Abstract
Lactic acid bacteria (LAB) have historically been used in fermentation processes, playing a key role in the development of foods with health benefits. Understanding the factors that affect LAB functionality is essential for optimizing their application. During fermentation processes, LAB produce different metabolites of interest, such as lactic acid, gamma-aminobutyric acid (GABA), and short-chain fatty acids, whose production is influenced by conditions such as temperature and pH. Although LAB exhibit optimal growth ranges, their ability to adapt to moderate variations makes them particularly valuable in various applications. Currently, the impact of these LAB metabolites on human physiology is being actively investigated, especially for modulation of the Microbiota–Gut–Brain axis. Certain compounds derived from LAB have been shown to contribute to neurological, immunological, and metabolic processes, opening new perspectives for the design of functional foods. This article provides a comprehensive overview of the importance of lactic acid bacteria in human health and highlights their potential for the development of innovative strategies to promote well-being through diet.
1. Introduction
The Microbiota–Gut–Brain axis (MGBA) is an intricate and highly dynamic bidirectional communication network that integrates the central nervous system (CNS), the enteric nervous system (ENS), and the gut microbiota. Far beyond a simple exchange, this axis orchestrates an elaborate dialogue involving neural, endocrine, immune, and metabolic pathways [1]. Emerging evidence highlights that changes in the composition or activity of the gut microbiota can profoundly impact brain function, influencing emotional regulation, cognitive performance, and stress responses [2]. Dysfunctions within this axis have been increasingly been associated with neuropsychiatric and gastrointestinal disorders, underscoring the pivotal role of the gut ecosystem in maintaining both mental and intestinal health.
Among the diverse microbial groups inhabiting the gut, lactic acid bacteria (LAB) stand out due to their remarkable ability to interact with the host at multiple levels. LAB are Gram-positive, non-sporulating micro-organisms commonly found in fermented foods and the human gastrointestinal tract, where they contribute to gut homeostasis [3]. In the context of the MGBA, LAB have drawn particular attention for their potential as modulators of neurobehavioral processes. Notably, certain LAB strains have been classified as “psychobiotics,” which are live micro-organisms that, when administered in adequate amounts, confer mental health benefits through mechanisms such as the stimulation of vagal pathways, modulation of immune responses, and production of neuroactive compounds [1]. Experimental studies in both animal models and clinical settings increasingly support the idea that LAB supplementation can mitigate symptoms of anxiety, depression, and stress, offering a promising adjunctive strategy for neuropsychiatric interventions.
Central to the modulatory effects exerted by LAB and other gut micro-organisms is the production of intestinal metabolites. These small molecules, generated through microbial fermentation processes, serve as critical messengers within the MGBA. Short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate are among the most extensively studied; they not only fuel colonocytes but also influence blood–brain barrier integrity, regulate systemic inflammation, and modulate neurotransmitter systems by affecting microglial activation [4]. Beyond SCFAs, LAB are capable of synthesizing key neurotransmitters like γ-aminobutyric acid (GABA) and serotonin, directly impacting neuronal excitability and mood regulation [2]. Additionally, their contribution to the production of vitamins essential for neural function, such as folate and vitamin B12, further emphasizes the interconnectedness between microbial metabolism and brain health. Understanding these LAB metabolic pathways offers a valuable framework for developing microbiota-targeted therapies aimed at enhancing mental well-being through gut microbiome modulation.
The scientific literature was searched in databases such as PubMed, Scopus, ScienceDirect, and Google Scholar. The search focused on publications from the last 10 years using the following combined keywords: “lactic acid bacteria”, “metabolites”, “gut–brain axis”, “psychobiotics”, and “microbiota modulation”. Articles were selected based on their relevance to the roles of LAB-derived metabolites in modulating gut microbiota and their potential impact on neurophysiological functions. Preference was given to original research articles and systematic reviews published in peer-reviewed journals.
2. Lactic Acid Bacteria
Currently, various species of LAB are being extensively studied. These bacteria are Gram-positive, exhibit cocci or bacilli morphology, and are resistant to acidic pH conditions [5]. They are non-motile, facultative anaerobic micro-organisms that obtain energy through phosphorylation [6]. One of the most remarkable characteristics of LAB is their ability to hydrolyze large molecules, which facilitate digestion. Moreover, these bacteria produce bioactive compounds, such as fatty acids, which have been the focus of increased interest, in particular in investigating their effects on food and food processing residues [7]. Some lactic acid bacteria (LAB) are classified as GRAS (Generally Recognized As Safe) due to their widespread use in food processing and production, their high tolerance to a low pH, and their non-pathogenic nature.
Some lactic acid bacteria (LAB) are considered probiotics due to factors such as their high survival rate in the intestinal tract, their adhesion capacity, and their ability to inhibit micro-organisms that may negatively affect gut health. Through these mechanisms, they can exert beneficial effects on digestive health. However, there is evidence indicating that not all LAB have positive effects; therefore, it cannot be asserted that all LAB possess probiotic or beneficial properties [8]. It is estimated that by 2028, the market for probiotic-containing products will reach approximately USD 95.52 billion [9]. Due to their capacity to modify and process food, LAB enhances nutrient absorption and facilitate digestion, in part through their prebiotic activity [10]. For this reason, the food industry has shown a growing interest in the development of products with probiotic properties, positioning LAB as one of the most relevant microbial groups in this field [11].
2.1. Main LAB Genera and Species
The use of LAB is widely recognized for its role in food fermentation. The Lactobacillus genus is the most well-known and globally utilized [12]. Other important genera are: Lacticaseibacillus, Limosilactobacillus, Leuconostoc, Bifidobacterium, and Enterococcus. The following Table 1 shows examples of LAB applications on various food matrices and their resulting products, which have shown positive effects on human health.

Table 1.
Lactic acid bacteria, substrate types, and associated fermentation effects.
2.2. Fermentation Mechanisms and Metabolite Production
LAB have been used for centuries during the fermentation of food products, and currently account for nearly 80% of all fermentations performed in the food industry. Their use dates back to ancient civilizations, such as China, Palestine, Egypt, Abyssinia, and Babylon [33]. Among the metabolites produced during fermentation are peptides, bacteriocins, enzymes, and lactic acid. However, it is important to note that not all LAB strains have the capacity to produce all of these compounds [8]. Although further studies are required to fully understand the fermentation mechanisms of LAB, it is known that glucose is their primary substrate. During this process, two molecules of pyruvate are generated via the Embden–Meyerhof–Parnas glycolytic pathway, producing two ATP and two NADH per molecule of glucose metabolized. Subsequently, pyruvate is reduced to lactic acid through the action of the enzyme lactate dehydrogenase, regenerating NAD+ and thus sustaining the glycolytic cycle.
There are two main types of lactic fermentation: homolactic and heterolactic. In heterolactic fermentation, lactose is hydrolyzed by the enzyme β-galactosidase into glucose and galactose, which are then fermented to produce lactic acid, contributing to the reduction in the medium’s pH [34]. Some of the most significant products derived from LAB fermentation are postbiotics, which have beneficial health effects due to their content of organic acids, carbohydrates, vitamins, and bacteriocins [8]. In addition to modifying flavor, the fermentation process can confer functional and medicinal properties to fruits and vegetables. It has been observed that certain vegetables exhibit increased levels of organic acids, lipids, and organoheterocyclic compounds following fermentation [35]. Furthermore, increases in the concentration of flavonoids and volatile compounds have also been reported as a result of LAB fermentation [15]. This effect is attributed to the ability of LAB to cleave macromolecular bonds, releasing smaller bioactive molecules and enhancing their functional bioavailability [12].
In the pursuit of development and innovation for obtaining important metabolites, unconventional methods, such as mutagenesis, as well as ultraviolet and gamma irradiation, have been employed to obtain bacteria with enhanced yields of various metabolites or traits [36]. Random mutagenesis or non-recombinant strategies, adaptive laboratory evolution, homologous recombination, genome engineering, and recombineering have been used to modify lactic acid bacteria (LAB), improving different characteristics, such as the production of compounds including organic acids, vitamins, bacteriocins, and exopolysaccharides (EPSs), as well as nutritional and sensory enhancements of foods, such as protein digestibility and metabolite production from aromatic amino acids [37]. One of the most advanced genetic editing techniques is CRISPR/Cas (Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated proteins), which are used to identify, cut, and modify DNA fragments. This technology has revolutionized the improvement of LAB for metabolite production, allowing for their application in biopreservation, and overcoming the inherent limitations of these bacteria [38].
Recently, submerged fermentation has been investigated as a strategy to process agro-industrial waste to extract compounds with antioxidant activity and assess their potential antidiabetic effects [14]. A noteworthy example is the production of the non-protein amino acid γ-aminobutyric acid (GABA), synthesized from glutamic acid via the action of the enzyme glutamate decarboxylase. This postbiotic is recognized for its neuroprotective and antidiabetic properties, sparking interest in identifying LAB strains with a high GABA production capacity during fermentation [39].
2.3. Factors Influencing Metabolite Production
As previously mentioned, LAB are capable of reaching the intestinal tract, and most strains can survive at temperatures ranging from 30 to 45 °C, with some tolerating up to 60 °C [33]. Temperature is a critical factor during fermentation processes. An optimal temperature (with a variation range of ±5 °C) promotes the production of flavor- and aroma-enhancing compounds, making the product more acceptable to consumers. Conversely, temperatures above the optimal range can inhibit fermentation, negatively altering the organoleptic profile and reducing the concentration of key metabolites such as organic acids [10].
For preservation purposes, techniques such as lyophilization at −80 °C for 40 h—entailing freezing and drying steps—have been evaluated. For example, Lactobacillus bulgaricus CFL1 has shown a high sensitivity to thermal stress, whereas L. plantarum WCFS1 demonstrated resistance to osmotic and mechanical stress but was susceptible to heat stress [40]. The type of substrate also plays a significant role in metabolite production. Sugars are essential for LAB growth and metabolism, as they are required for biotransformation processes. During this process, an increase in the production of certain compounds, such as organic acids, as well as a rise in the total acidity, can be observed [13].
Although LAB are generally considered anaerobic, this characteristic is species-dependent. Some strains exhibit tolerance to oxygen exposure; however, such an exposure can lead to the accumulation of toxic metabolites, resulting in cellular fragmentation and reduced bacterial viability [33].
3. Metabolites Produced by LAB and the Microbiota–Gut–Brain Axis
The Microbiota–Gut–Brain axis represents a bidirectional communication network between the gut and the brain, involving various physiological systems such as the circulatory, endocrine, and immune systems, in addition to regulatory axes like the hypothalamic–pituitary–adrenal (HPA) axis (Figure 1) [41]. One of the most critical components of this network is the vagus nerve, which acts as a primary conduit for neural signaling between the gastrointestinal tract and the central nervous system (CNS). This interaction is further mediated by enteroendocrine cells (EECs), a specialized subset of intestinal epithelial cells that, despite comprising only ~1% of the epithelium, play an essential role in sensing microbial signals and regulating physiological responses such as food intake, nutrient absorption, and homeostasis [41].
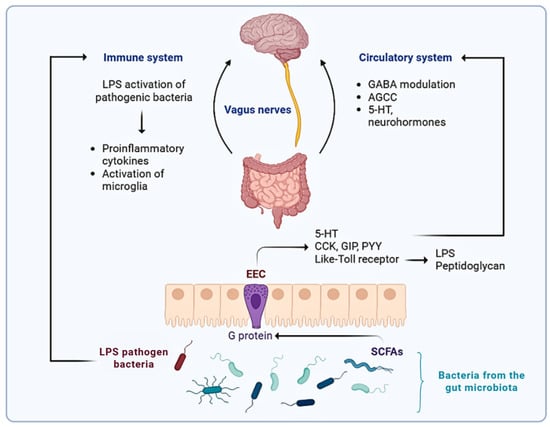
Figure 1.
Enteroendocrine cells (EECs), despite constituting only ~1% of the intestinal epithelium, play a pivotal role in gut–brain communication. Through microbial sensing and the release of serotonin and intestinal hormones such as Cholecystokinin (CCK), Gastric Inhibitory Peptide (GIP), and Peptide YY (PYY), EECs signal to the vagus nerve, modulating central nervous system activity and physiological responses. Short-chain fatty acids (SCFAs), produced by microbial fermentation, further influence this axis by activating receptors on EECs and vagal afferents, regulating inflammation, gut permeability, and neuroendocrine function. Additionally, microbial components like Lipopolysaccharide (LPS) can trigger immune responses that reach the brain via cytokine signaling and microglial activation, integrating neuroimmune communication within the gut–brain axis.
In this context, microbial metabolites produced in the gut act as key signaling molecules, communicating with the CNS via two main routes. The first involves the translocation of metabolites, such as short-chain fatty acids (SCFAs) and bacterially derived neurotransmitters, across the intestinal mucosa into the systemic, enterohepatic, and pulmonary circulation, enabling them to eventually reach the brain through the blood–brain barrier. The second route relies on the activation of neuroenteric circuits, wherein chemical and mechanical signals generated within the gut stimulate submucosal neurons, which then transmit afferent signals to the CNS via the vagus nerve [9].
3.1. Lactic Acid
Among the primary metabolites produced by LAB in the gut is lactic acid, synthesized via the fermentation of carbohydrates. In humans, lactic acid exists as two stereoisomers, L-lactic acid and D-lactic acid, which differ in their metabolic fates and systemic effects. L-lactate, primarily generated through glycolysis from carbohydrate and amino acid catabolism, is endogenously integrated into human energy metabolism, where it supports neuronal oxidative metabolism, enhances synaptic plasticity, and facilitates protein synthesis, which is essential for learning and memory [42].
Conversely, D-lactate, which is produced in smaller amounts from carbohydrate and lipid metabolism, is poorly metabolized by mammals due to the lack of D-lactate-specific dehydrogenases. Under normal physiological conditions, it does not enter systemic circulation. However, in cases of increased intestinal permeability or mucosal damage, D-lactate may translocate into the bloodstream, where its accumulation can lead to metabolic acidosis and adverse neurophysiological effects. This occurs partly through competitive inhibition of neuronal L-lactate uptake, which impairs brain energy homeostasis [42].
Moreover, lactic acid has been associated with the upregulation of tight junction proteins in the colon, which decreases intestinal permeability and provides protection against the translocation of pro-inflammatory components into the bloodstream and brain [43]. Preclinical studies have demonstrated that this property exerts both neuroprotective and immunomodulatory effects. For instance, Watanabe et al. (2009) reported that in a rat model of intestinal injury, lactic acid produced by Lactobacillus casei reduced neutrophil infiltration and the expression of pro-inflammatory cytokines, thereby mitigating neuroinflammation [44,45]. Nevertheless, alterations in the composition of gut microbiota—such as the overgrowth of lactic acid-producing strains—may lead to excessive lactate production. When this is coupled with impaired gut barrier integrity, systemic lactate overflow may occur, contributing to a spectrum of clinical manifestations, many of which are associated with neuropsychiatric disorders [45,46].
3.2. Short-Chain Fatty Acids (SCFAs)
Although the primary metabolic function of lactic acid bacteria (LAB) is the production of lactic acid, several strains are also capable of synthesizing short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate, particularly when cultured in media enriched with fermentable polysaccharides and dietary fibers [46]. SCFAs are aliphatic molecules composed of carbon chains ranging from two to six atoms, and they represent the most abundant microbial metabolites in the colon. These molecules have the ability to cross the blood–brain barrier and interact with various cell types, including neurons, immune cells, endocrine cells, and intestinal epithelial cells [47].
Beyond their role in modulating the intestinal microenvironment (e.g., pH, epithelial integrity, and immune function), SCFAs also act as signaling molecules in the central nervous system (CNS) through multiple pathways. One of the main mechanisms involves neuronal signaling via the vagus nerve, where SCFAs interact with enteroendocrine cells (EECs) and other intestinal epithelial cells expressing G-protein-coupled receptors (GPCRs). This interaction triggers the release of neuropeptides and signals that modulate stress responses, mood, and appetite [48].
Another relevant pathway includes immune signaling, whereby SCFAs regulate the proliferation of pro-inflammatory cells, such as Th17, and modulate microglial activation, leading to a reduction in chronic neuroinflammation, which is a process implicated in neurodegenerative disorders such as depression, Parkinson’s disease, and Alzheimer’s disease [49]. SCFAs are also involved in neuroendocrine regulation (Table 2), as their interaction with receptors on EECs promotes the secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY). Additionally, SCFA-mediated signaling in pancreatic β-cells enhances insulin secretion, and these molecules also influence the release of ghrelin, serotonin, and leptin, all of which are involved in energy homeostasis and feeding behavior [4,50]. Lastly, SCFAs play a role in neurochemical signaling through the regulation of neurotrophic factors, particularly brain-derived neurotrophic factor (BDNF), which is critical for memory, learning, neurogenesis, and synaptic plasticity [51,52].

Table 2.
Short-chain fatty acids with effects on the Microbiota–Gut–Brain axis.
3.3. Tryptophan Metabolites
Tryptophan-derived metabolites are bioactive compounds involved in the regulation of biological processes. This essential amino acid can be metabolized through three main pathways: the kynurenine (KYN) pathway, the serotonin pathway, and the indole pathway, with the latter being highly dependent on the microbial metabolism present in the human gut [59].
Various studies have documented the role of indole metabolites as mediators in both the prevention and progression of several diseases, such as Alzheimer’s disease and inflammatory bowel disease [60,61]. In this context, certain LAB strains, such as L. reuteri, possess specialized enzymes that enable them to transform tryptophan into indole derivatives such as indole-3-propionic acid (IPA), indole-3-acetic acid (IAA), and indole-3-lactic acid (ILA). These compounds have been shown in various studies to influence human immunity and participate in neurotrophic and hormonal signaling pathways [58,59,62,63]. Experimental models have demonstrated that these compounds act on the Microbiota–Gut–Brain axis by indirectly modulating the synthesis of intestinal serotonin and promoting neuronal signaling through the vagus nerve. Moreover, LAB-derived indoles can mitigate microglial overactivation, support synaptic recovery in cases of brain injury, and increase the expression of brain-derived neurotrophic factors (BDNFs) in key regions such as the hippocampus. These actions are further enhanced by the activation of the aryl hydrocarbon receptor (AhR), which is highly expressed in both intestinal immune cells and neurons, thereby facilitating the integrated regulation of neuronal plasticity and immune homeostasis [62,63].
These findings highlight the emerging role of LAB as producers of tryptophan-derived metabolites, showing a promising avenue for the intervention of neurological, inflammatory, and behavioral disorders through their probiotic properties.
3.4. Gamma-Aminobutyric Acid (GABA)
Gamma-aminobutyric acid (GABA) is a non-proteinogenic four-carbon amino acid synthesized through the decarboxylation of L-glutamic acid via a reaction catalyzed by the enzyme glutamate decarboxylase (GAD) [64]. In mammals, GABA functions as the principal inhibitory neurotransmitter in the central nervous system (CNS), although it also plays modulatory roles within both excitatory and inhibitory neuronal circuits [65]. Recent evidence has demonstrated that GABA biosynthesis is not restricted to the nervous system. Several strains of lactic acid bacteria (LAB), including Lactiplantibacillus plantarum, L. brevis, and L. rhamnosus, as well as some species of Bifidobacterium, possess a functional GAD system that enables them to convert L-glutamic acid into GABA through the same enzymatic pathway [66,67].
Bacterially derived GABA has attracted growing interest due to its role in modulating the Microbiota–Gut–Brain axis (MGBA). It has been shown that this neurotransmitter can cross the intestinal barrier and activate GABAergic receptors expressed on intestinal epithelial cells, triggering afferent signaling pathways to the CNS via the vagus nerve, which have been associated with behavioral fermentum alterations [68]. According to Blanco et al. (2020), this signaling may attenuate activation of the hypothalamic–pituitary–adrenal (HPA) axis, leading to a reduction in circulating cortisol levels under stress conditions [69]. Furthermore, recent experimental data suggest that microbial GABA can induce the expression of brain-derived neurotrophic factor (BDNF) in the hippocampus, promoting synaptic plasticity and neuronal regeneration [70].
Additionally, GABA has been shown to modulate the expression of mucins and tight junction proteins, thereby contributing to the maintenance of intestinal barrier integrity. This mechanism may indirectly influence the bidirectional communication within the MGBA by modulating vagal nerve activity [71].
3.5. Exopolysaccharides
Exopolysaccharides (EPSs) are polysaccharide compounds synthesized and secreted to the exterior of the cell, where they are either attached to the cell surface or freely released into the medium, by various micro-organisms such as fungi, yeasts, algae, and especially bacteria. EPSs are classified as homopolysaccharides (composed of a single type of sugar unit) or heteropolysaccharides (composed of different types of monomeric residues) depending on their structure, and they may or may not include non-carbohydrate residues such as phosphate, acetyl, or amine groups [72]. In bacteria, EPSs play a key role in the formation and maintenance of complex microbial communities [73]. In the case of lactic acid bacteria (LAB), EPS production enhances the characteristics of fermented foods by improving their rheological properties such as viscosity, which is a desirable trait in certain food groups like yogurts. However, in recent years, EPSs have also been shown to contribute significantly to the nutritional value of foods, as well as to exert important biological activities, including antioxidant, antitumor, immunomodulatory, and antibacterial effects [74].
Several studies have explored the potential protective effects of LAB against Alzheimer’s disease; however, a recent study specifically attributed these effects to the EPSs produced by these bacteria through mechanisms such as free radical scavenging, modulation of peroxidation products, and apoptosis signaling [75]. The biological activity of EPSs depends on factors such as the bacterial strain, degree of polymerization, and molecular size. It has been reported that EPSs containing more than 50% mannose are considered biologically active [76], and that those with a higher molecular weight exhibit significant biological activities due to their reduced ability to cross cell membranes [77].
EPSs are currently widely used for various applications, and several LAB strains have been identified as producers of bioactive EPSs, including Limosilactobacillus fermentum Lf2, MTCC 25067, and PS150 (Table 3). The latter has recently been considered a postbiotic due to its ability to remodel the microbiota and improve sleep disorders, with its effects attributed to its high EPS production compared to other strains such as L. fermentum GR1009 [78]. Among the main EPSs produced by LAB are dextran, synthesized by genera such as Leuconostoc, Streptococcus, Weissella, Pediococcus, and Lactobacillus [1]; levan, produced by Leuconostoc and Limosilactobacillus; inulin, synthesized by Leuconostoc, Lentilactobacillus, and Lactobacillus; and reuteran, produced by Limosilactobacillus [79].

Table 3.
The microbial metabolites of some LAB and the mechanism they use to control diseases by affecting the MGBA.
4. Health Implications and Therapeutic Applications
Lactic acid bacteria (LAB) are a group of Gram-positive micro-organisms that have been recognized as safe by the United States Food and Drug Administration (FDA) and are therefore widely used as probiotics and biopreservatives in food products [87]. LAB produce a variety of bioactive metabolites, including lactic acid, 3-phenyllactic acid, B-group vitamins, bacteriocins, gamma-aminobutyric acid (GABA), ornithine, mannitol, exopolysaccharides, and 2-hydroxyisocaproic acid, all of which have been shown to confer health benefits [88]. As a result, they exhibit multiple metabolic and protechnological properties, such as acidifying, proteolytic, and lipolytic activities, as well as antioxidant capacity and flavor enhancement. Certain LAB strains have been reported to enhance the intestinal mucosal barrier against pathogenic micro-organisms by stimulating the immune system. Furthermore, SCFAs, such as acetate, propionate, and butyrate, have been reported to regulate gene expression, epigenetic mechanisms, and neuroplasticity [89,90].
The human microbiota communicates with the gut–brain axis through chemical, neurological, and immunological pathways, playing a fundamental role in the maintenance of health and overall well-being [90]. This communication through microbial metabolites occurs via two main mechanisms: (1) intestinal microbial metabolites reach the submucosal layer of the gut, enter the enterohepatic, pulmonary, and systemic circulation, and eventually reach the brain; and (2) intestinal signals stimulate the submucosal nerve plexus and propagate via the vagus nerve to the central nervous system [91].
These findings have led to the proposal of using probiotics (preparations of live micro-organisms) as a therapeutic approach for neurodevelopmental and mental disorders characterized by increased intestinal permeability, such as depression, anxiety, autism, and schizophrenia, due to their ability to suppress inflammation and inhibit the induction of the IL-8 cytokine in the gut [92]. Table 4 presents studies in which specific LAB strains have been used, showing positive effects on certain neuropsychiatric disorders. Additionally, the table highlights how the development of these conditions is closely linked to the composition and functionality of the gut microbiota.

Table 4.
Main characteristics of studies using LAB in the treatment of neuropsychiatric disorders.
5. Therapeutic Application of LAB
LAB and their metabolites in the treatment of mental and neurodevelopmental disorders, such as depression, anxiety, autism, and schizophrenia, have gained increasing attention. This is particularly relevant given that, in conditions like depression, nearly 30% of patients exhibit resistance to conventional therapies [103]. Common pharmacological treatments include monoamine oxidase inhibitors, tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), non-selective serotonin–norepinephrine reuptake inhibitors (SNRIs), selective norepinephrine reuptake inhibitors, and serotonin modulators. Many of these drugs are associated with significant adverse effects such as tachycardia, hypotension, sexual dysfunction, drowsiness, vomiting, nausea, diarrhea, hyponatremia, urinary retention, dry mouth and eyes, and gastrointestinal bleeding, among others [104]. In this context, Miyaoka et al. (2018) reported that administration of Clostridium butyricum as a complementary therapy in patients with treatment-resistant major depressive disorder resulted in significant improvements, with a 70% response rate [105].
In addition to the previously mentioned metabolites, LAB strains such as Streptococcus thermophilus, Lactobacillus delbrueckii subsp. bulgaricus, and L. bulgaricus CRL 871 have been identified as folate producer compounds derived from folic acid that are essential for numerous critical biological processes, including DNA synthesis and methylation. Consequently, folate-producing LAB have recently been proposed for their use during the fermentation of food products to enhance vitamin content [106].
The targeted production of bioactive compounds and molecules relevant to the gut–brain axis and related diseases has become an increasingly important research focus. One prominent example is gamma-aminobutyric acid (GABA), whose bacterial synthesis offers promising applications in both the food and pharmaceutical industries [107].
6. Future Perspectives
Probiotic intervention is emerging as a promising approach for improving metabolism and body weight regulation in humans. Multiple studies have identified lactic acid bacteria (LAB) as key modulators within the GMBA, owing to their capacity to influence central nervous system (CNS) functions through various mechanisms involving the immune system, the enteric nervous system, and the vagus nerve [108]. Specific strains, such as L. plantarum, have demonstrated, in preclinical models of Alzheimer’s disease, the ability to reduce both cerebral and colonic inflammation, thereby enhancing intestinal barrier integrity and attenuating cognitive decline [X]. Similarly, L. rhamnosus has shown the potential to modulate GABA receptor expression in the brain, producing anxiolytic and antidepressant effects that are primarily mediated through vagal pathways [109]. Moreover, certain LAB strains have been associated with improvements in memory, reduced stress responsiveness, and better sleep quality, positioning them as promising candidates for therapeutic interventions in neurological disorders such as depression and Alzheimer’s disease. Emerging evidence also suggests potential applications in Parkinson’s disease and ADHD.
Nevertheless, despite the reported beneficial effects of LAB and their metabolites on the GMBA in both preclinical and clinical studies, further research is needed to elucidate the specific molecular mechanisms underlying their roles in mental and metabolic disorders. Additional studies are also required to improve our understanding of how specific dietary components influence the composition of the gut microbiota and its subsequent effects on host health, particularly considering the highly individualized nature of dietary responses [110]. Although these mechanisms remain to be fully defined, characterizing a healthy gut microbiota may allow for the early prediction of metabolic and neuropsychiatric disorders [111].
Taken together, these findings support the development of emerging therapeutic strategies that include dietary interventions as adjuncts to pharmacological treatments, disease prevention through targeted microbiota modulation, and the biotechnological production of bioactive compounds derived from LAB fermentation. Furthermore, the design of personalized psychobiotics is highlighted as an innovative complementary therapeutic strategy for the modulation of the Microbiota–Gut–Brain axis in various neurological and psychiatric conditions.
7. Conclusions
Lactic acid bacteria (LAB) are important in both the food industry and health due to their ability to produce beneficial bioactive metabolites. Their classification as GRAS and use as probiotics and bio-preservatives make them valuable in developing functional foods. LAB also play a key role in the gut–brain connection through metabolites like short-chain fatty acids (SCFAs) and GABA, which help regulate brain function, immunity, and neuroplasticity. These properties suggest that LAB could be useful in treating neuropsychiatric disorders such as depression, anxiety, and autism, especially in patients who do not respond to traditional therapies. However, more research is needed to understand the mechanisms behind these disorders and the influence of diet on the gut microbiota. This opens up opportunities for new therapeutic strategies that combine microbiota modulation and dietary interventions to complement traditional treatments and help prevent diseases.
Author Contributions
Conceptualization, Y.L.A.-G. and R.R.-H.; methodology, N.C.C.-A.; software, R.M.S.-S.; validation, R.R.-H.; formal analysis, R.R.-H.; investigation, A.R.C.-L.; resources, R.R.-H.; data curation, S.D.C.R.-O.; writing—original draft preparation, Y.L.A.-G. and R.R.-H.; writing—review and editing, S.D.C.R.-O.; visualization, S.D.C.R.-O.; supervision, R.R.-H.; project administration, S.D.C.R.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota–gut–brain axis. Physiol. Rev. 2023, 103, 277–320. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Heeney, D.D.; Gareau, M.G.; Marco, M.L. Intestinal Lactobacillus in health and disease, a driver or just along for the ride? Curr. Opin. Biotechnol. 2018, 49, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 508738. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Santander-Cortés, A.I.; Cástro-Rosas, J. Isolation of lactic acid bacteria with probiotic potential from typical fermented Mexican food: A review. Pädi Boletín Científico Cienc. Básicas E Ing. Del ICBI 2024, 11, 59–68. [Google Scholar] [CrossRef]
- Yang, X.; Hong, J.; Wang, L.; Cai, C.; Mo, H.; Wang, J.; Fang, X.; Liao, Z. Effect of Lactic Acid Bacteria Fermentation on Plant-Based Products. Fermentation 2024, 10, 48. [Google Scholar] [CrossRef]
- Abdul Hakim, B.N.; Xuan, N.J.; Hazwani Oslan, S.N. A Comprehensive Review of Bioactive Compounds from Lactic Acid Bacteria: Potential Functions as Functional Food in Dietetics and the Food Industry. Foods 2023, 12, 2850. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Cui, Y.; Wang, L.; Duan, J.; Yang, X.; Liu, X.; Zhang, S.; Sun, B.; Yu, H.; et al. Characteristics of Lactic Acid Bacteria as Potential Probiotic Starters and Their Effects on the Quality of Fermented Sausages. Foods 2024, 13, 198. [Google Scholar] [CrossRef]
- Ran, J.; Tang, Y.; Mao, W.; Meng, X.; Jiao, L.; Li, Y.; Zhao, R.; Zhou, H. Optimization of the fermentation process and antioxidant activity of mixed lactic acid bacteria for honeysuckle beverage. Front. Microbiol. 2024, 15, 1364448. [Google Scholar] [CrossRef]
- Asha, M.N.; Chowdhury, M.S.R.; Hossain, H.; Rahman, M.A.; Emon, A.A.; Tanni, F.Y.; Islam, M.R.; Hossain, M.M.; Rahman, M.M. Antibacterial potential of lactic acid bacteria isolated from raw cow milk in Sylhet district, Bangladesh: A molecular approach. Vet. Med. Sci. 2024, 10, e1463. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Li, W.; Li, J.; Zeng, S.; Chen, Z.; Gao, J.; Zheng, H.; Lin, H.; Zhu, G.; Qin, X.; et al. Dynamic changes of zinc chemical speciation and zinc-containing peptides release in oysters (Crassostrea hongkongensis) during enzymatic hydrolysis. Food Biosci. 2024, 58, 103649. [Google Scholar] [CrossRef]
- Wang, H.; He, X.; Li, J.; Wu, J.; Jiang, S.; Xue, H.; Zhang, J.; Jha, R.; Wang, R. Lactic acid bacteria fermentation improves physicochemical properties, bioactivity, and metabolic profiles of Opuntia ficus-indica fruit juice. Food Chem. 2024, 453, 139646. [Google Scholar] [CrossRef] [PubMed]
- Razola-Díaz, M.D.C.; De Montijo-Prieto, S.; Aznar-Ramos, M.J.; Jiménez-Valera, M.; Ruiz-Bravo, A.; Verardo, V.; Gómez-Caravaca, A.M. Effect of lactic acid bacteria fermentation on the polar compounds content with antioxidant and antidiabetic activity of avocado seed extracts. Fermentation 2023, 9, 420. [Google Scholar] [CrossRef]
- He, Y.; Zhu, Y.; Lv, J.; Gu, Y.; Wang, T.; Chen, J. Effects of lactic acid bacteria fermentation on the bioactive composition, volatile compounds and antioxidant activity of Huyou (Citrus aurantium ‘Changshan-huyou’) peel and pomace. Food Qual. Saf. 2023, 7, fyad003. [Google Scholar] [CrossRef]
- Leksono, B.Y.; Cahyanto, M.N.; Rahayu, E.S.; Yanti, R.; Utami, T. Enhancement of antioxidant activities in black soy milk through isoflavone aglycone production during indigenous lactic acid bacteria fermentation. Fermentation 2022, 8, 326. [Google Scholar] [CrossRef]
- Zhang, P.; Tang, F.; Cai, W.; Zhao, X.; Shan, C. Evaluating the effect of lactic acid bacteria fermentation on quality, aroma, and metabolites of chickpea milk. Front. Nutr. 2022, 9, 1069714. [Google Scholar] [CrossRef]
- Abd Al-Halim, L.R.; Nasr, N.M. Quality of new Lactobaillus rhamnosus like-yoghurt and the effect of using gum Arabic as prebiotic. Fayoum J. Agric. Res. Dev. 2024, 38, 381–391. [Google Scholar] [CrossRef]
- Colmenares-Cuevas, S.I.; Contreras-Oliva, A.; Salinas-Ruiz, J.; Hidalgo-Contreras, J.V.; Flores-Andrade, E.; García-Ramírez, E.J. Development and study of the functional properties of marshmallow enriched with bee (Apis mellifera) honey and encapsulated probiotics (Lactobacillus rhamnosus). Front. Nutr. 2024, 11, 1353530. [Google Scholar] [CrossRef]
- Shori, A.B. Comparative analysis of Lactobacillus starter cultures in fermented camel milk: Effects on viability, antioxidant properties, and sensory characteristics. Foods 2024, 13, 2711. [Google Scholar] [CrossRef]
- Ngamsamer, C.; Muangnoi, C.; Tongkhao, K.; Sae-Tan, S.; Treesuwan, K.; Sirivarasai, J. Potential health benefits of fermented vegetables with additions of Lacticaseibacillus rhamnosus GG and polyphenol vitexin based on their antioxidant properties and prohealth profiles. Foods 2024, 13, 982. [Google Scholar] [CrossRef] [PubMed]
- Karimkhani, M.M.; Jamshidi, A.; Nasrollahzadeh, M.; Armin, M.; Jafari, S.M.; Zeinali, T. Fermentation of Rubus dolichocarpus juice using Lactobacillus gasseri and Lacticaseibacillus casei and protecting phenolic compounds by Stevia extract during cold storage. Sci. Rep. 2024, 14, 5711. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.D.L.; Monteiro, S.S.; Pasquali, M.A.D.B. Study of fermentation strategies by Lactobacillus gasseri for the Production of probiotic food using passion fruit juice combined with green tea as raw material. Foods 2022, 11, 1471. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Jiang, Y.; Zhang, X.; Cui, S.; Liu, X.; Zhao, J.; Zhang, H.; Chen, W. Comparative peptidomics analysis of Milk Fermented by Lactobacillus helveticus. Foods 2022, 11, 3885. [Google Scholar] [CrossRef]
- Skrzypczak, K.; Gustaw, W.; Fornal, E.; Kononiuk, A.; Michalak-Majewska, M.; Radzki, W.; Waśko, A. Functional and technological potential of whey protein isolate in production of milk beverages fermented by new strains of lactobacillus helveticus. Appl. Sci. 2020, 10, 7089. [Google Scholar] [CrossRef]
- Hovhannisyan, H.G.; Danielyan, L.V.; Chichoyan, N.B.; Pashayan, M.M.; Baghdasaryan, L.G.; Melkumyan, I.E.; Barseghyan, A.H. Improvement of functional and sensory properties of fermented dairy drink Narine using raw apricot gum. Funct. Foods Health Dis. 2024, 14, 600–614. [Google Scholar] [CrossRef]
- Gugel, I.; Marchetti, F.; Costa, S.; Gugel, I.; Baldini, E.; Vertuani, S.; Manfredini, S. 2G-lactic acid from olive oil supply chain waste: Olive leaves upcycling via Lactobacillus casei fermentation. Appl. Microbiol. Biotechnol. 2024, 108, 379. [Google Scholar] [CrossRef]
- Lugo-Zarate, L.; Delgado-Olivares, L.; Cruz-Cansino, N.D.S.; González-Olivares, L.G.; Castrejón-Jiménez, N.S.; Estrada-Luna, D.; Jiménez-Osorio, A.S. Blackberry juice fermented with two consortia of lactic acid bacteria and isolated whey: Physicochemical and antioxidant properties during storage. Int. J. Mol. Sci. 2024, 25, 8882. [Google Scholar] [CrossRef]
- Jeong, D.-E.; Kim, J.; Kim, B.; Cho, Y. Effects on antioxidant, whitening, and anti-wrinkle omprovement of ethanol extracts from fermented Trigonotis radicans var. sericea by Lactobacillus brevis. Food Sci. Preserv. 2024, 31, 173–182. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, C.; Guo, Z.; Li, S.; Zhu, Z.; Grimi, N.; Xiao, J. Fermentation of Betaphycus gelatinum using lactobacillus brevis: Growth of probiotics, total polyphenol content, polyphenol profile, and antioxidant capacity. Foods 2023, 12, 3334. [Google Scholar] [CrossRef]
- De Montijo-Prieto, S.; Razola-Díaz, M.D.C.; Barbieri, F.; Tabanelli, G.; Gardini, F.; Jiménez-Valera, M.; Ruiz-Bravo, A.; Verardo, V.; Gómez-Caravaca, A.M. Impact of lactic acid bacteria fermentation on phenolic compounds and antioxidant activity of avocado leaf extracts. Antioxidants 2023, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Neylon, E.; Nyhan, L.; Zannini, E.; Monin, T.; Münch, S.; Sahin, A.W.; Arendt, E.K. Food ingredients for the future: In-depth analysis of the effects of lactic acid bacteria fermentation on spent barley rootlets. Fermentation 2023, 9, 78. [Google Scholar] [CrossRef]
- Sionek, B.; Szydłowska, A.; Trząskowska, M.; Kołożyn-Krajewska, D. The impact of physicochemical conditions on lactic acid bacteria survival in food products. Fermentation 2024, 10, 298. [Google Scholar] [CrossRef]
- Fazio, A.; La Torre, C.; Caroleo, M.C.; Caputo, P.; Cannataro, R.; Plastina, P.; Cione, E. Effect of addition of pectins from jujubes (Ziziphus jujuba Mill.) on vitamin C production during heterolactic fermentation. Molecules 2020, 25, 2706. [Google Scholar] [CrossRef]
- Hou, F.; Cai, Y.; Wang, J. Antioxidant capacity changes and untargeted metabolite profile of broccoli during lactic acid bacteria fermentation. Fermentation 2023, 9, 474. [Google Scholar] [CrossRef]
- Abdel-Salam, S.W.; Abdel-Aziz, M.H.; Abdel-Razik, M.; Mattar, Z.A.; Abdel-Aziz, A.B.; Makharitta, R.R. Advanced in the production of L-Ascorbic acid using biotechnological processes. Int. J. Sci. Res. Arch. 2024, 3, 546–556. [Google Scholar] [CrossRef]
- Xie, Z.; McAuliffe, O.; Jin, Y.S.; Miller, M.J. Invited review: Genomic modifications of lactic acid bacteria and their applications in dairy fermentation. J. Dairy Sci. 2024, 107, 8749–8764. [Google Scholar] [CrossRef]
- Dong, H.; Wang, H.; Fu, S.; Zhang, D. CRISPR/Cas tools for enhancing the biopreservation ability of lactic acid bacteria in aquatic products. Front. Bioeng. Biotechnol. 2022, 10, 1114588. [Google Scholar] [CrossRef]
- Icer, M.A.; Sarikaya, B.; Kocyigit, E.; Atabilen, B.; Celik, M.N.; Capasso, R.; Agagündüz, D.; Budán, F. Contributions of Gamma-Aminobutyric Acid (GABA) produced by lactic acid bacteria on food quality and human health: Current applications and futes prospects. Foods 2024, 13, 2437. [Google Scholar] [CrossRef]
- Gagneten, M.; Passot, S.; Cenard, S.; Ghorbal, S.; Schebor, C.; Fonseca, F. Mechanistic study of the differences in lactic acid bacteria resistance to freeze or spray drying and storage. Appl. Microbiol. Biotechnol. 2024, 108, 361. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, W.; Li, Y.; Cong, Y. Enteroendocrine cells: Sensing gut microbiota and regulating inflammatory bowel diseases. Inflamm. Bowel Dis. 2019, 26, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Scavuzzo, C.J.; Rakotovao, I.; Dickson, C.T. Differential effects of L-and D-lactate on memory encoding and consolidation: Potential role of HCAR1 signaling. Neurobiol. Learn. Mem. 2020, 168, 107151. [Google Scholar] [CrossRef] [PubMed]
- Luiz, J.; Manassi, C.; Magnani, M.; Cruz, A.; Pimentel, T.; Verruck, S. Lactiplantibacillus plantarum as a promising adjuvant for neurological disorders therapy through the brain-gut axis and related action pathways. Crit. Rev. Food Sci. Nutr. 2025, 65, 715–727. [Google Scholar] [CrossRef]
- Watanabe, T.; Nishio, H.; Tanigawa, T.; Yamagami, H.; Okazaki, H.; Watanabe, K.; Arakawa, T. Probiotic Lactobacillus casei strain Shirota prevents indomethacin-induced small intestinal injury: Involvement of lactic acid. Am. J. Physiol.-Gastrointest. Liver Physiol. 2009, 297, G506–G513. [Google Scholar] [CrossRef] [PubMed]
- Muto, M.; Kaji, T.; Onishi, S.; Yano, K.; Yamada, W.; Ieiri, S. An overview of the current management of short-bowel syndrome in pediatric patients. Surg. Today 2022, 52, 12–21. [Google Scholar] [CrossRef]
- Bamigbade, G.B.; Abdin, M.; Subhash, A.; Arachchi, M.P.; Ullah, N.; Gan, R.Y.; Ali, A.; Kamal-Eldin, A.; Ayyash, M. Plant polysaccharide-capped nanoparticles: A sustainable approach to modulate gut microbiota and advance functional food applications. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70156. [Google Scholar] [CrossRef]
- Averina, O.V.; Zorkina, Y.A.; Yunes, R.A.; Kovtun, A.S.; Ushakova, V.M.; Morozova, A.Y.; Kostyuk, G.P.; Danilenko, V.N.; Chekhonin, V.P. Bacterial metabolites of human gut microbiota correlating with depression. Int. J. Mol. Sci. 2020, 21, 9234. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Erny, D.; Dokalis, N.; Mezö, C.; Castoldi, A.; Mossad, O.; Staszewski, O.; Frosch, M.; Villa, M.; Fuchs, V.; Mayer, A.; et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab. 2021, 33, 2260–2276.e7. [Google Scholar] [CrossRef]
- Solsona, R. Impact of the Short-Chain Fatty Acids on the Microbiota-Gut-Brain Axis. 2021. Available online: https://diposit.ub.edu/dspace/handle/2445/179149 (accessed on 13 June 2025).
- Stilling, R.M.; Van De Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Qian, X.H.; Xie, R.Y.; Liu, X.L.; Chen, S.D.; Tang, H.D. Mechanisms of short-chain fatty acids derived from gut microbiota in Alzheimer’s disease. Aging Dis. 2022, 13, 1252. [Google Scholar] [CrossRef] [PubMed]
- Barki, N.; Bolognini, D.; Börjesson, U.; Jenkins, L.; Riddell, J.; Hughes, D.I.; Ulven, T.; Hudson, B.D.; Ulven, E.R.; Dekker, N.; et al. Chemogenetics defines a short-chain fatty acid receptor gut–brain axis. eLife 2022, 11, e73777. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.; Venkatesh, I.; Basu, A. Short-Chain Fatty Acids in the Microbiota-Gut-Brain Axis: Role in Neurodegenerative Disorders and Viral Infections. ACS Chem. Neurosci. 2023, 14, 1045–1062. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.F.; Miao, J.; Zheng, R.F.; Li, J.Y. Short-chain fatty acids: Important components of the gut-brain axis against AD. Biomed. Pharmacother. 2024, 175, 116601. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Noto, D.; Hoshino, Y.; Mizuno, M.; Miyake, S. Butyrate suppresses demyelination and enhances remyelination. J. Neuroinflamm. 2019, 16, 165. [Google Scholar] [CrossRef]
- Hoyles, L.; Snelling, T.; Umlai, U.; Nicholson, J.; Carding, S.; Glen, R.; McArthur, S. Microbiome–host systems interactions: Protective effects of propionate upon the blood–brain barrier. Microbiome 2018, 6, 55. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, S.; Liu, L.; Mao, A.; Kan, H.; Yu, F.; Zhou, T. The gut microbiota-derived metabolite indole-3-propionic acid enhances leptin sensitivity by targeting STAT3 against diet-induced obesity. Clin. Transl. Med. 2024, 14, e70053. [Google Scholar] [CrossRef]
- Pan, T.; Pei, Z.; Fang, Z.; Wang, H.; Zhu, J.; Zhang, H.; Lu, W. Uncovering the specificity and predictability of tryptophan metabolism in lactic acid bacteria with genomics and metabolomics. Front. Cell. Infect. Microbiol. 2023, 13, 1154346. [Google Scholar] [CrossRef]
- Flannigan, K.L.; Nieves, K.M.; Szczepanski, H.E.; Serra, A.; Lee, J.W.; Alston, L.A.; Ramay, H.; Mani, S.; Hirota, S.A. The pregnane X receptor and indole-3-propionic acid shape the intestinal mesenchyme to restrain inflammation and fibrosis. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 765–795. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.; Kong, Y.; Ye, T.; Yu, Q.; Satyanarayanan, S.K.; Su, K.P.; Liu, J. Microbiota-derived metabolite Indoles induced aryl hydrocarbon receptor activation and inhibited neuroinflammation in APP/PS1 mice. Brain Behav. Immun. 2022, 106, 76–88. [Google Scholar] [CrossRef]
- Zheng, L.; Xin, J.; Ye, H.; Sun, N.; Gan, B.; Gong, X.; Zhang, T. Lactobacillus Johnsonii YH1136 alleviates schizophrenia-like behavior in mice: A gut–microbiota–brain axis hypothesis study. BMC Microbiol. 2025, 25, 191. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, P.; Cai, Z.; He, P.; Wang, J.; He, H.; Zhao, J. Buqi-Huoxue-Tongnao decoction drives gut microbiota-derived indole lactic acid to attenuate ischemic stroke via the gut-brain axis. Chin. Med. 2024, 19, 126. [Google Scholar] [CrossRef] [PubMed]
- Sahab, N.R.; Subroto, E.; Balia, R.L.; Utama, G.L. γ-Aminobutyric acid found in fermented foods and beverages: Current trends. Heliyon 2020, 6, e05526. [Google Scholar] [CrossRef] [PubMed]
- Jena, R.; Choudhury, P.K. Lactic acid bacteria in fermented dairy foods: Gamma-aminobutyric acid (GABA) production and its therapeutic implications. Food Biosci. 2024, 62, 105276. [Google Scholar] [CrossRef]
- Gutiérrez, L.C.D. Development of a Microencapsulated Functional Ingredient Formulated with the Neurotransmitter GABA and the Lactiplantibacillus plantarum K16 Produced Through Biotechnological. Ph.D. Thesis, Universidad del País Vasco-Euskal Herriko Unibertsitatea, Vitoria-Gasteiz, Spain, 2023. [Google Scholar]
- Salvador, A.G.; Antolinez, S.Q.; Furundarena, I.H.; Aróstegui, S.; de Cerio, F.G. Mental illness and healthy nutrition. New alternatives for its treatment. Rev. Esp. Nutr. Comunitaria 2021, 27, 70–81. [Google Scholar]
- Ikegami, M.; Narabayashi, H.; Nakata, K.; Yamashita, M.; Sugi, Y.; Fuji, Y.; Matsufuji, H.; Harata, G.; Yoda, K.; Miyazawa, K.; et al. Intervention in gut microbiota increases intestinal γ-aminobutyric acid and alleviates anxiety behavior: A possible mechanism via the action on intestinal epithelial cells. Front. Cell. Infect. Microbiol. 2024, 14, 1421791. [Google Scholar] [CrossRef]
- Martínez Blanco, P. Microorganismos Como Medicamentos, ¿Podemos Gestionar la Microbiota? 2020. Available online: http://hdl.handle.net/10902/19434 (accessed on 13 June 2025).
- Lacouture, A.T. Relación de la Microbiota Intestinal con el Desarrollo de Depresión y Ansiedad. Bachelor’s Thesis, Universidad Vasco de Quiroga, Morelia, Michoacán, 2023. [Google Scholar]
- Conn, K.; Borsom, E.; Cope, E. Implications of microbe-derived ɣ-aminobutyric acid (GABA) in gut and brain barrier integrity and GABAergic signaling in Alzheimer’s disease. Gut Microbes 2024, 16, 2371950. [Google Scholar] [CrossRef]
- Nguyen, H.-T.; Pham, T.-T.; Nguyen, P.-T.; Le-Buanec, H.; Rabetafika, H.N.; Razafindralambo, H.L. Advances in microbial exopolysaccharides: Present and future applications. Biomolecules 2024, 14, 1162. [Google Scholar] [CrossRef]
- Yegorenkova, I.V.; Tregubova, K.V.; Matora, L.Y.; Burygin, G.L.; Ignatov, V.V. Biofilm formation by Paenibacillus polymyxa strains differing in the production and rheological properties of their exopolysaccharides. Curr. Microbiol. 2011, 62, 1554–1559. [Google Scholar] [CrossRef]
- Li, S.; Huang, R.; Shah, N.P.; Tao, X.; Xiong, Y.; Wei, H. Antioxidant and antibacterial activities of exopolysaccharides from Bifidobacterium bifidum WBIN03 and Lactobacillus plantarum R315. J. Dairy Sci. 2014, 97, 7334–7343. [Google Scholar] [CrossRef]
- Sirin, S.; Aslim, B. Characterization of lactic acid bacteria derived exopolysaccharides for use as a defined neuroprotective agent against amyloid beta1–42-induced apoptosis in SH-SY5Y cells. Sci. Rep. 2020, 10, 8124. [Google Scholar] [CrossRef]
- Gientka, I.; Błażejak, S.; Stasiak-Różańska, L.; Chlebowska-Śmigiel, A. Exopolysaccharides from yeast: Insight into optimal conditions for biosynthesis, chemical composition and functional properties-review. Acta Sci. Pol. Technol. Aliment. 2015, 14, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Ayyash, M.; Abu-Jdayil, B.; Itsaranuwat, P.; Galiwango, E.; Tamiello-Rosa, C.; Abdullah, H.; Esposito, G.; Hunashal, Y.; Obaid, R.S.; Hamed, F. Characterization, bioactivities, and rheological properties of exopolysaccharide produced by novel probiotic Lactobacillus plantarum C70 isolated from camel milk. Int. J. Biol. Macromol. 2020, 144, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Hsieh, Y.-H.; Hsu, C.-C.; Lin, C.-H.; Chen, Y.-H. Exopolysaccharide is the potential effector of Lactobacillus fermentum PS150, a hypnotic psychobiotic strain. Front. Microbiol. 2023, 14, 1209067. [Google Scholar] [CrossRef]
- Flores-Maciel, M.G.; Ochoa-Martínez, A.; Rutiaga-Quiñones, O.M.; Rutiaga-Quiñones, J.G. Importance of lactic acid bacteria as producers of exopolysaccharides. Agraria 2024, 1, 2. [Google Scholar]
- Zhang, J.; He, J.; Hu, J.; Ji, Y.; Lou, Z. Exploring the role of gut microbiota in depression: Pathogenesis and therapeutic insights. Asian J. Psychiatry 2025, 105, 104411. [Google Scholar] [CrossRef]
- Utama, G.L.; Sahab, N.R.M.; Nurmilah, S.; Yarlina, V.P.; Subroto, E.; Balia, R.L. Unveiling microbial dynamics in terasi spontaneous fermentation: Insights into glutamate and GABA production. Curr. Res. Food Sci. 2025, 10, 100950. [Google Scholar] [CrossRef]
- Chintakovid, N.; Singkhamanan, K.; Yaikhan, T.; Nokchan, N.; Wonglapsuwan, M.; Jitpakdee, J.; Kantachote, D.; Surachat, K. Probiogenomic analysis of Lactiplantibacillus plantarum SPS109: A potential GABA-producing and cholester-ol-lowering probiotic strain. Heliyon 2024, 10, e33823. [Google Scholar] [CrossRef]
- Karpiński, P.; Żebrowska-Różańska, P.; Kujawa, D.; Łaczmański, Ł.; Samochowiec, J.; Jabłoński, M.; Plichta, P.; Piotrowski, P.; Bielawski, T.; Misiak, B. Gut microbiota alterations in schizophrenia might be related to stress exposure: Findings from the machine learning analysis. Psychoneuroendocrinology 2023, 155, 106335. [Google Scholar] [CrossRef]
- Malan-Müller, S.; Martín-Hernández, D.; Caso, J.R.; Matthijnssens, J.; Rodríguez-Urrutia, A.; Lowry, C.A.; Leza, J.C. Metagenomic symphony of the intestinal ecosystem: How the composition affects the mind. Brain Behav. Immun. 2025, 123, 510–523. [Google Scholar] [CrossRef]
- Feng, J.; Cen, Q.; Cui, Y.; Hu, X.; Li, M.; Wang, L.; Wei, J.; Sun, N.; Wang, J.; Zhang, A. Lactobacillus rhamnosus: An emerging probiotic with therapeutic potential for depression. Pharmacol. Res. 2025, 211, 107541. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Vemuganti, V.; Kuehn, J.F.; Ulland, T.K.; Rey, F.E.; Bendlin, B.B. Gut microbial metabolism in Alzheimer’s disease and related dementias. Neurotherapeutics 2024, 21, e00470. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Nasser, A.; Hathout, A.S.; Badr, A.N.; Barakat, O.S.; Fathy, H.M. Extraction and characterization of bioactive secondary metabolites from lactic acid bacteria and evaluating their antifungal and antiaflatoxigenic activity. Biotechnol. Rep. 2023, 38, e00799. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.M.; Maione, A.; Buonanno, A.; Guida, M.; Andolfi, A.; Salvatore, F.; Galdiero, E. Biological activities, biosynthetic capacity and metabolic interactions of lactic acid bacteria and yeast strains from traditional home-made kefir. Food Chem. 2025, 470, 142657. [Google Scholar] [CrossRef]
- De Luca, L.; Pizzolongo, F.; Calabrese, M.; Blaiotta, G.; Aponte, M.; Romano, R. Addition of glutamine to milk during fermentation by individual strains of lactic acid bacteria and the effects on pyroglutamic and butyric acid. J. Food Compos. Anal. 2024, 130, 106175. [Google Scholar] [CrossRef]
- Singh, H.; Chopra, C.; Singh, H.; Malgotra, V.; Khurshid Wani, A.; Singh Dhanjal, D.; Sharma, I.; Nepovimova, E.; Alomar, S.; Singh, R.; et al. Gut-brain axis and Alzheimer’s disease: Therapeutic interventions and strategies. J. Funct. Foods 2024, 112, 105915. [Google Scholar] [CrossRef]
- Liang, Y.; Cui, L.; Gao, J.; Zhu, M.; Zhang, Y.; Zhang, H.-L. Gut Microbial Metabolites in Parkinson’s Disease: Implications of Mitochondrial Dysfunction in the Pathogenesis and Treatment. Mol. Neurobiol. 2021, 58, 3745–3758. [Google Scholar] [CrossRef]
- Alli, S.R.; Gorbovskaya, I.; Liu, J.C.W.; Kolla, N.J.; Brown, L.; Müller, D.J. The gut microbiome in depression and potential benefit of prebiotics, probiotics and synbiotics: A systematic review of clinical trials and observational studies. Int. J. Mol. Sci. 2022, 23, 4494. [Google Scholar] [CrossRef]
- Misera, A.; Kaczmarczyk, M.; Łoniewski, I.; Liśkiewicz, P.; Podsiadło, K.; Misiak, B.; Skonieczna-Żydecka, K.; Samochowiec, J. Comparative analysis of gut microbiota in major depressive disorder and schizophrenia during hospitalisation—The case-control, post hoc study. Psychoneuroendocrinology 2025, 171, 107208. [Google Scholar] [CrossRef]
- Merchak, A.R.; Wachamo, S.; Brown, L.C.; Thakur, A.; Moreau, B.; Brown, R.M.; Rivet-Noor, C.R.; Raghavan, T.; Gaultier, A. Lactobacillus from the Altered Schaedler Flora maintain IFNγ homeostasis to promote behavioral stress resilience. Brain Behav. Immun. 2024, 115, 458–469. [Google Scholar] [CrossRef]
- Zhang, P.; Jin, W.; Lyu, Z.; Lyu, X.; Li, L. Study on the mechanism of gut microbiota in the pathogenetic interaction between depression and Parkinson ‘s disease. Brain Res. Bull. 2024, 215, 111001. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, Y.; Wang, Z.; Wang, R.; Dong, Y. Lactiplantibacillus plantarum CR12 attenuates chronic unforeseeable mild stress induced anxiety and depression-like behaviors by modulating the gut microbiota-brain axis. J. Funct. Foods 2023, 107, 105710. [Google Scholar] [CrossRef]
- Qian, X.; Li, Q.; Zhu, H.; Chen, Y.; Lin, G.; Zhang, H.; Chen, W.; Wang, G.; Tian, P. Bifidobacteria with indole-3-lactic acid-producing capacity exhibit psychobiotic potential via reducing neuroinflammation. Cell Rep. Med. 2024, 5, 101798. [Google Scholar] [CrossRef] [PubMed]
- Sai, Y.; Ge, W.; Zhong, L.; Zhang, Q.; Xiao, J.; Shan, Y.; Ye, W.; Liu, H.; Liu, S.; Ye, F.; et al. The role of the gut microbiota and the nicotinate/nicotinamide pathway in rotenone-induced neurotoxicity. Curr. Res. Toxicol. 2025, 8, 100212. [Google Scholar] [CrossRef]
- Wang, L.; Tsai, C.; Chou, W.; Kuo, H.; Huang, Y.; Lee, S.; Dai, H.; Yang, C.; Li, C.; Yeh, Y. Add-On Bifidobacterium bifidum supplement in children with attention-deficit/hyperactivity disorder: A 12-Week randomized double-blind placebo-controlled clinical trial. Nutrients 2024, 16, 2260. [Google Scholar] [CrossRef]
- Tanure, Y.C.B.; Mafra, A.C.M.; Guimarães, B.L.M.; Magalhães, R.C.; Fagundez, C.; Nascimento, I.J.B.D.; Brito, J.C.M. Potential benefits of kefir and its compounds on Alzheimer’s Disease: A systematic review. Brain Behav. Immun. Integr. 2025, 10, 100115. [Google Scholar] [CrossRef]
- Dhami, M.; Raj, K.; Singh, S. Relevance of gut microbiota to Alzheimer’s Disease (AD): Potential effects of probiotic in management of AD. Aging Health Res. 2023, 3, 100128. [Google Scholar] [CrossRef]
- Kong, Q.; Chen, Q.; Mao, X.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium longum CCFM1077 ameliorated neurotransmitter disorder and neuroinflammation closely linked to regulation in the kynurenine pathway of autistic-like rats. Nutrients 2022, 14, 1615. [Google Scholar] [CrossRef]
- Miyanishi, H.; Nitta, A. A role of BDNF in the depression pathogenesis and a potential target as antidepressant: The modulator of stress sensitivity “Shati/Nat8l-BDNF system” in the dorsal striatum. Pharmaceuticals 2021, 14, 889. [Google Scholar] [CrossRef]
- Vergel Hernández, J.; Barrera Robledo, M.E. Management of depressive disorder: Which treatment should I choose? Med. J. Risaralda 2021, 27, 24637. [Google Scholar] [CrossRef]
- Miyaoka, T.; Kanayama, M.; Wake, R.; Hashioka, S.; Hayashida, M.; Nagahama, M.; Okazaki, S.; Yamashita, S.; Miura, S.; Miki, H.; et al. Clostridium butyricum MIYAIRI 588 as adjunctive therapy for treatment-resistant major depressive disorder: A prospective open-label trial. Clin. Neuropharmacol. 2018, 41, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Carrizo, S.L.; de Moreno de LeBlanc, A.; LeBlanc, J.G.; Rollán, G.C. Quinoa pasta fermented with lactic acid bacteria prevents nutritional deficiencies in mice. Food Res. Int. 2020, 127, 108735. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Liu, Z.; Xie, F.; Bilal, M.; Liu, L.; Yang, R.; Wang, Z. Microbial production of gamma-aminobutyric acid: Applications, state-of-the-art achievements, and future perspectives. Crit. Rev. Biotechnol. 2021, 41, 491–512. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, C.; D’Antongiovanni, V.; Benvenuti, L.; d’Amati, A.; Ippolito, C.; Segnani, C.; Pierucci, C.; Bellini, G.; Annese, T.; Virgintino, D.; et al. Lactiplantibacillus plantarum HEAL9 attenuates cognitive impairment and progression of Alzheimer’s disease and related bowel symptoms in SAMP8 mice by modulating microbiota-gut-inflammasome-brain axis. Food Funct. 2024, 15, 10323–10338. [Google Scholar] [CrossRef]
- Bravo, J.; Forsythe, P.; Chew, M.; Escaravage, E.; Savignac, H.; Dinan, T.; Bienenstock, J.; Cryan, J. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Van Son, J.; Koekkoek, L.L.; La Fleur, S.E.; Serlie, M.J.; Nieuwdorp, M. The role of the gut microbiota in the gut–brain axis in obesity: Mechanisms and future implications. Int. J. Mol. Sci. 2021, 22, 2993. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Geurts, L.; Hoyles, L.; Iozzo, P.; Kraneveld, A.D.; La Fata, G.; Miani, M.; Patterson, E.; Pot, B.; Shortt, C.; et al. The microbiota–gut–brain axis: Pathways to better brain health. Perspectives on what we know, what we need to investigate and how to put knowledge into practice. Cell. Mol. Life Sci. 2022, 79, 80. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).