The Effect of ESTAAN on Must Browning Induced by Fungal Disease (Botrytis cinerea) That Affects Grapes
Abstract
1. Introduction
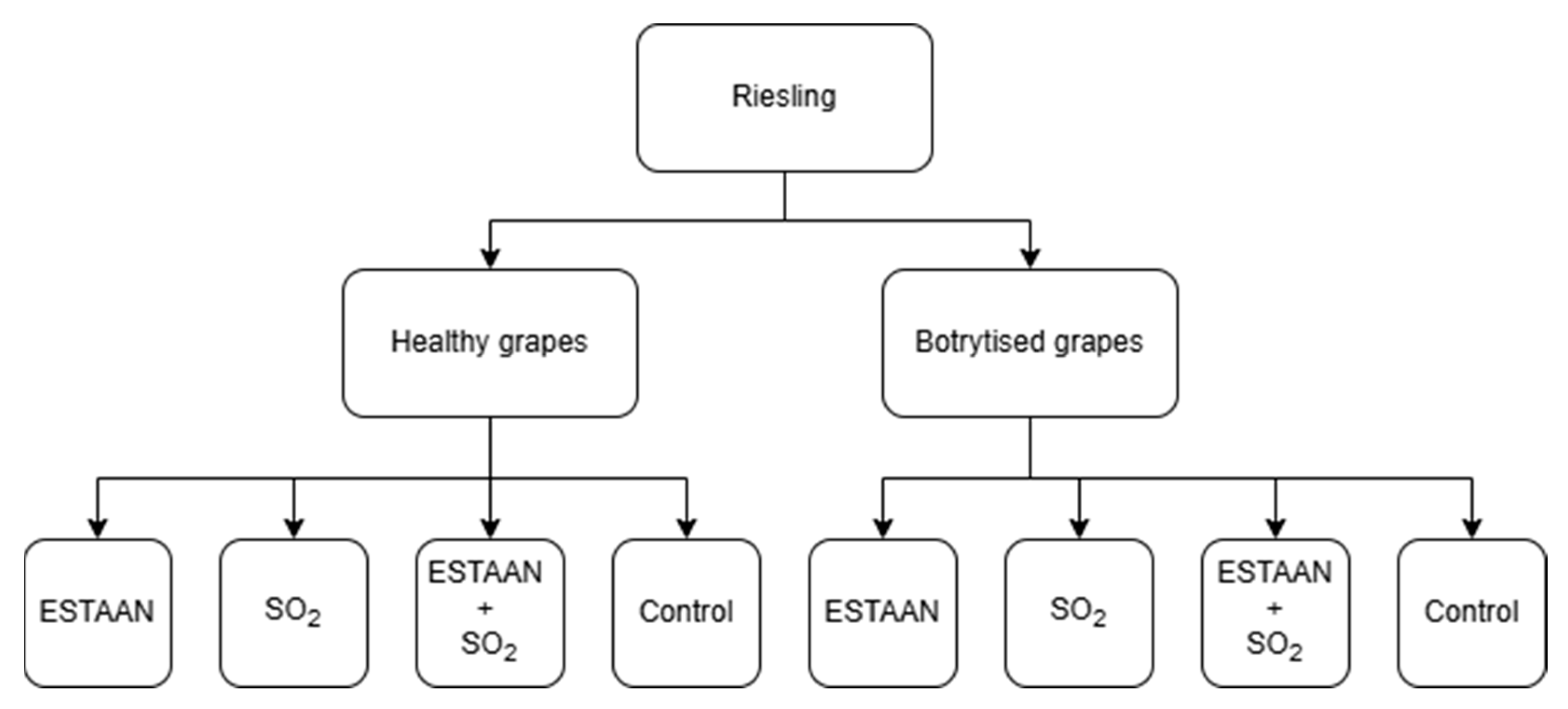
2. Materials and Methods
- Variant (1) Healthy grapes: (1a) 2 mL·L−1 ESTAAN; (1b) 40 mg·L−1 SO2; (1c) 1 mL·L−1 ESTAAN + 20 mg·L−1 SO2; (1d) CONTROL (no additives), all in triplicate.
- Variant (2) Infected grapes: (2a) 2 mL·L-1 ESTAAN; (2b) 40 mg·L−1 SO2; (2c) 1 mL·L−1 ESTAAN + 20 mg·L−1 SO2; (2d) CONTROL (no additives), all in triplicate.
2.1. Determination of Glucose, Fructose, and Organic Acids by HPLC
2.2. Determination of Laccase Activity
2.3. Determination of Total Phenolic Compounds
2.4. Determination of Acetaldehyde by HPLC
2.5. Determination of Geraniol and Fatty Acid Esters by GC-MS
2.6. Determination of Total Sulfur Dioxide Content (Iodometric Titration)
2.7. Sensory Analysis
2.8. Statistical Evaluation
3. Results
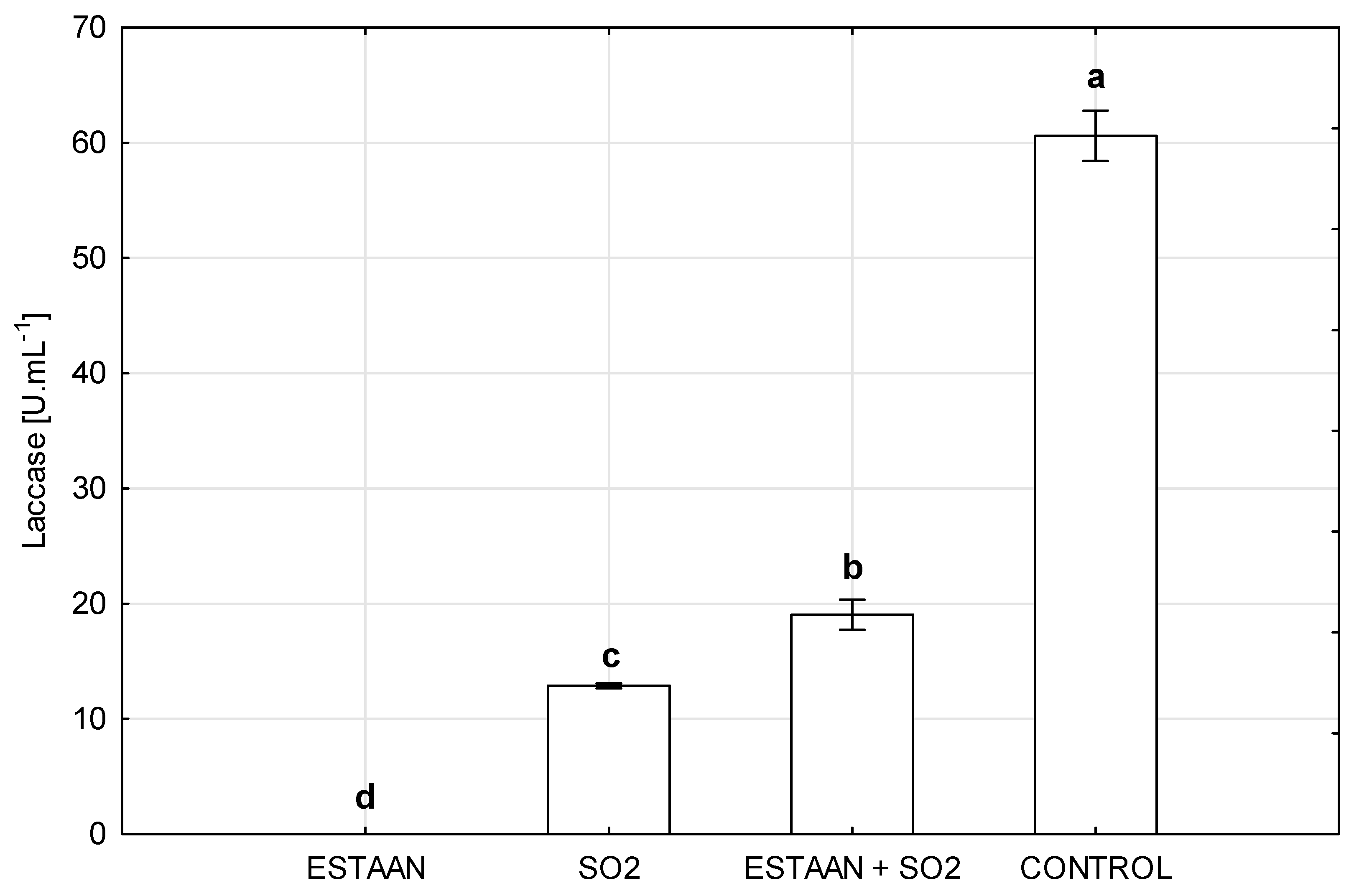
3.1. Activity of Laccase in Botrytized Must
3.2. Total Polyphenol Content in Musts from Healthy and Botrytized Grapes
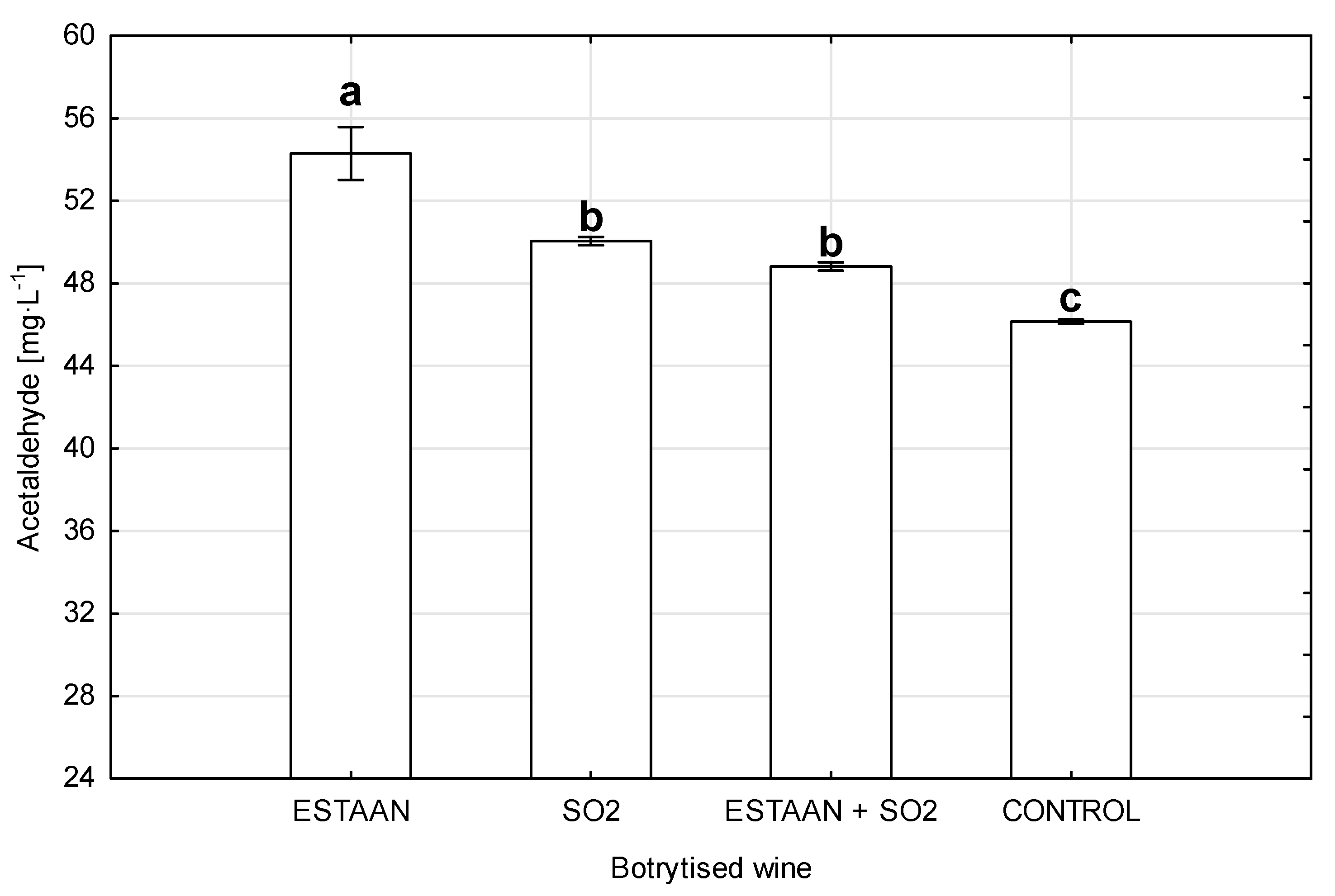
3.3. Acetaldehyde Content in Wines from Healthy and Botrytized Grapes
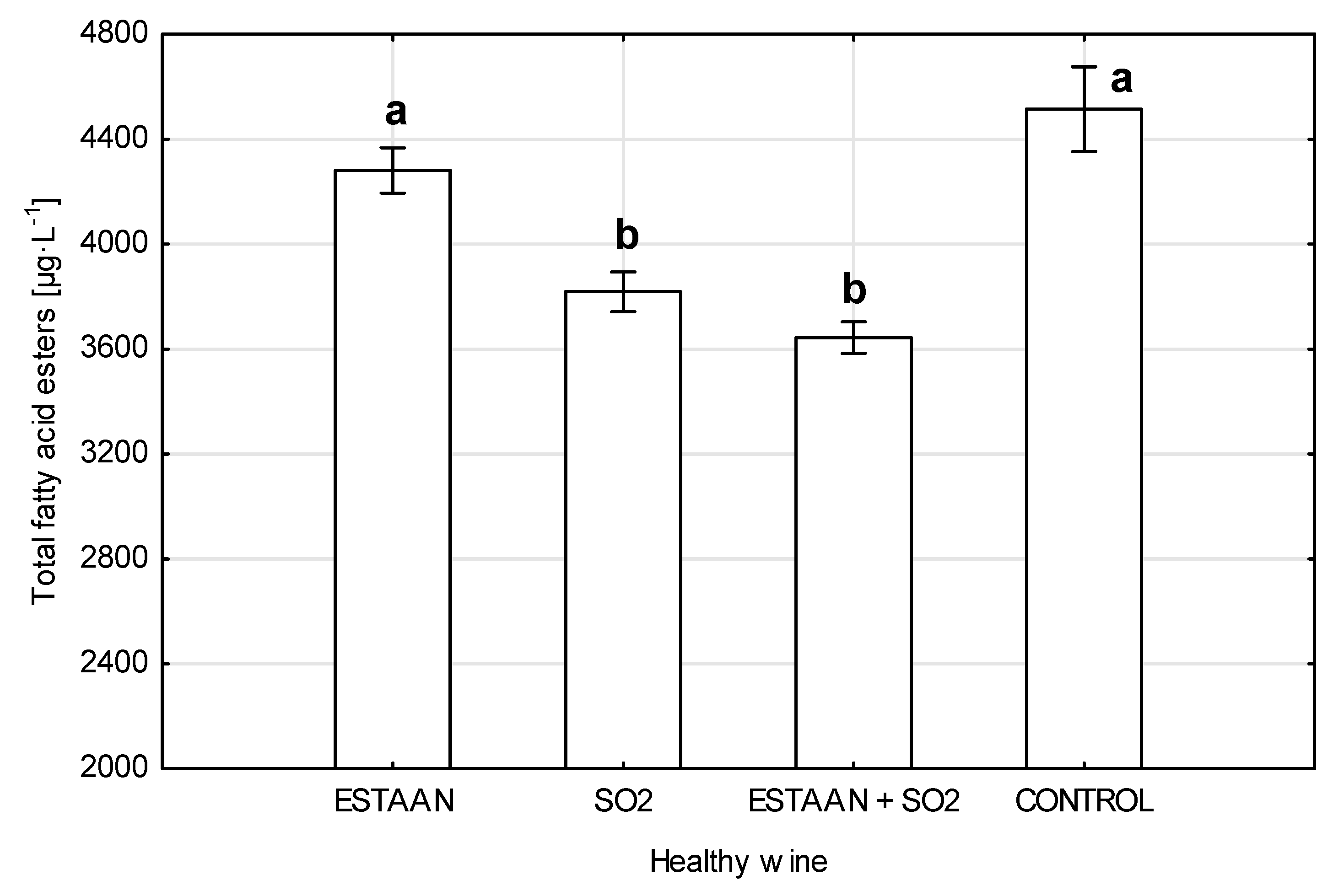
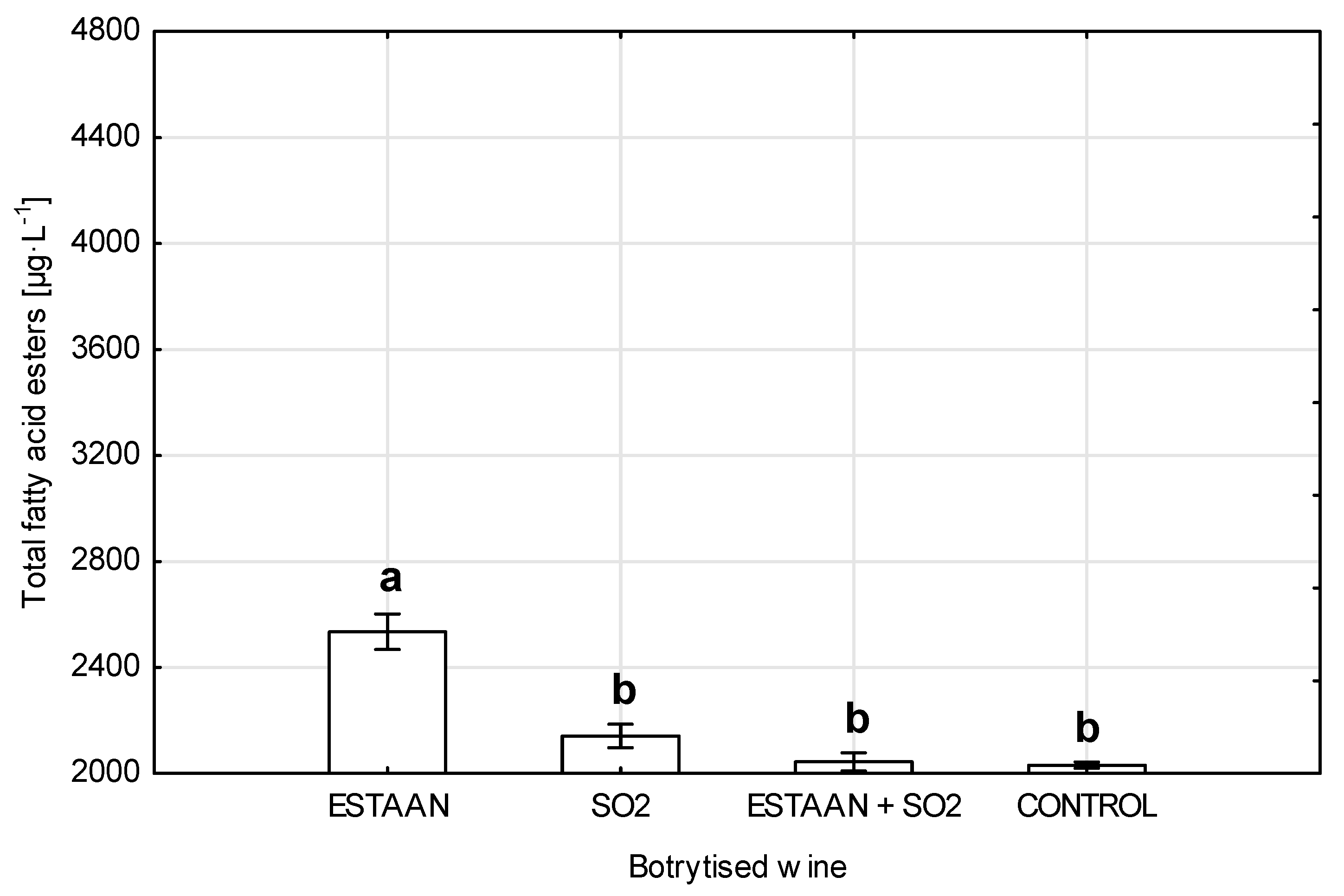
3.4. Fatty Acid Ester Content in Wine from Healthy and Botrytized Grapes
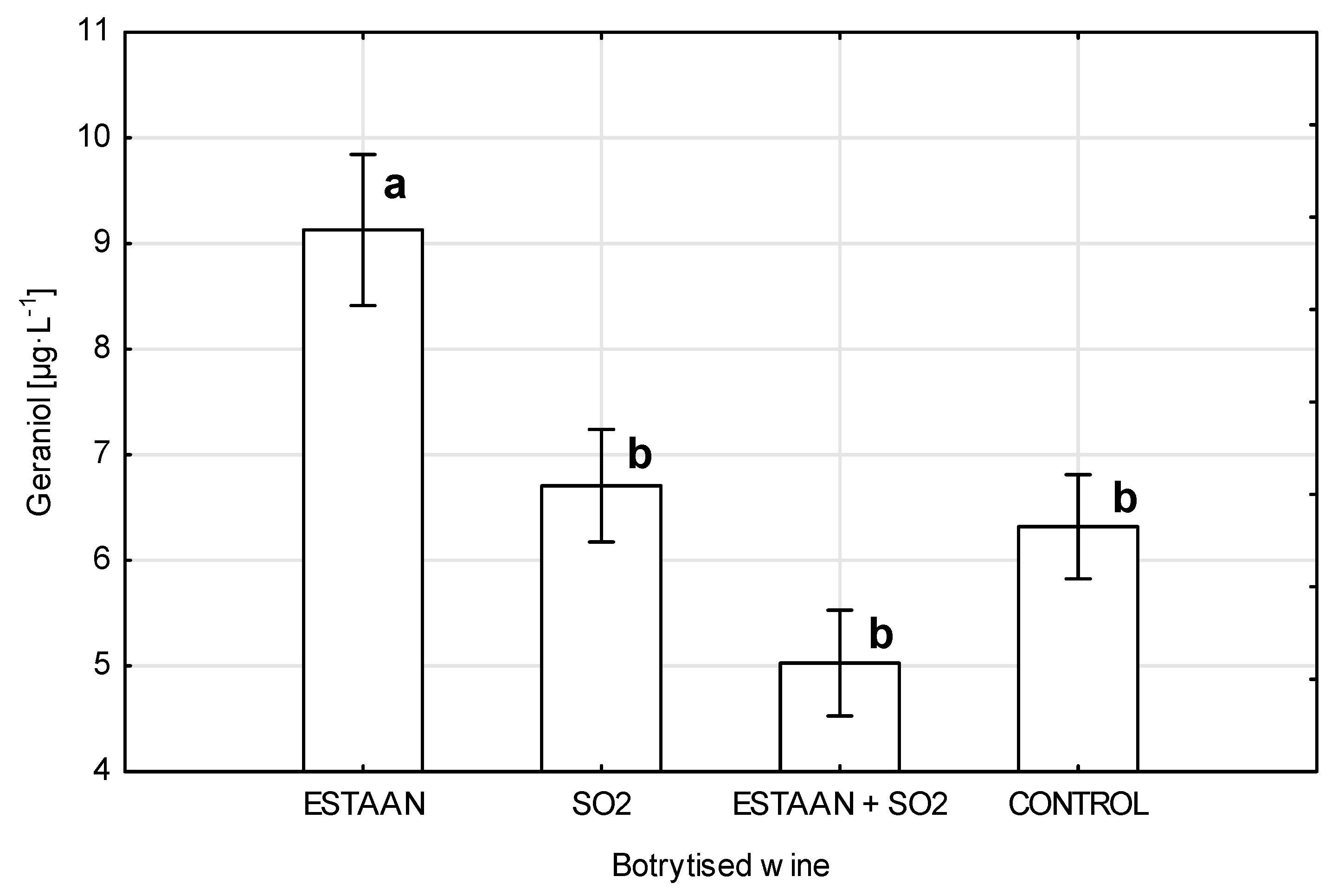
3.5. Geraniol Content in Wine from Healthy and Botrytized Grapes
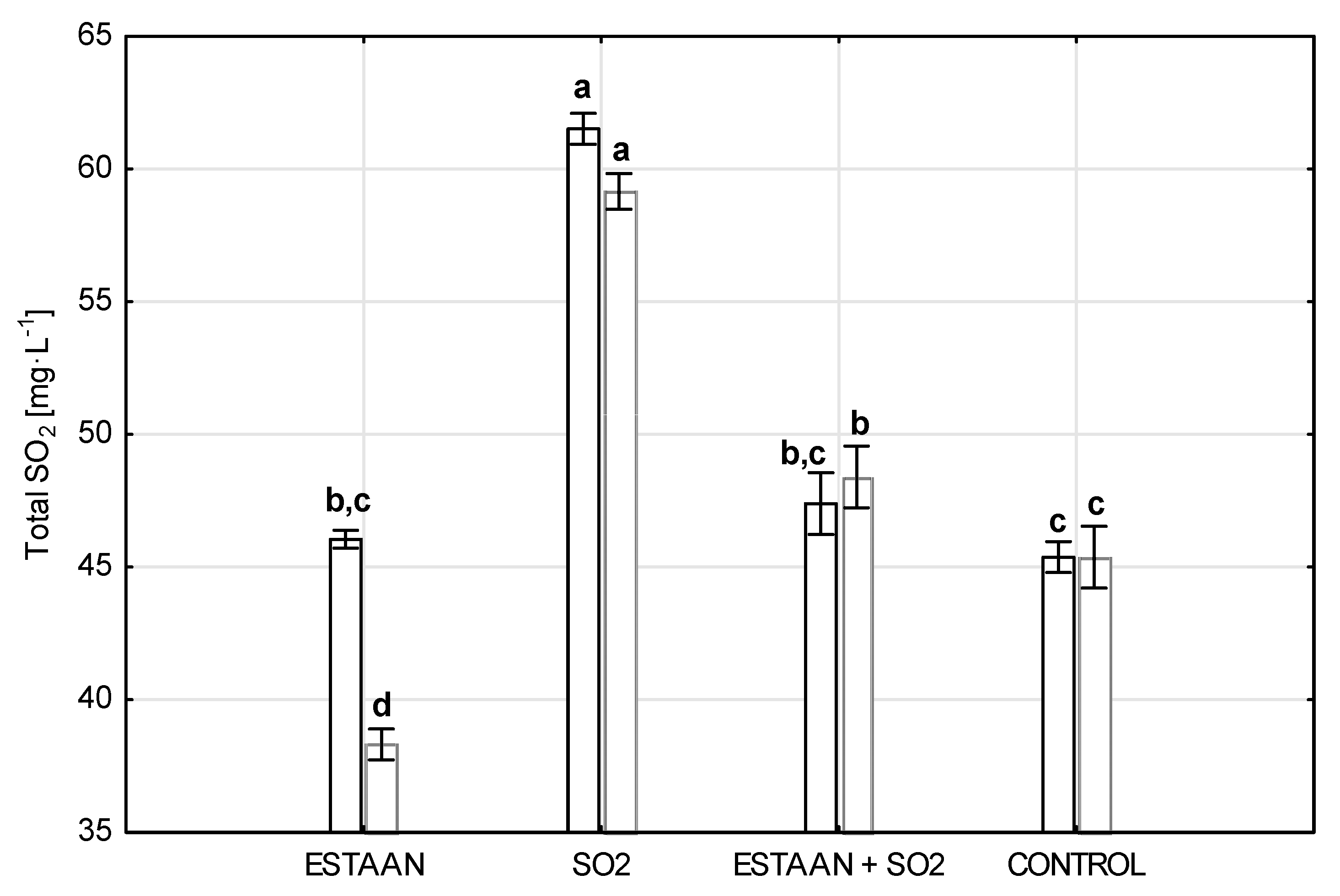
3.6. Total SO2 Content in Wine from Healthy and Botrytised Grapes
3.7. Sensory Analysis of Wine from Healthy and Botrytised Grapes
3.7.1. The Varietal Aroma of Riesling
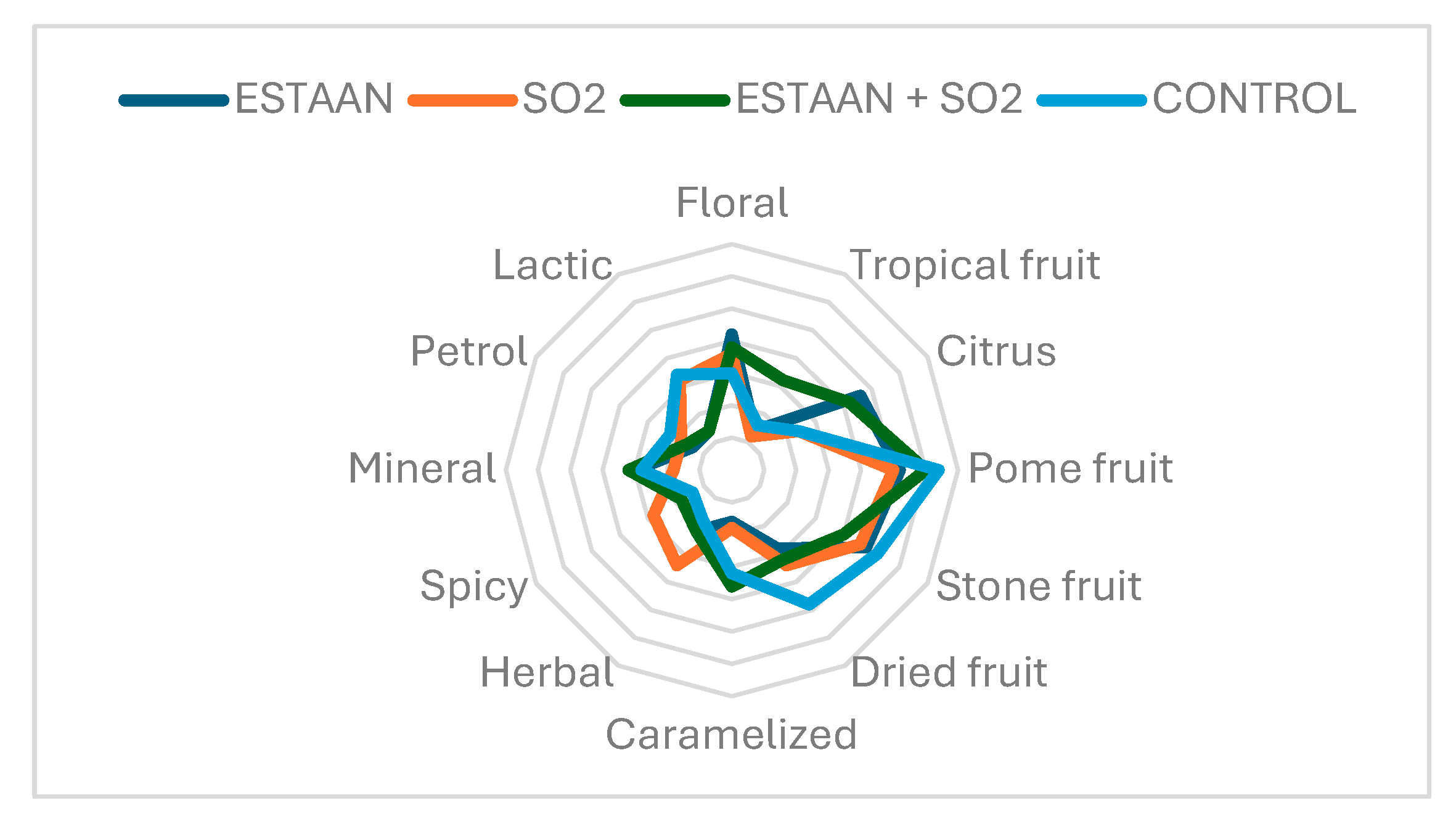
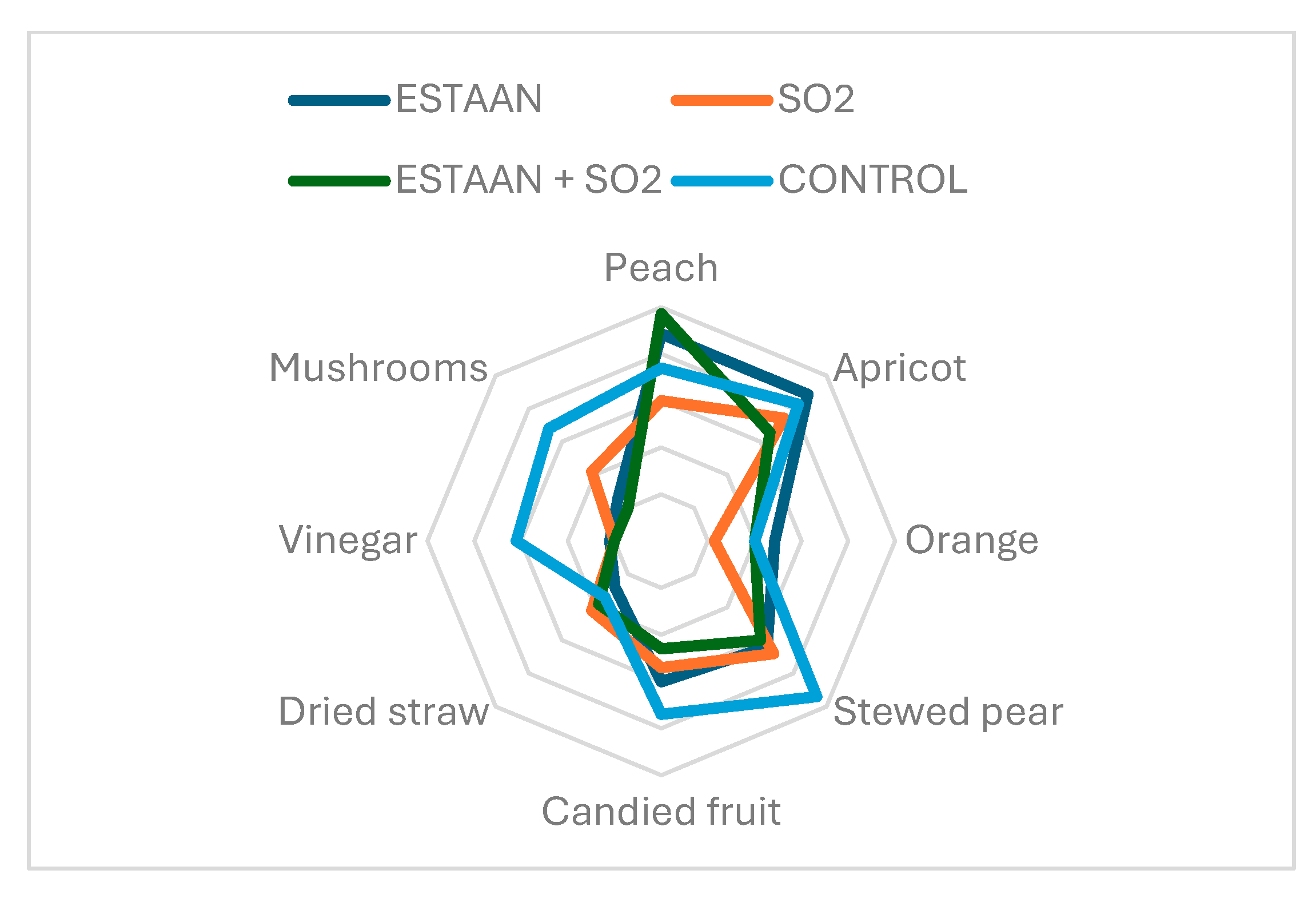
3.7.2. Botrytized Wine Profile
3.7.3. 100-Point Scale Wine Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zimdars, S.; Hitschler, J.; Schieber, A.; Weber, F. Oxidation of Wine Polyphenols by Secretomes of Wild Botrytis cinerea Strains from White and Red Grape Varieties and Determination of Their Specific Laccase Activity. J. Agric. Food Chem. 2017, 65, 10582–10590. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.; Giménez, P.; Just-Borràs, A.; Solé-Clua, I.; Gombau, J.; Heras, J.M.; Sieczkowski, N.; Gil, M.; Canals, J.M.; Zamora, F. Inoculation with a selected strain of Metschnikowia pulcherrima as a bioprotective alternative to sulphites for preventing browning of white grape must. OENO One 2024, 58. [Google Scholar] [CrossRef]
- Giménez, P.; Just-Borràs, A.; Gombau, J.; Canals, J.M.; Zamora, F. Effects of laccase from Botrytis cinerea on the oxidative degradation of anthocyanins. OENO One 2023, 57, 243–253. [Google Scholar] [CrossRef]
- Li, H.; Guo, A.; Wang, H. Mechanisms of oxidative browning of wine. Food Chem. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- García-Roldán, A.; Canalda-Sabaté, A.; Gombau-Roigé, J.; Bustamante-Quiñones, M.; Just-Borras, A.; Heras, J.M.; Sieczkowski, N.; Canals, J.M.; Zamora, F. Development of a model to study browning caused by tyrosinase in grape must. Food Chem. 2025, 463, 141457. [Google Scholar] [CrossRef]
- Giménez, P.; Just-Borras, A.; Pons, P.; Gombau, J.; Heras, J.M.; Sieczkowski, N.; Canals, J.M.; Zamora, F. Biotechnological tools for reducing the use of sulfur dioxide in white grape must and preventing enzymatic browning: Glutathione; inactivated dry yeasts rich in glutathione; and bioprotection with Metschnikowia pulcherrima. Eur. Food Res. Technol. 2023, 249, 1491–1501. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Cantos-Villar, E. Demonstrating the efficiency of sulphur dioxide replacements in wine: A parameter review. Trends Food Sci. Technol. 2015, 42, 27–43. [Google Scholar] [CrossRef]
- Giménez, P.; Anguela, S.; Just-Borras, A.; Pons-Mercadé, P.; Vignault, A.; Canals, J.M.; Teissedre, P.-L.; Zamora, F. Development of a synthetic model to study browning caused by laccase activity from Botrytis cinerea. LWT 2022, 154, 112871. [Google Scholar] [CrossRef]
- Stockley, C.; Paschke-Kratzin, A.; Teissedre, P.-L.; Restani, P.; Tejedor, N.G.; Quini, C. SO2 and Wine: A Review; International Organisation of Vine and Wine (OIV): Paris, France, 2021. [Google Scholar]
- Lisanti, M.T.; Blaiotta, G.; Nioi, C.; Moio, L. Alternative Methods to SO2 for Microbiological Stabilization of Wine. Compr. Rev. Food Sci. Food Saf. 2019, 18, 455–479. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion related to a notification from the Oenological Products and Practices International Association (OENOPPIA) on lysozyme from hen’s egg to be used in the manufacture of wine as an anti-microbial stabilizer/additive pursuant to Article 6, paragraph 11 of Directive 2000/13/EC—For permanent exemption from labelling. EFSA J. 2011, 9, 2386. [Google Scholar]
- Tedesco, F.; Pietrafesa, R.; Siesto, G.; Scieuzo, C.; Salvia, R.; Falabella, P.; Capece, A. Antimicrobial Activity of Chitosan from Different Sources Against Non-Saccharomyces Wine Yeasts as a Tool for Producing Low-Sulphite Wine. Beverages 2024, 10, 105. [Google Scholar] [CrossRef]
- Bustamante, M.; Giménez, P.; Just-Borràs, A.; Solé-Clua, I.; Gombau, J.; Heras, J.M.; Sieczkowski, N.; Gil, M.; Pérez-Navarro, J.; Gómez-Alonso, S.; et al. Use of Glutathione, Pure or as a Specific Inactivated Yeast, as an Alternative to Sulphur Dioxide for Protecting White Grape Must from Browning. Foods 2024, 13, 310. [Google Scholar] [CrossRef]
- Europe, B. Estaan®—Plant-Based Sulphite Replacement for Wine. 2021. Available online: https://biolethics.com/estaan/ (accessed on 15 March 2025).
- Bonich, F.S.; Freund, M.; Tarasov, A.; Bollinetti, M.; Ovadenko, O.; Christmann, M. Evaluation of plant phenolic extracts as an alternative to sulfur dioxide for the control of Oenococcus oeni and Brettanomyces bruxellensis. BIO Web Conf. 2023, 68, 02027. [Google Scholar]
- Dubourdieu, D.; Grassin, C.; Deruche, C.; Ribéreau-Gayon, P. Mise au point d’une mesure rapide de l’activité laccase dans les moûts et dans les vins par la méthode a la syringaldazine. Application à l’appréciation de l’état sanitaire des vendanges. OENO One 1984, 18, 237. [Google Scholar] [CrossRef]
- Grassin, C.; Dubourdieu, D. Optimisation de la méthode de dosage de l’activité laccase de Botrytis cinerea par la syringaldazine. OENO One 1986, 20, 125. [Google Scholar] [CrossRef]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites; Wiley-Blackwell Scientific Publications: Oxford, UK, 1994. [Google Scholar]
- International Organisation of Vine and Wine. OIV Standard for International Wine and Spirituous Beverages of Vitivinicultural Origin Competitions; International Organisation of Vine and Wine: Dijon, France, 2021. [Google Scholar]
- Magyar, I.; Soós, J. Botrytized Wines—Current Perspectives. Int. J. Wine Res. 2016, 8, 29–39. [Google Scholar] [CrossRef]
- Sun, Q.; Cui, R.; Zhao, Y. Regional aroma characteristics of spontaneously fermented Cabernet Sauvignon wines produced from seven sub-regions in Shangri-La of China. Sci. Rep. 2024, 14, 24566. [Google Scholar] [CrossRef]
- Stevenson, T. Riesling’s Character and ‘Petrol’ Aromas. 2008. Available online: https://wine-pages.com/columnist-articles/tom-stevenson-ponders-on-rieslings-character-and-petrol-aromas/ (accessed on 27 March 2025).
- Styger, G.; Prior, B.; Bauer, F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef]
- Steyer, D.; Erny, C.; Claudel, P.; Riveill, G.; Karst, F.; Legras, J.-L. Genetic analysis of geraniol metabolism during fermentation. Food Microbiol. 2013, 33, 228–234. [Google Scholar] [CrossRef]
- Mikami, N.; Konya, M.; Enoki, S.; Suzuki, S. Geraniol as a Potential Stimulant for Improving Anthocyanin Accumulation in Grape Berry Skin through ABA Membrane Transport. Plants 2022, 11, 1694. [Google Scholar] [CrossRef]
- Karampatea, A.; Skendi, A.; Irakli, M.; Bouloumpasi, E. Replacement of Sulfur Dioxide in White, Rosé, and Red Wines by a Blend of Tannins Extracted from Multiple Plant Materials. Beverages 2024, 10, 110. [Google Scholar] [CrossRef]
- Prusova, B.; Licek, J.; Kumsta, M.; Baron, M.; Sochor, J. Effect of New Methods for Inhibiting Malolactic Fermentation on the Analytical and Sensory Parameters of Wines. Fermentation 2024, 10, 122. [Google Scholar] [CrossRef]
- Kelly, M. Botrytis Bunch Rot: Winemaking Implications and Considerations. 2018. Available online: https://psuwineandgrapes.wordpress.com/2018/06/01/botrytis-bunch-rot-winemaking-implications-and-considerations/ (accessed on 27 March 2025).
- Tosi, E.; Fedrizzi, B.; Azzolini, M.; Finato, F.; Simonato, B.; Zapparoli, G. Effects of noble rot on must composition and aroma profile of Amarone wine produced by the traditional grape withering protocol. Food Chem. 2012, 130, 370–375. [Google Scholar] [CrossRef]
- Claus, H.; Sabel, A.; König, H. Wine Phenols and Laccase: An Ambivalent Relationship. In Wine—Phenolic Composition, Classification and Health Benefits; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2014; pp. 155–185. [Google Scholar]
- Kelly, J.; Inglis, D.; Dowling, L.; Pickering, G. Impact of Botrytis cinerea-infected grapes on quality parameters of red wine made from withered grapes. Aust. J. Grape Wine Res. 2022, 28, 439–449. [Google Scholar] [CrossRef]
- Santos, H.; Augusto, C.; Reis, P.; Rego, C.; Figueiredo, A.C.; Fortes, A.M. Volatile Metabolism of Wine Grape Trincadeira: Impact of Infection with Botrytis cinerea. Plants 2022, 11, 141. [Google Scholar] [CrossRef]
- Wang, X.-J.; Tao, Y.-S.; Wu, Y.; An, R.-Y.; Yue, Z.-Y. Aroma compounds and characteristics of noble-rot wines of Chardonnay grapes artificially botrytized in the vineyard. Food Chem. 2017, 226, 41–50. [Google Scholar] [CrossRef]
- Canals, J.M.; Giménez, P.; Anguela, S.; Just-Borras, A.; Pons-Mercadé, P.; Gombau, J.; Vignault, A.; Teissedre, J.M.C.P.-L.; Zamora, F. Inhibitory effect of sulfur dioxide, ascorbic acid and glutathione on browning caused by laccase activity. In Proceedings of the Macrowine 2021, Virtual, 23–30 June 2021. [Google Scholar]
- (OIV), International organization of vine and wine. International Oenological Codex; OIV: Paris, France, 2025. [Google Scholar]





| Variant | Glucose | Fructose | Tartaric Acid | Malic Acid | Acetic Acid |
|---|---|---|---|---|---|
| Healthy must | 89.6 | 90.7 | 4.15 | 2.13 | 0.05 |
| Botrytised must | 87.1 | 97.7 | 3.54 | 4.15 | 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novosad, M.; Licek, J.; Kumsta, M.; Baron, M.; Sochor, J. The Effect of ESTAAN on Must Browning Induced by Fungal Disease (Botrytis cinerea) That Affects Grapes. Fermentation 2025, 11, 360. https://doi.org/10.3390/fermentation11070360
Novosad M, Licek J, Kumsta M, Baron M, Sochor J. The Effect of ESTAAN on Must Browning Induced by Fungal Disease (Botrytis cinerea) That Affects Grapes. Fermentation. 2025; 11(7):360. https://doi.org/10.3390/fermentation11070360
Chicago/Turabian StyleNovosad, Martin, Josef Licek, Michal Kumsta, Mojmir Baron, and Jiri Sochor. 2025. "The Effect of ESTAAN on Must Browning Induced by Fungal Disease (Botrytis cinerea) That Affects Grapes" Fermentation 11, no. 7: 360. https://doi.org/10.3390/fermentation11070360
APA StyleNovosad, M., Licek, J., Kumsta, M., Baron, M., & Sochor, J. (2025). The Effect of ESTAAN on Must Browning Induced by Fungal Disease (Botrytis cinerea) That Affects Grapes. Fermentation, 11(7), 360. https://doi.org/10.3390/fermentation11070360






