Application of Commercial Pectinase as a Biocatalyst During Self-Induced Anaerobic Fermentation of Coffee (Coffea arabica L. var. Typica)
Abstract
1. Introduction
2. Materials and Methods
2.1. Coffee Sample
2.2. Coffee Fermentation Processing
2.3. Drying, Roasting, and Grinding Coffee
2.4. Obtaining Extracts from the Pulp, Green, and Roasted Beans
2.5. Monitoring Mass Loss (ML), pH, Total Soluble Solids (TSSs), Instrumental Color, and Total Reducing Sugar
2.6. Enzymatic Activity
2.6.1. Polygalacturonase Activity
2.6.2. Pectin Lyase Activity
2.7. Determination of Phenolic Content and Antioxidant Capacity Assays
2.8. Bioactive Compound Assay
2.9. Volatile Compounds by GC-MS-HS
2.10. Statistical Analysis
3. Results
3.1. Physicochemical Monitoring During Coffee Fermentation
3.2. Reducing Sugar and Enzyme Activity
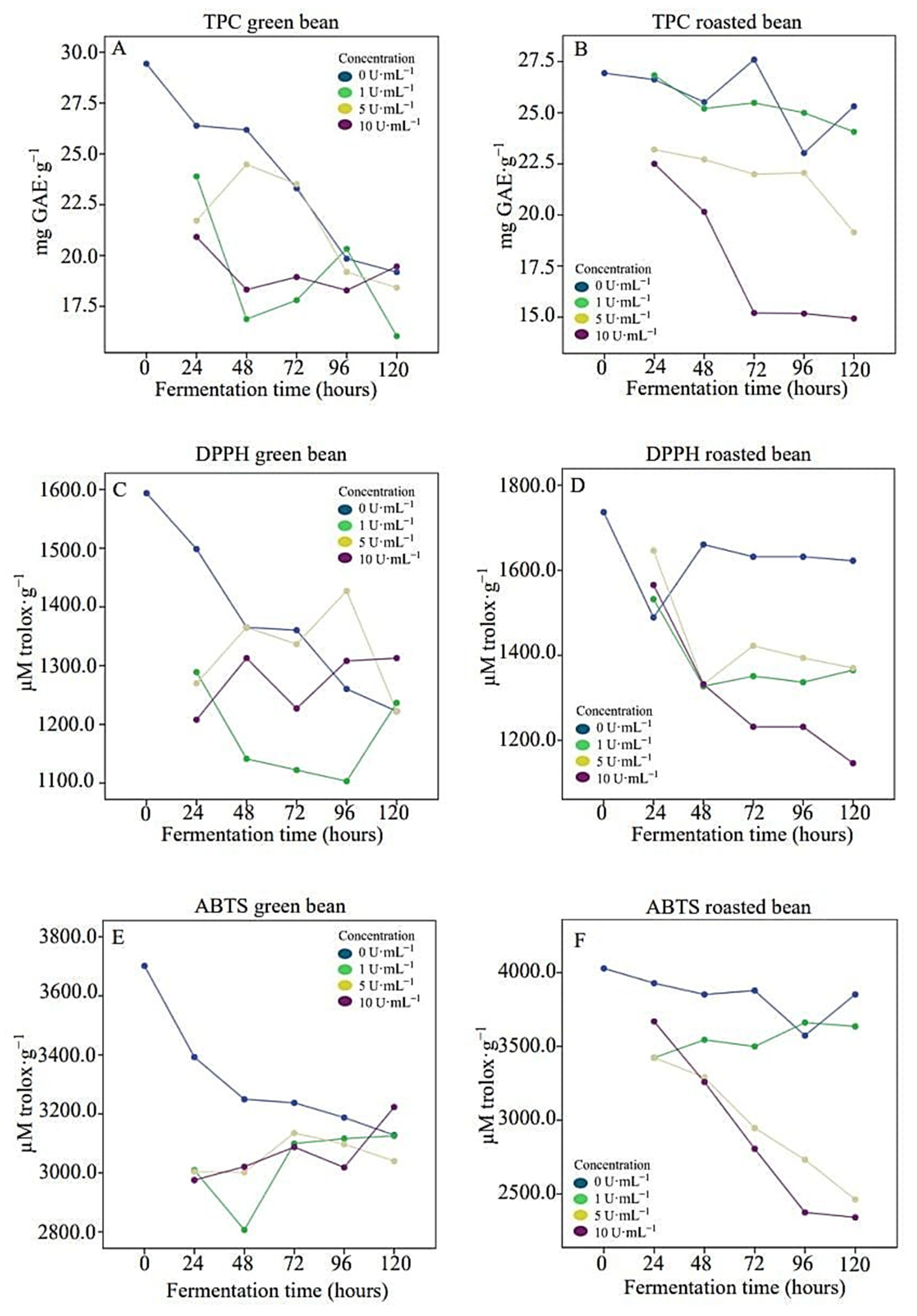
3.3. Phenolic Compounds and Antioxidant Activity
3.4. Bioactive Compounds by HPLC
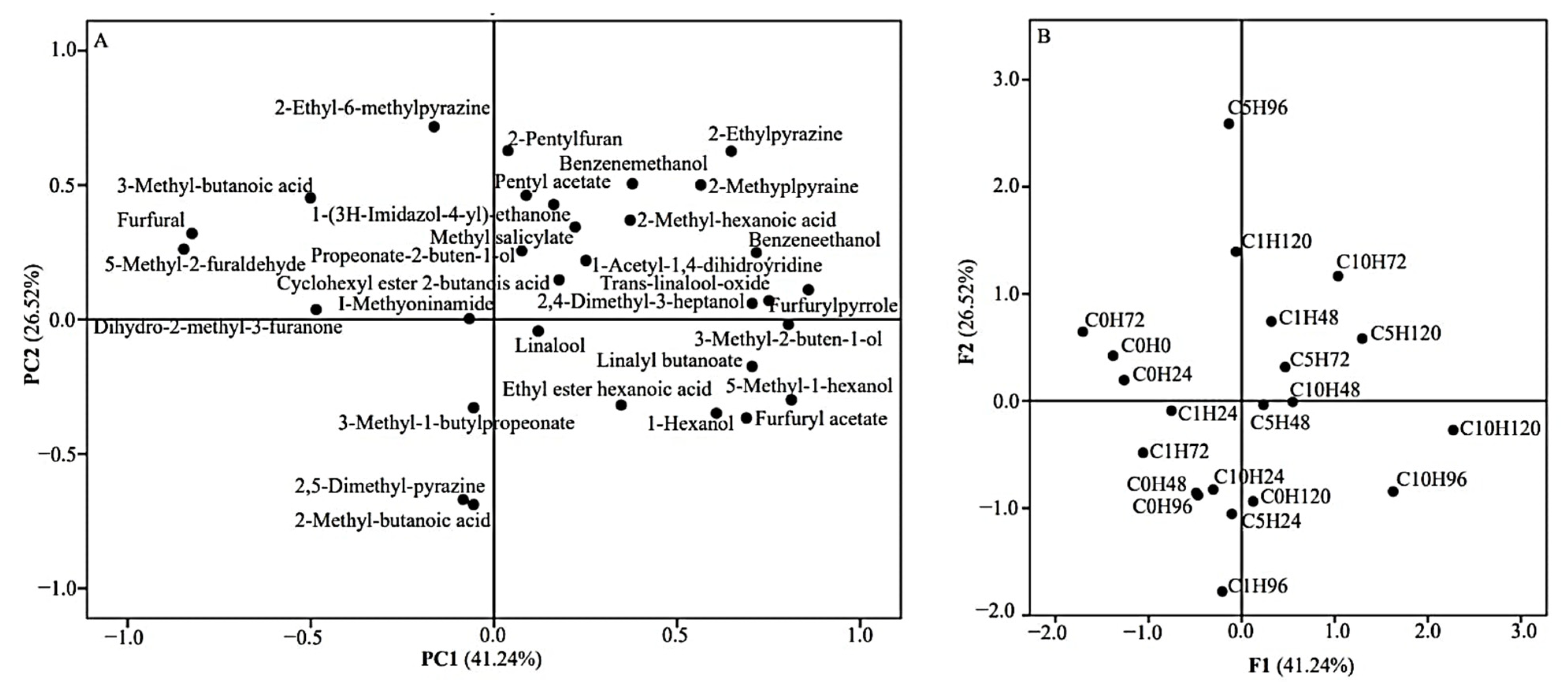
3.5. Volatile Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SIAF | Self-induced anaerobic fermentation |
| 5CQA | 5-O-caffeoylquinic acid |
| MS | Mass loss |
| TSSs | Total soluble solids |
| DNSA | Dinitrosalicylic acid |
| TPC | Total phenolic content |
| PCA | Principal component analysis |
| C0H0 | Enzymatic concentration of 0 U·mL−1 and 0 h of fermentation |
| C0H24 | Enzymatic concentration of 0 U·mL−1 and 24 h of fermentation |
| C0H48 | Enzymatic concentration of 0 U·mL−1 and 48 h of fermentation |
| C0H72 | Enzymatic concentration of 0 U·mL−1 and 72 h of fermentation |
| C0H96 | Enzymatic concentration of 0 U·mL−1 and 96 h of fermentation |
| C0H120 | Enzymatic concentration of 0 U·mL−1 and 120 h of fermentation |
| C1H24 | Enzymatic concentration of 1 U·mL−1 and 24 h of fermentation |
| C1H48 | Enzymatic concentration of 1 U·mL−1 and 48 h of fermentation |
| C1H72 | Enzymatic concentration of 1 U·mL−1 and 72 h of fermentation |
| C1H96 | Enzymatic concentration of 1 U·mL−1 and 96 h of fermentation |
| C1H120 | Enzymatic concentration of 1 U·mL−1 and 120 h of fermentation |
| C5H24 | Enzymatic concentration of 5 U·mL−1 and 24 h of fermentation |
| C5H48 | Enzymatic concentration of 5 U·mL−1 and 48 h of fermentation |
| C5H72 | Enzymatic concentration of 5 U·mL−1 and 72 h of fermentation |
| C5H96 | Enzymatic concentration of 5 U·mL−1 and h96 h of fermentation |
| C5H120 | Enzymatic concentration of 5 U·mL−1 and 120 h of fermentation |
| C10H24 | Enzymatic concentration of 10 U·mL−1 and 24 h of fermentation |
| C10H48 | Enzymatic concentration of 10 U·mL−1 and 48 h of fermentation |
| C10H72 | Enzymatic concentration of 10 U·mL−1 and 72 h of fermentation |
| C10H96 | Enzymatic concentration of 10 U·mL−1 and 96 h of fermentation |
| C10H120 | Enzymatic concentration of 10 U·mL−1 and 120 h of fermentation |
References
- Molet-Rodríguez, A.; Méndez, D.A.; López-Rubio, A.; Fabra, M.A.; Martínez-Sanz, M.; Salvia-Trujillo, L.; Martín-Belloso, O. Emulsification capacity of pectin extracts from persimmon waste: Effect of structural characteristics and pectin-polyphenol interactions. Food Hydrocoll. 2025, 158, 110553. [Google Scholar] [CrossRef]
- Ferreira, L.J.C.; Gomes, M.S.; Oliveira, L.M.; Santos, L.D. Coffee fermentation process: A review. Food Res. Int. 2023, 169, 112793. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.V.M.; Sampaio, V.M.; Wiele, N.; Vale, A.S.; Neto, D.P.C.; Souza, A.F.D.; Santos, D.V.N.; Ruiz, I.R.; Rogez, H.; Soccol, C.R. How yeast has transformed the coffee market by creating new flavors and aromas through modern post-harvest fermentation systems. Trends Food Sci. Technol. 2024, 151, 104641. [Google Scholar] [CrossRef]
- Zafar, T.; Hadri, S.H.; Imram, M.; Asad, M.J.; Saba; Athar, I.; Mahmood, T. Production, purification and characterization of endo-polygalacturonase using novel strain of Bacillus pumillus through RSM. Process Biochem. 2024, 146, 188–194. [Google Scholar] [CrossRef]
- Yadav, K.; Dwivedi, S.; Gupta, S.; Tanveer, A.; Yadav, S.; Yadav, P.K.; Anand, G.; Yadav, D. Recent insights into microbial pectin lyases: A review. Process Biochem. 2023, 134, 199–217. [Google Scholar] [CrossRef]
- Sánchez-Riaño, A.M.; Vega-Oliveros, C.; Ladino-Garzón, W.L.; Orozco-Blanco, D.A.; Bahamón-Monje, A.F.; Gutiérrez-Guzmán, N.; Amorocho-Cruz, C.M. Effects of cherries sanitization methods and fermentation times on quality parameters of coffee beans. Heliyon 2024, 10, e33508. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.C.B.; Batista, N.N.; Dias, D.R.; Schwan, R.F. Impact of microbial self-induced anaerobiosis fermentation (SIAF) on coffee quality. Food Biosci. 2022, 47, 101640. [Google Scholar] [CrossRef]
- Tai, E.S.; Hsieh, P.C.; Sheu, S.C. Effect of polygalacturonase and feruloyl esterase from Aspergillus tubingensis on demucilage and quality of coffee beans. Process Biochem. 2014, 49, 1274–1280. [Google Scholar] [CrossRef]
- Elhalis, H.; Cox, J.; Zhao, J. Yeasts are essential for mucilage degradation of coffee beans during wet fermentation. Yeast 2023, 40, 425–436. [Google Scholar] [CrossRef]
- Febrianto, N.A.; Zhu, F. Coffee bean processing: Emerging methods and their effects on chemical, biological and sensory properties. Food Chem. 2023, 412, 135489. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Miguel, M.G.C.P.; Evangelista, S.R.; Martins, P.M.M.; Mullem, J.V.; Belizario, M.H.; Schwan, R.F. Behavior of yeast inoculated during semi-dry coffee fermentation and the effect on chemical and sensorial properties of the final beverage. Food Res. Int. 2017, 92, 26–32. [Google Scholar] [CrossRef]
- Adolfo Lutz Institute. Analytical Standards of the Adolfo Lutz Institute, 4th ed.; Adolfo Lutz Institute: São Paulo, Brazil, 2008. [Google Scholar]
- Kim, W.; Kim, S.Y.; Kim, D.O.; Kim, B.Y.; Baik, M.Y. Puffing, a novel coffee bean processing technique for the enhancement of extract yield and antioxidant capacity. Food Chem. 2018, 240, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Perrone, D.; Donangelo, C.; Farah, A. Fast simultaneous analysis of Caffeine, Trigonelline, nicotinic acid and sucrose in coffee by liquid chromatography-mass spectrometry. Food Chem. 2008, 110, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Albersheim, P. Pectin lyase from fungi. Meth. Enzymol. 1966, 8, 628–631. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Raventos, R.M.L. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent. Sci. Hortic. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Moraes, S.M.; Sampaio, C.G.; Jimenez, J.P.; Saura-Calixto, F.D. Scientific Methodology: Determination of Total Antioxidant Activity in Fruits by DPPH Free Radical Capture. 2007. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/CNPAT/10224/1/Cot_127.pdf (accessed on 5 November 2024).
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Medic. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Vandendool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Ferrreira, L.J.C.; Casé, I.N.; Bertarini, P.L.L.; Oliveira, L.M.; Santos, L.D. Impact of immature coffee fruit and water addition during spontaneous fermentation process: Chemical composition and sensory profile. Electron. J. Biotechnol. 2024, 69, 21–29. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W.H. Antioxidant Activity, Total Polyphenol, Flavonoid and Tannin Contents of Fermented Green Coffee Beans with Selected Yeasts. Fermentation 2019, 5, 29. [Google Scholar] [CrossRef]
- Silva, M.E.S.; Oliveira, R.L.; Moraes, M.M.; Camara, C.A.G.; Arruda, L.L.A.L.; Silva, S.P.; Porto, T.S. Impact of fermentation time on the bioactive and volatile composition of coffee: Insights for producers and researchers. Food Chem. 2025, 490, 145067. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.M.M.; Batista, N.N.; Naves, J.A.O.; Dias, D.R.; Schwan, R.F. Use of microencapsulated starter cultures by spray drying on coffee under self-induced anaerobiosis fermentation (SIAF). Food Res. Int. 2023, 172, 113189. [Google Scholar] [CrossRef] [PubMed]
- Cassimiro, D.M.J.; Batista, N.N.; Fonseca, H.C.; Naves, J.A.O.; Coelho, J.M.; Bernardes, P.C.; Dias, D.R.; Schwan, R.F. Wet fermentation of Coffea canephora by lactic acid bacteris and yeasts using the self-induced Anaerobic fermentation (SIAF) method enhances the coffee quality. Food Microbiol. 2023, 110, 104161. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.L.A.; Oliveira, L.S.C.; Silva, F.L.H.; Amorim, B.C. Caracterização da poligalacturonase produzida por fermentação semi-sólida utilizando resíduo do maracujá como substrato. Rev. Bras. Eng. Agríc. Ambient. 2010, 14, 987–992. [Google Scholar] [CrossRef]
- Navina, B.; Huthaash, K.K.; Velmurugan, N.K.; Korumili, T. Insights into recent innovations in anti-browning strategies for fruit and vegetable preservation. Trends Food Sci. Technol. 2023, 139, 104128. [Google Scholar] [CrossRef]
- Elhalis, H.; Cox, J.; Frank, D.; Zhao, J. Microbiological and biochemical performances of six yeast species as potential starter culture for wet fermentation of coffee beans. LWT 2021, 137, 110430. [Google Scholar] [CrossRef]
- Oliveira, R.L.; Dias, J.L.; Silva, O.S.; Porto, T.S. Immobilization of pectinase from Aspergillus aculeatus in alginate beads and clarification of apple and umbu juices in a packed bed reactor. Food Bioprod. Process. 2018, 109, 9–18. [Google Scholar] [CrossRef]
- Uenojo, M.; Pastore, G.M. Pectinolytic enzymes: Industrial application and future perspectives. Quím. Nova 2007, 30, 388–394. [Google Scholar] [CrossRef]
- Luo, P.; Ai, J.; Wang, Q.; Luo, Y.; Liao, Z.; Giampieri, F.; Battino, M.; Sieniawska, E.; Bai, W.; Tian, L. Enzymatic treatment shapes in vitro digestion pattern of phenolic compounds in mulberry juice. Food Chem. 2025, 469, 142555. [Google Scholar] [CrossRef]
- Wei, X.; Hao, J.; Xiong, K.; Guo, H.; Xue, S.; Dai, Y.; Zhang, Y.; Chen, Y.; Zhang, S. Effect of pectinase produced by Bacillus velezensis w17-6 on methanol content and overall quality of kiwifruit wine. Food Biosci. 2024, 59, 104180. [Google Scholar] [CrossRef]
- Abduh, M.Y.; Merari, G.; Angkasa, M.D.; Pangastuti, W.I.R.; Rahmawati, A.; Taufik, I.; Tan, M.I.; Rosmiati, M. Effects of fermentation using Aspergillus spp. Towards extractions yield and bioactivity of coffee pulp extract. Biomass Conserv. Biorefinery 2025, 15, 15087–15099. [Google Scholar] [CrossRef]
- Therdtatha, P.; Jareontanahum, N.; Chaisuwan, W.; Yakul, K.; Paemanee, A.; Manassa, A.; Moukamnerd, W.; Phimolsiripol, Y.; Sommano, S.R.; Seesuriyachan, P. Production of functional Arabica and Robusta green coffee beans: Optimization of fermentation with microbial cocktails to improve antioxidant activity and metabolomic profiles. Biocatal. Agric. Biotechnol. 2023, 53, 102869. [Google Scholar] [CrossRef]
- Silva, M.E.S.; Oliveira, R.L.; Sousa, T.C.A.; Grisi, C.V.; Ferreira, V.C.S.; Porto, T.S.; Madruga, M.S.; Silva, S.P.; Silva, F.A.P. Microencapsulated phenolic-rich extract from juice processing grape pomace (Vittis labrusca. Isabela Var): Effects on oxidative stability of raw and pre-cooked bovine burger. Food Biosci. 2022, 50, 102212. [Google Scholar] [CrossRef]
- Zhou, M.-Z.; Yan, C.-Y.; Zeng, Z.; Luo, L.; Zeng, W.; Huang, Y.-H. N-Methyltransferases of caffeine biosynthetic pathway in plant. J. Agric. Food. Chem. 2020, 68, 15359–15372. [Google Scholar] [CrossRef] [PubMed]
- Maia Júnior, C.R.L.; Batista, N.N.; Martinez, S.J.; Bressani, A.P.P.; Dias, D.R.; Schwan, R.F. Impact of scaling up on coffee fermentation using starter cultures. Appl. Food Res. 2024, 4, 100611. [Google Scholar] [CrossRef]
- Wang, X.; Hu, S.; Wan, X.; Pan, C. Effect of microbial fermentation on caffeine content of tea leaves. J. Agric. Food Chem. 2005, 53, 7238–7242. [Google Scholar] [CrossRef]
- Bressani, A.P.P.; Batista, N.N.; Ferreira, G.; Martinez, S.J.; Simão, J.B.P.; Dias, D.R.; Schwan, R.F. Characterization of bioactive, chemical, and sensory compounds from fermented coffees with different yeasts species. Food Res. Int. 2021, 150, 110755. [Google Scholar] [CrossRef]
- Ban, Y.; Park, H.; Hong, S.J.; Yu, S.Y.; Moon, H.S.; Shin, E.-C. Sensomics and chemometrics approaches of differential brewed and roasted coffee (Coffea arabica) from Ethiopia using biomimetic sensory-based machine perception techniques: Effect of caffeine on bitter taste and the generation of volatiles. Food Chem. 2025, 476, 143407. [Google Scholar] [CrossRef]
- Shen, X.; Wang, Q.; Wang, H.; Fang, G.; Li, Y.; Zhang, J.; Liu, K. Microbial characteristics and functions in coffee fermentation: A review. Fermentation 2025, 11, 5. [Google Scholar] [CrossRef]
- Borém, F.M.; Rabelo, M.H.S.; Alves, A.P.C.; Santos, C.M.; Pieroni, R.S.; Nakajima, M.; Sugino, R. Fermentation of coffee fruit sequential inoculation of Lactiplantibacillus plantarum and Saccharomyces cerevisiae: Effect in sensory attributes and chemical composition of the beans. Food Chem. 2024, 446, 138820. [Google Scholar] [CrossRef]
- Matinez, S.J.; Batista, N.N.; Bressani, A.P.P.; Leite, S.; Naves, J.A.O.; Dias, D.R.; Schwan, R.F. Volatile and sensory mapping of SIAF fermentation with selected coffee yeasts from different Brazilian regions. Food Biosci. 2024, 62, 105551. [Google Scholar] [CrossRef]
- Debona, D.G.; Lyrio, M.V.V.; Luz, J.M.R.; Frinhani, R.Q.; Araújo, B.Q.; Oliveira, E.C.S.; Agnoletti, B.Z.; Coura, M.R.; Pereira, L.L.; Castro, E.V.R. Comprehensive evaluation of Volatile compounds and sensory profiles of coffee throughout the roasting process. Food Chem. 2025, 478, 143586. [Google Scholar] [CrossRef]
- Rosário, D.K.A.; Mutz, Y.S.; Vieira, K.M.; Schwan, R.F.; Bernardes, P.C. Effect os self-induced anaerobiosis fermentation (SIAF) in the volatile compounds and sensory quality of coffee. Eur. Food Res. Technol. 2024, 250, 667–675. [Google Scholar] [CrossRef]
- Lopes, A.C.A.; Andrade, R.P.; Casagrande, M.R.; Santiago, W.D.; Resende, M.L.V.; Cardoso, M.G.; Vilanova, M.; Duarte, W.F. Production and characterization of a new distilled beverage from green coffee seed residue. Food Chem. 2022, 377, 131960. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, N.A.; Mangunwardoyo, W.; Santoso, T.J.; Yasman. Investigation of Volatile compounds of Liberica coffee beans fermented at varying degrees of roasting. Food Res. 2024, 8, 43–53. [Google Scholar]
- Rivera, A.M.P.; Silva, J.L.; Torres-Valenzuela, L.; Plaza-Dorado, J.L. From waste to taste: Coffee by-products as starter cultures for sustainable fermentation and improved coffee quality. Sustainability 2024, 16, 10763. [Google Scholar] [CrossRef]
- Galarza, G.; Figueroa, J.G. Volatile compound characterization of coffee (Coffea arabica) Processed at different fermentation times using SPME-GC-MS. Molecules 2022, 27, 2004. [Google Scholar] [CrossRef]
- Várady, M.; Tauchen, J.; Franková, A.; Kloucek, P.; Popelka, P. Effect of method of processing specialty coffee beans (natural, washed, honey, fermentation, maceration) on bioactive and volatile compounds. LWT 2022, 172, 114245. [Google Scholar] [CrossRef]
- Tang, V.C.Y.; Sun, J.; Cornuz, M.; Yu, B.; Lassabliere, B. Effect of solid-state fungal fermentation on the non-volatiles content and volatiles composition of Coffea canephora (Robusta) coffee beans. Food Chem. 2021, 337, 128023. [Google Scholar] [CrossRef]

| Physical–Chemical Parameter | Time (h) | Enzyme Solution Concentration (U·ML−1) | |||
|---|---|---|---|---|---|
| 0 | 1 | 5 | 10 | ||
| Colorimetric parameter L | 0 | 10.17 ± 0.07E | - | - | - |
| 24 | 12.17 ± 0.43DAB | 13.43 ± 1.44AA | 11.60 ± 0.26AAB | 10.97 ± 0.12AB | |
| 48 | 18.40 ± 1.15BA | 13.60 ± 0.83AB | 11.27 ± 0.15ABC | 11.07 ± 0.09AC | |
| 72 | 21.10 ± 2.40AA | 10.63 ± 0.18BB | 11.27 ± 0.19AB | 11.00 ± 0.06AB | |
| 96 | 11.30 ± 0.35DA | 12.57 ± 0.80ABA | 11.50 ± 0.38AA | 11.17 ± 0.18AA | |
| 120 | 14.40 ± 1.11CA | 11.43 ± 0.03ABB | 11.97 ± 0.94AB | 11.30 ± 0.95AB | |
| Colorimetric parameter a* | 0 | 3.17 ± 0.81C | - | - | - |
| 24 | 3.30 ± 0.23CAB | 5.07 ± 0.42AA | 3.23 ± 0.34AAB | 2.50 ± 0.40AB | |
| 48 | 5.80 ± 1.56BCA | 5.07 ± 1.41AA | 2.20 ± 0.21AB | 0.67 ± 0.32AB | |
| 72 | 11.07 ± 1.66AA | 6.30 ± 0.21AB | 2.07 ± 0.41AC | 0.40 ± 0.15AC | |
| 96 | 4.17 ± 0.97CB | 6.90 ± 0.52AA | 3.57 ±0.60ABC | 1.67 ± 0.29AC | |
| 120 | 7.23 ± 0.52BA | 4.80 ± 0.78AB | 2.73 ± 0.58ABC | 1.13 ± 0.37AC | |
| Colorimetric parameter b* | 0 | 1.47 ± 0.66B | - | - | - |
| 24 | −4.50 ± 0.67DD | 2.47 ± 0.37BA | 1.93 ± 0.95ABB | 0.87 ± 0.41ACC | |
| 48 | 5.57 ± 0.50AA | 4.47 ± 0.72AA | 2.83 ± 0.19AB | −1.23 ± 0.07DC | |
| 72 | 6.77 ± 0.54AA | 2.77 ± 0.17BB | 1.17 ± 0.66BC | −1.47 ± 0.07DD | |
| 96 | 1.07 ± 0.34BCB | 4.03 ± 0.38ABA | 1.10 ± 0.97BB | −0.17 ± 0.09CDB | |
| 120 | −0.47 ± 0.52CC | 3.83 ± 0.24ABA | 0.60 ± 0.70BC | 0.97 ± 0.07AB | |
| TSSs (°Brix) | 0 | 15.20 ± 0.15A | - | - | - |
| 24 | 14.53 ± 0.26ABA | 13.40 ± 0.21ABB | 11.00 ± 0.29AC | 10.13 ± 0.19AD | |
| 48 | 13.97 ± 0.50BCA | 13.43 ± 0.07AA | 10.53 ± 0.48ABB | 10.00 ± 0.06AB | |
| 72 | 13.33 ± 0.67CDA | 12.67 ± 0.17ABA | 10.50 ± 0.29ABB | 10.13 ± 0.15AB | |
| 96 | 13.00 ± 0.06DA | 12.60 ± 0.15BA | 10.33 ± 0.17ABCB | 9.67 ± 0.34AB | |
| 120 | 12.00 ± 0.29EA | 12.67 ± 0.18ABA | 9.67 ± 0.09CB | 9.63 ± 0.22AB | |
| pH | 0 | 5.23 ± 0.03A | - | - | - |
| 24 | 4.49 ± 0.01BA | 4.58 ± 0.05AA | 4.28 ± 0.08AB | 4.19 ± 0.07AB | |
| 48 | 4.11 ± 0.02CA | 4.16 ± 0.01BA | 4.13 ± 0.04BA | 4.20 ± 0.01AA | |
| 72 | 4.13 ± 0.02CAB | 4.07 ± 0.01BB | 4.15 ± 0.02BAB | 4.17 ± 0.04AA | |
| 96 | 3.98 ± 0.01DB | 4.07 ± 0.02BAB | 4.10 ± 0.06BA | 4.04 ± 0.02BAB | |
| 120 | 3.99 ± 0.01DAB | 3.95 ± 0.01CB | 4.06 ± 0.03BA | 3.90 ± 0.05CB | |
| Fermentation Time (Hours) | Enzymatic Concentration * | Caffeine (mg·100g−1) | Trigonelline (mg·100g−1) | Chlorogenic Acid (5CQA) (mg·100g−1) | |||
|---|---|---|---|---|---|---|---|
| Green | Roasted | Green | Roasted | Green | Roasted | ||
| 0 | 0 | 592.93 ± 27.2a | 873.63 ± 32.5a | 1028.75 ± 31.4a | 914.79 ± 44.0bc | 423.46 ± 40.3a | 185.85 ± 2.5a |
| 24 | 0 | 506.98 ± 36.1Cb | 894.40 ± 52.4Aa | 859.25 ± 8.4Bb | 1047.48 ± 35.2Aa | 374.24 ± 15.9Ab | 196.19 ± 16.1Aa |
| 1 | 645.07 ± 24.0Bab | 902.38 ± 10.3Aa | 910.88 ± 11.9Aa | 911.75 ± 37.7Ba | 262.97 ± 7.9Ca | 95.55 ± 7.9Ba | |
| 5 | 742.51 ± 16.7Aa | 890.18 ± 19.1Aa | 847.97 ±11.2Ba | 790.36 ± 37.8Ca | 302.38 ± 4.7Ba | 63.81 ± 1.3BCa | |
| 10 | 648.60 ± 9.2Ba | 795.68 ± 12.1Ba | 716.84 ± 9.8Ca | 687.30 ± 32.5Dab | 168.96 ± 5.6Da | 55.96 ± 15.0Ca | |
| 48 | 0 | 593.25 ± 13.6Ba | 866.01 ± 46.2Aab | 857.71 ± 8.9Ab | 1069.03 ± 38.8Aa | 335.80 ± 2.1Ac | 189.08 ± 14.4Aa |
| 1 | 568.26 ± 19.3Bc | 826.88 ± 7.6Ab | 820.93 ± 4.9Ab | 897.84 ± 15.7Ba | 220.28 ± 1.4Bc | 92.84 ± 7.6Bab | |
| 5 | 685.36 ± 25.8Aab | 857.57 ± 3.0Ab | 768.28 ± 29.6Bb | 769.96 ± 4.3Cab | 310.80 ± 9.5Aa | 69.38 ± 0.2Ba | |
| 10 | 595.80 ± 2.1Bab | 693.24 ± 21.8Bb | 686.66 ± 0.1Ca | 737.90 ± 11.2Ca | 156.54 ± 7.3Cab | 26.83 ± 11.4Ca | |
| 72 | 0 | 546.88 ± 8.9Bab | 832.24 ± 13.0Aab | 844.96 ± 2.1Ab | 993.97 ± 5.9Aab | 364.41 ± 0.7Abc | 181.43 ± 12.5Aa |
| 1 | 694.90 ± 6.2Aa | 792.92 ± 2.5Ab | 808.48 ± 20.0Abc | 847.72 ± 9.7Bab | 256.71 ± 11.3Cab | 81.18 ± 1.7Bab | |
| 5 | 722.09 ± 3.7Aa | 773.55 ± 5.5Ab | 801.71 ± 21.9Aab | 691.26 ± 38.0Cbc | 312.83 ± 4.8Ba | 49.87 ± 11.BCab | |
| 10 | 592.95 ±14.7Bab | 683.22 ± 9.0Bb | 676.15 ± 8.6Ba | 738.86 ± 42.9Ca | 148.20 ± 5.0Dabc | 42.77 ± 9.9Ca | |
| 96 | 0 | 540.69 ± 19.3Bab | 808.61 ± 24.3Ab | 742.48 ± 9.6Ac | 936.50 ± 3.3Abc | 236.88 ± 1.4Ad | 145.33 ± 23.2Ab |
| 1 | 627.60 ± 40.3Ab | 806.52 ± 7.6Ab | 767.68 ± 26.5Ac | 813.87 ± 11.0Bb | 226.75 ± 8.1Abc | 65.75 ± 7.5Bab | |
| 5 | 642.11 ± 10.2Abc | 786.39 ± 14.7Ab | 787.81 ± 5.2Ab | 716.09 ± 29.5Cabc | 259.23 ± 7.8Ab | 47.87 ± 7.1Bab | |
| 10 | 556.41 ± 11.3Bb | 635.56 ± 20.4Bbc | 625.58 ± 1.5Bb | 564.26 ± 54.6Dc | 125.58 ± 11.2Bbc | nd | |
| 120 | 0 | 557.99 ± 5.6Bab | 856.90 ± 20.9Aab | 752.06 ± 4.3Ac | 871.57 ± 15.4Ac | 243.91 ± 1.8Ad | 105.58 ± 1.0Ac |
| 1 | 643.91 ± 32.9Aab | 808. 46 ± 18.3Bb | 783.03 ± 27.8Abc | 770.99 ± 21.1Bb | 228.25 ± 13.3Abc | 62.89 ± 6.7Bb | |
| 5 | 616.28 ± 2.9Ac | 701.59 ± 16.3Cc | 691.76 ± 17.8Bc | 644.68 ± 1.3Cc | 213.22 ± 6.7Ac | 30.53 ± 2.6Bb | |
| 10 | 487.83 ± 21.5Cc | 605.49 ± 21.9Dc | 614.45 ± 12.7Cb | 609.95 ± 5.5Cbc | 121.76 ± 6.5Bc | nd | |
| Volatile Compounds | Treatment (RPA *) | ||||
|---|---|---|---|---|---|
| C10H24 | C10H48 | C10H72 | C10H96 | C10H120 | |
| Amide | |||||
| l-Methioninamide | nd | 1.11 | 0.59 | 0.45 | nd |
| Pyrazines | |||||
| 2-Methylpyrazine | 3.70 | 5.04 | 5.50 | 6.00 | 5.36 |
| 2,5-Dimethyl-Pyrazine | 2.75 | nd | nd | 1.49 | 1.13 |
| 2-Ethyl-6-methylpyrazine | 0.15 | 0.22 | 0.76 | 0.71 | 0.67 |
| 2-Ethylpyrazine | nd | 1.89 | 2.39 | 1.86 | 2.03 |
| Pyrroles | |||||
| 1-furfuryl-Pyrrole | 0.39 | 0.55 | 0.94 | 1.11 | 1.39 |
| Furans | |||||
| Furfural | 17.36 | 15.19 | 15.68 | 10.15 | 12.17 |
| 5-Methyl-2-furaldehyde | 8.44 | 8.20 | 7.48 | 3.83 | 5.54 |
| 2-Pentylfuran | 0.29 | 0.40 | 0.46 | 0.37 | nd |
| Furfuryl acetate | 5.19 | 6.77 | 6.41 | 9.98 | 8.53 |
| Dihydro-2-methyl-3-furanone | 1.68 | 1.55 | 1.06 | 0.74 | nd |
| Esters | |||||
| Pentyl acetate | nd | nd | 0.72 | nd | nd |
| 3-Methyl-1-butyl propanoate | nd | nd | nd | 0.69 | 0.73 |
| Methyl salicylate | 0.20 | 0.35 | 0.45 | 0.44 | 0.55 |
| Acids | |||||
| 3-methyl-Butanoic acid | 2.20 | 1.93 | 2.76 | 2.11 | 1.55 |
| 2-methyl-Hexanoic acid | nd | nd | 0.62 | nd | 0.43 |
| 2-methyl-Butanoic acid | 43.60 | 40.62 | 40.04 | 45.31 | 41.06 |
| Ethyl ester Hexanoic acid | 1.06 | 1.35 | 1.79 | 1.66 | 1.82 |
| Cyclohexyl ester 2-Butenoic acid | 0.26 | 0.61 | 0.60 | 0.54 | 0.40 |
| Pyridine | |||||
| 1-Acetyl-1,4-dihydropyridine | 0.75 | 0.86 | 0.98 | 0.64 | 0.57 |
| Alcohol | |||||
| Benzeneethanol | 0.36 | 0.37 | 0.66 | 0.55 | 0.92 |
| Benzenemethanol | 0.18 | nd | 0.31 | nd | 0.28 |
| 3-Methyl-2-buten-1-ol | 0.54 | 0.56 | 0.96 | 1.67 | 1.74 |
| Propanoate-2-Buten-1-ol | 0.42 | 0.55 | nd | nd | nd |
| 1-Hexanol | 1.51 | 1.72 | 1.55 | 1.71 | 2.04 |
| 5-methyl-1-Hexanol | 0.90 | 1.20 | 0.78 | 1.45 | 1.38 |
| 2,4-Dimethyl-3-heptanol | nd | 0.33 | 0.29 | 0.34 | 0.67 |
| Terpenes | |||||
| Linalool | 0.18 | 0.85 | nd | nd | nd |
| Trans-Linalool oxide | 0.18 | 0.21 | 0.24 | 0.24 | 0.34 |
| Linalyl butanoate | 0.21 | 0.24 | 0.62 | 0.93 | 1.04 |
| Imidazole | |||||
| 1-(3H-Imidazol-4-yl)-ethanone | 3.72 | 5.48 | 4.45 | 3.87 | 4.03 |
| Volatile Compounds | Treatment (RPA *) | ||||
|---|---|---|---|---|---|
| C5H24 | C5H48 | C5H72 | C5H96 | C5H120 | |
| Amide | |||||
| l-Methioninamide | 1.08 | 0.89 | 1.03 | 0.96 | 0.51 |
| Pyrazines | |||||
| 2-Methylpyrazine | 4.94 | 6.66 | 6.24 | 6.22 | 7.84 |
| 2,5-Dimethyl-Pyrazine | 2.3 | nd | nd | nd | nd |
| 2-Ethyl-6-methylpyrazine | 0.65 | 0.88 | 0.84 | 1.95 | 1.08 |
| 2-Ethylpyrazine | nd | 2.09 | 2.19 | 3.08 | 3.72 |
| Pyrroles | |||||
| 1-furfuryl-Pyrrole | 0.26 | 0.43 | 0.55 | 0.60 | 0.75 |
| Furans | |||||
| Furfural | 13.94 | 13.67 | 14.41 | 17.64 | 11.26 |
| 5-Methyl-2-furaldehyde | 8.01 | 7.41 | 8.00 | 8.90 | 5.39 |
| 2-Pentylfuran | nd | 0.33 | 0.36 | 5.25 | 0.55 |
| Furfuryl acetate | 5.71 | 7.14 | 8.75 | 1.02 | 8.49 |
| Dihydro-2-methyl-3-furanone | 1.86 | 1.95 | 1.74 | 1.78 | 1.18 |
| Esters | |||||
| Pentyl acetate | nd | nd | 0.58 | nd | nd |
| 3-Methyl-1-butyl propanoate | 0.77 | nd | nd | nd | 0.65 |
| Methyl salicylate | 0.18 | nd | 0.33 | 0.46 | 0.61 |
| Acids | |||||
| 3-methyl-Butanoic acid | 2.02 | 2.28 | 2.53 | 2.91 | 2.71 |
| 2-methyl-Hexanoic acid | nd | nd | nd | nd | nd |
| 2-methyl-Butanoic acid | 45.51 | 42.73 | 38.92 | 37.07 | 37.47 |
| Ethyl ester Hexanoic acid | 1.69 | 1.99 | 1.99 | nd | 2.71 |
| Cyclohexyl ester 2-Butenoic acid | 0.41 | 0.18 | 0.43 | 0.56 | 0.85 |
| Pyridine | |||||
| 1-Acetyl-1,4-dihydropyridine | 0.57 | 0.89 | 0.63 | 0.55 | 0.64 |
| Alcohol | |||||
| Benzeneethanol | 0.30 | 0.28 | 0.37 | 0.60 | nd |
| Benzenemethanol | nd | nd | nd | 0.36 | nd |
| 3-Methyl-2-buten-1-ol | nd | nd | nd | nd | 1.68 |
| Propanoate-2-Buten-1-ol | 0.33 | nd | nd | 0.57 | nd |
| 1-Hexanol | 1.83 | 1.76 | 1.82 | 1.61 | 1.37 |
| 5-methyl- 1-Hexanol | 1.08 | 0.97 | 1.14 | 0.66 | 1.12 |
| 2,4-Dimethyl-3-heptanol | nd | nd | nd | nd | nd |
| Terpenes | |||||
| Linalool | nd | 0.20 | nd | nd | nd |
| Trans-Linalool oxide | 0.22 | 0.19 | 0.23 | 0.25 | 0.35 |
| Linalyl butanoate | 0.45 | 0.51 | 0.61 | nd | 1.26 |
| Imidazole | |||||
| 1-(3H-Imidazol-4-yl)-ethanone | 4.92 | 5.59 | 5.46 | 5.69 | 5.85 |
| Volatile Compounds | Treatment (RPA *) | ||||
|---|---|---|---|---|---|
| C1H24 | C1H48 | C1H72 | C1H96 | C1H120 | |
| Amide | |||||
| l-Methioninamide | 0.94 | 0.93 | 0.82 | 0.94 | 0.89 |
| Pyrazines | |||||
| 2-Methylpyrazine | 4.92 | 5.72 | 4.51 | 4.43 | 6.72 |
| 2,5-Dimethyl-Pyrazine | 1.63 | nd | 0.93 | 1.86 | nd |
| 2-Ethyl-6-methylpyrazine | 0.69 | 0.99 | 0.68 | 0.71 | 1.07 |
| 2-Ethylpyrazine | nd | 3.13 | 1.06 | nd | 2.18 |
| Pyrroles | |||||
| 1-furfuryl-Pyrrole | 0.33 | 0.43 | nd | 0.35 | 0.42 |
| Furans | |||||
| Furfural | 19.49 | 14.18 | 18.06 | 12.47 | 19.19 |
| 5-Methyl-2-furaldehyde | 10.01 | 8.47 | 8.71 | 7.96 | 10.39 |
| 2-Pentylfuran | nd | nd | nd | nd | nd |
| Furfuryl acetate | 4.26 | 6.76 | 3.21 | 6.90 | 6.94 |
| Dihydro-2-methyl-3-furanone | 2.11 | nd | 1.74 | 1.30 | 2.29 |
| Esters | |||||
| Pentyl acetate | nd | nd | nd | nd | 1.06 |
| 3-Methyl-1-butyl propanoate | nd | nd | 0.50 | 0.81 | nd |
| Methyl salicylate | 0.49 | 0.34 | nd | 0.24 | 0.50 |
| Acids | |||||
| 3-methyl-Butanoic acid | 3.01 | 2.65 | 2.81 | nd | 2.55 |
| 2-methyl-Hexanoic acid | nd | nd | nd | nd | 0.69 |
| 2-methyl-Butanoic acid | 40.20 | 40.34 | 47.81 | 51.72 | 32.09 |
| Ethyl ester Hexanoic acid | 1.55 | 2.21 | 1.05 | 1.66 | 1.85 |
| Cyclohexyl ester 2-Butenoic acid | 0.38 | 0.67 | 0.46 | 0.33 | 0.26 |
| Pyridine | |||||
| 1-Acetyl-1,4-dihydropyridine | 0.58 | 0.52 | 0.54 | nd | 0.37 |
| Alcohol | |||||
| Benzeneethanol | nd | 0.40 | nd | nd | nd |
| Benzenemethanol | nd | 0.23 | nd | nd | nd |
| 3-Methyl-2-buten-1-ol | nd | nd | nd | nd | nd |
| Propanoate-2-Buten-1-ol | nd | 1.69 | nd | nd | nd |
| 1-Hexanol | 1.66 | 1.78 | 1.52 | 1.99 | 1.59 |
| 5-methyl- 1-Hexanol | 0.86 | 0.76 | 0.57 | 0.75 | 0.82 |
| 2,4-Dimethyl-3-heptanol | nd | nd | nd | nd | 0.25 |
| Terpenes | |||||
| Linalool | nd | nd | nd | nd | nd |
| Trans-Linalool oxide | nd | nd | nd | nd | nd |
| Linalyl butanoate | 0.42 | 0.50 | nd | 0.56 | 0.54 |
| Imidazole | |||||
| 1-(3H-Imidazol-4-yl)-ethanone | 6.19 | 5.65 | 4.63 | 4.55 | 6.26 |
| Volatile Compounds | Treatment (RPA *) | |||||
|---|---|---|---|---|---|---|
| C0H0 | C0H24 | C0H48 | C0H72 | C0H96 | C0H120 | |
| Amide | ||||||
| l-Methioninamide | 0.24 | nd | 0.88 | 0.58 | 0.87 | 1.02 |
| Pyrazines | ||||||
| 2-Methylpyrazine | 4.45 | 4.60 | 5.07 | 4.21 | 3.96 | 4.69 |
| 2,5-Dimethyl-Pyrazine | nd | nd | nd | 1.15 | 2.51 | 1.74 |
| 2-Ethyl-6-methylpyrazine | 0.80 | 1.46 | 0.61 | 1.40 | nd | nd |
| 2-Ethylpyrazine | nd | nd | nd | nd | nd | nd |
| Pyrroles | ||||||
| 1-furfuryl-Pyrrole | 0.26 | 0.26 | 0.24 | 0.28 | 0.71 | 0.42 |
| Furans | ||||||
| Furfural | 24.24 | 22.52 | 14.28 | 25.39 | 19.75 | 14.16 |
| 5-Methyl-2-furaldehyde | 12.17 | 10.63 | 6.81 | 11.3 | 10.18 | 8.35 |
| 2-Pentylfuran | 0.43 | nd | nd | nd | nd | nd |
| Furfuryl acetate | 3.67 | 2.38 | 4.30 | 2.33 | 6.24 | 12.77 |
| Dihydro-2-methyl-3-furanone | 2.55 | nd | 1.90 | 2.01 | 1.49 | 1.90 |
| Esters | ||||||
| Pentyl acetate | nd | nd | nd | nd | nd | nd |
| 3-Methyl-1-butyl propanoate | nd | 1.76 | nd | 0.39 | 1.02 | nd |
| Methyl salicylate | 0.41 | 0.30 | nd | 0.61 | 0.6 | 0.56 |
| Acids | ||||||
| 3-methyl-Butanoic acid | 3.32 | 4.11 | 2.41 | 3.94 | 3.40 | 2.90 |
| 2-methyl-Hexanoic acid | nd | nd | nd | nd | nd | nd |
| 2-methyl-Butanoic acid | 38.16 | 41.89 | 51.48 | 38.44 | 38.54 | 37.43 |
| Ethyl ester Hexanoic acid | 1.26 | 1.41 | 1.75 | 1.58 | 1.92 | 3.00 |
| Cyclohexyl ester 2-Butenoic acid | 0.43 | 0.54 | 0.37 | 0.55 | 0.68 | 0.84 |
| Pyridine | ||||||
| 1-Acetyl-1,4-dihydropyridine | 0.54 | 0.47 | 0.94 | 0.38 | 0.47 | nd |
| Alcohol | ||||||
| Benzeneethanol | nd | nd | nd | nd | nd | nd |
| Benzenemethanol | nd | nd | nd | nd | nd | nd |
| 3-Methyl-2-buten-1-ol | nd | nd | nd | nd | nd | nd |
| Propanoate-2-Buten-1-ol | nd | nd | nd | nd | nd | nd |
| 1-Hexanol | 1.29 | 1.16 | 1.61 | 1.08 | 1.53 | 1.80 |
| 5-methyl-1-Hexanol | 0.70 | 0.63 | 0.82 | nd | 0.80 | 1.09 |
| 2,4-Dimethyl-3-heptanol | nd | nd | nd | nd | nd | nd |
| Terpenes | ||||||
| Linalool | nd | nd | nd | nd | nd | nd |
| Trans-Linalool oxide | nd | nd | nd | nd | nd | 0.35 |
| Linalyl butanoate | nd | nd | 0.49 | nd | 1.10 | nd |
| Imidazole | ||||||
| 1-(3H-Imidazol-4-yl)-ethanone | 4.39 | 3.88 | 3.86 | 4.36 | 4.19 | 5.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.E.d.S.; Oliveira, R.L.d.; Moraes, M.M.d.; Camara, C.A.G.d.; Silva, S.P.d.; Porto, T.S. Application of Commercial Pectinase as a Biocatalyst During Self-Induced Anaerobic Fermentation of Coffee (Coffea arabica L. var. Typica). Fermentation 2025, 11, 361. https://doi.org/10.3390/fermentation11070361
Silva MEdS, Oliveira RLd, Moraes MMd, Camara CAGd, Silva SPd, Porto TS. Application of Commercial Pectinase as a Biocatalyst During Self-Induced Anaerobic Fermentation of Coffee (Coffea arabica L. var. Typica). Fermentation. 2025; 11(7):361. https://doi.org/10.3390/fermentation11070361
Chicago/Turabian StyleSilva, Marcelo Edvan dos Santos, Rodrigo Lira de Oliveira, Marcilio Martins de Moraes, Claudio Augusto Gomes da Camara, Suzana Pedroza da Silva, and Tatiana Souza Porto. 2025. "Application of Commercial Pectinase as a Biocatalyst During Self-Induced Anaerobic Fermentation of Coffee (Coffea arabica L. var. Typica)" Fermentation 11, no. 7: 361. https://doi.org/10.3390/fermentation11070361
APA StyleSilva, M. E. d. S., Oliveira, R. L. d., Moraes, M. M. d., Camara, C. A. G. d., Silva, S. P. d., & Porto, T. S. (2025). Application of Commercial Pectinase as a Biocatalyst During Self-Induced Anaerobic Fermentation of Coffee (Coffea arabica L. var. Typica). Fermentation, 11(7), 361. https://doi.org/10.3390/fermentation11070361







