Its Potential as Fermentation Feedstock. Appl. Microbiol. Biotechnol. 2020, 104, 6115–6128. [Google Scholar] [CrossRef]
- Lamsal, B.P.; Pathirapong, P.; Rakshit, S. Microbial Growth and Modification of Corn Distillers Dried Grains with Solubles during Fermentation. Ind. Crops Prod. 2012, 37, 553–559. [Google Scholar] [CrossRef]
- Murthy, P.S.; Madhava Naidu, M. Sustainable Management of Coffee Industry By-Products and Value Addition—A Review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Bhutada, G. Visualizing Global Coffee Production in 2024. Available online: https://www.visualcapitalist.com/visualizing-global-coffee-production-in-2024/ (accessed on 1 April 2025).
- Alves, R.C.; Rodrigues, F.; Antónia Nunes, M.; Vinha, A.F.; Oliveira, M.B.P.P. State of the Art in Coffee Processing By-Products. In Handbook of Coffee Processing By-Products; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–26. ISBN 978-0-12-811290-8. [Google Scholar]
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, Composition, and Application of Coffee and Its Industrial Residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Bomfim, A.; Oliveira, D.; Voorwald, H.; Benini, K.; Dumont, M.-J.; Rodrigue, D. Valorization of Spent Coffee Grounds as Precursors for Biopolymers and Composite Production. Polymers 2022, 14, 437. [Google Scholar] [CrossRef]
- Montemurro, M.; Casertano, M.; Vilas-Franquesa, A.; Rizzello, C.G.; Fogliano, V. Exploitation of Spent Coffee Ground (SCG) as a Source of Functional Compounds and Growth Substrate for Probiotic Lactic Acid Bacteria. LWT 2024, 198, 115974. [Google Scholar] [CrossRef]
- Cheese|USDA Foreign Agricultural Service. Available online: https://www.fas.usda.gov/data/production/commodity/0240000 (accessed on 30 April 2025).
- Malos, I.G.; Ghizdareanu, A.-I.; Vidu, L.; Matei, C.B.; Pasarin, D. The Role of Whey in Functional Microorganism Growth and Metabolite Generation: A Biotechnological Perspective. Foods 2025, 14, 1488. [Google Scholar] [CrossRef]
- Panesar, P.; Kennedy, J.; Gandhi, D.; Bunko, K. Bioutilisation of Whey for Lactic Acid Production. Food Chem. 2007, 105, 1–14. [Google Scholar] [CrossRef]
- Global Wood Pulp Production 2022|Statista. Available online: https://www.statista.com/statistics/240570/consumption-and-production-of-fibrous-material-worldwide/ (accessed on 2 May 2025).
- Mayo, B.; Flórez, A.B. Lactic Acid Bacteria: Lactobacillus Plantarum. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2022; pp. 206–217. ISBN 978-0-12-818767-8. [Google Scholar]
- BDS ISO 7169:1989; Products from Processed Fruits and Vegetables. Determination of Sugar’s Content. Bulgarian Institute for Standardization: Sofia, Bulgaria, 2025.
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Van ’T Riet, K. Modeling of the Bacterial Growth Curve. Appl. Env. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Luedeking, R.; Piret, E.L. A Kinetic Study of the Lactic Acid Fermentation. Batch Process at Controlled pH. J. Biochem. Microbiol. Technol. Eng. 1959, 1, 393–412. [Google Scholar] [CrossRef]
- Zaini, N.A.M.; Charalampopoulos, D.; Chatzifragkou, A.A. Lactic Acid Production from Dried Distiller’s Grains with Solubles Hydrolysate via Co-Fermentation of Lactobacillus Pentosus and Lactobacillus Coryniformis. Malays. Appl. Biol. 2018, 47, 173–179. [Google Scholar]
- Zaini, N.A.B.M.; Chatzifragkou, A.; Charalampopoulos, D. Microbial Production of D-lactic Acid from Dried Distiller’s Grains with Solubles. Eng. Life Sci. 2019, 19, 21–30. [Google Scholar] [CrossRef]
- Wang, C.; Su, W.; Zhang, Y.; Hao, L.; Wang, F.; Lu, Z.; Zhao, J.; Liu, X.; Wang, Y. Solid-State Fermentation of Distilled Dried Grain with Solubles with Probiotics for Degrading Lignocellulose and Upgrading Nutrient Utilization. AMB Express 2018, 8, 188. [Google Scholar] [CrossRef]
- Hudeckova, H.; Neureiter, M.; Obruca, S.; Frühauf, S.; Marova, I. Biotechnological Conversion of Spent Coffee Grounds into Lactic Acid. Lett. Appl. Microbiol. 2018, 66, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.-C.; Kim, Y.Y.; Yang, J.E.; Lee, H.M.; Hwang, I.M.; Park, H.W.; Kim, H.M. Utilization of Coffee Waste as a Sustainable Feedstock for High-Yield Lactic Acid Production through Microbial Fermentation. Sci. Total Environ. 2024, 912, 169521. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Jang, Y.W.; Lee, J.; Kim, S.; Park, C.; Yoo, H.Y. Statistical Optimization of Alkali Pretreatment to Improve Sugars Recovery from Spent Coffee Grounds and Utilization in Lactic Acid Fermentation. Processes 2021, 9, 494. [Google Scholar] [CrossRef]
- Kopp, D.; Willows, R.D.; Sunna, A. Cell-Free Enzymatic Conversion of Spent Coffee Grounds Into the Platform Chemical Lactic Acid. Front. Bioeng. Biotechnol. 2019, 7, 389. [Google Scholar] [CrossRef]
- Turner, T.L.; Kim, E.; Hwang, C.; Zhang, G.-C.; Liu, J.-J.; Jin, Y.-S. Conversion of Lactose and Whey into Lactic Acid by Engineered Yeast. J. Dairy Sci. 2017, 100, 124–128. [Google Scholar] [CrossRef]
- Alonso, S.; Herrero, M.; Rendueles, M.; Díaz, M. Residual Yoghurt Whey for Lactic Acid Production. Biomass Bioenergy 2010, 34, 931–938. [Google Scholar] [CrossRef]
- Taleghani, H.G.; Najafpour, G.D.; Ghoreyshi, A.A. A Study on the Effect of Parameters on Lactic Acid Production from Whey. Pol. J. Chem. Technol. 2016, 18, 58–63. [Google Scholar] [CrossRef]
- Panesar, P.S.; Kennedy, J.F.; Knill, C.J.; Kosseva, M. Production of L(+) Lactic Acid Using Lactobacillus casei from Whey. Braz. Arch. Biol. Technol. 2010, 53, 219–226. [Google Scholar] [CrossRef]
- Buyondo, J.P.; Liu, S. Lactic Acid Production by Lactobacillus Pentosus from Wood Extract Hydrolysates. J. Sci. Technol. For. Prod. Process. 2011, 1, 38–47. [Google Scholar]
- Kuo, Y.-C.; Yuan, S.-F.; Wang, C.-A.; Huang, Y.-J.; Guo, G.-L.; Hwang, W.-S. Production of Optically Pure l -Lactic Acid from Lignocellulosic Hydrolysate by Using a Newly Isolated and d -Lactate Dehydrogenase Gene-Deficient Lactobacillus Paracasei Strain. Bioresour. Technol. 2015, 198, 651–657. [Google Scholar] [CrossRef]
- Yuan, S.-F.; Hsu, T.-C.; Wang, C.-A.; Jang, M.-F.; Kuo, Y.-C.; Alper, H.S.; Guo, G.-L.; Hwang, W.-S. Production of Optically Pure l(+)-Lactic Acid from Waste Plywood Chips Using an Isolated Thermotolerant Enterococcus Faecalis SI at a Pilot Scale. J. Ind. Microbiol. Biotechnol. 2018, 45, 961–970. [Google Scholar] [CrossRef]
- Eom, I.-Y.; Oh, Y.-H.; Park, S.J.; Lee, S.-H.; Yu, J.-H. Fermentative L-Lactic Acid Production from Pretreated Whole Slurry of Oil Palm Trunk Treated by Hydrothermolysis and Subsequent Enzymatic Hydrolysis. Bioresour. Technol. 2015, 185, 143–149. [Google Scholar] [CrossRef]
- Niemelä, K.; Tamminen, T.; Ohra-aho, T. Black Liquor Components as Potential Raw Materials. In Proceedings of the 14th International Symposium on Wood, Fibre, and Pulping Chemistry, Durban, South Africa, 25–28 June 2007. [Google Scholar]
- Pola, L.; Collado, S.; Oulego, P.; Díaz, M. Kraft Black Liquor as a Renewable Source of Value-Added Chemicals. Chem. Eng. J. 2022, 448, 137728. [Google Scholar] [CrossRef]
- Morya, R.; Kumar, M.; Tyagi, I.; Kumar Pandey, A.; Park, J.; Raj, T.; Sirohi, R.; Kumar, V.; Kim, S.-H. Recent Advances in Black Liquor Valorization. Bioresour. Technol. 2022, 350, 126916. [Google Scholar] [CrossRef]
- Brown, D.M.; Grunden, A.M.; Pawlak, J.J. Statistical Optimization of Black Liquor-Containing Media for Growth and Lactic Acid Production by Paenibacillus Glucanolyticus SLM1. Bioresour. Technol. Rep. 2021, 13, 100629. [Google Scholar] [CrossRef]
- Lagarias, J.C.; Reeds, J.A.; Wright, M.H.; Wright, P.E. Convergence Properties of the Nelder--Mead Simplex Method in Low Dimensions. SIAM J. Optim. 1998, 9, 112–147. [Google Scholar] [CrossRef]
- Popova-Krumova, P.; Danova, S.; Atanasova, N.; Yankov, D. Lactic Acid Production by Lactiplantibacillus Plantarum AC 11S—Kinetics and Modeling. Microorganisms 2024, 12, 739. [Google Scholar] [CrossRef] [PubMed]
- Kostov, G.; Angelov, M.; Denkova, Z.; Dobrev, I.; Goranov, B. Lactic Acid Production with Lactobacillus casei ssp. Rhamnosus NBIMCC 1013: Modeling and Optimization of the Nutrient Medium. Eng. Life Sci. 2011, 11, 517–527. [Google Scholar] [CrossRef]
- Fu, W.; Mathews, A.P. Lactic Acid Production from Lactose by Lactobacillus Plantarum: Kinetic Model and Effects of pH, Substrate, and Oxygen. Biochem. Eng. J. 1999, 3, 163–170. [Google Scholar] [CrossRef]
- Biazar, J.; Tango, M.; Babolian, E.; Islam, R. Solution of the Kinetic Modeling of Lactic Acid Fermentation Using Adomian Decomposition Method. Appl. Math. Comput. 2003, 144, 433–439. [Google Scholar] [CrossRef]
- Amrane, A.; Prigent, Y. Mathematical Model for Lactic Acid Production from Lactose in Batch Culture: Model Development and Simulation. J. Chem. Technol. Biotechnol. 1994, 60, 241–246. [Google Scholar] [CrossRef]
- Altıok, D.; Tokatlı, F.; Harsa, Ş. Kinetic Modelling of Lactic Acid Production from Whey by Lactobacillus casei (NRRL B-441). J. Chem. Technol. Biotechnol. 2006, 81, 1190–1197. [Google Scholar] [CrossRef]
- Balannec, B.; Bouguettoucha, A.; Amrane, A. Unstructured Model for Batch Cultures without pH Control of Lactobacillus Helveticus—Inhibitory Effect of the Undissociated Lactic Acid. Biochem. Eng. J. 2007, 35, 289–294. [Google Scholar] [CrossRef]
- Naomi David, A.; Sewsynker-Sukai, Y.; Gueguim Kana, E.B. Co-Valorization of Corn Cobs and Dairy Wastewater for Simultaneous Saccharification and Lactic Acid Production: Process Optimization and Kinetic Assessment. Bioresour. Technol. 2022, 348, 126815. [Google Scholar] [CrossRef] [PubMed]
| Component | Percentage | Method | |
|---|---|---|---|
| DDGS | SCG | ||
| Moisture | 5.4 ± 0.4 | 6.7 ± 0.3 | LAP-001 |
| Total solids | 94.6 ± 0.4 | 93.3 ± 0.3 | LAP-001 |
| Total carbohydrates | 49.2 ± 1.6 | 52.9 ± 1.6 | LAP-002 |
| Lignin | 30.5 ± 0.4 | 31.5 ± 0.6 | LAP-003 |
| Extractives | 20.3 ± 1.2 | 15.6 ± 1.0 | LAP-010 |
| Ash | 4.7 ± 0.5 | 1.4 ± 0.2 | LAP-005 |
| No | Strains | Collection | Origin | Media and T °C |
|---|---|---|---|---|
| 1 | Lactiplantibacillus plantarum SCC1 | IMicB | Artisanal white brained cheese | MRS at 37 °C |
| 2 | Lactiplantibacillus plantarum RL29 | IMicB/ | Artisanal white brained cheese | MRS at 37 °C |
| 3 | Lactiplantibacillus plantarum 2HS | IMicB | Human origin | MRS at 37 °C |
| 4 | Ligilactobacillus salivarius 1S | IMicB/ | Human origin | MRS at 37 °C |
| 5 | Lactiplantibacillus plantarum f53 | IMicB | Human origin | MRS at 37 °C |
| 6 | Lactobacillus delbrueckii subsp. lactis | IMicB | Home-made yogurt | MRS at 37 °C |
| 7 | Lactobacillus helveticus NS | IMicB | Home-made yogurt | MRS at 37 °C |
| Strain | Model Parameters | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µmax | A | λ | YX/S | YP/S | α | β | Q1 | Q2 | R12 | R22 | ||
| L. plantarum f53 | 1 | 0.513 | 14.531 | 13.866 | 0.276 | 2.573 | 7.022 | 0.009 | 0.1895 | 0.2837 | 0.9950 | 0.9986 |
| 2 | 0.472 | 13.985 | 12.569 | 0.156 | 2.361 | 5.142 | 0.010 | 0.0696 | 0.5467 | 0.9984 | 0.9984 | |
| 3 | 0.394 | 13.427 | 13.492 | 0.157 | 5.407 | 12.564 | 0.009 | 0.0309 | 0.0219 | 0.9885 | 0.9998 | |
| 4 | 1.174 | 12.623 | 24.249 | 0.580 | 4.493 | 3.470 | 0.029 | 0.1013 | 0.0335 | 0.9914 | 0.9986 | |
| 5 | 0.490 | 12.864 | 15.504 | 0.313 | 2.312 | 7.156 | 0.008 | 0.1231 | 1.2410 | 0.9950 | 0.9861 | |
| L. plantarum 2HS | 1 | 0.383 | 14.583 | 10.922 | 0.250 | 2.393 | 7.025 | 0.011 | 0.215 | 0.2347 | 0.9973 | 0.9989 |
| 2 | 0.405 | 13.129 | 10.580 | 0.455 | 3.541 | 7.701 | 0.018 | 0.1505 | 0.9457 | 0.9989 | 0.9920 | |
| 3 | 0.376 | 12.590 | 12.975 | 0.191 | 4.905 | 10.349 | 0.006 | 0.1326 | 0.0212 | 0.9912 | 0.9998 | |
| 4 | 1.033 | 11.769 | 24.332 | 0.349 | 4.731 | 5.712 | 0.016 | 0.0421 | 0.0706 | 0.9866 | 0.9963 | |
| 5 | 0.544 | 11.978 | 17.448 | 0.2488 | 1.122 | 7.992 | 0.006 | 0.0874 | 1.2025 | 0.9920 | 0.9844 | |
| Microorganism | Substrate | Growth Model | μmax (h−1) | Reference |
|---|---|---|---|---|
| L. casei | Lactose 20 g/L | 0.511 | [49] | |
| L. plantarum | Lactose 40 g/L | 0.364 (pH 6.0) | [50] | |
| L. helveticus | Lactose 50 g/L | 0.25 | [51] | |
| L. helveticus | Whey ultrafiltrate powder | 0.56 | [52] | |
| L. casei | Whey lactose | 0.265 | [53] | |
| L. helveticus | Whey lactose | 0.64 | [54] | |
| L. plantarum | Dairy wastewater | 0.35 | [55] | |
| L. plantarum | Lactose | 0.24 | [10] | |
| L. plantarum | DDGS | 0.23 | [10] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naydenova, G.; Dobreva, L.; Danova, S.; Popova-Krumova, P.; Yankov, D. Comparison of Lactic Acid Production from Different Agro-Industrial Waste Materials. Fermentation 2025, 11, 437. https://doi.org/10.3390/fermentation11080437
Naydenova G, Dobreva L, Danova S, Popova-Krumova P, Yankov D. Comparison of Lactic Acid Production from Different Agro-Industrial Waste Materials. Fermentation. 2025; 11(8):437. https://doi.org/10.3390/fermentation11080437
Chicago/Turabian StyleNaydenova, Greta, Lili Dobreva, Svetla Danova, Petya Popova-Krumova, and Dragomir Yankov. 2025. "Comparison of Lactic Acid Production from Different Agro-Industrial Waste Materials" Fermentation 11, no. 8: 437. https://doi.org/10.3390/fermentation11080437
APA StyleNaydenova, G., Dobreva, L., Danova, S., Popova-Krumova, P., & Yankov, D. (2025). Comparison of Lactic Acid Production from Different Agro-Industrial Waste Materials. Fermentation, 11(8), 437. https://doi.org/10.3390/fermentation11080437






