Abstract
Dehydration and storage conditions used to preserve dairy cultures in the industry may negatively impact their viability and functionality. This study investigated the effects of freeze-drying and storage on the metabolic activity of Lacticaseibacillus rhamnosus 73 (L73). The strain’s viability after freeze-drying and storage, its metabolic activity in cultured milk, and its performance as a ripening agent in miniature cheeses were evaluated. Neither the freeze-drying process nor the storage conditions negatively affected its viability, as L73 maintained its initially high levels (>10 log cfu mL−1) throughout the storage period. L73 improved the overall quality of the cheeses, as a reduction in hydrophobic peptides (i.e., potential bitter peptides) was evidenced in cheese manufactured with L73. Furthermore, L73 exhibited protective properties, as evidenced by the decreased availability of compounds that could be used as energy sources by adventitious microorganisms (e.g., galactose, hippuric acid) and the increased production of lactic acid in both cultured milk and cheese.
1. Introduction
Lactic acid bacteria (LAB) have an important function in fermented dairy products, especially contributing to their safety, texture, flavor, and functionality [1]. They are part of the natural microbiota of raw milk and the dairy environment. In cheese manufacture, LAB are added as starter cultures or as secondary cultures. In particular, the starters are needed when milk is subjected to thermal treatment [2]. The starters (e.g., Lactobacillus helveticus, Lactococcus lactis, and Streptococcus thermophilus) are added to acidify the milk or curd during the initial hours of cheesemaking, while the second group is used to enhance or preserve specific quality attributes of the cheese. The most common goals of adjunct cultures are to improve texture and flavor and to control the cheese microbiota.
The adjunct cultures usually include non-starter lactic acid bacteria (NSLAB), since many strains of NSLAB have shown great potential as ripening agents (e.g., source of key enzymes that contribute to the aging process). A large number of studies focused on NSLAB mesophilic lactobacilli strains, such as Lacticaseibacillus casei, Lacticaseibacillus paracasei, Lactiplantibacillus plantarum, and Lacticaseibacillus rhamnosus [3]. Several strains, primarily isolated from dairy ingredients and products (e.g., raw milk and raw-milk cheeses) [4], but also from non-dairy sources [5], have been selected as flavor-producing and protective cultures.
While the enzymatic profile of selected bacteria is a critical factor in identifying potential adjunct cultures, it is equally important to evaluate their survival throughout industrial processing. Thus, studies of survival following preservation treatments such as freeze-drying, spray-drying, or freezing are required to commercialize dairy cultures. Although the freeze-drying method has a high investment cost, it is the most widely used in the dairy culture industry because it has advantages over other conservation technologies [6]. The general procedure for this method consists of freezing the cells at −80 °C and subjecting them to partial dehydration at low pressure in two stages: first by water sublimation and then by water desorption [7]. The survival of microorganisms after this process depends on several factors, such as the initial cell concentration, the medium and growth conditions of the cells, the dehydration matrix, and the rehydration conditions, among others [8]. In particular, the medium in which cells are resuspended before the freeze-drying process is one of the most influential factors. Numerous studies have reported that incorporating cryoprotective agents into the drying medium enhances the survival rate of microorganisms after lyophilization [9,10,11]. The type and extent of protection are dependent on the cryoprotectant used [12]. Cryoprotectants are grouped in low (e.g., glucose, sucrose, trehalose, lactose, and mannose) and high molecular weight (e.g., maltodextrin, alginate, wheat protein, gelatin, xanthan gum) [9,10,11]. Low molecular weight cryoprotectants stabilize the phospholipid layer of the cell membrane, while high molecular weight compounds form a viscous coating on the cell surface, preventing ice crystal growth [13].
Lacticaseibacillus rhamnosus 73 (L73) is an autochthonous mesophilic bacterium isolated from cheese as an NSLAB [14] with great potential as a secondary culture in cheesemaking. L73 has been characterized in in vitro studies as a peptidolytic and flavor-producing microorganism, due to its high levels of peptidase activity [15], as well as transaminase and glutamate dehydrogenase activities [16]. Furthermore, in situ studies in cheese have confirmed its ability to enhance the ripening process. Despite the great potential of this strain, to date, there are no studies that address its ability to withstand freeze-drying and storage conditions. This work aimed to elucidate the influence of both freeze-drying and storage on the viability and metabolic activity of L73. In addition to assessing the strain’s viability after freeze-drying and storage, its activity in cultured milk and its performance as a ripening culture in cheese were also assessed.
2. Materials and Methods
2.1. Strain
Lacticaseibacillus rhamnosus 73 (L73) belongs to the INLAIN collection and was routinely stored at −80 °C in MRS medium (Biokar, Beauvais, France) containing 15% (v/v) glycerol. It was reactivated in MRS at 37 °C for 16 h under microaerophilic conditions and then reinoculated (2% v/v) in a culture medium based on cheese whey permeate (Section 2.2).
2.2. Biomass Production, Freeze-Drying, and Storage
L73 was grown in triplicate in an optimized medium containing cheese whey permeate (12.86 g L−1) (Variolac 850, Arla Foods Ingredients S.A., Buenos Aires, Argentina) and yeast extract (0.353 g L−1) (Biokar, Beauvais, France) according to Batistela et al. [15]. Briefly, the activated cells in MRS broth (at 37 °C for 18 h) were inoculated at 2% (v/v) and incubated at 37 °C for 24 h under controlled pH and constant stirring (200 rpm) in a Sartorius Biostat A plus equipment (Goettingen, Germany). The pH was automatically maintained at 6.5 with sterile 2.3 N NaOH. A sterile food-grade antifoam (Sendeco S.A., Buenos Aires, Argentina) was added at 0.1% (v/v). At the end of fermentation, the cells were harvested by centrifugation (13,000× g, 30 min, 4 °C) and washed three times with 50 mM potassium phosphate buffer (pH 7) and resuspended in a cryoprotecting solution containing 10% (w/v) maltodextrin (GLOBE®, Ingredion Argentina S.R.L., Buenos Aires, Argentina) and 3% (w/v) sucrose (Cicarelli, Santa Fe, Argentina).
Two milliliters of cell suspension were distributed in glass vials, frozen at −80 °C for 24 h, and then freeze-dried using a Christ Alpha 1-4 LD plus equipment (Osterode am Harz, Germany) according to Oddi et al. [10]. Afterward, the vials were vacuum-sealed with a rubber stopper and aluminium cap and stored at 5 °C for 6 months (m).
2.3. Analysis of the Freeze-Dried Samples
Plate counts in MRS medium were carried out immediately before freeze-drying and for the powders at 0, 2, 4, and 6 m of storage at 5 °C. The powders were rehydrated with sterile water to their original volume prior to lyophilization, and the results were expressed as colony-forming units (cfu) per milliliter of the rehydrated solution. The plates were incubated at 37 °C for 48 h under microaerophilic conditions.
2.4. Cultured Milk
The ability of L73 to grow in milk was evaluated in cultured milk (CM) made with L73 freeze-dried cells stored at 5 °C. Cultured milks CM 2m, CM 4m, and CM 6m were prepared using the powders stored at 5 °C for 2, 4, and 6 m, respectively. First, milk was prepared by reconstitution of skim milk powder in distilled water at 10% (w/v) and then sterilized by autoclaving at 115 °C for 30 min. Then, cell suspensions were obtained by rehydrating the freeze-dried cells contained in a vial with 2 mL of sterile water. Then, sterilized milk fractioned in 100-mL screw-cap bottles was inoculated with the cell suspensions at 0.2% (v/v) (~7.3 log cfu mL−1) and incubated in a water bath at 37 °C for 24 h. A non-inoculated milk (NIM) was incubated under the same conditions as the cultured milks as a control. The experiment was performed in triplicate using independent cultures. Microbiological counts were determined in samples CM 2m, CM 4m, and CM 6m, while the determination of carbohydrates and organic acids by high-performance liquid chromatography (HPLC) was carried out on the CM 6m sample, as described in Section 2.6.
2.5. Cheesemaking
The performance of L73 freeze-dried cells as a ripening agent was studied at the end of a 6-month storage period at 5 °C. In this experiment, L73 vials stored at −20 °C for 6 m were also used as a ripening agent. Three semi-hard cheeses were made per treatment using the following ripening agents: (i) non-ripening agent (NRA), (ii) L73 cells stored at −20 °C for 6 m (RA −20); and (iii) L73 cells stored at 5 °C for 6 m (RA 5). The cheeses were manufactured at a 1-L scale using bovine milk as per Peralta and Bergamini [17]. A flowchart of the cheesemaking process is shown in Figure 1. Briefly, milk was pasteurized at 63 °C for 30 min and then fractionated into a 1-L cheese vat (glass beakers). After supplementing milk with calcium chloride (0.02% w/v) and keeping milk at 38 °C, Streptococcus thermophilus ST12 (Chr. Hansen, Hørsholm, Denmark) was resuspended in milk, and then it was added to all the cheese vats to achieve 1 × 106 cfu mL−1. At that time, the ripening agents mentioned above were added to the cheese vat (at 1 × 106 cfu mL−1). After adding the ChyMax coagulant (Chr. Hansen, 74 IMCU mL−1), milk was held quiescently for approximately 45 min. The curd was then cut and reduced to the size of corn kernels. Keeping gently stirring, the mixture of grains and whey was heated from 38 °C to 45 °C at a rate of 1 °C/min. The cheese vat was gently tilted several times to drain most of the whey, and then the curd was molded into 100-mL perforated collectors. A weight was placed on the top of each of the molded cheeses, and they were acidified in a chamber at 45 °C to reach a pH of 5.3 and immediately submerged in cold sterile brine (20% w/v). Cheeses were stored at 12 °C for 5 days, coated with a yellow commercial paint coating, vacuum-packed in plastic bags, and then returned to the maturation chambers to complete a 120-day ripening period.
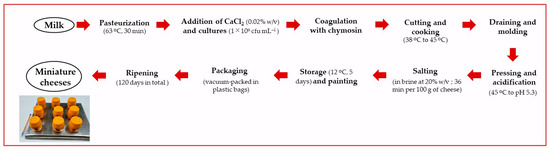
Figure 1.
Flowchart of the cheesemaking process.
2.6. Cheese Composition, pH Determination, and Microbiological Counts
Cheese samples were analyzed in duplicate at 120 days of maturation for moisture, fat, and protein using standardized methods [18,19,20]. The pH was assessed using a pH meter (Orion Research Incorporated, Boston, MA, USA) from a cheese suspension prepared by mixing 5 g of grated cheese with 5 mL of distilled water [21].
Microbiological counts were carried out in selective media according to Giménez et al. [22], total lactic bacteria (PCA milk, 37 °C, 48 h), mesophilic lactobacilli (MRS agar, 37 °C, 48 h), coliforms (VRBL agar, 32 °C, 24 h), yeasts and molds (YGC agar, 25 °C, 5 days) and enterococci (BEA agar, 37 °C, 48 h). All results were expressed as log colony-forming units (cfu) per gram of cheese. All media were purchased from Biokar (Beauvais, France).
2.7. Carbohydrates and Organic Acids of Cultured Milk and Cheese
The determination of carbohydrates (RI detector) and organic acids (UV detector, 210 nm) was performed by HPLC [23]. The HPLC system (Perkin Elmer, Norwalk, CT, USA) consisted of a quaternary pump, a vacuum degasser, a column oven (set at 65 °C), and UV/Vis (set at 210 nm) and RI (set at 37 °C) detectors. An Aminex HPX-87H column (300 mm × 7.8 mm) connected in line with a guard column (Aminex Cation-H, 30 × 4.6 mm) (Bio-Rad Laboratories, Hercules, CA, USA) was used. Chromatographic separation was carried out using an isocratic mobile phase of 0.01 M H2SO4 at a flow rate of 0.6 mL min−1. Results were expressed as milligrams of each compound per 100 g of cheese or milk, as applicable.
2.8. Peptide Profiles of the Soluble Fraction of Cheese
The peptide profiles were analyzed by reversed-phase HPLC as described in Milesi et al. [24]. Water-soluble extracts of the cheeses were prepared using an Ultraturrax® homogenizer (Model T25, IKA, Staufen, Germany) by mixing 5 g of cheese with 25 mL of water. The suspensions were treated at 40 °C for 60 min, centrifuged (10,000× g for 30 min), and the obtained supernatants were filtered through fast-flow filter paper and 0.45 μm membranes and stored at −20 °C until analysis. The same HPLC system detailed in Section 2.7 was used, but only the UV detector was set up at 214 nm, and the column oven was set at 40 °C. An Aquapore OD-300 C18 column (PerkinElmer, 220 mm × 4.6 mm, 7 μm pore size, 300 Å) was used. Separation was carried out at 1 mL min−1 using a linear gradient of increasing acetonitrile in water with trifluoroacetic acid. The area under the peptides in the chromatograms was integrated, and the results were expressed in arbitrary units.
2.9. Statistical Analyses
Data on viability in the freeze-drying process were analyzed by a T-test. Data on microbiological counts in the powders during storage (0, 2, 4, and 6 m) and in cultured milks were analyzed by one-way ANOVA followed by Fisher’s LSD test. A T-test was also used to analyze data on carbohydrates and organic acids in cultured milks. Cheese data of composition, pH, microbiological counts, carbohydrates, and organic acids were analyzed by one-way ANOVA followed by Fisher’s LSD test. All analyses of T-test and ANOVA were carried out in InfoStat version 12 software (InfoStat Group, National University of Córdoba, Argentina), and differences were considered significant at p < 0.05. Principal component analyses and hierarchical clustering heatmap of organic acids and peptide profiles data were performed using MetaboAnalyst 6.0 [25].
3. Results and Discussion
3.1. Microbiological Counts of Freeze-Dried Cultures
Viable counts of lactobacilli before (10.22 ± 0.07 log cfu mL−1) and after (10.14 ± 0.14 log cfu mL−1) freeze-drying did not show significant differences (p > 0.05). There were no significant differences (p > 0.05) among plate counts at 0, 2, 4, and 6 months of storage. Both results highlight the high capacity of L73 to withstand freeze-drying and storage at 5 °C under the conditions studied. The positive effect of freeze-drying on the viability and activity of lactic acid bacteria during storage has been well documented; indeed, it is the most widely used method to preserve lactic acid bacteria on an industrial scale [6]. Many parameters—including the growth medium, cell density, suspension medium, and freeze-drying conditions, among others—can affect a strain’s survival during the freeze-drying process [26,27].
One of the key components in the preservation of microorganisms is the type of cryoprotectant used as a carrier of bacteria during freeze-drying [8,28]. Several cryoprotectants, applied singly or combined, have been proposed to preserve the viability of microorganisms, including monosaccharides (e.g., glucose and fructose), disaccharides (e.g., lactose and sucrose), polysaccharides (e.g., maltodextrin), and other complex components (e.g., milk and whey proteins) [6]. In this study, the L73 cells were protected by an amorphous material containing a mixture of maltodextrin and sucrose. Maltodextrin 10% (w/v) was recently used to protect breast milk-derived bifidobacteria from spray-drying and along storage [29]. Sucrose, whether used alone or in combination with maltodextrin, also showed promising results as a cryoprotectant [30,31]. The combination of short- and long-chain carbohydrates has been widely reported as an effective strategy to enhance bacterial resistance to freeze-drying and storage [12,13,32,33,34]. Furthermore, the composition of the growth medium is crucial for protecting the cell membrane integrity in the subsequent processing. In this direction, it has been reported that the accumulation of compatible solutes into cells can increase cell resistance [33]. For example, Silva et al. [35] reported that, even though sucrose was not metabolized by Lactobacillus delbrueckii ssp. bulgaricus, its resistance to the spray-drying and storage process was enhanced because of the sucrose accumulation in the cells. It is worth noting that L73 was grown in a high-lactose level culture medium (~10 g 100 mL−1) previously optimized by Batistela et al. [15]. In this medium, the strain only partially metabolized lactose, leaving a high residual level (~7 g 100 mL−1). Therefore, it is highly probable that part of the residual lactose accumulates within the cells and concomitantly improves its resistance.
3.2. Growth and Activity in Cultured Milk
This study is the first report about L73’s growing capacity in milk. The freeze-dried cells successfully grew in cultured milk, reaching similar values to those observed in MRS. Regardless of the storage time, L73 level in the cultured milk was ~9 log cfu mL−1, and no significant differences were found among samples (i.e., CM 2m, CM 4m, and CM 6m). A similar concentration was reported for Lacticaseibacillus rhamnosus RO11 [36]. Even though L. rhamnosus strains may reach high levels; this outcome was not entirely expected, as this species usually exhibits slow growth in milk [37,38]. No growth was detected in the non-inoculated milk.
The results of carbohydrates and organic acids concentrations in the cultured milk inoculated with the cells stored for 6 m at 5 °C (CM 6m) and in the non-inoculated milk (NIM) are shown in Figure 2. Both lactose and galactose can be metabolized by L. rhamnosus [39]. The concentration of lactose in NIM and CM 6m was high (~5000 mg 100 mL−1), and only slight numerical differences between the cultured milk and the control were found (p > 0.05). The high level of lactose in fermented milks inoculated with probiotic bacteria (e.g., L. rhamnosus and bifidobacteria) is expected since they usually exhibit slow lactose metabolism capacity and low lactic acid production [37,38]. On the other hand, the concentration of galactose was significantly lower in CM 6m compared to NIM (p < 0.05), highlighting the ability of L73 to metabolize galactose. Low levels of galactose (<50 mg mL−1) were naturally present in control milk.
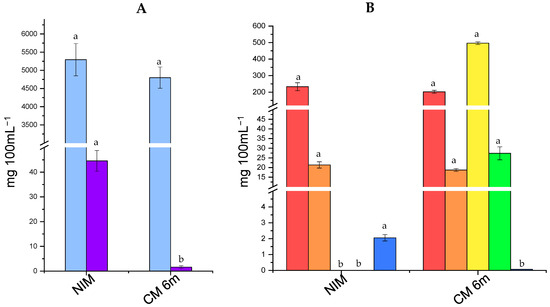
Figure 2.
Concentration of carbohydratese4 (A) and organic acids (B) in non-inoculated milk (NIM) and cultured milk inoculated with L73 freeze-dried cultures stored for 6 months (CM 6m). Lactose ( ), galactose (
), galactose ( ), citric acid (
), citric acid ( ), orotic acid (
), orotic acid ( ), lactic acid (
), lactic acid ( ), acetic acid (
), acetic acid ( ), and hippuric acid (
), and hippuric acid ( ). Different letters for the same compound indicate statistically significant differences (p > 0.05).
). Different letters for the same compound indicate statistically significant differences (p > 0.05).
 ), galactose (
), galactose ( ), citric acid (
), citric acid ( ), orotic acid (
), orotic acid ( ), lactic acid (
), lactic acid ( ), acetic acid (
), acetic acid ( ), and hippuric acid (
), and hippuric acid ( ). Different letters for the same compound indicate statistically significant differences (p > 0.05).
). Different letters for the same compound indicate statistically significant differences (p > 0.05).
The results for organic acids showed that neither citric acid nor orotic acid was metabolized by L73. Determining the organic acids naturally present in milk is important because some microorganisms can utilize them as an energy source for growth [40]. Furthermore, citrate metabolism is usually associated with the production of flavor compounds, particularly diacetyl, which is characterized by a butter-like aroma [41]. On the other hand, the concentration of lactic acid and acetic acid was significantly higher in CM 6m than in NIM. L. rhamnosus is a facultative heterofermentative microorganism [42], and it produces the L-lactic acid isomer [43]. The level of lactic acid was approximately 500 mg 100 mL−1, while the concentration of acetic acid was relatively low (~25 mg 100 mL−1). As expected, neither of the two acids was detected in NIM. Hippuric acid is another organic acid naturally present in milk [40]. Its concentration was lower in CM 6m in comparison to NIM, suggesting the ability of L73 to metabolize it. The ability to reduce energy substrates (i.e., galactose and hippuric acid) after the six-month storage, as well as its ability to produce antimicrobial components (i.e., lactic acid and acetic acid), reveals the potential of L73 as a protective culture in cheese [3].
3.3. Cheese Composition, pH Values, and Microbiological Counts
Results of composition, pH, and plate counts of cheese samples (NRA, RA -20, and RA 5) at the end of maturation are reported in Table 1. No significant differences (p > 0.05) were found among cheeses for moisture, fat, and protein content. On the contrary, both RA-20 and RA 5 presented lower (p < 0.05) pH values compared to NRA.

Table 1.
Mean (±SD) of composition and pH of cheeses at 120 days of maturation.
The results of chemical composition and pH are similar to those reported by Giménez et al. [22], who assessed the potential of 11 mesophilic lactobacilli strains, including L73, as secondary cultures in semi-hard cheese. Giménez et al. [22] used fresh L73 grown in MRS medium. In contrast, in the present study, the L73 cells were grown in a more cost-effective culture medium and subjected to a common preservation method usually applied at an industrial scale. Thus, neither freeze-drying nor storage affected the ability to reduce the cheese pH values. It is important to note that low pH values are not directly associated with defects in this type of cheese. For example, a recently published study [44] using the same cheese model, but applying Lactiplantibacillus plantarum 29 as secondary culture, reported the highest overall flavor scores, despite the low pH (4.8).
Results of plate counts of cheese samples are shown in Table 2. Although slight numerical differences in total LAB counts were observed between cheeses with and without the ripening agent, these differences were not statistically significant (p > 0.05). The levels of total LAB are in line with those reported by Giménez et al. [22] and Prada [44], who used the same cheese model. The population of the starter culture is generally associated with the total LAB count; however, it is not a selective medium for starter cultures. The dominant microbiota during the cheesemaking was Streptococcus thermophilus since it was added to the cheese milk at a high level (106 cfu mL−1). Within the first hours of manufacture, the starter culture population in the cheese matrix reaches approximately 109 cfu g−1 [45], as a result of both microbial proliferation and cell concentration in the curd after whey drainage. It is well established that bacteria in milk become physically entrapped within the curd matrix during coagulation, and only those located on the exposed cut surfaces are released into the whey [46].

Table 2.
Mean (±SD) of microbiological counts of cheeses at 120 days of maturation.
On the other hand, the NSLAB increases from low to a high level within approximately 3 months of ripening [5]. However, the levels of NSLAB depend on many factors, including milk microbiota, cheese making and ripening conditions, interactions with starter culture, and salt content, among others. The level of Lactobacillus grown in acidified MRS agar was relatively high (>7 log cfu g−1) in RA -20 and RA 5. The acidification of the MRS to 5.4 is used to inhibit the growth of Streptococcus thermophilus [47], allowing the estimation of lactobacilli level more accurately. Giménez et al. [22] also reported a high level of lactobacilli in cheeses manufactured with fresh L73. On the contrary, the Lactobacillus levels in NRA (i.e., NSLAB level) were not detected in the lowest dilution performed (<4 log cfu g−1). In addition, low levels of Enterococcus, molds and yeasts, and coliforms were also found in all cheeses. The low level of these microorganisms could be associated with the good microbiological quality of the raw milk and the excellent hygienic cheesemaking conditions. Although cheese models at laboratory scale represent the real matrix of cheese [48], they may not fully represent the hygienic conditions found in industrial settings.
3.4. Carbohydrates and Organic Acids in Cheese
Figure 3 shows the concentration of carbohydrates and organic acids in cheeses. The concentration of lactose and galactose in cheeses ranged between 200 and 600 mg 100 g−1 and 400 and 1000 mg 100 g−1, respectively. Statistical differences (p < 0.05) were observed for both sugars, which presented a lower concentration in RA -20 and RA 5 compared to NRA. On the other hand, the concentration of lactic acid was significantly higher in RA -20 and RA 5 (>2500 mg 100 g−1) than NRA (~1250 mg 100 g−1). During the first hours of cheesemaking, lactose is metabolized by the starter culture to lactic acid, contributing to developing the desired texture as well as acidity of the cheese [45]. Residual lactose and galactose may be metabolized by NSLAB or secondary cultures if they are added. In this context, the lower concentration of both lactose and galactose was attributed to the L73’s ability to metabolize this sugar in cheese [22]. The high level of galactose in NRA was expected as most S. thermophilus strains cannot use galactose as an energy source, and it is accumulated in the cheese during the ripening [45,49]. This accumulation can be detrimental to cheese quality since both residual lactose and galactose can be fermented by potential spoilage microorganisms [50]. For example, galactose-positive heterofermentative NSLAB (e.g., Lactobacillus wasatchensis) may cause a defect known as late gas formation in Cheddar cheese [51,52]. Therefore, the L73 capacity to metabolize galactose in the cheese environment highlights its potential to maintain cheese quality during ripening. Furthermore, the production of antimicrobial compounds such as lactic acid also contributes to its protective role. Interestingly, the incorporation of the ripening agents probably controlled or limited the growth of acetic acid-producing bacteria from the NSLAB group, since the level of acetic acid in NRA (~350 mg 100 g−1) was higher than the rest of the cheeses (~240 mg 100 g−1). It was not surprising that the levels of acetic acid in the miniature cheeses with the ripening agent did not increase, in contrast to the observations in cultured milk. Cheese is a more complex system in which matrix composition, microbiota, and long ripening times, among other factors, could have influenced the final concentration of this acid [53].
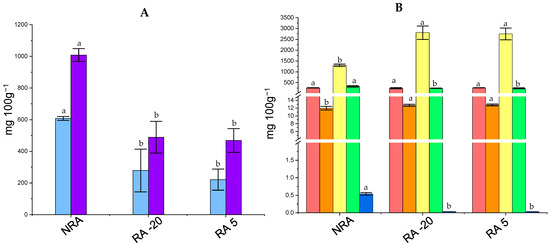
Figure 3.
Concentration of carbohydrates (A)
and organic acids (B) in cheeses ripened for 120 days. Lactose ( ), galactose (
), galactose ( ), citric acid (
), citric acid ( ), orotic acid (
), orotic acid ( ), lactic acid (
), lactic acid ( ), acetic acid (
), acetic acid ( ), and hippuric acid (
), and hippuric acid ( ). Abbreviations: NRA,
cheese with no ripening agent; RA -20, cheese manufactured with L73
freeze-dried cells stored at −20 °C for 6 months as a ripening agent; RA 5,
cheese manufactured with L73 freeze-dried cells stored at 5 °C for 6 months as
a ripening agent. Bars for the same compound with the same letter did not
differ significantly (p > 0.05).
). Abbreviations: NRA,
cheese with no ripening agent; RA -20, cheese manufactured with L73
freeze-dried cells stored at −20 °C for 6 months as a ripening agent; RA 5,
cheese manufactured with L73 freeze-dried cells stored at 5 °C for 6 months as
a ripening agent. Bars for the same compound with the same letter did not
differ significantly (p > 0.05).
 ), galactose (
), galactose ( ), citric acid (
), citric acid ( ), orotic acid (
), orotic acid ( ), lactic acid (
), lactic acid ( ), acetic acid (
), acetic acid ( ), and hippuric acid (
), and hippuric acid ( ). Abbreviations: NRA,
cheese with no ripening agent; RA -20, cheese manufactured with L73
freeze-dried cells stored at −20 °C for 6 months as a ripening agent; RA 5,
cheese manufactured with L73 freeze-dried cells stored at 5 °C for 6 months as
a ripening agent. Bars for the same compound with the same letter did not
differ significantly (p > 0.05).
). Abbreviations: NRA,
cheese with no ripening agent; RA -20, cheese manufactured with L73
freeze-dried cells stored at −20 °C for 6 months as a ripening agent; RA 5,
cheese manufactured with L73 freeze-dried cells stored at 5 °C for 6 months as
a ripening agent. Bars for the same compound with the same letter did not
differ significantly (p > 0.05).
No significant differences were found for citric acid among cheese samples, and its concentration was ~250 mg 100 g−1. The concentrations of orotic acid and hippuric acid were very low, and both showed significant differences between cheese with and without a ripening agent. These three organic acids are naturally present in milk [40] and could be metabolized by microorganisms during cheese ripening. Citrate-positive bacteria could produce some important compounds for cheese flavor (e.g., diacetyl) [41]. The inability to metabolize citric acid is consistent with the results observed in cultured milk and also with previous studies that addressed the use of fresh L73 cells as secondary culture in semi-hard cheese [22].
Finally, a principal component analysis (PCA) was used to map the relationships among cheese samples (Figure 4). The scores plot for the first two principal components (PC), which explained 93.2% of the total variance, revealed a clear separation between cheeses with and without the ripening agent. The three replicates of the NRA samples were grouped on the positive side of PC 1 and were associated with acetic acid, lactose, galactose, and hippuric acid, as can be seen in the loading plot. On the contrary, replicates of RA -20 and RA 5 were grouped on the negative side of PC1 and were associated with lactic acid and orotic acid.
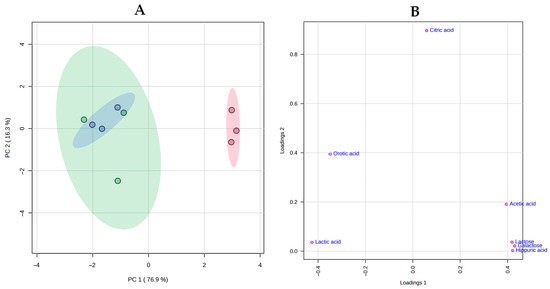
Figure 4.
Principal Component Analysis of the levels of carbohydrates and organic acids in cheeses. Scores plot (A)
and loading plot (B) of cheeses ripened for 120 days. Abbreviations: NRA
( ), cheese with no ripening agent; RA -20 (
), cheese with no ripening agent; RA -20 ( ), cheese
manufactured with L73 freeze-dried cells stored at −20 °C for 6 months as
ripening agent; RA 5 (
), cheese
manufactured with L73 freeze-dried cells stored at −20 °C for 6 months as
ripening agent; RA 5 ( ), cheese manufactured with L73 freeze-dried cells stored
at 5 °C for 6 months as ripening agent.
), cheese manufactured with L73 freeze-dried cells stored
at 5 °C for 6 months as ripening agent.
 ), cheese with no ripening agent; RA -20 (
), cheese with no ripening agent; RA -20 ( ), cheese
manufactured with L73 freeze-dried cells stored at −20 °C for 6 months as
ripening agent; RA 5 (
), cheese
manufactured with L73 freeze-dried cells stored at −20 °C for 6 months as
ripening agent; RA 5 ( ), cheese manufactured with L73 freeze-dried cells stored
at 5 °C for 6 months as ripening agent.
), cheese manufactured with L73 freeze-dried cells stored
at 5 °C for 6 months as ripening agent.
3.5. Peptide Profiling of Cheese
Chromatographic profiles of soluble peptides in cheeses are shown in Figure 5. The high number of peptides was expected as cheeses were ripened for a long period (120 days). There are some visual differences between cheeses, especially between samples with and without the ripening agent. The area of 46 peaks of peptide profiles was used to make a PCA (Figure 6). The first and second PCs accounted for 84.7% and 4.9% of the total variation in the dataset, respectively. The three replicates of the NRA samples were grouped on the negative side of PC1 and were associated with a mixture of late and early eluted peptides in the chromatographic runs. On the contrary, the replicates of both RA -20 and RA 5 were grouped on the positive side of PC1 and were only associated with early eluted peptides (2, 7, 11, 12, 14, and 15). Furthermore, a hierarchical clustering heatmap (Figure 7) was made using sugars, organic acids, and peptide profiles data. The up dendrogram depicts the separation of the NRA replicates from the rest of the cheeses, highlighting the effects of the adjunct culture on these variables.
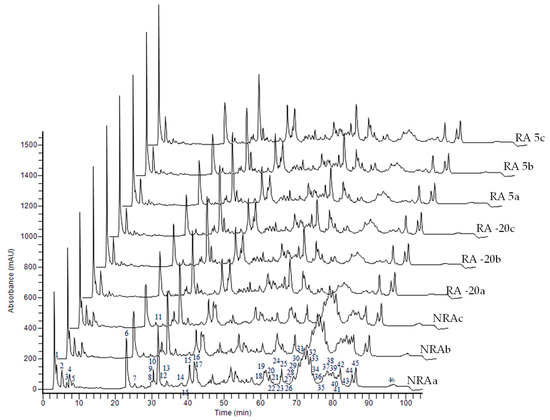
Figure 5.
Peptide profiling of cheeses. The numbers in the chromatogram indicate the peaks selected for the PCA. Abbreviations: NRA, cheese with no ripening agent; RA -20, cheese manufactured with L73 freeze-dried cells stored at −20 °C for 6 months as ripening agent; RA 5, cheese manufactured with L73 freeze-dried cells stored at 5 °C for 6 months as ripening agent. The letters a, b, and c in the code of cheeses indicate the independent trials.
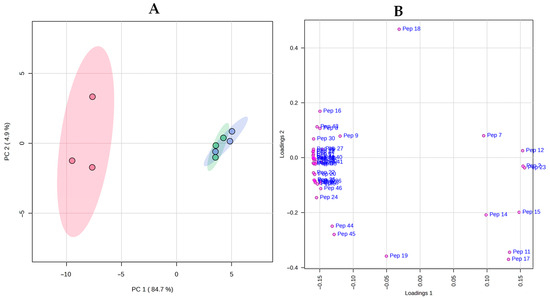
Figure 6.
Principal Component Analysis of
peptide profiling of cheeses. Scores plot (A) and loading plot (B)
of cheeses ripened for 120 days. Abbreviations: NRA ( ), cheese with no
ripening agent; RA -20 (
), cheese with no
ripening agent; RA -20 ( ), cheese manufactured with L73 freeze-dried cells
stored at −20 °C for 6 months as ripening agent; RA 5 (
), cheese manufactured with L73 freeze-dried cells
stored at −20 °C for 6 months as ripening agent; RA 5 ( ),
cheese manufactured with L73 freeze-dried cells stored at 5 °C for 6 months as
ripening agent.
),
cheese manufactured with L73 freeze-dried cells stored at 5 °C for 6 months as
ripening agent.
 ), cheese with no
ripening agent; RA -20 (
), cheese with no
ripening agent; RA -20 ( ), cheese manufactured with L73 freeze-dried cells
stored at −20 °C for 6 months as ripening agent; RA 5 (
), cheese manufactured with L73 freeze-dried cells
stored at −20 °C for 6 months as ripening agent; RA 5 ( ),
cheese manufactured with L73 freeze-dried cells stored at 5 °C for 6 months as
ripening agent.
),
cheese manufactured with L73 freeze-dried cells stored at 5 °C for 6 months as
ripening agent.
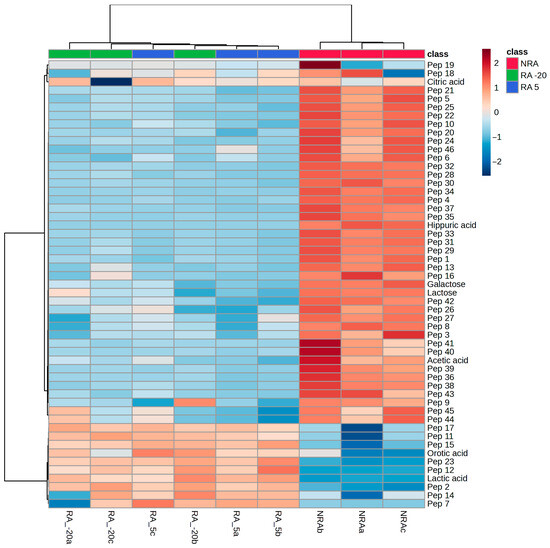
Figure 7.
Hierarchical clustering heatmap. Abbreviations: NRA, cheese with no ripening agent; RA -20, cheese manufactured with L73 freeze-dried cells stored at −20 °C for 6 months as ripening agent; RA 5, cheese manufactured with L73 freeze-dried cells stored at 5 °C for 6 months as ripening agent. The letters a, b, and c in the code of cheeses indicate the replicates.
Proteolysis during aging plays a key role in most cheese varieties [54]. This biochemical event contributes to the development of texture and flavor in cheese. Coagulant breaks down large peptides, which are further broken down by starter and NSLAB enzymes (proteinases and various peptidases) to small peptides and amino acids [55,56]. In particular, microbial peptidases are essential to degrade bitter peptides, which may be generated by the coagulant or by certain microbial proteases. Peptides that elute late in the profiles are hydrophobic peptides, which have been generally associated with bitterness [57]. The results obtained showed the ability of L73 freeze-dried cells to reduce the level of hydrophobic peptides, which might have a positive impact on the flavor of cheeses. In addition, L73 had shown an interesting peptidase profile after growing in the culture medium based on cheese whey permeate [15]. Furthermore, its contribution to proteolysis was verified in situ in different cheese varieties [22,23,24]. The increase in amino acids is important since they are precursors of numerous volatile compounds. Microbial aminotransferases from lactic cultures or NSLAB would be the limiting factor in the degradation of these amino acids into volatile compounds [58]. It is interesting to highlight that L73 presents a high level of aminotransferases [16]. In fact, the ability to produce flavor compounds was previously confirmed in vitro [15] and in situ in semi-hard cheeses [22,24].
Finally, it is worth mentioning that the ripening agents contributed to cheese ripening not only by supplying proteolytic enzymes but also by enhancing calcium solubilization. It is well established that cheese texture is influenced by the balance between soluble and insoluble calcium, and that greater acidification promotes calcium solubilization [59]. Although the levels of soluble and insoluble calcium were not directly measured, the lower pH values observed in cheeses with ripening agents could indicate a shift in the ratio between these calcium forms, and a concomitant modification of their texture properties.
4. Conclusions
In conclusion, this study brings to light the potential of L73 as a ripening agent in cheese after undergoing both freeze-drying and a long storage period. The viability of L73 was not negatively affected by either the freeze-drying process or subsequent storage, as it consistently maintained high counts throughout the storage period. The L73 metabolic activity was verified at the end of the six-month storage period in situ in cultured milk and miniature cheeses. Its potential as a ripening agent was preserved, as its ability to reduce compounds that could be used as energy sources by adventitious microorganisms and to produce antimicrobial compounds during ripening was not affected by the freeze-drying process and storage. In addition, its contribution to proteolysis, by hydrolyzing hydrophobic peptides, was preserved as well. These findings may benefit starter culture producers, as the freeze-drying and storage conditions applied to L73 could be effective in preserving other strains as well.
Author Contributions
Conceptualization, G.H.P.; investigation, G.H.P., M.E.B., E.C.A., and C.V.B.; writing—original draft preparation, G.H.P.; writing—review and editing, G.H.P., M.E.B., E.C.A., and C.V.B.; supervision, G.H.P.; project administration, G.H.P. and C.V.B.; funding acquisition, G.H.P. and C.V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Agencia Santafesina de Ciencia, Tecnología e Innovación de la Provincia de Santa Fe-ASaCTeI (PEICID-2022-037), Agencia Nacional de Promoción Científica y Tecnólogica-ANPCyT (PICT-2018-01334; PICT-2019-01633).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the findings are available from the corresponding author upon reasonable request.
Acknowledgments
The authors acknowledge Milkaut S.A., and Arla Foods for their generous donations of raw milk and cheese whey permeate, respectively.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gänzle, M.G.; Monnin, L.; Zheng, J.; Zhang, L.; Coton, M.; Sicard, D.; Walter, J. Starter culture development and innovation for novel fermented foods. Annu. Rev. Food Sci. Technol. 2024, 15, 211–239. [Google Scholar] [CrossRef] [PubMed]
- Wouters, J.T.M.; Ayad, E.H.E.; Hugenholtz, J.; Smit, G. Microbes from raw milk for fermented dairy products. Int. Dairy J. 2002, 12, 91–109. [Google Scholar] [CrossRef]
- Bintsis, T.; Papademas, P. The application of protective cultures in cheese: A review. Fermentation 2024, 10, 117. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Mancini, L.; Fox, P.F. Pros and cons for using non-starter lactic acid bacteria (NSLAB) as secondary/adjunct starters for cheese ripening. Trends Food Sci. Technol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- Ciocia, F.; Mcsweeney, P.L.H.; Piraino, P.; Parente, E. Use of dairy and non-dairy Lactobacillus plantarum, Lactobacillus paraplantarum and Lactobacillus pentosus strains as adjuncts in cheddar cheese. Dairy Sci. Technol. 2015, 93, 623–640. [Google Scholar] [CrossRef]
- Peighambardoust, S.H. Application of spray drying for preservation of lactic acid starter cultures: A review. Trends Food Sci. Technol. 2011, 22, 215–224. [Google Scholar] [CrossRef]
- Aschenbrenner, M.; Foerst, P.; Kulozik, U. Freeze-drying of probiotics. In Advances in Probiotic Technology; Foerst, P., Santivarangkna, C., Eds.; CRC Press: Boca Ratón, FL, USA, 2015; pp. 213–241. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Silva, J.; Ho, P.; Teixeira, P.; Malcata, F.X.; Gibbs, P. Relevant factors for the preparation of freeze-dried lactic acid bacteria. Int. Dairy J. 2004, 14, 835–847. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q.; Chen, W.; Du, G.; Chen, J. Short communication: Protection of lyophilized milk starter Lactobacillus casei Zhang by glutathione. J. Dairy Sci. 2016, 99, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- Oddi, S.; Binetti, A.; Burns, P.; Cuatrin, A.; Reinheimer, J.; Salminen, S.; Vinderola, G. Occurrence of bacteria with technological and probiotic potential in Argentinian human breast-milk. Benef. Microbes 2020, 11, 685–702. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.S.; Silva, J.; Ho, P.; Teixeira, P.; Malcata, F.X. Survival of freeze-dried Lactobacillus plantarum and Lactobacillus rhamnosus during storage in the presence of protectants. Biotechnol. Lett. 2002, 24, 1587–1591. [Google Scholar] [CrossRef]
- Oluwatosin, S.O.; Tai, S.L.; Fagan-endres, M.A. Sucrose, maltodextrin and inulin efficacy as cryoprotectant, preservative and prebiotic—Towards a freeze dried Lactobacillus plantarum topical probiotic. Biotechnol. Rep. 2022, 33, e00696. [Google Scholar] [CrossRef] [PubMed]
- Tyutkov, N.; Zhernyakova, A.; Birchenko, A.; Eminova, E.; Nadtochii, L.; Baranenko, D. Probiotics viability in frozen food products. Food Biosci. 2022, 50, 101996. [Google Scholar] [CrossRef]
- Ugarte, M.B.; Guglielmotti, D.; Giraffa, G.; Reinheimer, J.; Hynes, E. Nonstarter lactobacilli isolated from soft and semi-hard argentinean cheeses: Genetic characterization and resistance to biological barriers. J. Food Prot. 2006, 69, 2983–2991. [Google Scholar] [CrossRef] [PubMed]
- Batistela, M.E.; Bergamini, C.V.; Ale, E.C.; Renzo, S.; Peralta, G.H. Culture medium based on whey permeate for biomass production of lactobacilli with technological aptitudes. Food Biosci. 2024, 62, 105445. [Google Scholar] [CrossRef]
- Peralta, G.H.; Bergamini, C.V.; Hynes, E.R. Aminotransferase and glutamate dehydrogenase activities in lactobacilli and streptococci. Braz. J. Microbiol. 2016, 47, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Peralta, G.H.; Bergamini, C.V. Mini-cheese models. In Dairy Foods Processing; Gomes da Cruz, A., Colombo Pimentel, T., Esmerino, E.A., Verruck, S., Eds.; Methods and Protocols in Food Science; Humana: New York, NY, USA, 2025; pp. 151–162. [Google Scholar] [CrossRef]
- ISO 5534:2004; Cheese and Processed Cheese: Determination of the Total Solids Content (5534/IDF 4, Reference method). International Organisation for Standardisation: Geneva, Switzerland, 2004.
- ISO 3433:2008; Cheese: Determination of Fat Content: Van Gulik Method. (3433/IDF 222). International Organisation for Standardisation: Geneva, Switzerland, 2018.
- ISO 8968-1:2014; Milk and Milk Products: Determination of Nitrogen Content: Part 1: Kjeldahl Principle and Crude Protein Calculation. (8968/IDF 20). International Organisation for Standardisation: Geneva, Switzerland, 2014.
- Ardö, Y.; Polychroniadou, A. Laboratory Manual for Chemical Analysis Of Cheese: Improvement of the Quality of the Production of Raw Milk Cheeses; Publications Office: Luxembourg, 1999. [Google Scholar]
- Giménez, P.; Peralta, G.H.; Wolf, I.V.; Hynes, E.R.; Bergamini, C.V. Adjunct cultures of autochthonous Lactobacillus strains to diversify and improve cheese maturation. Int. J. Dairy Technol. 2014, 78, 13111. [Google Scholar] [CrossRef]
- Giménez, P.; Peralta, G.H.; Batistela, M.E.; George, G.A.; Ale, E.C.; Quintero, J.P.; Hynes, E.R.; Bergamini, C.V. Impact of the use of skim milk powder and adjunct cultures on the composition, yield, proteolysis, texture and melting properties of Cremoso cheese. Int. Dairy J. 2023, 140, 105595. [Google Scholar] [CrossRef]
- Milesi, M.; Vinderola, G.; Sabbag, N.; Meinardi, C.; Hynes, E. Influence on cheese proteolysis and sensory characteristics of non-starter lactobacilli strains with probiotic potential. Food Res. Int. 2009, 42, 1186–1196. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Champagne, C.P.; Gardner, N.; Brochu, E.; Beaulieu, Y. The freeze-drying of lactic acid bacteria. a review. Can. Inst. Food Sci. Technol. J. 1991, 24, 118–128. [Google Scholar] [CrossRef]
- Morgan, C.A.; Herman, N.; White, P.A.; Vesey, G. Preservation of microorganisms by drying; a review. J. Microbiol. Methods 2006, 66, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, F.; Girardeau, A.; Passot, S. Freeze-drying of lactic acid bacteria: A stepwise approach for developing a freeze-drying protocol based on physical properties. In Cryopreservation and Freeze-Drying Protocols; Wolkers , W.F., Oldenhof, H., Eds.; Springer Nature: New York, NY, USA, 2021; pp. 703–720. [Google Scholar]
- Senovieski, M.L.; Loyeau, P.A.; Puntillo, M.A.; Binetti, A.G.; Vinderola, C.G. Cheese whey for production of breast milk-derived bifidobacteria: Influence of fermentation conditions on the survival to spray drying and storage. Int. J. Dairy Technol. 2024, 77, 853–861. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D.; Park, H. Effects of protectant and rehydration conditions on the survival rate and malolactic fermentation efficiency of freeze-dried Lactobacillus plantarum JH287. Appl. Microbiol. Biotechnol. 2016, 100, 7853–7863. [Google Scholar] [CrossRef] [PubMed]
- Bai, S. Enhancing the Stability of Probiotics: Freeze-Drying and Encapsulation. Doctoral Thesis, Lund University, Lund, Sweden, 2024. [Google Scholar]
- Lodato, P.; Segovia de Huergo, M.; Buera, M.P. Viability and thermal stability of a strain of Saccharomyces cerevisiae freeze-dried in different sugar and polymer matrices. Appl. Microbiol. Biotechnol. 1999, 52, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Santivarangkna, C.Ã.; Higl, B.; Foerst, P. Protection mechanisms of sugars during different stages of preparation process of dried lactic acid starter cultures. Food Microbiol. 2008, 25, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Li, Y.; Kang, W.; Han, Y.; Yin, B.; Yang, R.; Tang, R.; Pan, L.; Wang, J.; Li, W.; et al. Synergistic combination of cryoprotectants improved freeze-dried survival rate and viable counts of Lactiplantibacillus plantarum. Int. J. Dairy Technol. 2024, 77, 348–357. [Google Scholar] [CrossRef]
- Silva, J.; Silva, J.; Sofia, A.; Helena, C.; Teixeira, P.; Gibbs, P.A. Induction of stress tolerance in Lactobacillus delbrueckii ssp. bulgaricus by the addition of sucrose to the growth medium. J. Dairy Res. 2004, 71, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Gaudreau, H.; Champagne, C.P.; Jelen, P. The use of crude cellular extracts of Lactobacillus delbrueckii ssp. bulgaricus 11842 to stimulate growth of a probiotic Lactobacillus rhamnosus culture in milk. Enzym. Microb. Technol. 2005, 36, 83–90. [Google Scholar] [CrossRef]
- Shah, N. Probiotic bacteria: Selective enumeration and survival in dairy foods. J. Dairy Sci. 2000, 83, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, H.; Qiao, Y.; Liu, G.; Leng, C.; Zhang, Y.; Lv, X.; Feng, Z. The nutrient requirements of Lactobacillus rhamnosus GG and their application to fermented milk. J. Dairy Sci. 2019, 102, 5971–5978. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Christine, K.W.; Shah, N.P. Towards galactose accumulation in dairy foods fermented by conventional starter cultures: Challenges and strategies. Trends Food Sci. Technol. 2015, 41, 24–36. [Google Scholar] [CrossRef]
- Tormo, M.; Izco, J.M. Alternative reversed-phase high-performance liquid chromatography method to analyse organic acids in dairy products. J. Chromatogr. A 2004, 1033, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.; Winter, C.K. Diacetyl in foods: A review of safety and sensory characteristics. Compr. Rev. Food Sci. Food Saf. 2015, 14, 634–643. [Google Scholar] [CrossRef]
- Calasso, M.; Gobbetti, M. Lactic acid bacteria|Lactobacillus spp.: Other species. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2011; pp. 125–131. [Google Scholar] [CrossRef]
- Hatti-kaul, R.; Chen, L.; Dishisha, T.; Enshasy, H.E. Lactic acid bacteria: From starter cultures to producers of chemicals. FEMS Microbiol. Lett. 2018, 365, fny213. [Google Scholar] [CrossRef] [PubMed]
- Prada, S. Uso de Homogeneización de Alta Presión y Lisozima Para Mejorar la Actividad Metabólica de Lactiplantibacillus plantarum 29 en Queso Semiduro sin sal Añadida. Bachelor’s Thesis, Universidad Nacional del Litoral, Santa Fe, Argentina, 2025. [Google Scholar]
- Johnson, M.E. Mesophilic and thermophilic cultures used in traditional cheesemaking. Microbiol. Spectr. 2013, 1, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Kindstedt, P.S. The Basics of cheesemaking. Microbiol. Spectr. 2013, 1, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Galat, A.; Dufresne, J.; Combrisson, J.; Thépaut, J.; Boumghar-Bourtchai, L.; Boyer, M.; Fourmestraux, C. Novel method based on chromogenic media for discrimination and selective enumeration of lactic acid bacteria in fermented milk products. Food Microbiol. 2016, 55, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Ur-Rehman, S.; Fox, P.F.; McSweeney, P.L.H.; Madkor, S.A.; Farkye, N.Y. Alternatives to pilot plant experiments in cheese-ripening studies. Int. J. Dairy Technol. 2001, 54, 121–126. [Google Scholar] [CrossRef]
- Hutkins, R.; Halambeck, S.M.; Morris, H.A. Use of galactose-fermenting Streptococcus thermophilus in the manufacture of Swiss, mozzarella, and short-method cheddar cheese. J. Dairy Sci. 1986, 69, 1–8. [Google Scholar] [CrossRef]
- Green, I.R.; Oberg, C.J.; McMahon, D.J. Galactose-positive adjunct cultures prevent gas formation by Paucilactobacillus wasatchensis WDC04 in a model gas production test. J. Dairy Sci. 2021, 104, 10540–10549. [Google Scholar] [CrossRef] [PubMed]
- Ortakci, F.; Broadbent, J.R.; Oberg, C.J.; McMahon, D.J. Growth and gas production of a novel obligatory heterofermentative Cheddar cheese nonstarter lactobacilli species on ribose and galactose. J. Dairy Sci. 2015, 98, 3645–3654. [Google Scholar] [CrossRef] [PubMed]
- Ortakci, F.; Broadbent, J.R.; Oberg, C.J.; McMahon, D.J. Late blowing of Cheddar cheese induced by accelerated ripening and ribose and galactose supplementation in presence of a novel obligatory heterofermentative nonstarter Lactobacillus wasatchensis. J. Dairy Sci. 2015, 98, 7460–7472. [Google Scholar] [CrossRef] [PubMed]
- Neviani, E.; Gatti, M.; Gardini, F.; Levante, A. Microbiota of cheese ecosystems: A perspective on cheesemaking. Foods 2025, 14, 830. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, V.K.; McSweeney, P.L.H.; Magboul, A.A.A.; Fox, P.F. Proteolysis in cheese during ripening. In Cheese: Chemistry, Physics and Microbiology; Elsevier: Amsterdam, The Netherlands, 2004; pp. 391–433. [Google Scholar] [CrossRef]
- Fox, P.F.; Singh, T.K.; McSweeney, P.L.H. Proteolysis in cheese during ripening. In Biochemistry of Milk Products; Andrews, A.T., Varley, J., Eds.; Woodhead Publishing: Sawston, UK, 2005; pp. 1–31. [Google Scholar] [CrossRef]
- Ardö, Y.; McSweeney, P.L.H.; Magboul, A.A.A.; Upadhyay, V.K.; Fox, P.F. Biochemistry of cheese ripening: Proteolysis. In Cheese Chemistry, Physics and Microbiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 445–482. [Google Scholar] [CrossRef]
- Fallico, V.; McSweeney, P.L.H.; Horne, J.; Pediliggieri, C.; Hannon, J.A.; Carpino, S.; Licitra, G. Evaluation of bitterness in Ragusano cheese. J. Dairy Sci. 2005, 88, 1288–1300. [Google Scholar] [CrossRef] [PubMed]
- Ardö, Y. Flavour formation by amino acid catabolism. Biotechnol. Adv. 2006, 24, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Mahony, J.A.O.; Lucey, J.A.; McSweeney, P.L.H. Chymosin-mediated proteolysis, calcium solubilization, and texture development during the ripening of Cheddar cheese. J. Dairy Sci. 2005, 88, 3101–3114. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).