Lactic Acid Bacteria as Probiotics Improve Bioactive Compounds in Radix Angelica gigas (Danggui) via Solid-State Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Probiotics Screening for SSF
2.3. Characteristics of Cell Proliferation in SSF Using LAB
2.4. Quantification of Bioactive Compounds
2.5. Statistical and Correlation Analysis
3. Results
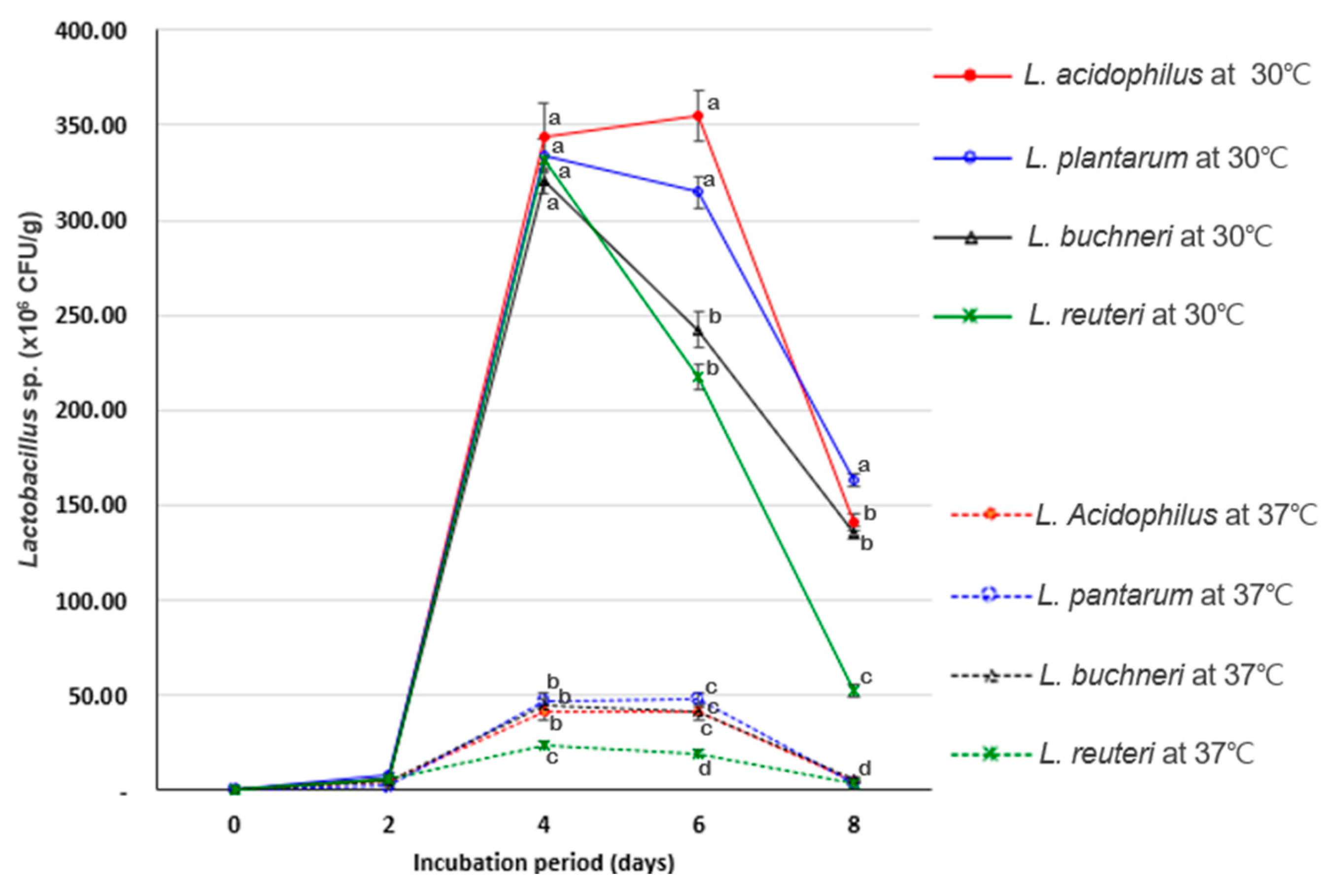
3.1. Evaluation of Probiotic Growth in SSF Conditions
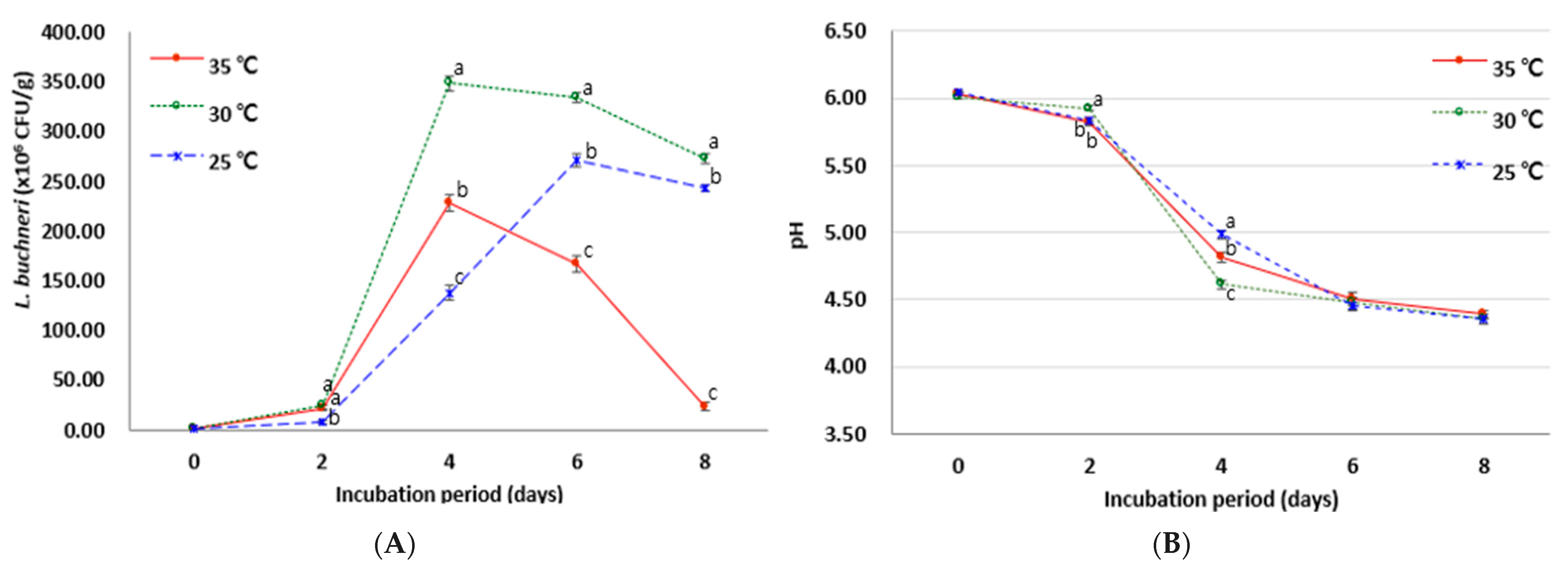
3.2. Effects of Culture Period and Temperature on Cell Growth and Antioxidant Activities of SSF Using L. buchneri
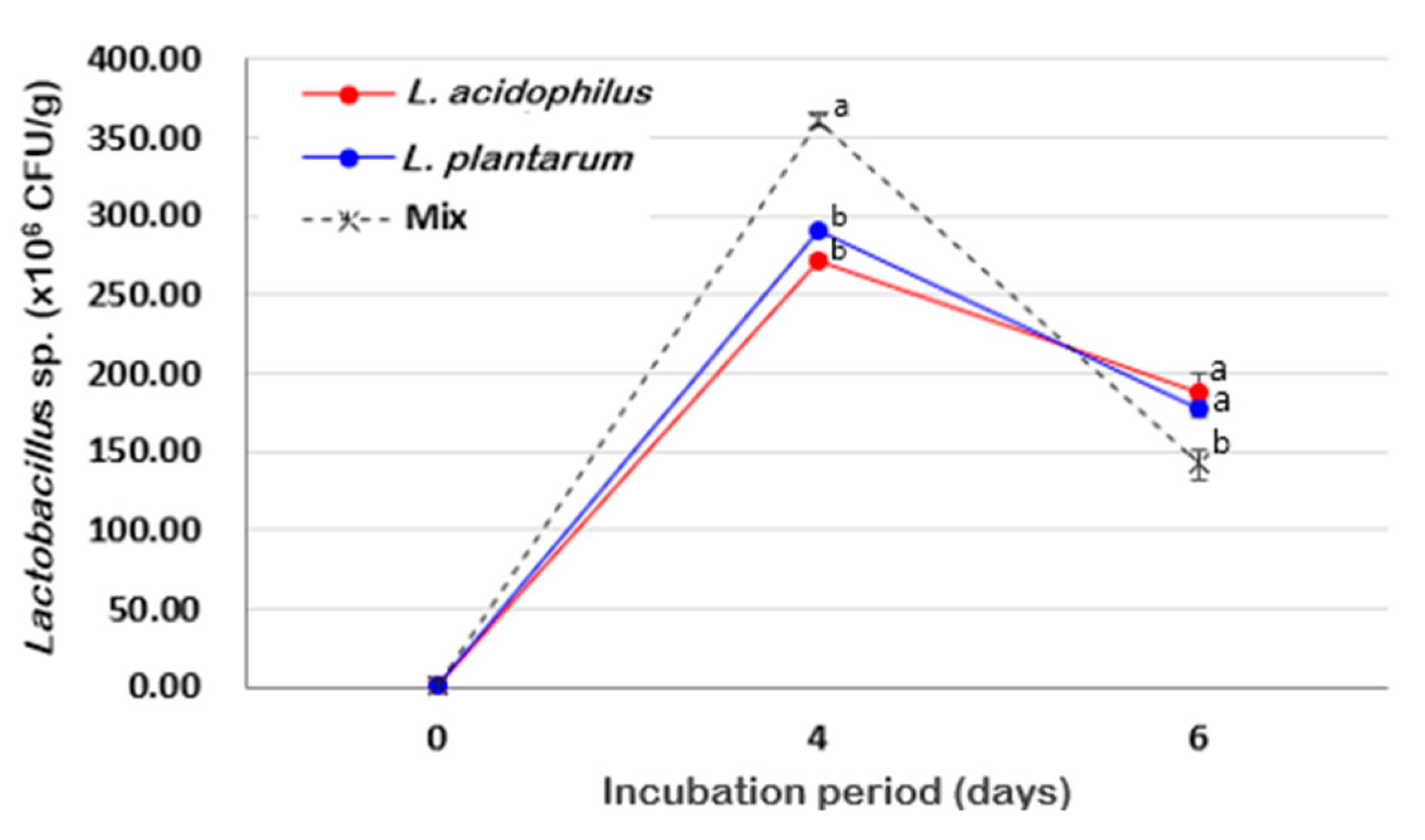
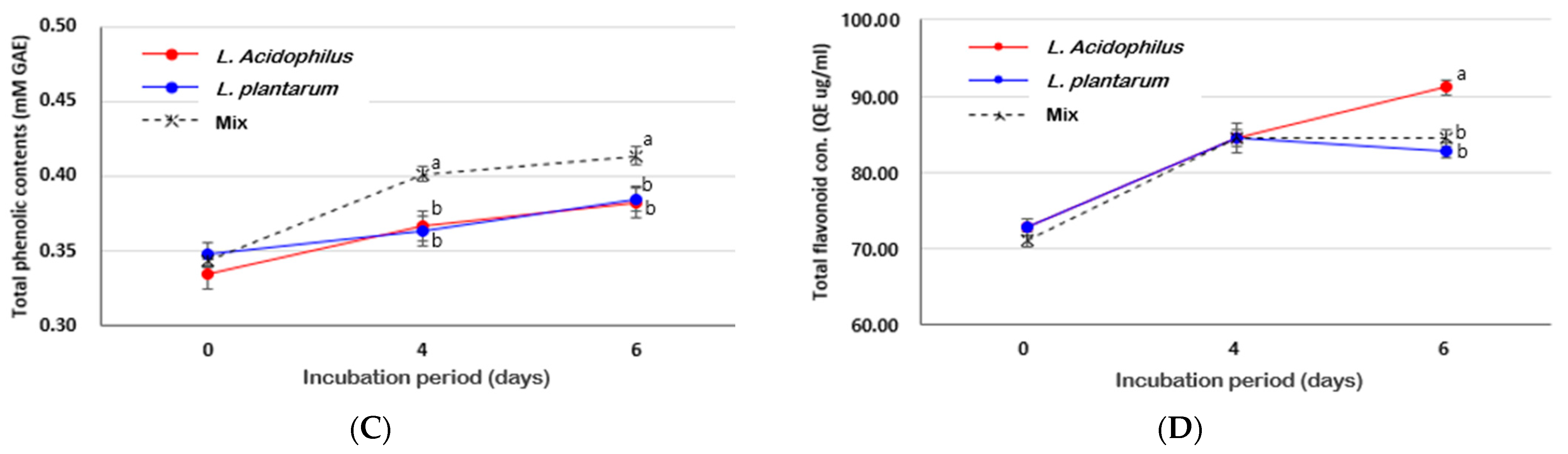
3.3. Effects of SSF Using Different LAB on Antioxidant Activities of Radix Angelica gigas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SSF | Solid-state fermentation |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| GAE | Gallic acid equivalents |
| QE | Quercetin equivalent |
| LAB | Lactic acid bacteria |
| CFU | Colony-forming unit |
References
- Park, Y.; Park, P.S.; Jeong, D.H.; Sim, S.; Kim, N.; Park, H.; Jeon, K.S.; Um, Y.; Kim, M.-J. The characteristics of the growth and the active compounds of Angelica gigas Nakai in cultivation sites. Plants 2020, 9, 823. [Google Scholar] [CrossRef]
- He, Z.; Wang, Y.; Chen, Y.; Geng, F.; Jiang, Z.; Li, X. Angelica gigas Nakai: An overview on its chemical composition and pharmacological activity. Biochem. Syst. Ecol. 2023, 111, 104717. [Google Scholar] [CrossRef]
- Zhang, X.; Miao, Q.; Pan, C.; Yin, J.; Wang, L.; Qu, L.; Yin, Y.; Wei, Y. Research advances in probiotic fermentation of Chinese herbal medicines. iMeta 2023, 2, e93. [Google Scholar] [CrossRef]
- Yang, B.; Xie, Y.; Guo, M.; Rosner, M.H.; Yang, H.; Ronco, C. Nephrotoxicity and Chinese herbal medicine. Clin. J. Am. Soc. Nephrol. 2018, 13, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Charen, E.; Harbord, N. Toxicity of herbs, vitamins, and supplements. Adv. Chronic Kidney Dis. 2020, 27, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, L.; Fan, W.; Jiang, Y.; Zhang, C.; Li, J.; Peng, W.; Wu, C. The application of fermentation technology in traditional Chinese medicine: A review. Am. J. Chin. Med. 2020, 48, 899–921. [Google Scholar] [CrossRef]
- Wu, T.; Wang, N.; Zhang, Y.; Xu, X. Advances in the study on microbial fermentation and transformation of traditional Chinese medicine. Afr. J. Microbiol. Res. 2013, 7, 1644–1650. [Google Scholar]
- Bose, S.; Song, M.-Y.; Nam, J.-K.; Lee, M.-J.; Kim, H. In vitro and in vivo protective effects of fermented preparations of dietary herbs against lipopolysaccharide insult. Food Chem. 2012, 134, 758–765. [Google Scholar] [CrossRef]
- Ma, J.; Wang, J.; Wan, Y.; Wang, S.; Jiang, C. Probiotic-fermented traditional Chinese herbal medicine, a promising approach to maintaining the intestinal microecology. J. Ethnopharmacol. 2025, 337, 118815. [Google Scholar] [CrossRef]
- Lee, J.-J.; Kwon, H.; Lee, J.-H.; Kim, D.-G.; Jung, S.-H.; Ma, J.Y. Fermented Soshiho-tang with Lactobacillus plantarum enhances the antiproliferative activity in vascular smooth muscle cell. BMC Complement. Altern. Med. 2014, 14, 78. [Google Scholar] [CrossRef]
- Garrido-Galand, S.; Asensio-Grau, A.; Calvo-Lerma, J.; Heredia, A.; Andrés, A. The potential of fermentation on nutritional and technological improvement of cereal and legume flours: A review. Food Res. Int. 2021, 145, 110398. [Google Scholar] [CrossRef] [PubMed]
- Canoy Postigo, L.O.; Jacobo-Velázquez, D.A.; Guajardo-Flores, D.; Amezquita, L.E.G.; García-Cayuela, T. Solid-state fermentation for enhancing the nutraceutical content of agrifood byproducts: Recent advances and its industrial feasibility. Food Biosci. 2021, 41, 100926. [Google Scholar]
- Arora, S.; Rani, R.; Ghosh, S. Bioreactors in solid state fermentation technology: Design, applications and engineering aspects. J. Biotechnol. 2018, 269, 16–34. [Google Scholar] [CrossRef]
- Lee, H.-S.; Kim, M.-R.; Park, Y.; Park, H.J.; Chang, U.J.; Kim, S.Y.; Suh, H.J. Fermenting red ginseng enhances its safety and efficacy as a novel skin care anti-aging ingredient: In vitro and animal study. J. Med. Food 2012, 15, 1015–1023. [Google Scholar] [CrossRef]
- Carneiro, A.A.J.; Ferreira, I.C.F.R.; Duenas, M.; Barros, L.; Silva, R.; Gomes, E.; Santos-Buelga, C. Chemical composition and antioxidant activity of dried powder formulations of Agaricus blazei and Lentinus edodes. Food Chem. 2013, 138, 2168–2173. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Park, Y.S.; Jung, S.T.; Kang, S.G.; Heo, B.K.; Arancibia-Avila, P.; Toledo, F.; Drzewiecki, J.; Namiesnik, J.; Gorinstein, S. Antioxidants and proteins in ethylene-treated kiwifruits. Food Chem. 2008, 107, 640–648. [Google Scholar] [CrossRef]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar]
- R Core Team. R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Lixia, Z.; Gao, W.; Wang, H. Review of traditional Chinese medicine processed by fermentation. China J. Chin. Mater. Medica 2012, 37, 3695–3700. [Google Scholar]
- Kim, S.-K.; Guevarra, R.B.; Kim, Y.-T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.-H. Role of probiotics in human gut microbiome-associated diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- Anee, I.J.; Alam, S.; Begum, R.A.; Shahjahan, R.M.; Khandaker, A.M. The role of probiotics on animal health and nutrition. J. Basic. Appl. Zool. 2021, 82, 52. [Google Scholar] [CrossRef]
- Ng, C.-C.; Wang, C.-Y.; Wang, Y.-P.; Tzeng, W.-S.; Shyu, Y.-T. Lactic acid bacterial fermentation on the production of functional antioxidant herbal Anoectochilus formosanus Hayata. J. Biosci. Bioeng. 2011, 111, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-M.; Hong, C.-H.; Kang, S.-H.; Seo, D.-S.; Kim, S.-O.; Lee, H.-Y.; Sim, H.-J.; An, H.-J. Anti-photoaging effect of plant extract fermented with Lactobacillus buchneri on CCD-986 fibroblasts and HaCaT keratinocytes. J. Funct. Biomater. 2020, 11, 3. [Google Scholar] [CrossRef]
- Wang, J.-H.; Bose, S.; Kim, H.-G.; Han, K.-S.; Kim, H. Fermented Rhizoma Atractylodis macrocephalae alleviates high fat diet-induced obesity in association with regulation of intestinal permeability and microbiota in rats. Sci. Rep. 2015, 5, 8391. [Google Scholar] [CrossRef]
- Huang, C.-C.; Lai, T.-H.; Huang, R.-Y.; Su, K.-W.; Lai, S.-R.; Lan, A. Effect of an herbal preparation fermented by Lactobacillus reuteri LR107 in preventing periodontal inflammation in an experimental gingivitis model. Asian J. Complement. Altern. Med. 2014, 2, 12–18. [Google Scholar]
- Eikmeyer, F.G.; Kofinger, P.; Poschenel, A.; Junemann, S.; Zakrzewski, M.; Heinl, S.; Mayrhuber, E.; Grabherr, R.; Puhler, A.; Schwab, H.; et al. Metagenome analyses reveal the influence of the inoculant Lactobacillus buchneri CD034 on the microbial community involved in grass ensiling. J. Biotechnol. 2013, 167, 334–343. [Google Scholar] [CrossRef]
- Eikmeyer, F.G.; Heinl, S.; Marx, H.; Puhler, A.; Grabherr, R.; Schluter, A. Identification of oxygen-responsive transcripts in the silage inoculant Lactobacillus buchneri CD034 by RNA sequencing. PLoS ONE 2015, 10, e0134149. [Google Scholar] [CrossRef]
- Oude Elferink, S.J.; Krooneman, J.; Gottschal, J.C.; Spoelstra, S.F.; Faber, F.; Driehuis, F. Anaerobic conversion of lactic acid to acetic acid and 1,2-propanediolby Lactobacillus buchneri. Appl. Environ. Microbiol. 2001, 67, 125–132. [Google Scholar] [CrossRef]
- Heinl, S.; Grabherr, R. Systems biology of robustness and flexibility: Lactobacillus buchneri—A show case. J. Biotechnol. 2017, 257, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [PubMed]
- König, H.; Berkelmann-Löhnertz, B. Maintenance of wine-associated microorganisms. In Biology of Microorganisms on Grapes, in Must and in Wine; König, H., Gottfried, U., Fröhlich, J., Eds.; Springer: Cham, Switzerland, 2017; pp. 549–572. ISBN 9783319600215. [Google Scholar]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Sionek, B.; Szydłowska, A.; Skowska, M.T.; Kołożyn-Krajewska, D. The impact of physicochemical conditions on lactic acid bacteria survival in food products. Fermentation 2024, 10, 298. [Google Scholar] [CrossRef]
- Slizewska, K.; Chlebicz-Wójcik, A. Growth kinetics of probiotic Lactobacillus strains in the alternative, cost-efficient semi-solid fermentation medium. Biology 2020, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.R.; Chang, J.Y.; Chang, H.C. Production of γ-Aminobutyric Acid (GABA) by Lactobacillus buchneri Isolated from Kimchi and its Neuroprotective Effect on Neuronal Cells. J. Microbiol. Biotechnol. 2007, 17, 104–109. [Google Scholar]
- Sridevi, N.; Vishwe, P.; Prabhune, A. Hypocholesteremic effect of bile salt hydrolase from Lactobacillus buchneri ATCC 4005. Food Res. Int. 2009, 42, 516–520. [Google Scholar] [CrossRef]
- Hlahla, L.N.; Mudau, F.N.; Mariga, I.K. Effect of fermentation temperature and time on the chemical composition of bush tea (Athrixia phylicoides DC.). J. Med. Plant Res. 2010, 4, 824–829. [Google Scholar]
- Coulon, S.; Chemardin, P.; Gueguen, Y.; Arnaud, A.; Galzy, P. Purification and characterization of an intracellular β-glucosidase from Lactobacillus casei ATCC 393. Appl. Biochem. Biotechnol. 1998, 74, 105–114. [Google Scholar] [CrossRef]
- Erskine, E.; Ozkan, G.; Lu, B.; Capanoglu, E. Effects of fermentation process on the antioxidant capacity of fruit byproducts. ACS Omega 2023, 8, 4543–4553. [Google Scholar] [CrossRef]
- Sarıta, S.; Portocarrero, A.C.M.; López, J.M.M.; Lombardo, M.; Koch, W.; Raposo, A.; El-Seedi, H.R.; Alves, J.L.B.; Esatbeyoglu, T.; Sercan Kara, S.; et al. The impact of fermentation on the antioxidant activity of food products. Molecules 2024, 29, 3941. [Google Scholar] [CrossRef]
- Zhao, Y.-S.; Eweys, A.S.; Zhang, J.-Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.-B.; Xiao, X. Fermentation affects the antioxidant activity of plant-based food material through the release and production of bioactive components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Lee, Y.-C.; Kim, S.-S.; Hong, H.-D.; Kim, K.-T. Quality and antioxidant activity of ginseng seed processed by fermentation strains. J. Ginseng Res. 2015, 39, 178–182. [Google Scholar] [CrossRef]
- Qiao, H.; Zhang, X.; Shi, H.; Song, Y.; Bian, C.; Guo, A. Assessment of the physicochemical properties and bacterial composition of Lactobacillus plantarum and Enterococcus faecium-fermented Astragalus membranaceus using single molecule, real-time sequencing technology. Sci. Rep. 2018, 8, 11862. [Google Scholar] [CrossRef]
- Seong, J.S.; Xuan, S.H.; Park, S.H.; Lee, K.S.; Park, Y.M.; Park, S.N. Antioxidative and antiaging activities and component analysis of Lespedeza cuneata G. Don extracts fermented with Lactobacillus pentosus. J. Microbiol. Biotechnol. 2017, 27, 1961–1970. [Google Scholar] [CrossRef]
- Wen, K.C.; Lin, S.-P.; Yu, C.-P.; Chen, H.-M. Comparison of Puerariae Radix and its hydrolysate on stimulation of hyaluronic acid production in NHEK cells. Am. J. Chin. Med. 2010, 38, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Chen, X.; Jassbi, A.R.; Xiao, J. Microbial biotransformation of bioactive flavonoids. Biotechnol. Adv. 2015, 33, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Bose, S.; Wang, J.-H.; Yadav, M.K.; Mahajan, G.B.; Kim, H. Fermentation, a feasible strategy for enhancing bioactivity of herbal medicines. Food Res. Int. 2016, 81, 1–16. [Google Scholar] [CrossRef]
- Chapman, C.M.C.; Gibson, G.R.; Rowland, I. Health benefits of probiotics: Are mixtures more effective than single strains? Eur. J. Nutr. 2011, 50, 1–17. [Google Scholar] [CrossRef]
- Liu, H.; Ni, Y.; Qun Yu, Q.; Fan, L. Evaluation of co-fermentation of L. plantarum and P. kluyveri of a plant-based fermented beverage: Physicochemical, functional, and sensory properties. Food Res. Int. 2023, 172, 113060. [Google Scholar] [CrossRef]
- Li, X.; Gao, W.; Wang, L.; Chen, Y.; Cai, Z.; Wu, D.; Chen, N.; Jiang, Q.; Zheng, Z.; Zhu, J.; et al. Co-fermentation of Lactobacillus plantarum and Lactobacillus casei improves in vitro antioxidant capacity and quality of apple juice. Fermentation 2025, 11, 161. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Jafarpour, D. Fermentation of bergamot juice with Lactobacillus plantarum strains in pure and mixed fermentations: Chemical composition, antioxidant activity and sensorial properties. LWT Food Sci. Technol. 2020, 131, 109803. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Y.; Wu, Y.; Liu, Y.; Wu, Z. Fermentation and complex enzyme hydrolysis for improving the total soluble phenolic contents, flavonoid aglycones contents and bio-activities of guava leaves tea. Food Chem. 2018, 264, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, T.; Hong, Y.; Qiao, T.; Wang, Y.; Li, W.; Tang, S.; Yang, X.; Li, J.; Li, X.; et al. Mixture of five fermented herbs (Zhihuasi Tk) alters the intestinal microbiota and promotes the growth performance in piglets. Front. Microbiol. 2021, 12, 725196. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Su, Y.; An, Z.; Zhang, P.; Yue, Q.; Zhao, C.; Sun, X.; Zhang, S.; Liu, X.; Li, K.; et al. Fermentation products of Danshen relieved dextran sulfate sodium-induced experimental ulcerative colitis in mice. Sci. Rep. 2021, 11, 16210. [Google Scholar] [CrossRef]
- Jin, S.; Luo, M.; Wang, W.; Zhao, C.; Gu, C.; Li, C.; Zu, Y.; Fu, Y.; Guan, Y. Biotransformation of polydatin to resveratrol in Polygonum cuspidatum roots by highly immobilized edible Aspergillus niger and yeast. Bioresour. Technol. 2013, 136, 766–770. [Google Scholar] [CrossRef]

| L. rhamnosus | L. acidophilus | L. buchneri | L. plantarum | L. reuteri | B. subtilis | S. cerevisiae | |
|---|---|---|---|---|---|---|---|
| Bacterial growth (1) | 2.80 ± 0.36 f | 627.00 ± 24.64 b | 706.76 ± 51.32 a | 630.00 ± 20.00 b | 453.00 ± 15.72 c | 58.00 ± 20.00 de | 37.33 ± 13.01 e |
| pH change (2) | 0.35 ± 0.08 c | 1.23 ± 0.11 a | 1.02 ± 0.04 b | 1.27 ± 0.02 a | 1.25 ± 0.01 a | 0.42 ± 0.15 c | 0.39 ± 0.06 c |
| Temperature | Period of Incubation (Days) | ||||
|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | |
| DPPH radical scavenging activity (%) | |||||
| 25 °C | 17.26 ± 1.61 Ae | 20.02 ± 1.72 Bd | 23.24 ± 1.55 Bc | 33.19 ± 1.88 Ab | 36.32 ± 1.79 Aa |
| 30 °C | 18.10 ± 1.14 Ad | 25.68 ± 1.83 Ac | 33.45 ± 1.45 Ab | 33.49 ± 1.79 Ab | 38.02 ± 1.56 ABa |
| 35 °C | 17.13 ± 1.31 Ad | 26.75 ± 1.62 Ac | 33.00 ± 1.82 Ab | 33.09 ± 1.67 Ab | 39.45 ± 1.68 Aa |
| ABTS radical scavenging activity (%) | |||||
| 25 °C | 54.73 ± 0.30 Aa | 56.42 ± 0.37 Ca | 56.52 ± 0.54 Aa | 55.39 ± 1.07 Aa | 55.66 ± 0.85 Ba |
| 30 °C | 54.09 ± 0.30 Ab | 60.12 ± 0.18 Aa | 55.70 ± 0.99 Ab | 55.53 ± 0.73 Ab | 55.19 ± 0.47 Bb |
| 35 °C | 54.93 ± 0.30 Ab | 58.24 ± 1.14 Ba | 56.25 ± 0.67 Aab | 56.61 ± 0.18 Aab | 58.15 ± 0.95 Aa |
| Total phenolic content (mM gallic acid equivalent, mM GAE) | |||||
| 25 °C | 0.26 ± 0.001 Ac | 0.27 ± 0.005 Bc | 0.27 ± 0.002 Cc | 0.33 ± 0.006 Bb | 0.38 ± 0.006 Aa |
| 30 °C | 0.27 ± 0.001 Ae | 0.31 ± 0.003 Ad | 0.34 ± 0.001 Bc | 0.35 ± 0.007 Ab | 0.37 ± 0.007 Aa |
| 35 °C | 0.27 ± 0.008 Ac | 0.31 ± 0.005 Ab | 0.35 ± 0.006 Aa | 0.36 ± 0.013 Aa | 0.37 ± 0.020 Aa |
| Total flavonoid content (μg/mL quercetin equivalent, μg/mL QE) | |||||
| 25 °C | 46.93 ± 1.70 Ac | 48.74 ± 2.31 Bb | 48.89 ± 0.89 Cb | 55.78 ± 1.92 Aa | 57.26 ± 1.28 ca |
| 30 °C | 46.15 ± 1.85 Ac | 54.59 ± 2.33 Ab | 56.89 ± 1.00 Bab | 56.52 ± 0.94 Aab | 59.11 ± 1.00 Ba |
| 35 °C | 47.30 ± 1.89 Ad | 53.93 ± 0.64 Ac | 59.48 ± 2.31 Aab | 57.63 ± 1.70 Abc | 63.19 ± 0.64 Aa |
| Antioxidant Activity Assay | Temperature | Growth | pH | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 °C | 30 °C | 35 °C | 25 °C | 30 °C | 35 °C | ||||||||
| 0–4 d | 6–8 d | 0–4 d | 6–8 d | 0–4 d | 6–8 d | 0–4 d | 6–8 d | 0–4 d | 6–8 d | 0–4 d | 6–8 d | ||
| DPPH | 25 °C | 0.762 (0.017) | −0.963 (0.002) | 0.784 (0.012) | −0.942 (0.005) | 0.782 (0.013) | −0.899 (0.015) | −0.839 (0.005) | −0.949 (0.004) | −0.78 (0.013) | −0.912 (0.011) | −0.835 (0.005) | −0.888 (0.018) |
| 30 °C | 0.876 (0.002) | −0.925 (0.008) | 0.892 (0.001) | −0.895 (0.016) | 0.892 (0.001) | −0.81 (0.051) | −0.932 (0) | −0.897 (0.015) | −0.888 (0.001) | −0.833 (0.04) | −0.928 (0) | −0.805 (0.054) | |
| 35 °C | 0.812 (0.008) | −0.992 (0) | 0.822 (0.007) | −0.969 (0.001) | 0.831 (0.005) | −0.951 (0.004) | −0.878 (0.002) | −0.989 (0) | −0.822 (0.007) | −0.942 (0.005) | −0.869 (0.002) | −0.929 (0.007) | |
| ABTS | 25 °C | 0.503 (0.168) | −0.265 (0.612) | 0.521 (0.151) | −0.09 (0.865) | 0.528 (0.144) | −0.136 (0.798) | −0.592 (0.093) | −0.165 (0.755) | −0.522 (0.15) | −0.094 (0.859) | −0.587 (0.097) | −0.063 (0.906) |
| 30 °C | −0.324 (0.395) | 0.241 (0.646) | −0.305 (0.425) | 0.217 (0.679) | −0.292 (0.446) | 0.265 (0.611) | 0.203 (0.601) | 0.272 (0.602) | 0.304 (0.427) | 0.221 (0.675) | 0.213 (0.583) | 0.251 (0.631) | |
| 35 °C | −0.055 (0.887) | −0.63 (0.18) | −0.048 (0.902) | −0.723 (0.104) | −0.027 (0.945) | −0.753 (0.084) | −0.049 (0.901) | −0.686 (0.132) | 0.05 (0.898) | −0.747 (0.088) | −0.035 (0.928) | −0.75 (0.086) | |
| Phenol | 25 °C | 0.511 (0.16) | −0.912 (0.011) | 0.517 (0.154) | −0.982 (0) | 0.522 (0.15) | −0.967 (0.002) | −0.557 (0.119) | −0.963 (0.002) | −0.519 (0.152) | −0.981 (0.001) | −0.549 (0.126) | −0.981 (0.001) |
| 30 °C | 0.872 (0.002) | −0.604 (0.204) | 0.879 (0.002) | −0.487 (0.328) | 0.888 (0.001) | −0.546 (0.262) | −0.926 (0) | −0.576 (0.232) | −0.878 (0.002) | −0.477 (0.338) | −0.92 (0) | −0.484 (0.33) | |
| 35 °C | 0.854 (0.003) | −0.381 (0.456) | 0.858 (0.003) | −0.547 (0.261) | 0.871 (0.002) | −0.562 (0.246) | −0.91 (0.001) | −0.499 (0.314) | −0.86 (0.003) | −0.609 (0.199) | −0.902 (0.001) | −0.636 (0.175) | |
| Flavonoid | 25 °C | 0.473 (0.198) | −0.578 (0.229) | 0.494 (0.177) | −0.582 (0.225) | 0.498 (0.172) | −0.449 (0.372) | −0.573 (0.107) | −0.534 (0.275) | −0.485 (0.186) | −0.503 (0.309) | −0.561 (0.116) | −0.458 (0.361) |
| 30 °C | 0.663 (0.052) | −0.91 (0.012) | 0.679 (0.044) | −0.823 (0.044) | 0.686 (0.041) | −0.841 (0.036) | −0.743 (0.022) | −0.867 (0.025) | −0.677 (0.045) | −0.808 (0.052) | −0.737 (0.023) | −0.784 (0.065) | |
| 35 °C | 0.831 (0.006) | −0.924 (0.009) | 0.836 (0.005) | −0.937 (0.006) | 0.848 (0.004) | −0.921 (0.009) | −0.889 (0.001) | −0.948 (0.004) | −0.834 (0.005) | −0.915 (0.011) | −0.88 (0.002) | −0.914 (0.011) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, J.; Ham, Y.-K.; Choi, A.Y.; Yoon, H.; Sung, H.G. Lactic Acid Bacteria as Probiotics Improve Bioactive Compounds in Radix Angelica gigas (Danggui) via Solid-State Fermentation. Fermentation 2025, 11, 342. https://doi.org/10.3390/fermentation11060342
Heo J, Ham Y-K, Choi AY, Yoon H, Sung HG. Lactic Acid Bacteria as Probiotics Improve Bioactive Compounds in Radix Angelica gigas (Danggui) via Solid-State Fermentation. Fermentation. 2025; 11(6):342. https://doi.org/10.3390/fermentation11060342
Chicago/Turabian StyleHeo, Jeong, Youn-Kyung Ham, Ah Yeong Choi, Hyouk Yoon, and Ha Gyun Sung. 2025. "Lactic Acid Bacteria as Probiotics Improve Bioactive Compounds in Radix Angelica gigas (Danggui) via Solid-State Fermentation" Fermentation 11, no. 6: 342. https://doi.org/10.3390/fermentation11060342
APA StyleHeo, J., Ham, Y.-K., Choi, A. Y., Yoon, H., & Sung, H. G. (2025). Lactic Acid Bacteria as Probiotics Improve Bioactive Compounds in Radix Angelica gigas (Danggui) via Solid-State Fermentation. Fermentation, 11(6), 342. https://doi.org/10.3390/fermentation11060342







