Anaerobic Digestion of Food Waste and Granular Inoculum: Study on Temperature Effect and Substrate-to-Inoculum Ratio on Biogas Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Lab-Scale Installation
2.2. Process Description and Material Characteristics
2.2.1. Process Description
2.2.2. Food Mixture Characteristics
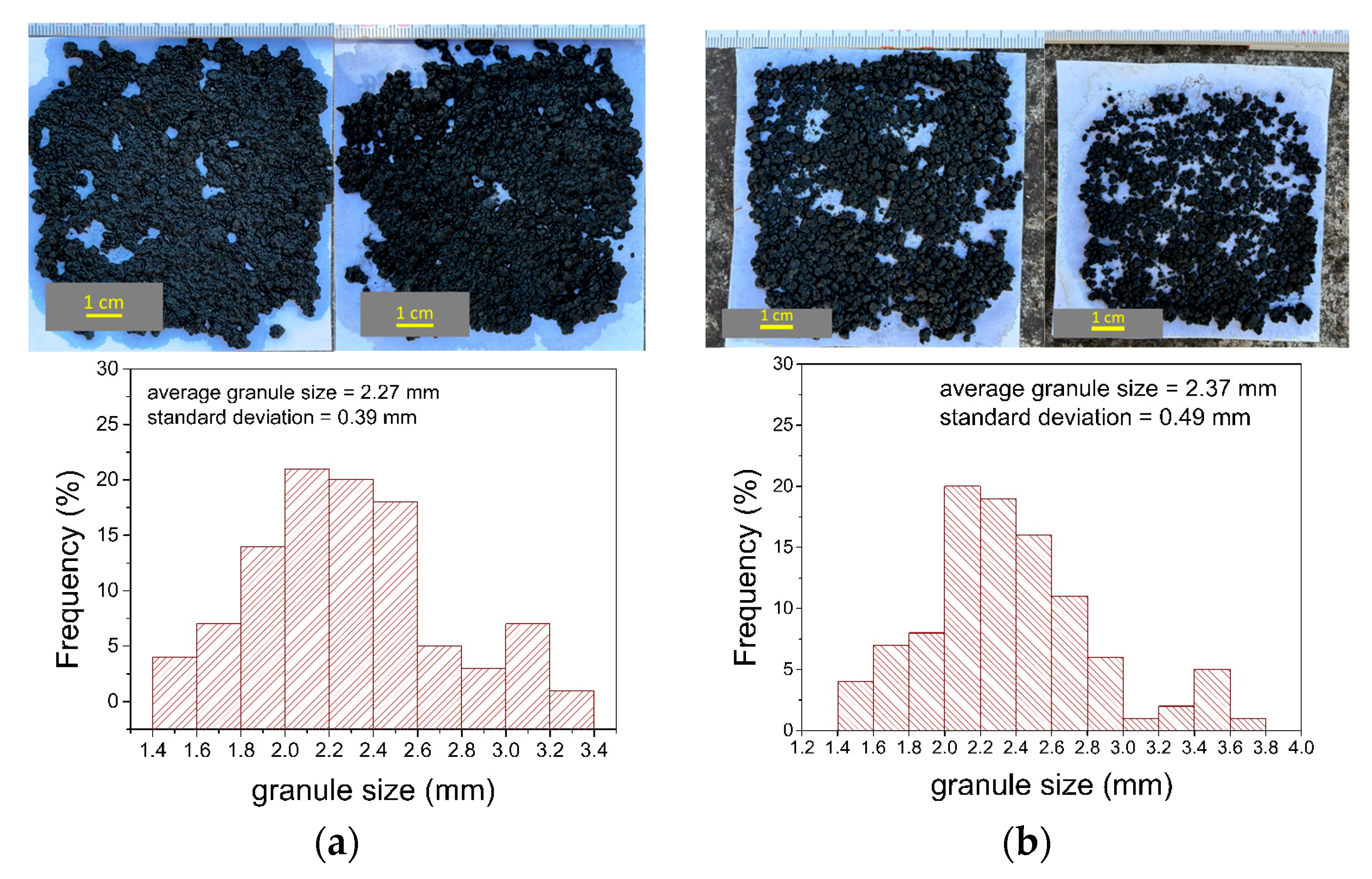
2.2.3. Biological and Morphological Characterization of Inoculum
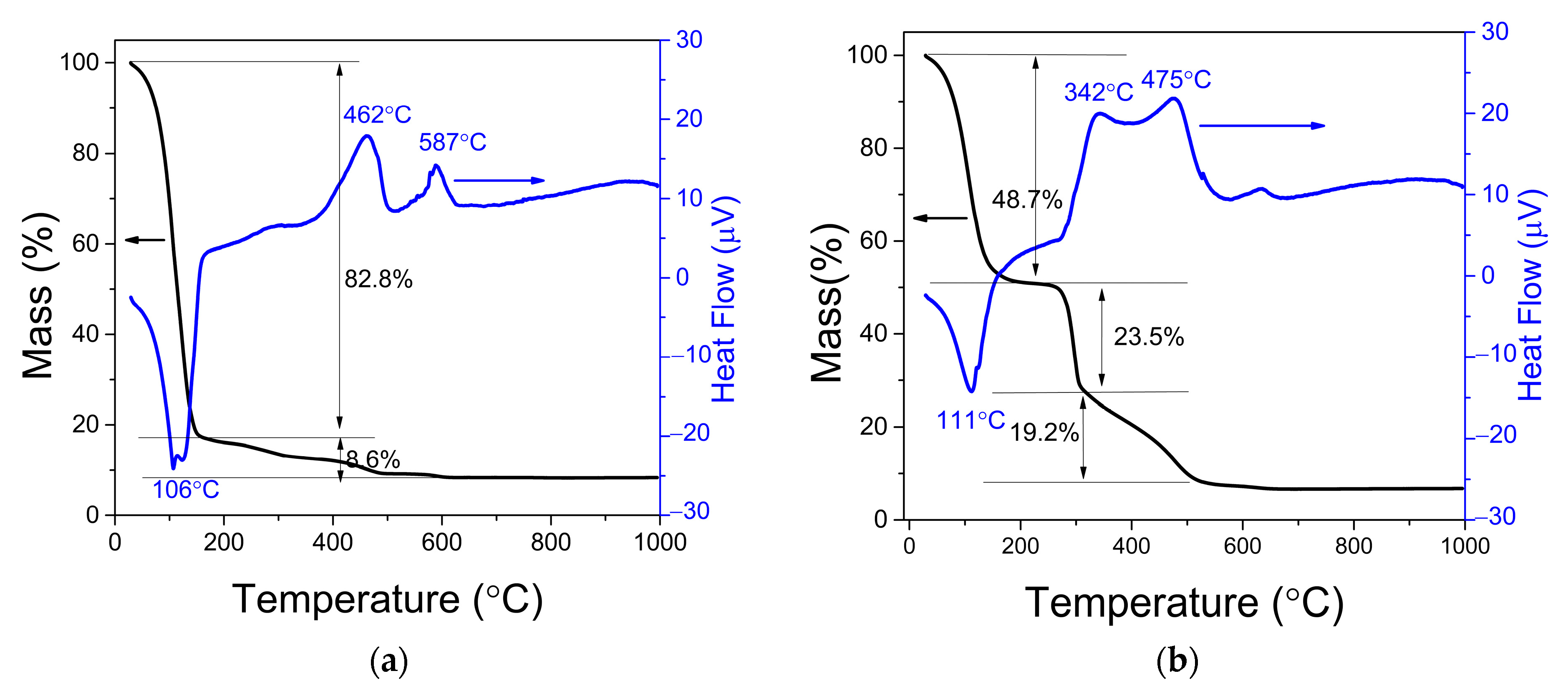
2.2.4. TG-DTA of Food Mixture and Inoculum
2.3. Analysis of Process Performances
2.3.1. Theoretical Production of CH4 and CO2
2.3.2. Evaluation of Experimental Biogas Production and Its Chemical Composition
2.3.3. CH4 and Organic Carbon Yield Conversion
- CH4exp and CO2exp is the amount of CH4 and CO2 produced in the digestion anaerobic batch experiments, determined as described in Section 2.3.2;
- CH4th and CO2th is the maximum theoretical amount of CH4 and CO2, calculated as presented in Section 2.3.1.
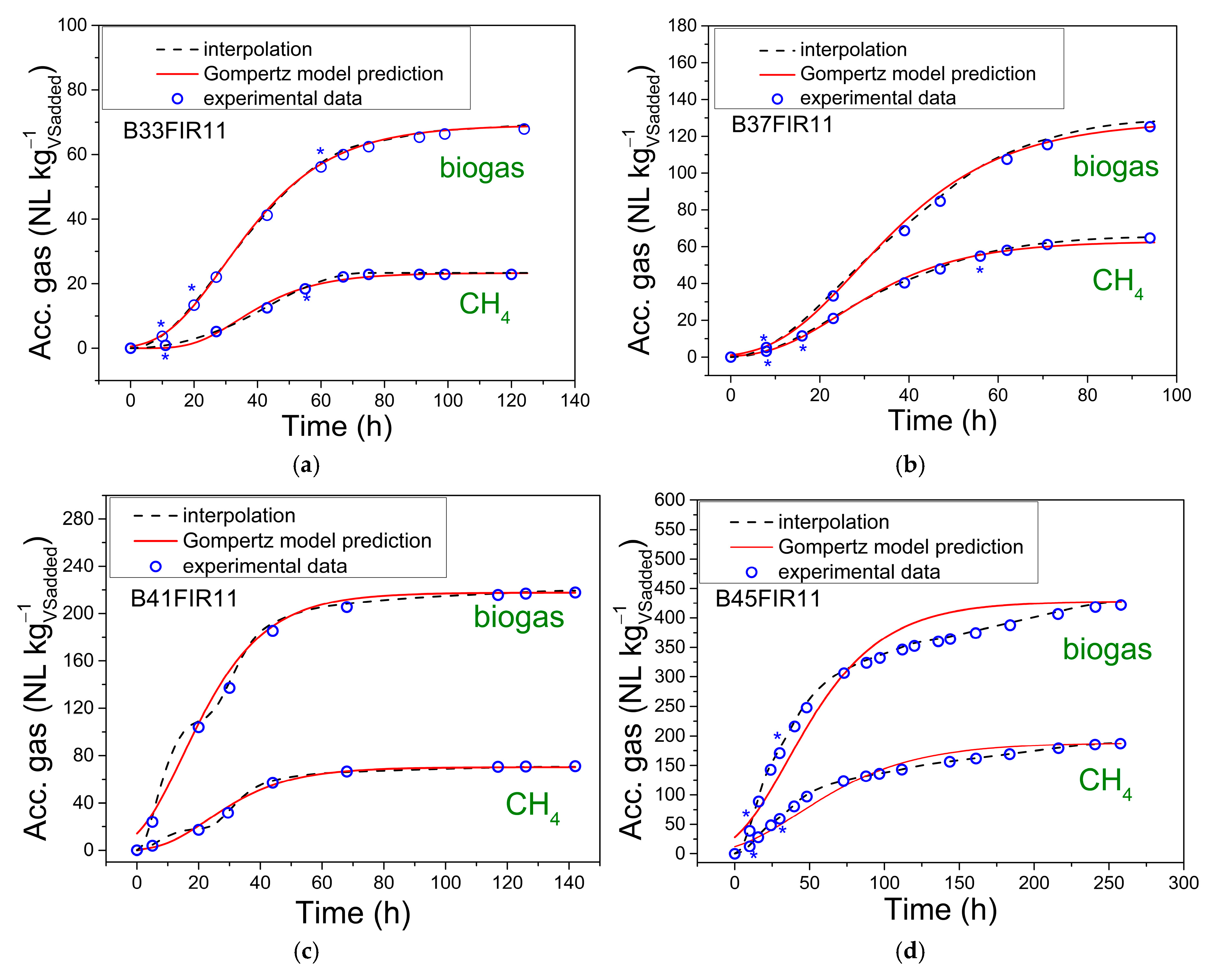
2.3.4. Gompertz Model Prediction—Kinetic Study of Accumulated Gas Production
- Pgas(t) is the production of accumulated gas in time;
- Md is the value on the y axis of the upper asymptote and represents the maximum accumulated gas.
- Rm is the kinetic parameter, the absolute growth rate, which affects the slope of the tangent to the curve.
- tlag is the value of time when the process starts to generate gas, obtained at the intersection of the tangent to the curve with the x axis. This value can be located at 6.6% of the upper asymptote on the y axis [40].
3. Results
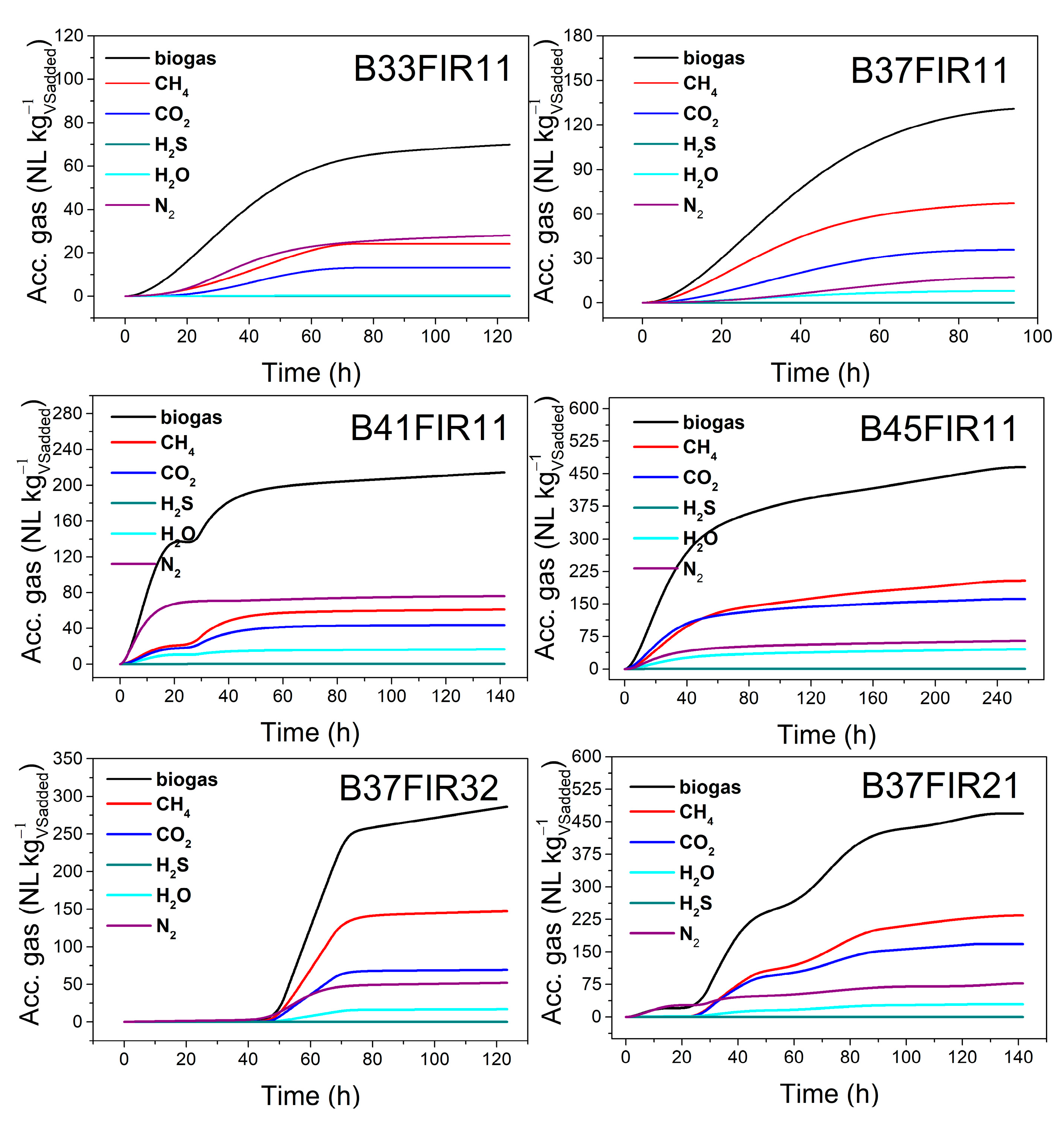
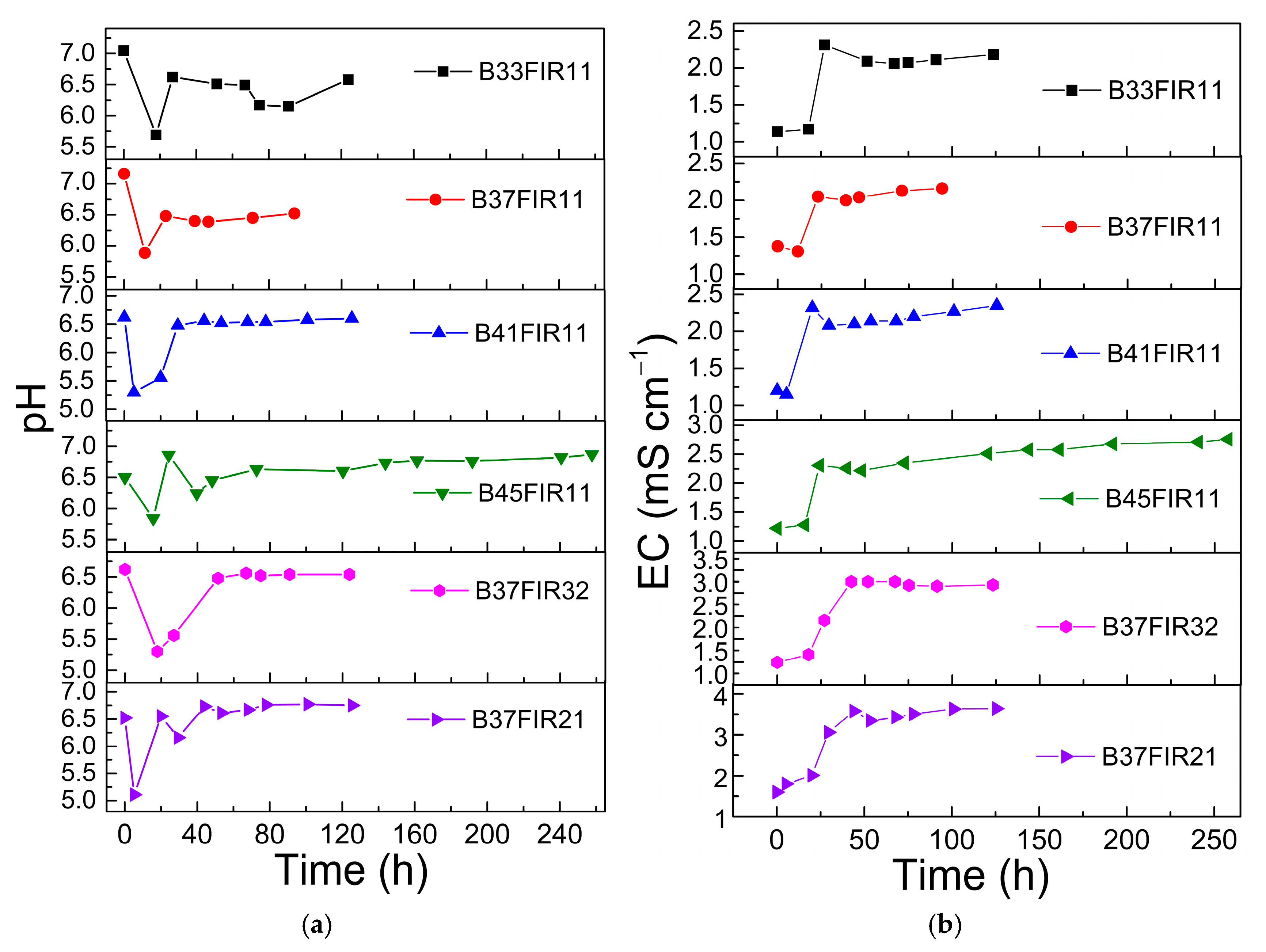
3.1. Evaluation of Process Performances: Biogas Production and Conversion Yield of CH4 and Organic Carbon
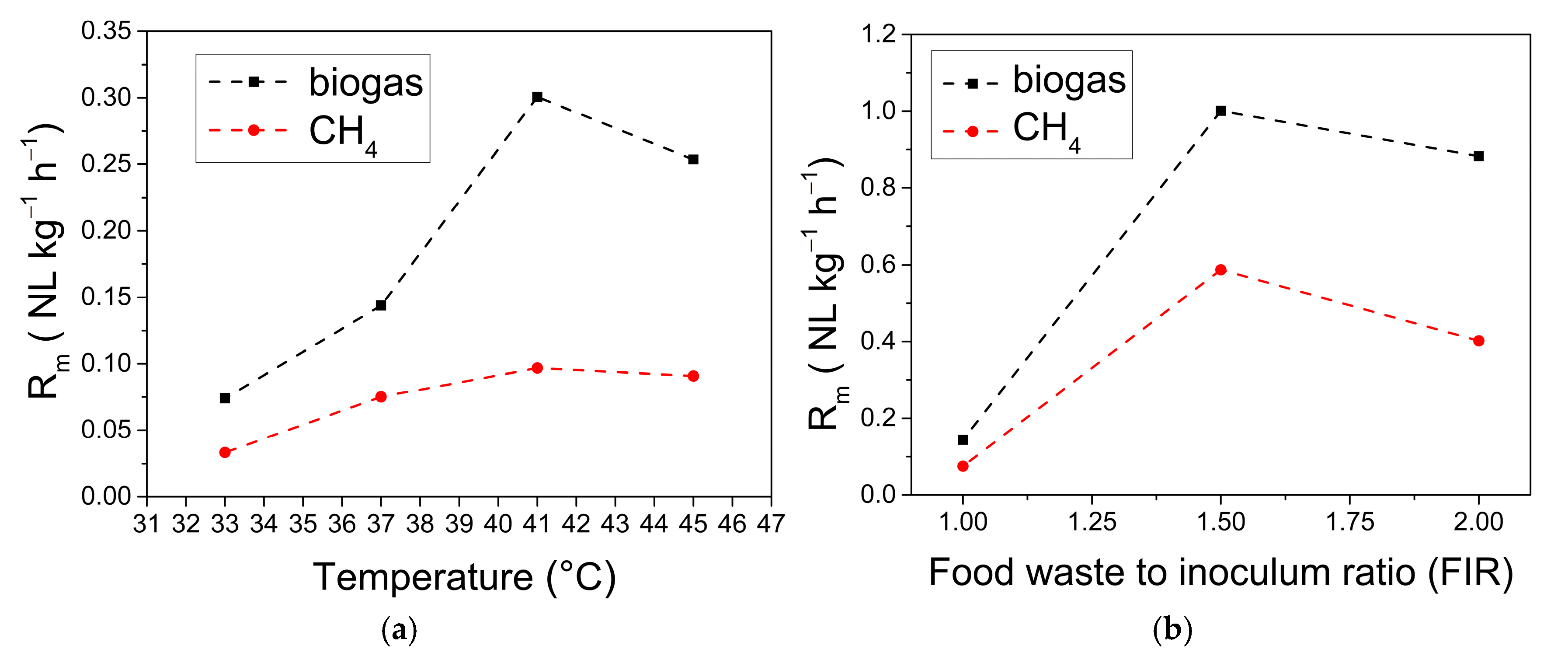
3.2. Gompertz Model Prediction
3.3. Morphological and Biological Characterization of the Inoculum
3.4. TG-DTA of Food Mixture and Inoculum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EUR-Lex: EU Law. European Commission, Secretariat-General. Communication from the Commission to the European Parlia-ment, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions REPowerEU Plan. 2022. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A52012DC0673 (accessed on 2 April 2025).
- Atchike, D.W.; Irfan, M.; Ahmad, M.; Rehman, M.A. Waste-to-Renewable Energy Transition: Biogas Generation for Sustainable Development. Front. Environ. Sci. 2022, 10, 840588. [Google Scholar] [CrossRef]
- Chowdhury, T.H. Technical-economical analysis of anaerobic digestion process to produce clean energy. Energy Rep. 2021, 7, 247–253. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Tabatabaei, M.; Aghbashlo, M. Biogas production from food wastes: A review on recent developments and future perspectives. Bioresour. Technol. Rep. 2019, 7, 100202. [Google Scholar] [CrossRef]
- Zupančič, M.; Možic, V.; Može, M.; Cimerman, F.; Golobič, I. Current Status and Review of Waste-to-Biogas Conversion for Selected European Countries and Worldwide. Sustainability 2022, 14, 1823. [Google Scholar] [CrossRef]
- Patel, C.; Kumar, G.; Upadhyay, A.K. Production of bio-gas (In urban areas using different types of wastes). Recent. Res. Sci. Technol. 2014, 6, 126–127. [Google Scholar]
- Morales-Polo, C.; Del Mar Cledera-Castro, M.; Moratilla Soria, B.Y. Reviewing the Anaerobic Digestion of Food Waste: From Waste Generation and Anaerobic Process to Its Perspectives. Appl. Sci. 2018, 8, 1804. [Google Scholar] [CrossRef]
- Chew, K.R.; Leong, H.Y.; Khoo, K.S.; Vo, D.V.N.; Anjum, H.; Chang, C.K.; Show, P.L. Effects of anaerobic digestion of food waste on biogas production and environmental impacts: A review. Environ. Chem. Lett. 2021, 19, 2921–2939. [Google Scholar] [CrossRef]
- Eurostat. Food Waste and Food Waste Prevention by NACE Rev. 2 Activity—Tonnes of Fresh Mass. Eurostat, 2023. Available online: https://ec.europa.eu/eurostat/databrowser/product/page/ENV_WASFW (accessed on 2 April 2025).
- European Environment Agency. Bio-Waste in Europe: Turning Challenges into Opportunities; Publications Office: Luxembourg, 2020; Available online: https://www.eea.europa.eu/en/analysis/publications/bio-waste-in-europe (accessed on 2 April 2025).
- Yasin, N.H.M.; Mumtaz, T.; Hassan, M.A.; Abd Rahman, N. Food waste and food processing waste for biohydrogen production: A review. J. Environ. Manag. 2013, 130, 375–385. [Google Scholar] [CrossRef]
- Rodríguez-Jiménez, L.M.; Pérez-Vidal, A.; Torres-Lozada, P. Research trends and strategies for the improvement of anaerobic digestion of food waste in psychrophilic temperatures conditions. Heliyon 2022, 8, 11174. [Google Scholar] [CrossRef]
- Conrad, Z.; Niles, M.T.; Neher, D.A.; Roy, E.D.; Tichenor, N.E.; Jahns, L. Relationship between food waste, diet quality, and environmental sustainability. PLoS ONE 2018, 13, 0195405. [Google Scholar] [CrossRef]
- Lau, K.Q.; Sabran, M.R.; Shafie, S.R. Utilization of Vegetable and Fruit By-products as Functional Ingredient and Food. Front. Nutr. 2021, 8, 661693. [Google Scholar] [CrossRef]
- Miramontes-Martínez, L.R.; Rivas-García, P.; Albalate-Ramírez, A.; Botello-Álvarez, J.E.; Escamilla-Alvarado, C.; Gomez-Gonzalez, R.; Alcalá-Rodríguez, M.M.; Valencia-Vázquez, R.; Santos-López, I.A. Anaerobic co-digestion of fruit and vegetable waste: Synergy and process stability analysis. J. Air Waste Manag. Assoc. 2021, 71, 620–632. [Google Scholar] [CrossRef]
- Ji, C.; Kong, C.X.; Mei, Z.L.; Li, J. A Review of the Anaerobic Digestion of Fruit and Vegetable Waste. Appl. Biochem. Biotechnol. 2017, 183, 906–922. [Google Scholar] [CrossRef]
- Tratsch, M.V.M.; Ceretta, C.A.; Silva, L.S.D.; Ferreira, P.A.A.; Brunetto, G. Composition and mineralization of organic compost derived from composting of fruit and vegetable waste. Rev. Ceres. 2019, 66, 307–315. [Google Scholar] [CrossRef]
- Latif, M.A.; Mehta, C.M.; Batstone, D.J. Influence of low pH on continuous anaerobic digestion of waste activated sludge. Water Res. 2017, 113, 42–49. [Google Scholar] [CrossRef]
- Salangsang, M.C.D.; Sekine, M.; Akizuki, S.; Sakai, H.D.; Kurosawa, N.; Toda, T. Effect of carbon to nitrogen ratio of food waste and short resting period on microbial accumulation during anaerobic digestion. Biomass Bioenergy 2022, 162, 106481. [Google Scholar] [CrossRef]
- Choi, Y.; Ryu, J.; Lee, S.R. Influence of carbon type and carbon to nitrogen ratio on the biochemical methane potential, pH, and ammonia nitrogen in anaerobic digestion. J. Anim. Sci. Technol. 2020, 62, 74–83. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Rene, E.R.; Dupont, C.; Wongrod, S.; Van Hullebusch, E.D. Anaerobic Digestion of Fruit Waste Mixed With Sewage Sludge Digestate Biochar: Influence on Biomethane Production. Front. Energy Res. 2020, 8, 31. [Google Scholar] [CrossRef]
- Deressa, L.; Libsu, S.; Chavan, R.B.; Manaye, D.; Dabassa, A. Production of Biogas from Fruit and Vegetable Wastes Mixed with Different Wastes. Environ. Ecol. Res. 2015, 3, 65–71. [Google Scholar] [CrossRef]
- Yen, S.; Preston, T.R.; Thi Thuy, N. Biogas production from fruit wastes combined with manure from pigs or buffaloes in an in vitro biodigester system. Livest. Res. Rural Dev. 2017, 29, 151. [Google Scholar]
- Alvarez, R.; Lidén, G. Semi-continuous co-digestion of solid slaughterhouse waste, manure, and fruit and vegetable waste. Renew. Energy 2008, 33, 726–734. [Google Scholar] [CrossRef]
- Park, N.D.; Thring, R.W.; Garton, R.P.; Rutherford, M.P.; Helle, S.S. Increased biogas production in a wastewater treatment plant by anaerobic co-digestion of fruit and vegetable waste and sewer sludge—A full scale study. Water Sci. Technol. 2011, 64, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- Arhoun, B.; Villen-Guzman, M.D.; Vereda-Alonso, C.; Rodriguez-Maroto, J.M.; Garcia-Herruzo, F.; Gómez-Lahoz, C. Anaerobic co-digestion of municipal sewage sludge and fruit/vegetable waste: Effect of different mixtures on digester stability and methane yield. J. Environ. Sci. Health Part A 2019, 54, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Mkhize, N.; Mjoli, N.S.; Khumalo, S.M.; Tettteh, E.K.; Mahlangu, T.P.; Rathilal, S. Enhanced Biogas Production through Anaerobic Co-Digestion of Agricultural Wastes and Wastewater: A Case Study in South Africa. Int. J. Energy Prod. Manag. 2023, 8, 123–131. [Google Scholar] [CrossRef]
- Chaurasia, A.K.; Siwach, P.; Shankar, R.; Mondal, P. Effect of pre-treatment on mesophilic anaerobic co-digestion of fruit, food and vegetable waste. Clean Technol. Environ. Policy 2021, 25, 603–616. [Google Scholar] [CrossRef]
- Zainudin Siti, F.; Anuar Aznah, N.; Yuzir Ali, M.; Sharani Noor, A.; Aris Alijah, M.; Khor Bee, C. The Granular Sludge Properties and Performance Evaluation of Upflow Anaerobic Sludge Blanket Reactor as a Pre-Treatment for Domestic Wastewater. Chem. Eng. Trans. 2023, 106, 1189–1194. [Google Scholar]
- Owusu-Agyeman, I.; Eyice, Ö.; Cetecioglu, Z.; Plaza, E. The study of structure of anaerobic granules and methane producing pathways of pilot-scale UASB reactors treating municipal wastewater under sub-mesophilic conditions. Bioresour. Technol. 2019, 290, 121733. [Google Scholar] [CrossRef]
- Mu, H.; Li, Y.; Zhao, Y.; Zhang, X.; Hua, D.; Xu, H.; Jin, F. Microbial and nutritional regulation of high-solids anaerobic mono-digestion of fruit and vegetable wastes. Environ. Technol. 2018, 39, 405–413. [Google Scholar] [CrossRef]
- Yazidi, H.; Thanikal, J.V. Non-linear kinetic modelling of anaerobic biodegradation of fruit and vegetable waste together with cooked oil. Int. J. Adv. Res. Biol. Sci. 2016, 3, 109–121. [Google Scholar]
- Gaur, R.Z.; Suthar, S. Anaerobic digestion of activated sludge, anaerobic granular sludge and cow dung with food waste for enhanced methane production. J. Clean. Prod. 2017, 164, 557–566. [Google Scholar] [CrossRef]
- Santos, L.A.D.; Valença, R.B.; Silva, L.C.S.D.; Holanda, S.H.D.B.; Silva, A.F.V.D.; Jucá, J.F.T.; Santos, A.F.M.S. Methane generation potential through anaerobic digestion of fruit waste. J. Clean. Prod. 2020, 256, 120389. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Yu, Z.; Lu, J.; Li, D.; Wang, G.; Li, Y.; Wu, Y.; Li, S.; Xu, F.; et al. Effect of inoculum and substrate/inoculum ratio on the performance and methanogenic archaeal community structure in solid state anaerobic co-digestion of tomato residues with dairy manure and corn stover. Waste Manag. 2018, 81, 117–127. [Google Scholar] [CrossRef] [PubMed]
- De La Rubia, M.A.; Villamil, J.A.; Rodriguez, J.J.; Mohedano, A.F. Effect of inoculum source and initial concentration on the anaerobic digestion of the liquid fraction from hydrothermal carbonisation of sewage sludge. Renew. Energy 2018, 127, 697–704. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, X.; Liu, Z.; Zhou, X.; Zhang, Y. Effect of inoculum sources on the anaerobic digestion of rice straw. Bioresour. Technol. 2018, 158, 149–155. [Google Scholar] [CrossRef]
- Li, Y.; Feng, L.; Zhang, R.; He, Y.; Liu, X.; Xiao, X.; Ma, X.; Chen, C.; Liu, G. Influence of Inoculum Source and Pre-incubation on Bio-Methane Potential of Chicken Manure and Corn Stover. Appl. Biochem. Biotechnol. 2013, 171, 117–127. [Google Scholar] [CrossRef]
- Demichelis, F.; Tommasi, T.; Deorsola, F.A.; Marchisio, D.; Fino, D. Effect of inoculum origin and substrate-inoculum ratio to enhance the anaerobic digestion of organic fraction municipal solid waste (OFMSW). J. Clean. Prod. 2022, 351, 131539. [Google Scholar] [CrossRef]
- Tjørve, K.M.C.; Tjørve, E. The use of Gompertz models in growth analyses, and new Gompertz-model approach: An addition to the Unified-Richards family. PLoS ONE 2017, 12, e0178691. [Google Scholar] [CrossRef]
- Siren, K.; Rosén, G.; Vad, J.; Nielsen, P.V. EXPERIMENTAL TECHNIQUES. In Industrial Ventilation Design Guidebook; Goodfellow, H., Tahti, E., Eds.; Academic Press: Cambridge, MA, USA, 2001; pp. 1105–1195. [Google Scholar]
- Doğu, D.; Demir, V. Investigation of pressure drop in main air line of pipeline milking system. Ege Üniversitesi Ziraat Fakültesi Derg. 2025, 62, 49–62. [Google Scholar] [CrossRef]
- Martinez-Fernandez, J.S.; Seker, A.; Davaritouchaee, M.; Gu, X.; Chen, S. Recovering Valuable Bioactive Compounds from Potato Peels with Sequential Hydrothermal Extraction. Waste Biomass Valoriz. 2021, 12, 1465–1481. [Google Scholar] [CrossRef]
- Bakshi, M.P.S.; Wadhwa, M.; Makkar, H.P.S. Waste to worth: Vegetable wastes as animal feed. CABI Rev. 2016, 6, 1–26. [Google Scholar] [CrossRef]
- Bugatti, V.; Brachi, P.; Viscusi, G.; Gorrasi, G. Valorization of Tomato Processing Residues Through the Production of Active Bio-Composites for Packaging Applications. Front Mater. 2019, 6, 34. [Google Scholar] [CrossRef]
- Achilonu, M.C.; Nwafor, I.C.; Umesiobi, D.O.; Sedibe, M.M. Biochemical proximates of pumpkin (Cucurbitaeae spp.) and their beneficial effects on the general well-being of poultry species. J. Anim. Physiol. Anim. Nutr. 2018, 102, 5–16. [Google Scholar] [CrossRef]
- Uzuazokaro, M.M.A.; Okwesili, F.C.N.; Chioma, A.A. Phytochemical and proximate composition of cucumber (Cucumis sativus) fruit from Nsukka, Nigeria. Afr. J. Biotechnol. 2018, 17, 1215–1219. [Google Scholar] [CrossRef]
- OECD. Safety Assessment of Foods and Feeds Derived from Transgenic Crops, Volume 3: Common Bean, Rice, Cowpea and Apple Compositional Considerations; Novel Food and Feed Safety; OECD Publishing: Paris, France, 2019; Available online: https://www.oecd-ilibrary.org/environment/safety-assessment-of-foods-and-feeds-derived-from-transgenic-crops-volume-3_f04f3c98-en (accessed on 2 April 2025).
- Omulo, G. Harnessing of banana ripening process for banana juice extraction in Uganda. Afr. J. Food Sci. Technol. 2015, 6, 108–117. [Google Scholar] [CrossRef]
- Verma, D.K.; Srivastav, P.P. Proximate Composition, Mineral Content and Fatty Acids Analyses of Aromatic and Non-Aromatic Indian Rice. Rice Sci. 2017, 24, 21–31. [Google Scholar] [CrossRef]
- Achinas, S.; Euverink, G.J.W. Theoretical analysis of biogas potential prediction from agricultural waste. Resour.-Effic. Technol. 2016, 2, 143–147. [Google Scholar]
- Roati, C.; Fiore, S.; Ruffino, B.; Marchese, F.; Novarino, D.; Zanetti, M.C. Preliminary Evaluation of the Potential Biogas Production of Food-Processing Industrial Wastes. Am. J. Environ. Sci. 2012, 8, 291–296. [Google Scholar]
- Ugwu, S.N.; Enweremadu, C.C. Biodegradability and kinetic studies on biomethane production from okra (Abelmoschus esculentus) waste. S. Afr. J. Sci. 2019, 115. [Google Scholar] [CrossRef]
- Jingura, R.M.; Kamusoko, R. Methods for determination of biomethane potential of feedstocks: A review. Biofuel Res. J. 2017, 4, 573–586. [Google Scholar] [CrossRef]
- Li, Y.; Alaimo, C.P.; Kim, M.; Kado, N.Y.; Peppers, J.; Xue, J.; Wan, C.; Green, P.G.; Zhang, R.; Jenkins, B.M.; et al. Composition and Toxicity of Biogas Produced from Different Feedstocks in California. Environ. Sci. Technol. 2019, 53, 11569–11579. [Google Scholar] [CrossRef]
- Chen, X.Y.; Vinh-Thang, H.; Ramirez, A.A.; Rodrigue, D.; Kaliaguine, S. Membrane gas separation technologies for biogas upgrading. RSC Adv. 2015, 5, 24399–24448. [Google Scholar] [CrossRef]
- Kujawa, J.; Kujawski, W. Driving force and activation energy in air-gap membrane distillation process. Chem. Pap. 2015, 69, 1438–1444. [Google Scholar] [CrossRef]
- Aceves-Lara, C.A.; Latrille, E.; Conte, T.; Steyer, J.P. Online estimation of VFA, alkalinity and bicarbonate concentrations by electrical conductivity measurement during anaerobic fermentation. Water Sci. Technol. 2012, 65, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Sikora, A.; Detman, A.; Chojnacka, A.; Blaszczyk, M.K. Anaerobic Digestion: I. A Common Process Ensuring Energy Flow and the Circulation of Matter in Ecosystems. II. A Tool for the Production of Gaseous Biofuels. In Fermentation Processes; Jozala, A.F., Ed.; InTech Open: London, UK, 2017; pp. 272–301. [Google Scholar]
- Khan, M.A.; Ashar, N.N.; Ganesh, A.G.; Rais, N.; Faheem, S.M.; Khan, S.T. Bacterial Community Structure in Anaerobic Digesters of a Full Scale Municipal Wastewater Treatment Plant—Case Study of Dubai, United Arab Emirates. J. Sustain. Dev. Energy Water Environ. Syst. 2019, 7, 385–398. [Google Scholar] [CrossRef]
- Mota-Panizio, R.; Hermoso-Orzáez, M.J.; Carmo-Calado, L.; Lourinho, G.; Brito, P.S.D.D. Biochemical Methane Potential of Cork Boiling Wastewater at Different Inoculum to Substrate Ratios. Appl. Sci. 2021, 11, 3064. [Google Scholar] [CrossRef]
- Syguła, E.; Świechowski, K.; Hejna, M.; Kunaszyk, I.; Białowiec, A. Municipal Solid Waste Thermal Analysis—Pyrolysis Kinetics and Decomposition Reactions. Energies 2021, 14, 4510. [Google Scholar] [CrossRef]
- Yao, C.; Wang, X.; Zhou, Y.; Jin, X.; Song, L.; Hu, Y.; Zeng, W. Thermogravimetric analysis and kinetics characteristics of typical grains. J. Therm. Anal. Calorim. 2021, 143, 647–659. [Google Scholar] [CrossRef]
- Font, R. Decomposition of Organic Wastes: Thermal Analysis and Evolution of Volatiles. In Handbook of Thermal Analysis and Calorimetry, 2nd ed.; Vyazovkin, S., Koga, N., Schick, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 6, pp. 339–397. [Google Scholar]
- Vakalis, S.; Sotiropoulos, A.; Moustakas, K.; Malamis, D.; Vekkos, K.; Baratieri, M. Characterization of Hotel Bio-waste by Means of Simultaneous Thermal Analysis. Waste Biomass Valoriz. 2016, 7, 649–657. [Google Scholar] [CrossRef]
- Kalogiannis, A.; Diamantis, V.; Eftaxias, A.; Stamatelatou, K. Long-Term Anaerobic Digestion of Seasonal Fruit and Vegetable Waste Using a Leach-Bed Reactor Coupled to an Upflow Anaerobic Sludge Bed Reactor. Sustainability 2023, 16, 50. [Google Scholar] [CrossRef]
- Zhu, Y.; Luan, Y.; Zhao, Y.; Liu, J.; Duan, Z.; Ruan, R. Current Technologies and Uses for Fruit and Vegetable Wastes in a Sustainable System: A Review. Foods 2023, 12, 1949. [Google Scholar] [CrossRef]
- Fernández, F.J.; Villaseñor, J.; Infantes, D. Kinetic and stoichiometric modelling of acidogenic fermentation of glucose and fructose. Biomass Bioenergy 2011, 35, 3877–3883. [Google Scholar] [CrossRef]
- Ali, S.; Dar, M.A.; Liaqat, F.; Sethupathy, S.; Rani, A.; Khan, M.I.; Rehan, M.; Zhu, D. Optimization of biomethane production from lignocellulosic biomass by a developed microbial consortium. Process Saf. Environ. Prot. 2024, 184, 1106–1118. [Google Scholar] [CrossRef]
- Charnier, C.; Latrille, E.; Lardon, L.; Miroux, J.; Steyer, J.P. Combining pH and electrical conductivity measurements to improve titrimetric methods to determine ammonia nitrogen, volatile fatty acids and inorganic carbon concentrations. Water Res. 2016, 95, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Tanko, S.F.; Ajibola, V.O.; Agbaji, E.A.; Mukailu, A.S.; Putshaka, J.D.; Amana, B.S. Effect of anaerobic treatment on sulphide concentration, TSS, TDS and electrical conductivity parameters of sulphide-lime unhairing wastewater. Bayero J. Pure Appl. Sci. 2019, 11, 260. [Google Scholar] [CrossRef]
- Robles, A.; Latrille, E.; Ribes, J.; Bernet, N.; Steyer, J.P. Electrical conductivity as a state indicator for the start-up period of anaerobic fixed-bed reactors. Water Sci. Technol. 2016, 73, 2294–2300. [Google Scholar] [CrossRef]
- Marín-Peña, O.; Alvarado-Lassman, A.; Vallejo-Cantú, N.A.; Juárez-Barojas, I.; Rodríguez-Jarquín, J.P.; Martínez-Sibaja, A. Electrical Conductivity for Monitoring the Expansion of the Support Material in an Anaerobic Biofilm Reactor. Processes 2020, 8, 77. [Google Scholar] [CrossRef]
- Gavala, H.N.; Yenal, U.; Skiadas, I.V.; Westermann, P.; Ahring, B.K. Mesophilic and thermophilic anaerobic digestion of primary and secondary sludge. Effect of pre-treatment at elevated temperature. Water Res. 2003, 37, 4561–4572. [Google Scholar] [CrossRef]
- Biyada, S.; Merzouki, M.; Dėmčėnko, T.; Vasiliauskienė, D.; Ivanec-Goranina, R.; Urbonavičius, J.; Marčiulaitienė, E.; Vasarevičius, S.; Benlemlih, M. Microbial community dynamics in the mesophilic and thermophilic phases of textile waste composting identified through next-generation sequencing. Sci. Rep. 2021, 11, 23624. [Google Scholar] [CrossRef]
- Steiniger, B.; Hupfauf, S.; Insam, H.; Schaum, C. Exploring Anaerobic Digestion from Mesophilic to Thermophilic Temperatures—Operational and Microbial Aspects. Fermentation 2023, 9, 798. [Google Scholar] [CrossRef]
- Rivière, D.; Desvignes, V.; Pelletier, E.; Chaussonnerie, S.; Guermazi, S.; Weissenbach, J.; Li, T.; Camacho, P.; Sghir, A. Towards the definition of a core of microorganisms involved in anaerobic digestion of sludge. ISME J. 2009, 3, 700–714. [Google Scholar] [CrossRef]
- Guo, J.; Peng, Y.; Ni, B.J.; Han, X.; Fan, L.; Yuan, Z. Dissecting microbial community structure and methane-producing pathways of a full-scale anaerobic reactor digesting activated sludge from wastewater treatment by metagenomic sequencing. Microb. Cell Factories 2015, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.-z.; Fang, S.; Zhang, L.; Huang, W.; Shao, Q.; Fang, F.; Feng, Q.; Cao, J.; Luo, J. Distribution patterns of functional microbial community in anaerobic digesters under different operational circumstances: A review. Bioresour. Technol. 2021, 341, 125823. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.R.; Anacleto, T.M.; Abreu, F.; Enrich-Prast, A. New insights into the factors influencing methanogenic pathways in anaerobic digesters. Anaerobe 2025, 91, 102925. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Angelidaki, I.; Zhang, Y. In situ Biogas Upgrading by CO2-to-CH4 Bioconversion. Trends Biotechnol. 2021, 39, 336–347. [Google Scholar] [CrossRef]
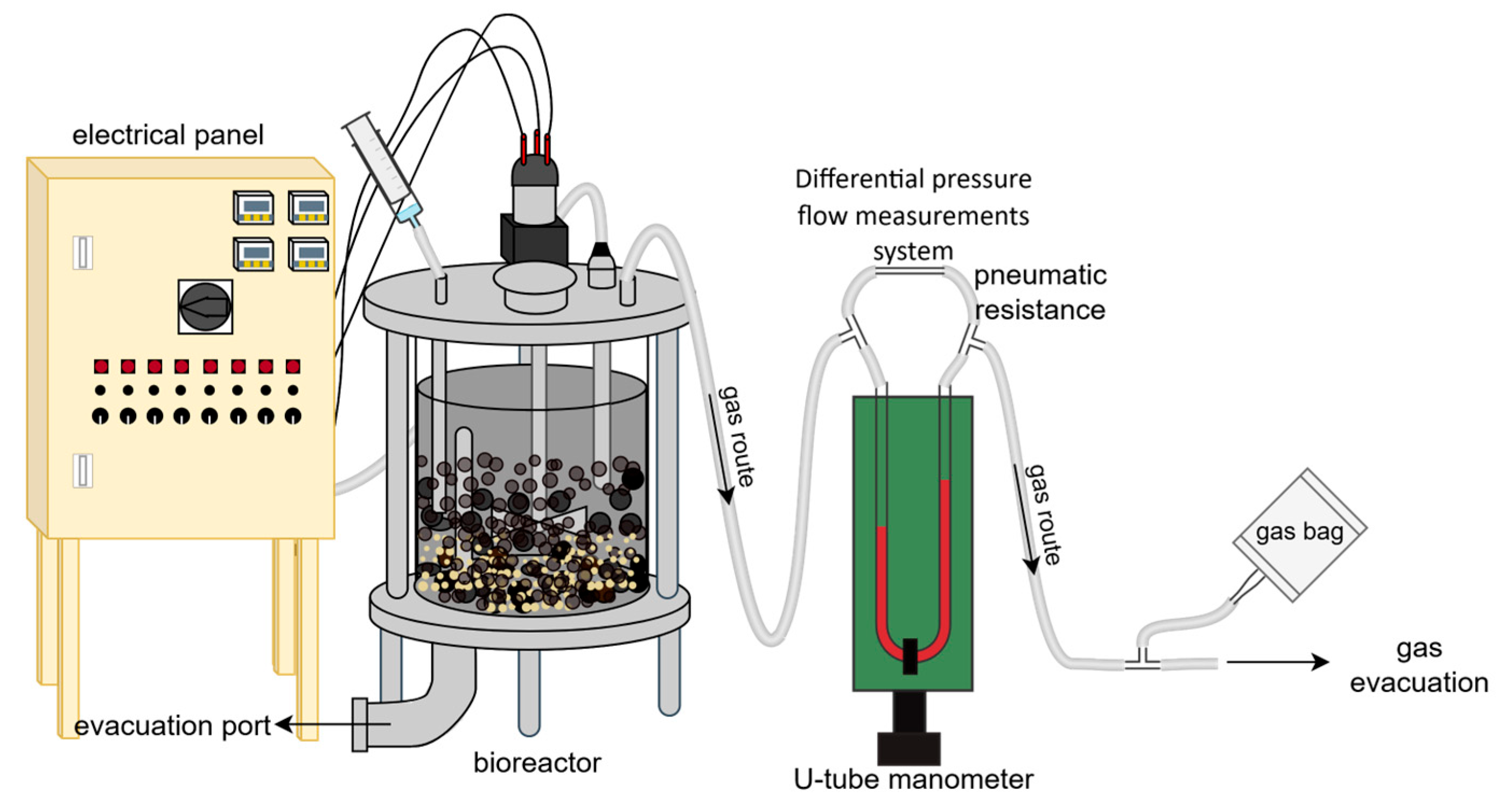



| No. | Batch Experiment Code | Temperature [°C] | Food Mixture to Inoculum Ratio (FIR) (w/w) |
|---|---|---|---|
| 1 | B33FIR11 | 33 | 1:1 |
| 2 | B37FIR11 | 37 | 1:1 |
| 3 | B41FIR11 | 41 | 1:1 |
| 4 | B45FIR11 | 45 | 1:1 |
| 5 | B37FIR32 | 37 | 3:2 |
| 6 | B37FIR21 | 37 | 2:1 |
| Food Component | Elemental Composition (%) | C/N Ratio | TS (%) | VS (%) | Water Content (%) | Ash Content (%) | Source | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O | |||||||
| Potato | 52.6 | 6.6 | 2.8 | 0.2 | 37.8 | 18.78 | 16.7 | 15.1 | 83.3 | 1.6 | [43] |
| Carrot | 13.1 | 21.4 | 3.5 | 0 | 62 | 3.74 | 9.5 | 8.79 | 90.5 | 0.07 | [44] |
| Tomato | 58.38 | 7.72 | 1.49 | 0 | 30.6 | 39.18 | 7.36 | 6.94 | 92.64 | 0.42 | [45] |
| Curcubita pepo * | 45.9 | 6.69 | 0.93 | 0.71 | 53.24 | 49.35 | 6.4 | 5.77 | 93.6 | 0.63 | [46] |
| Cucumber * | 45.06 | 6.67 | 3.06 | 2.33 | 48.97 | 14.72 | 5.8 | 4.86 | 94.2 | 0.94 | [47] |
| Apple * | 44.53 | 6.22 | 0.21 | 0.16 | 58.46 | 212.05 | 14.44 | 14.26 | 85.56 | 0.18 | [48] |
| Banana * | 44.49 | 6.24 | 0.51 | 0.39 | 57.66 | 87.23 | 25.09 | 24.26 | 74.91 | 0.83 | [49] |
| Boiled rice * | 44.33 | 6.25 | 1.12 | 0.85 | 56.25 | 39.58 | 31.65 | 31.48 | 68.34 | 0.17 | [50] |
| Food mixture | 43.46 | 8.65 | 1.85 | 0.64 | 49.94 | 23.49 | 14.89 | 14.21 | 85.11 | 0.59 | Calculated |
| Molecular Formula | C, H, N, S, O Coefficients | Carbohydrate, Lipid, and Protein Content (%) for [46,47,48,49,50]: | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O | Cucurbita Pepo | Cucumber | Apple | Banana | Rice | ||
| Carbohydrates | C6H10O6 | 6 | 10 | 6 | - | - | 75.81 | 61.93 | 96.92 | 94.15 | 89.37 |
| Lipids | C12H24O6 | 12 | 24 | 6 | - | - | 16.02 | 11.31 | 1.19 | 1.36 | 0.8 |
| Proteins | C13H25O7N3S | 13 | 25 | 7 | 3 | 1 | 8.16 | 26.75 | 1.82 | 4.49 | 9.82 |
| No. | Batch Experiments | Cumulated Volume (NL kg−1VSadded) | |||||
|---|---|---|---|---|---|---|---|
| Biogas | CH4 | CO2 | H2S | H2O | N2 | ||
| 1 | B33FIR11 | 69.88 | 24.08 | 13.08 | 43.23 × 10−3 | 0.350 | 28 |
| 2 | B37FIR11 | 130.7 | 67.19 | 35.85 | 62.01 × 10−3 | 8.18 | 17.31 |
| 3 | B41FIR11 | 214.43 | 60.97 | 43.83 | 193.56 × 10−3 | 16.64 | 75.91 |
| 4 | B45FIR11 | 465.37 | 203.03 | 160.44 | 698.36 × 10−3 | 44.9 | 64.62 |
| 5 | B37FIR32 | 286.25 | 147.2 | 69.29 | 93.03 × 10−3 | 16.92 | 51.93 |
| 6 | B37FIR21 | 468.59 | 233.95 | 168.37 | 298.41 × 10−3 | 29.84 | 77.4 |
| No. | Batch Experiments | Biogas (NL kg−1VSadded) | CH4 (%) | CO2(%) | H2S (ppm) | H2O (%) | N2 (%) |
|---|---|---|---|---|---|---|---|
| 1. | B33FIR11 | 69.88 | 34.39 | 18.73 | 619.05 | 5.03 | 39.95 |
| 2. | B37FIR11 | 130.7 | 51.32 | 27.34 | 474.2 | 6.26 | 13.25 |
| 3. | B41FIR11 | 214.43 | 28.49 | 20.42 | 907.56 | 7.77 | 35.46 |
| 4. | B45FIR11 | 465.37 | 43.64 | 34.47 | 1500.96 | 9.63 | 13.87 |
| 5. | B37FIR32 | 286.25 | 49.93 | 35.93 | 636.80 | 6.26 | 16.52 |
| 6. | B37FIR21 | 486.59 | 51.42 | 24.21 | 324.98 | 5.91 | 18.14 |
| No. | Batch Experiments | CH4th (NL kg−1VSadded) | CO2th (NL kg−1VSadded) | (%) | (%) |
|---|---|---|---|---|---|
| 1. | B33FIR11 | 604.82 | 291.22 | 3.98 | 4.15 |
| 2. | B37FIR11 | 11.11 | 11.5 | ||
| 3. | B41FIR11 | 10.08 | 11.69 | ||
| 4. | B45FIR11 | 33.57 | 40.56 | ||
| 5. | B37FIR32 | 642.38 | 253.89 | 22.91 | 24.15 |
| 6. | B37FIR21 | 655.47 | 240.78 | 36.42 | 44.89 |
| No. | Batch Experiment | Md (NL kg−1VSadded) | Rm (NL kg−1 h−1) | tlag (h) | R2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Biogas | CH4 | Biogas | CH4 | Biogas | CH4 | Biogas | CH4 | ||
| 1 | B33FIR11 | 68.73 | 23.28 | 0.0739 | 0.0333 | 10.6330 | 19.67 | 0.9997 | 0.9927 |
| 2 | B37FIR11 | 128.03 | 65.11 | 0.1428 | 0.0836 | 10.1433 | 9.28 | 0.9980 | 0.9949 |
| 3 | B41FIR11 | 217.69 | 70.22 | 0.3007 | 0.0968 | 0.1 | 8.133 | 0.9862 | 0.9875 |
| 4 | B45FIR11 | 427.05 | 188.46 | 0.2529 | 0.0908 | 0.1 | 0.1 | 0.9234 | 0.9440 |
| 5 | B37FIR32 | 308.9 | 156.14 | 1.0010 | 0.5874 | 47.4112 | 49.4466 | 0.9994 | 0.9991 |
| 6 | B37FIR21 | 519.03 | 237.22 | 0.8825 | 0.4019 | 17.4997 | 22.7862 | 0.9935 | 0.9935 |
| Substrate | Inoculum Type | Temperature and Substrate-to-Inoculum (S/I) Ratio | CH4 Production | Source |
|---|---|---|---|---|
| Fruit and vegetable waste | Granular activated sludge | 35 °C; 1:3 (kg/kg on VS basis) | 776.3 L kg−1VSadded | [15] |
| Fruit and vegetable waste (cabbage, lettuce, Chinese cabbage, tomato, carrot, cauliflower, etc.) | Anaerobic granular sludge | 37 °C; 4:1, 2:1, 1:1, 1:2, 1:5 (VS based) | 73.5 ÷ 262 L kg−1VSadded | [31] |
| Food waste: food from cooking (i.e., rice, breads, boiled egg yolks, potato, cabbage, cauliflower, noodles, lentils, etc.), peels of raw vegetables (carrot, cucumber, tomato, etc.), and stale food | Acclimatized anaerobic granular sludge | 35 °C; 1:3 (v/v) | 15.65 L kg−1VSadded | [33] |
| Passion fruit peel | Industrial granular anaerobic sludge | 37 °C; 5.4:1 (kg/kg on VS basis) | 115 NL kg−1VSadded | [34] |
| Orange bagasse | 37 °C; 5:1 (kg/kg on VS basis) | 128 NL kg−1VSadded | [34] | |
| Cashew bagasse | 37 °C; 4.13:1 (kg/kg on VS basis) | 62 NL kg−1VSadded | [34] | |
| Food waste (fruits and vegetables: banana, apple, tomato, potato, pumpkin, cucumber, carrot) and cereal food (boiled rice) | Industrial granular inoculum | 33 °C, 37 °C, 41 °C, 45 °C (at 1:1 w/w); 1:1, 2:1, 3:2 (w/w) at 37 °C | 24.08 ÷ 233.95 NL kg−1VSadded | this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanovici, M.; Dumitrel, G.-A.; Gherman, V.D.; Todinca, T.; Pana, A.-M.; Ordodi, V.L. Anaerobic Digestion of Food Waste and Granular Inoculum: Study on Temperature Effect and Substrate-to-Inoculum Ratio on Biogas Production. Fermentation 2025, 11, 348. https://doi.org/10.3390/fermentation11060348
Ivanovici M, Dumitrel G-A, Gherman VD, Todinca T, Pana A-M, Ordodi VL. Anaerobic Digestion of Food Waste and Granular Inoculum: Study on Temperature Effect and Substrate-to-Inoculum Ratio on Biogas Production. Fermentation. 2025; 11(6):348. https://doi.org/10.3390/fermentation11060348
Chicago/Turabian StyleIvanovici, Madalina, Gabriela-Alina Dumitrel, Vasile Daniel Gherman, Teodor Todinca, Ana-Maria Pana, and Valentin Laurentiu Ordodi. 2025. "Anaerobic Digestion of Food Waste and Granular Inoculum: Study on Temperature Effect and Substrate-to-Inoculum Ratio on Biogas Production" Fermentation 11, no. 6: 348. https://doi.org/10.3390/fermentation11060348
APA StyleIvanovici, M., Dumitrel, G.-A., Gherman, V. D., Todinca, T., Pana, A.-M., & Ordodi, V. L. (2025). Anaerobic Digestion of Food Waste and Granular Inoculum: Study on Temperature Effect and Substrate-to-Inoculum Ratio on Biogas Production. Fermentation, 11(6), 348. https://doi.org/10.3390/fermentation11060348







