Applied Bioelectrochemistry: Plastic Degradation and Energy Generation Using Klebsiella oxytoca in Microbial Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
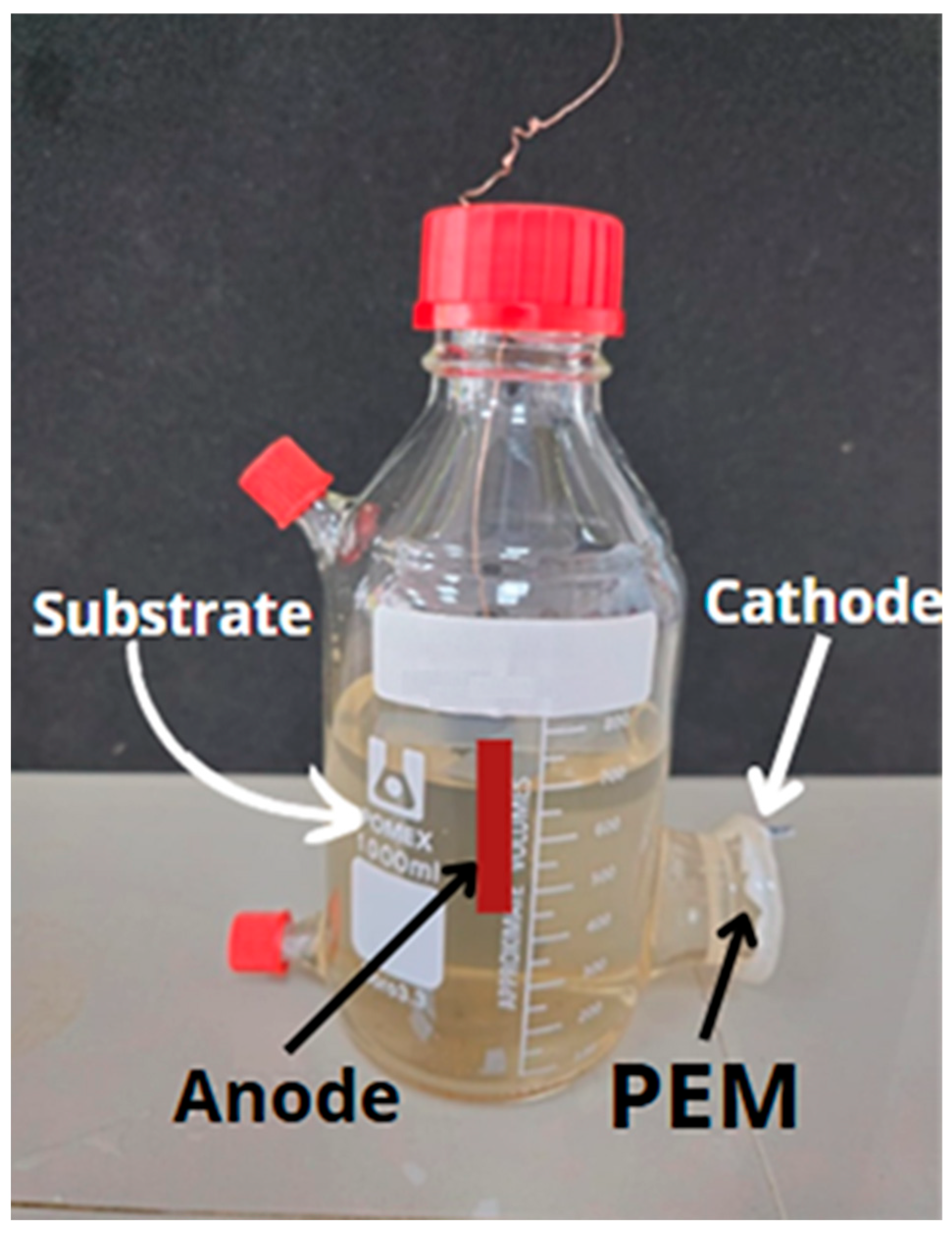
2.1. Assembly of the Single-Chamber Microbial Fuel Cells
2.2. Electrochemical and Morphological Tests
2.3. Collection of Samples, Isolation, and Selection of Klebsiella oxytoca
2.4. Microbial Fuel Cell Operation
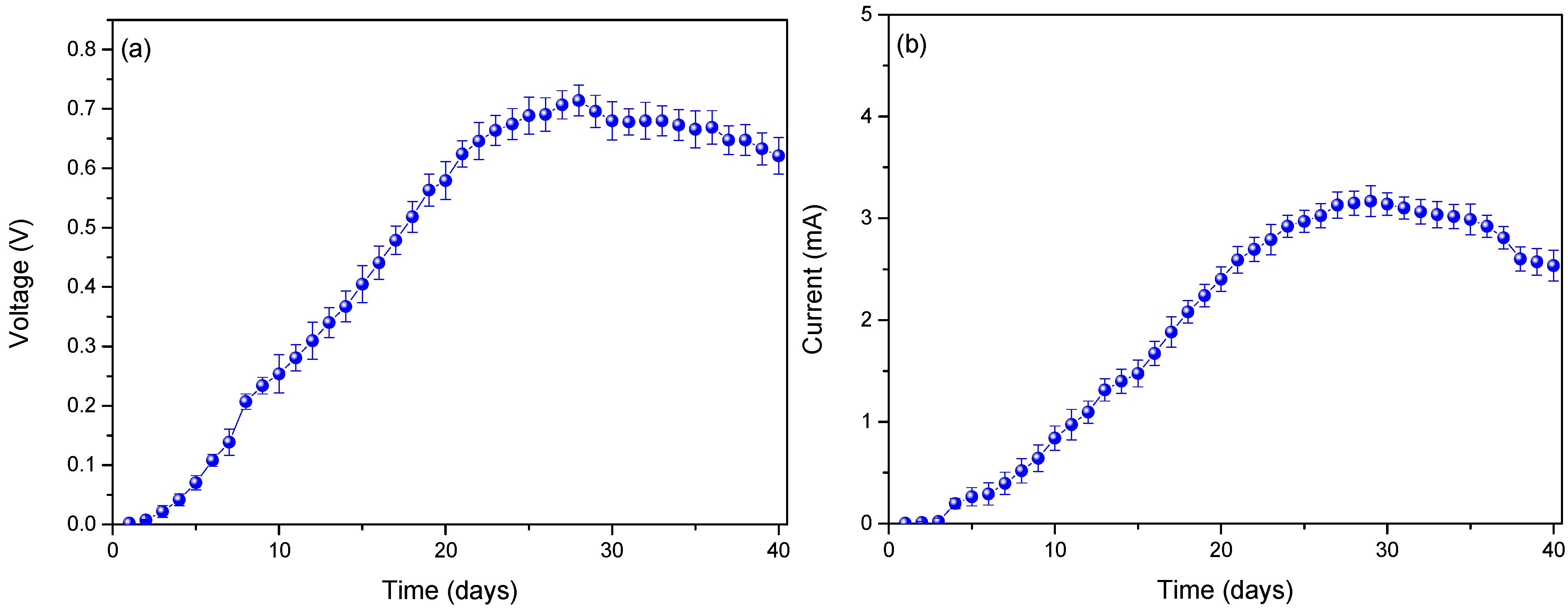
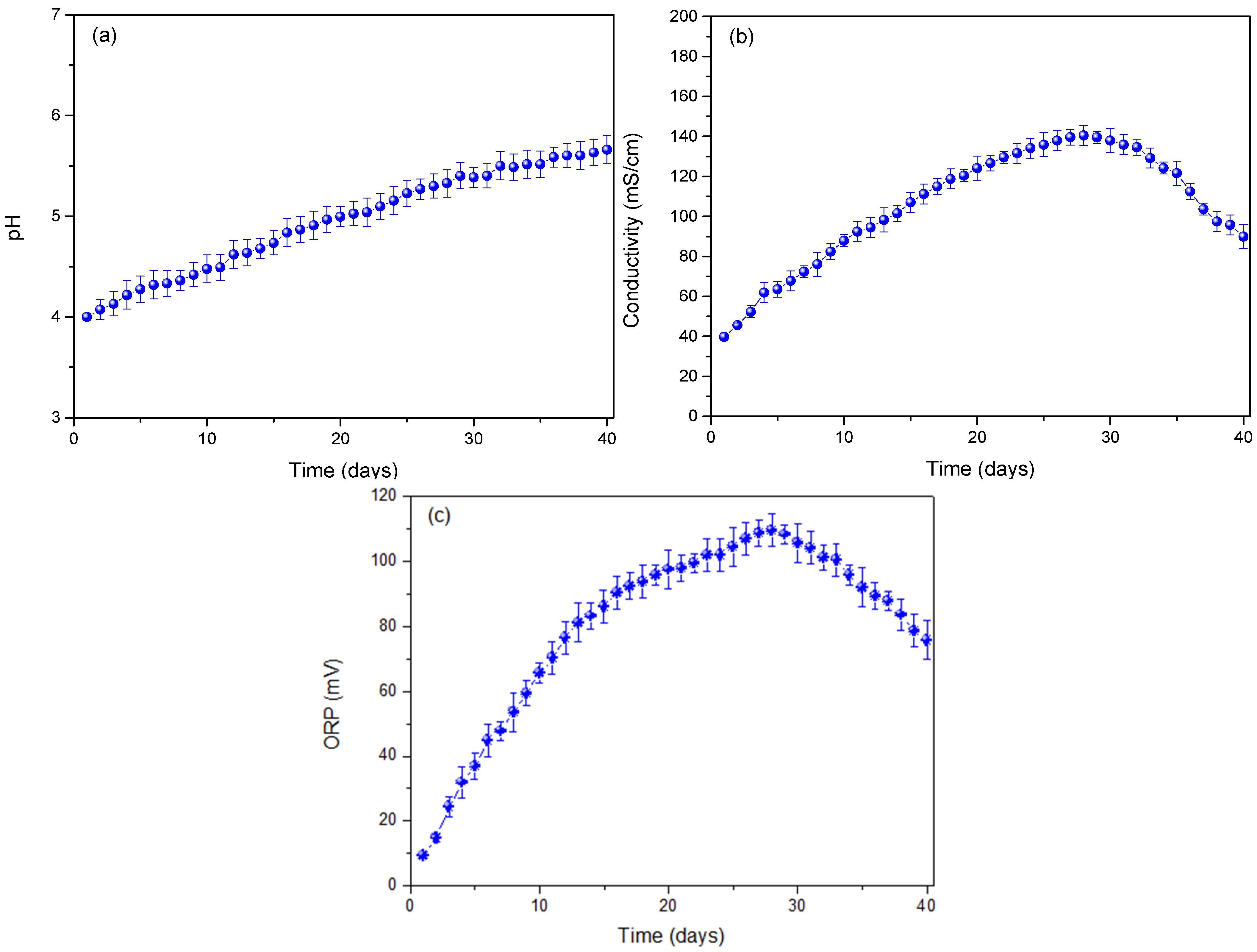
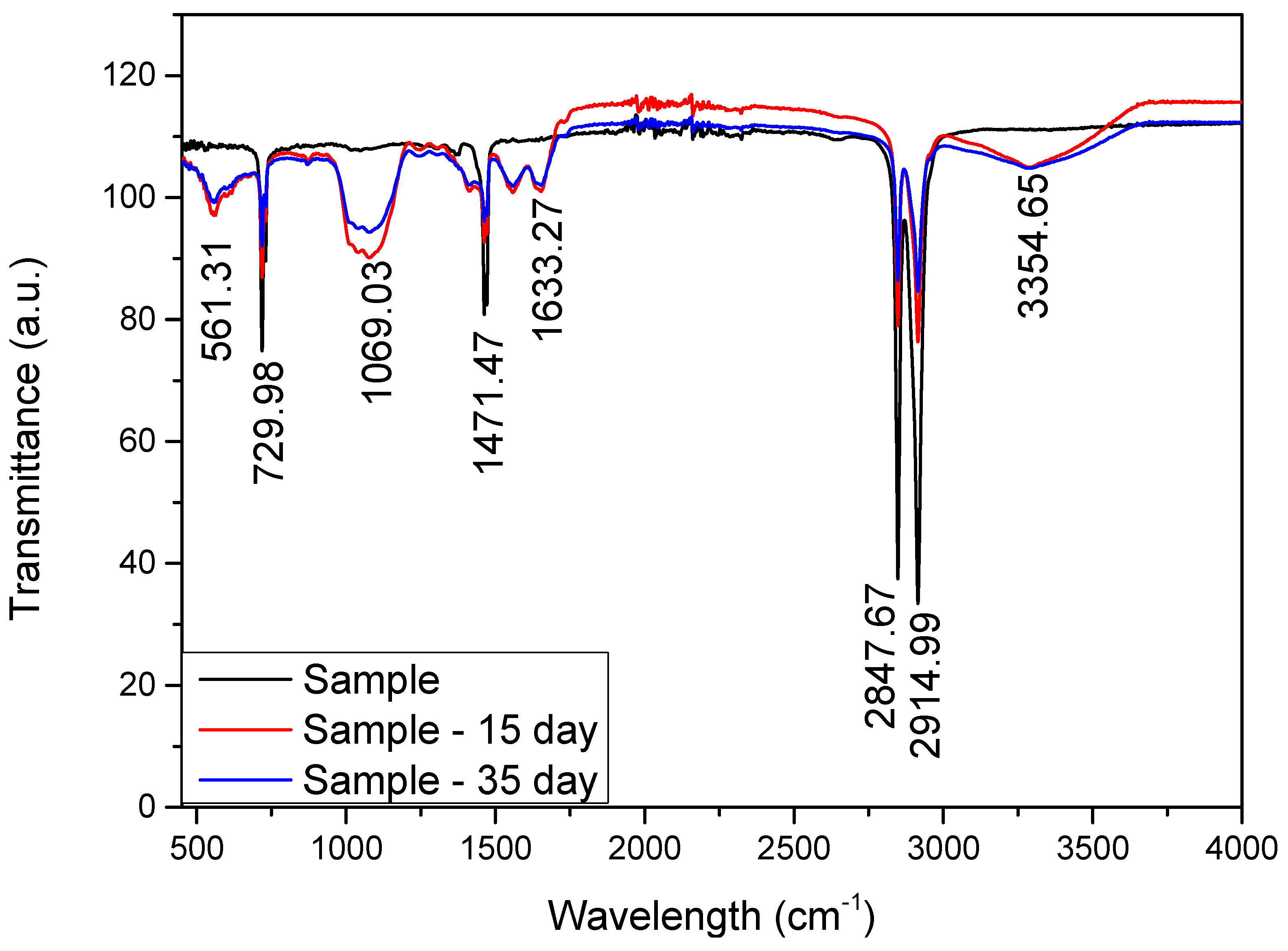
3. Results and Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Evode, N.; Qamar, S.A.; Bilal, M.; Barceló, D.; Iqbal, H.M. Plastic waste and its management strategies for environmental sustainability. Case Stud. Chem. Environ. Eng. 2021, 4, 100142. [Google Scholar] [CrossRef]
- Kibria, G.; Masuk, N.I.; Safayet, R.; Nguyen, H.Q.; Mourshed, M. Plastic Waste: Challenges and Opportunities to Mitigate Pollution and Effective Management. Int. J. Environ. Res. 2023, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H. Plastic waste as pyrolysis feedstock for plastic oil production: A review. Sci. Total Environ. 2023, 877, 162719. [Google Scholar] [CrossRef]
- Liang, Y.; Tan, Q.; Song, Q.; Li, J. An analysis of the plastic waste trade and management in Asia. Waste Manag. 2021, 119, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Nath, T.K.; Chong, S.; Foo, V.; Gibbins, C.; Lechner, A.M. The plastic waste problem in Malaysia: Management, recycling and disposal of local and global plastic waste. SN Appl. Sci. 2021, 3, 437. [Google Scholar] [CrossRef]
- Shams, M.; Alam, I.; Mahbub, S. Plastic pollution during COVID-19: Plastic waste directives and its long-term impact on the environment. Environ. Adv. 2021, 5, 100119. [Google Scholar] [CrossRef]
- Kalali, E.N.; Lotfian, S.; Shabestari, M.E.; Khayatzadeh, S.; Zhao, C.; Nezhad, H.Y. A critical review of the current progress of plastic waste recycling technology in structural materials. Curr. Opin. Green Sustain. Chem. 2023, 40, 100763. [Google Scholar] [CrossRef]
- Benson, N.U.; Bassey, D.E.; Palanisami, T. COVID pollution: Impact of COVID-19 pandemic on global plastic waste footprint. Heliyon 2021, 7, e06343. [Google Scholar] [CrossRef]
- Rinaldi, F.; Moghaddampoor, F.; Najafi, B.; Marchesi, R. Economic feasibility analysis and optimization of hybrid renewable energy systems for rural electrification in Peru. Clean Technol. Environ. Policy 2021, 23, 731–748. [Google Scholar] [CrossRef]
- Lillo, P.; Ferrer-Martí, L.; Juanpera, M. Strengthening the sustainability of rural electrification projects: Renewable energy, management models and energy transitions in Peru, Ecuador and Bolivia. Energy Res. Soc. Sci. 2021, 80, 102222. [Google Scholar] [CrossRef]
- Fernandez-Fuentes, M.H.; Eras-Almeida, A.A.; Egido-Aguilera, M.A. Characterization of Technological Innovations in Photovoltaic Rural Electrification, Based on the Experiences of Bolivia, Peru, and Argentina: Third Generation Solar Home Systems. Sustainability 2021, 13, 3032. [Google Scholar] [CrossRef]
- Canziani, F.; Vargas, R.; Gastelo-Roque, J.A. Hybrid Photovoltaic-Wind Microgrid With Battery Storage for Rural Electrification: A Case Study in Perú. Front. Energy Res. 2021, 8, 528571. [Google Scholar] [CrossRef]
- Quispe, J.C.; Obispo, A.E.; Alcantara, F.J. Economic feasibility assessment of microgrids with renewable energy sources in Peruvian rural areas. Clean Technol. Environ. Policy 2024, 26, 1415–1438. [Google Scholar] [CrossRef]
- James, A. Ceramic-microbial fuel cell (C-MFC) for waste water treatment: A mini review. Environ. Res. 2022, 210, 112963. [Google Scholar] [CrossRef]
- Huang, X.; Duan, C.; Duan, W.; Sun, F.; Cui, H.; Zhang, S.; Chen, X. Role of electrode materials on performance and microbial characteristics in the constructed wetland coupled microbial fuel cell (CW-MFC): A review. J. Clean. Prod. 2021, 301, 126951. [Google Scholar] [CrossRef]
- Afrin, A.; Swamy, P.C.A. Symphony of light: AIE and MFC in carbazole-based cyanostilbenes. J. Mater. Chem. C 2024, 12, 1923–1944. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Othman, M.H.D.; Liang, X.; Ayub, M.; Goh, H.H.; Kusworo, T.D.; Mohyuddin, A.; Chew, K.W. Microbial Fuel Cells (MFC): A Potential Game-Changer in Renewable Energy Development. Sustainability 2022, 14, 16847. [Google Scholar] [CrossRef]
- Ahmad, A.; Alshammari, M.B.; Ibrahim, M.N.M.; Dao, W.Y. Energy production with removal of lead and chromium from wastewater through microbial fuel cells energized by organic waste substrate. Biomass- Convers. Biorefinery 2024, 4, 1–13. [Google Scholar] [CrossRef]
- Misali, R.; Noor, N.N.M.; Oktavitri, N.I.; Kim, K. The impact of bottom water light exposure on electrical and sediment remediation performance of sediment microbial fuel cells. Chemosphere 2024, 362, 142720. [Google Scholar] [CrossRef]
- Chandra, S.; Pandit, S.; Deb, S.; Mohan, C.; Rajeev, M.; Ranjan, N.; Kumar, A.; Dikshit, P.K. Utilization of water chestnut waste for biohydrogen production and enhanced power generation by stacked microbial fuel cell. Biocatal. Agric. Biotechnol. 2024, 62, 103425. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, B.; Chi, Y.; Li, J.; Chi, Y.; Ji, M.; Zhai, H.; Wang, R.; Yuan, T.; Yu, H. Study on the treatment of simulated dye wastewater containing FMPs using the CW-MFC system. J. Water Process. Eng. 2024, 66, 105810. [Google Scholar] [CrossRef]
- Ramesh, G.S.; Nayak, S.; Sevda, S. Biodegradation Mechanisms and Bioremediation Applications for Plastic Waste. In Industrial Microbiology and Biotechnology: A New Horizon of the Microbial World; Springer: Berlin/Heidelberg, Germany, 2024; pp. 869–890. [Google Scholar] [CrossRef]
- Kim, H.; Nam, E.; An, K.; Lim, H. Laboratory-scale plastic upcycling and green growth: Evaluating the upcycling of plastic waste into carbon nanotubes from economic and environmental aspects. Chem. Eng. J. 2024, 495, 153300. [Google Scholar] [CrossRef]
- Das, L.; Tripathi, A.; Biswas, K.G.; Nigam, K.D.P. Plastic Waste and Wheat Straw into Biofuels and Conversion of Wastewater into Agricultural Use Water. In From Waste to Wealth; Springer Nature: Singapore, 2024; pp. 929–949. [Google Scholar] [CrossRef]
- Ferheen, I.; Spurio, R.; Marcheggiani, S. Emerging Issues on Antibiotic-Resistant Bacteria Colonizing Plastic Waste in Aquatic Ecosystems. Antibiotics 2024, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Mupamhadzi, T.L.; Machona, O.; Chidzwondo, F.; Mangoyi, R. Molecular Detection and Phylogenetic Analysis of the alkB Gene in Klebsiella oxytoca Strains Isolated from the Gut of Tenebrio molitor. Sci. World J. 2024, 2024, 3350591. [Google Scholar] [CrossRef]
- NTP 360.502, Water Quality, Peruvian, Peru, 2016. Available online: https://sni.org.pe/aprueban-normas-tecnicas-peruanas-sobre-carne-y-productos-carnicos-maiz-amilaceo-cebada-cerveza-aditivos-alimentarios-y-otros/ (accessed on 18 May 2025).
- Segundo, R.-F.; Magaly, D.L.C.-N.; Luis, C.-C.; Otiniano, N.M.; Soto-Deza, N.; Terrones-Rodriguez, N.; Mayra, D.L.C.-C. Obtaining Sustainable Electrical Energy from Pepper Waste. Sustainability 2024, 16, 3448. [Google Scholar] [CrossRef]
- Raaman, N.; Rajitha, N.; Jayshree, A.; Jegadeesh, R. Biodegradation of plastic by Aspergillus spp. isolated from polythene polluted sites around Chennai. J. Acad. Indus. Res. 2012, 1, 313–316. [Google Scholar] [CrossRef]
- La Cruz-Noriega, D.; Nazario-Naveda, R.; Benites, S.M.; Rojas-Flores, S.; Delfín-Narciso, D.; Rojas-Villacorta, W.; Diaz, F. Potential use of mango waste and microalgae Spirulina sp. for bioelectricity generation. Environ. Res. Eng. Manag. 2022, 78, 129. [Google Scholar] [CrossRef]
- Munir, E.; Harefa, R.S.M.; Priyani, N.; Suryanto, D. Plastic degrading fungi Trichoderma viride and Aspergillus nomius isolated from local landfill soil in Medan. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 126, p. 012145. [Google Scholar] [CrossRef]
- Asiandu, A.P.; Wahyudi, A.; Sari, S.W. A Review: Plastics Waste Biodegradation Using Plastics-Degrading Bacteria. J. Environ. Treat. Tech. 2021, 9, 148–157. [Google Scholar] [CrossRef]
- Sharma, H.; Neelam, D.K. Understanding challenges associated with plastic and bacterial approach toward plastic degradation. J. Basic Microbiol. 2023, 63, 292–307. [Google Scholar] [CrossRef]
- Pugazhendi, A.; Al-Mur, B.A.; Jeyakumar, R.B. Cosmetic industrial wastewater treatment and bioelectricity production in upflow microbial fuel cell (UMFC) using extremophilic bacterial consortium. J. Taiwan Inst. Chem. Eng. 2025, 166, 105438. [Google Scholar] [CrossRef]
- Patel, D.; Bapodra, S.L.; Madamwar, D.; Desai, C. Electroactive bacterial community augmentation enhances the performance of a pilot scale constructed wetland microbial fuel cell for treatment of textile dye wastewater. Bioresour. Technol. 2021, 332, 125088. [Google Scholar] [CrossRef] [PubMed]
- Fadzli, F.S.; Bhawani, S.A.; Mohammad, R.E.A. Microbial Fuel Cell: Recent Developments in Organic Substrate Use and Bacterial Electrode Interaction. J. Chem. 2021, 2021, 4570388. [Google Scholar] [CrossRef]
- Obileke, K.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial fuel cells, a renewable energy technology for bio-electricity generation: A mini-review. Electrochem. Commun. 2021, 125, 107003. [Google Scholar] [CrossRef]
- Guo, H.; Huang, C.; Geng, X.; Jia, X.; Huo, H.; Yue, W. Influence of the original electrogenic bacteria on the per-formance of oily sludge Microbial Fuel Cells. Energy Rep. 2022, 8, 14374–14381. [Google Scholar] [CrossRef]
- Truong, D.H.; Dam, M.S.; Bujna, E.; Rezessy-Szabo, J.; Farkas, C.; Vi, V.N.H.; Csernus, O.; Nguyen, V.D.; Gathergood, N.; Friedrich, L.; et al. In situ fabrication of electrically conducting bacterial cellulose-polyaniline-titanium-dioxide composites with the immobilization of Shewanella xiamenensis and its application as bioanode in microbial fuel cell. Fuel 2021, 285, 119259. [Google Scholar] [CrossRef]
- Gul, H.; Raza, W.; Lee, J.; Azam, M.; Ashraf, M.; Kim, K.-H. Progress in microbial fuel cell technology for wastewater treatment and energy harvesting. Chemosphere 2021, 281, 130828. [Google Scholar] [CrossRef]
- Mohyudin, S.; Farooq, R.; Jubeen, F.; Rasheed, T.; Fatima, M.; Sher, F. Microbial fuel cells a state-of-the-art technology for wastewater treatment and bioelectricity generation. Environ. Res. 2022, 204, 112387. [Google Scholar] [CrossRef] [PubMed]
- Daud, N.N.M.; Ahmad, A.; Yaqoob, A.A.; Ibrahim, M.N.M. Application of rotten rice as a substrate for bacterial species to generate energy and the removal of toxic metals from wastewater through microbial fuel cells. Environ. Sci. Pollut. Res. 2021, 28, 62816–62827. [Google Scholar] [CrossRef]
- Yaakop, A.S.; Ahmad, A.; Hussain, F.; Oh, S.-E.; Alshammari, M.B.; Chauhan, R. Domestic Organic Waste: A Potential Source to Produce the Energy via a Single-Chamber Microbial Fuel Cell. Int. J. Chem. Eng. 2023, 2023, 2425735. [Google Scholar] [CrossRef]
- Pan, P.; Bhattacharyya, N. Bioelectricity Production from Microbial Fuel Cell (MFC) Using Lysinibacillus xylanilyticus Strain nbpp1 as a Biocatalyst. Curr. Microbiol. 2023, 80, 252. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, L.; Hermanowicz, S.W. Influence of water transport characteristics on membrane internal conductive structure in forward osmosis microbial fuel cell. J. Mol. Liq. 2023, 380, 121704. [Google Scholar] [CrossRef]
- Abd-Elrahman, N.K.; Al-Harbi, N.; Basfer, N.M.; Al-Hadeethi, Y.; Umar, A.; Akbar, S. Applications of Nanomaterials in Microbial Fuel Cells: A Review. Molecules 2022, 27, 7483. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wu, X.; Shi, Z.; Li, X.; Qian, S.; Sun, X.; Sun, W.; Guo, C.; Li, C.M. Photoactive Manganese Ferrite-Modified Bacterial Anode to Simultaneously Boost Both Mediated and Direct Electron Transfer Processes in Microbial Fuel Cells. ACS Sustain. Chem. Eng. 2022, 10, 3355–3362. [Google Scholar] [CrossRef]
- Khater, D.Z.; Amin, R.S.; Fetohi, A.E.; El-Khatib, K.M.; Mahmoud, G.A.E. Microbial fuel cells: Biobattery for environmental bioremediation and bioelectricity generation. In Environmental Materials and Waste; Elsevier: Amsterdam, The Netherlands, 2024; pp. 813–833. [Google Scholar] [CrossRef]
- Rana, A.K.; Thakur, M.K.; Saini, A.K.; Mokhta, S.K.; Moradi, O.; Rydzkowski, T.; Alsanie, W.F.; Wang, Q.; Grammatikos, S.; Thakur, V.K. Recent developments in microbial degradation of polypropylene: Integrated approaches towards a sustainable environment. Sci. Total Environ. 2022, 826, 154056. [Google Scholar] [CrossRef]
- Abdullah; Krukiewicz, K. Development of electrically-conducting biohybrid materials based on electroactive bacteria and conjugated polymers: Review and perspectives. Electrochim. Acta 2023, 468, 143191. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Shah, M.P. (Eds.) Synergistic Approaches for Bioremediation of Environmental Pollutants: Recent Advances and Challenges; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar] [CrossRef]
- Rossi, R.; Logan, B.E. Using an anion exchange membrane for effective hydroxide ion transport enables high power densities in microbial fuel cells. Chem. Eng. J. 2021, 422, 130150. [Google Scholar] [CrossRef]
- Cao, B.; Zhao, Z.; Peng, L.; Shiu, H.-Y.; Ding, M.; Song, F.; Guan, X.; Lee, C.K.; Huang, J.; Zhu, D.; et al. Silver nanoparticles boost charge-extraction efficiency in Shewanella microbial fuel cells. Science 2021, 373, 1336–1340. [Google Scholar] [CrossRef]
- Arulmani, S.R.B.; Gnanamuthu, H.L.; Kandasamy, S.; Govindarajan, G.; Alsehli, M.; Elfasakhany, A.; Pugazhendhi, A.; Zhang, H. Sustainable bioelectricity production from Amaranthus viridis and Triticum aestivum mediated plant microbial fuel cells with efficient electrogenic bacteria selections. Process. Biochem. 2021, 107, 27–37. [Google Scholar] [CrossRef]
- Taşkan, B.; Bakır, M.; Taşkan, E. Enhanced power generation from algal biomass using multi-anode membrane-less sediment microbial fuel cell. Int. J. Energy Res. 2021, 45, 2011–2022. [Google Scholar] [CrossRef]
- Jang, Y.; Nyamjav, I.; Kim, H.R.; Suh, D.-E.; Park, N.; Lee, Y.E.; Lee, S. Identification of plastic-degrading bacteria in the human gut. Sci. Total Environ. 2024, 929, 172775. [Google Scholar] [CrossRef]
- Maidarjav, A.; Nyamjav, I.; Kim, H.R.; Suh, D.-E.; Lee, S. Biodegradation of Ethylene Vinyl Acetate Using Klebsiella aerogenes EM011 Isolated from Effective Microorganisms. J. Polym. Environ. 2024, 32, 5823–5836. [Google Scholar] [CrossRef]
- Metcalf, R.; Messer, L.F.; White, H.L.; Ormsby, M.J.; Matallana-Surget, S.; Quilliam, R.S. Evidence of interspecific plasmid uptake by pathogenic strains of Klebsiella isolated from microplastic pollution on public beaches. J. Hazard. Mater. 2024, 461, 132567. [Google Scholar] [CrossRef] [PubMed]
- Waqar, S.; Tariq, A.; Ullah, U.; Haleem, H.; Aimen, H.; Sattar, S.; Bostan, N. Arsenic efflux and bioremediation potential of Klebsiella oxytoca via the arsB gene. PLoS ONE 2025, 20, e0307918. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, X.; Lin, Y.; Gou, H.; Zhang, Y.; Yang, L. Degradation of polyethylene by Klebsiella pneumoniae Mk-1 isolated from soil. Ecotoxicol. Environ. Saf. 2023, 258, 114965. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Bhatti, Z.A.; Maqbool, F.; Siddiqui, M.F.; Zeb, S.; Zhao, Y.-G.; Xu, L.; Ahmad, S.; Hussain, Z. Effect of electrode in UASB-MFC reactor for nitrogen removal under anammox condition and its microbial community profile. Desalination Water Treat. 2024, 318, 100300. [Google Scholar] [CrossRef]
- Arulmani, S.R.B.; Dai, J.; Li, H.; Chen, Z.; Sun, W.; Zhang, H.; Yan, J.; Kandasamy, S.; Xiao, T. Antimony reduction by a non-conventional sulfate reducer with simultaneous bioenergy production in microbial fuel cells. Chemosphere 2022, 291, 132754. [Google Scholar] [CrossRef]
- Taşkan, B.; Taşkan, E. Inhibition of AHL-mediated quorum sensing to control biofilm thickness in microbial fuel cell by using Rhodococcus sp. BH4. Chemosphere 2021, 285, 131538. [Google Scholar] [CrossRef]


| BLAST Characterization | Length of Consensus Sequence (nt) | % Maximum Identification | Accession Number | Phylogeny |
|---|---|---|---|---|
| Klebsiella oxytoca | 1468 | 99.39 | NR_118853.1 | Cellular organisms; bacteria; Proteobacteria; Gammaproteobacteria; Enterobacterales; Enterobacteriaceae; Klebsiella |
| Author | Voltage (V) | PD (mW/cm2) | MFC Type | Organism |
|---|---|---|---|---|
| This research | 0.714 ± 0.026 | 11.391 ± 0.814 | single-chamber microbial fuel cells | Klebsiella oxytoca |
| Ahmad et al. (2024) [18] | 0.210 | 1.92 | single-chamber microbial fuel cells | Planctomycetes |
| Misali et al. (2024) [19] | 0.553 | 35.93 | dual-chamber microbial fuel cells | Consortium of microorganisms |
| Chandra et al. (2024) [20] | 0.21 | N/D | dual-chamber microbial fuel cells | Consortium of microorganisms |
| Arulmani et al. (2022) [62] | 0.721 | 194.45 | single-chamber microbial fuel cells | Amaranthus viridis, Triticum aestivum |
| Taşkan et al. (2021) [55] | 2.965 | 2965 | single-chamber microbial fuel cells | Gammaproteobacteria, Deltaproteobacteria, Alphaproteobacteria |
| Patel et al. (2021) [35] | 0.107 ± 10.40 | N/D | single-chamber microbial fuel cells | Exiguobacterium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segundo, R.-F.; Luis, C.-C.; Otiniano, N.M.; De La Cruz-Noriega, M.; Soto-Deza, N.; Alviz-Meza, A.; González-Delgado, Á.D. Applied Bioelectrochemistry: Plastic Degradation and Energy Generation Using Klebsiella oxytoca in Microbial Fuel Cells. Fermentation 2025, 11, 341. https://doi.org/10.3390/fermentation11060341
Segundo R-F, Luis C-C, Otiniano NM, De La Cruz-Noriega M, Soto-Deza N, Alviz-Meza A, González-Delgado ÁD. Applied Bioelectrochemistry: Plastic Degradation and Energy Generation Using Klebsiella oxytoca in Microbial Fuel Cells. Fermentation. 2025; 11(6):341. https://doi.org/10.3390/fermentation11060341
Chicago/Turabian StyleSegundo, Rojas-Flores, Cabanillas-Chirinos Luis, Nélida Milly Otiniano, Magaly De La Cruz-Noriega, Nancy Soto-Deza, Anibal Alviz-Meza, and Ángel Darío González-Delgado. 2025. "Applied Bioelectrochemistry: Plastic Degradation and Energy Generation Using Klebsiella oxytoca in Microbial Fuel Cells" Fermentation 11, no. 6: 341. https://doi.org/10.3390/fermentation11060341
APA StyleSegundo, R.-F., Luis, C.-C., Otiniano, N. M., De La Cruz-Noriega, M., Soto-Deza, N., Alviz-Meza, A., & González-Delgado, Á. D. (2025). Applied Bioelectrochemistry: Plastic Degradation and Energy Generation Using Klebsiella oxytoca in Microbial Fuel Cells. Fermentation, 11(6), 341. https://doi.org/10.3390/fermentation11060341








