Optimizing Caproic Acid Biosynthesis in Anaerobic Fermentation of Ethanol and Butanoic Acid: The Effects of C/N Ratio
Abstract
1. Introduction
2. Materials and Methods
2.1. Inoculum and Substrates
2.2. Experimental Setup
2.3. Analytical Methods
2.4. Microbial Analysis
2.5. Calculations
3. Results and Discussion
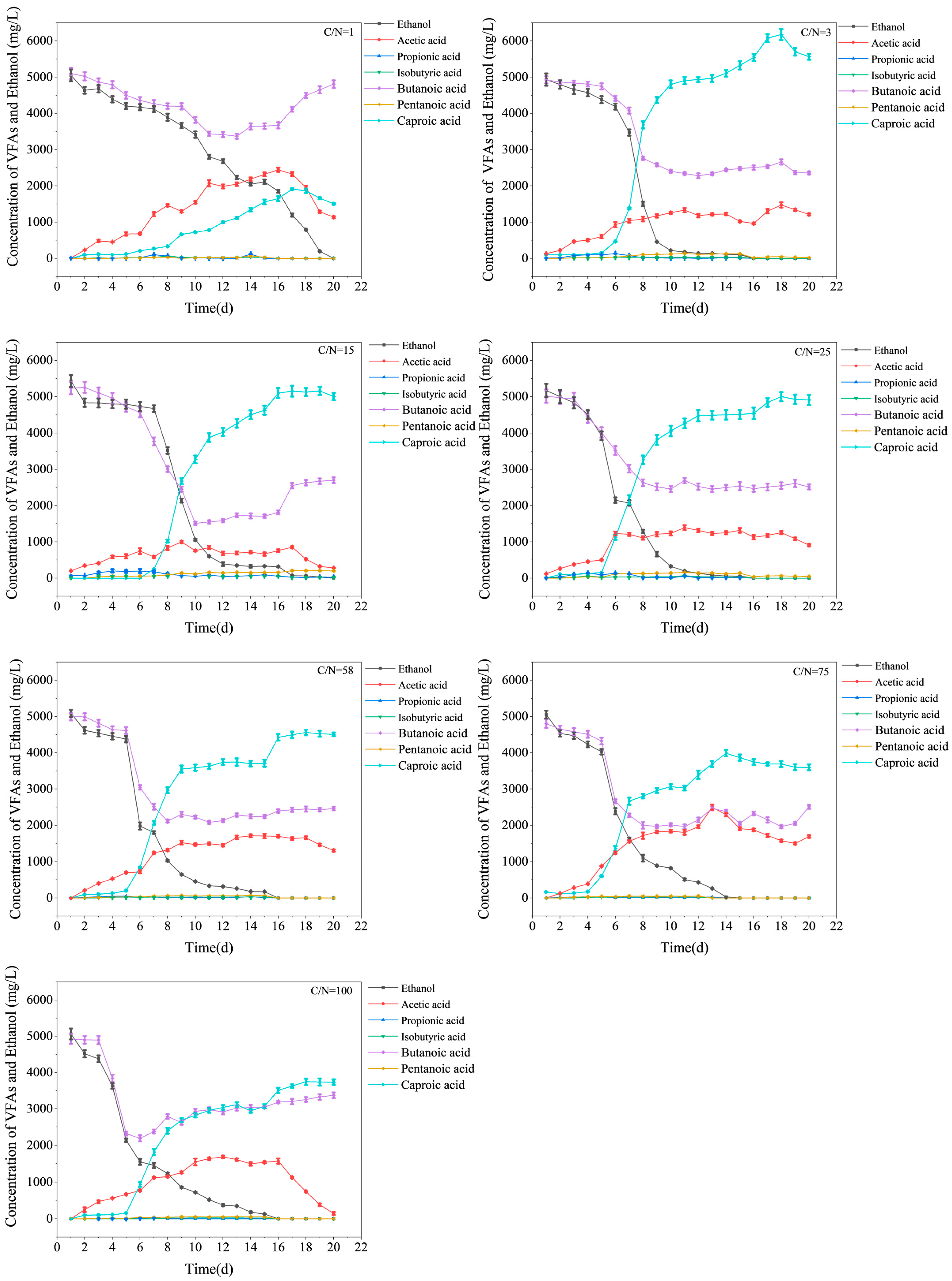
3.1. Variations in Substrates and Product Concentrations
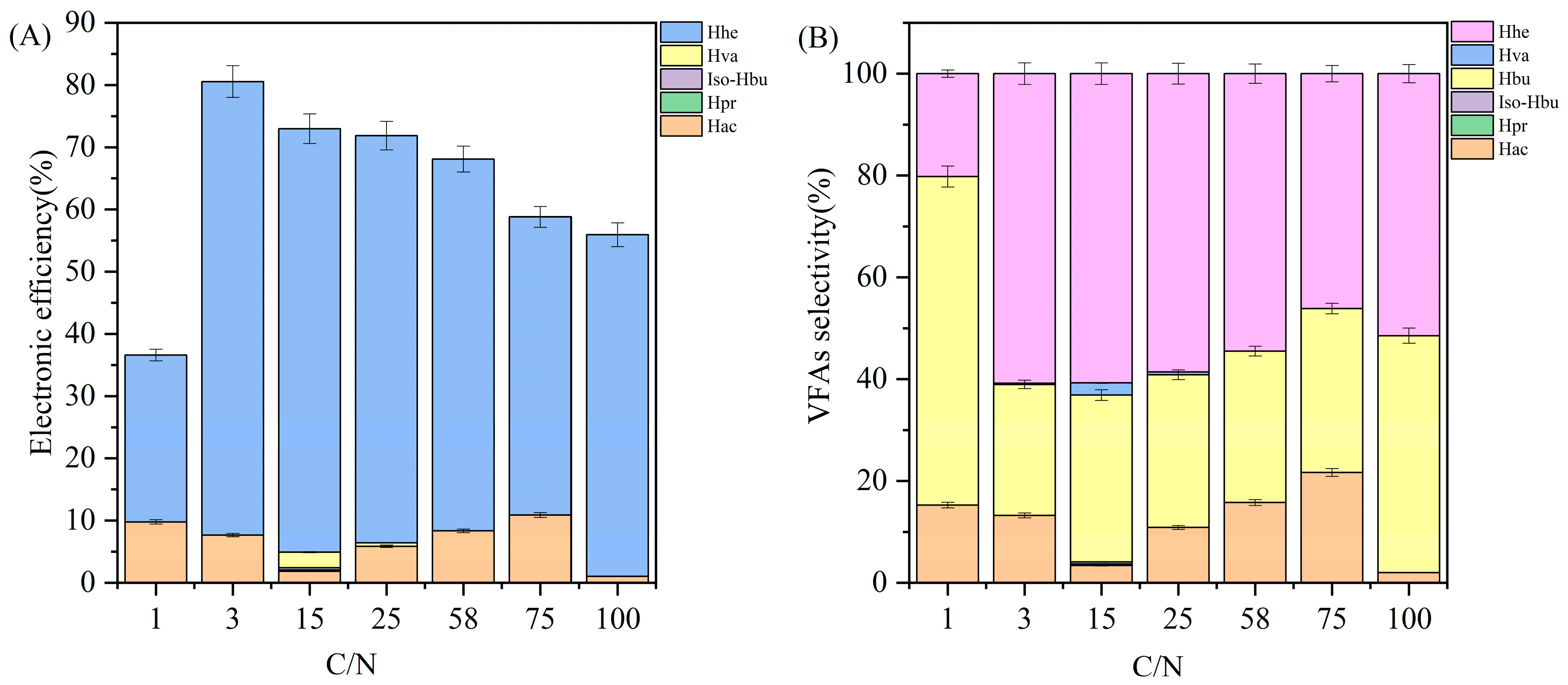
3.2. Electronic Efficiency and Product Selectivity
3.3. Distribution of Carbon in Products
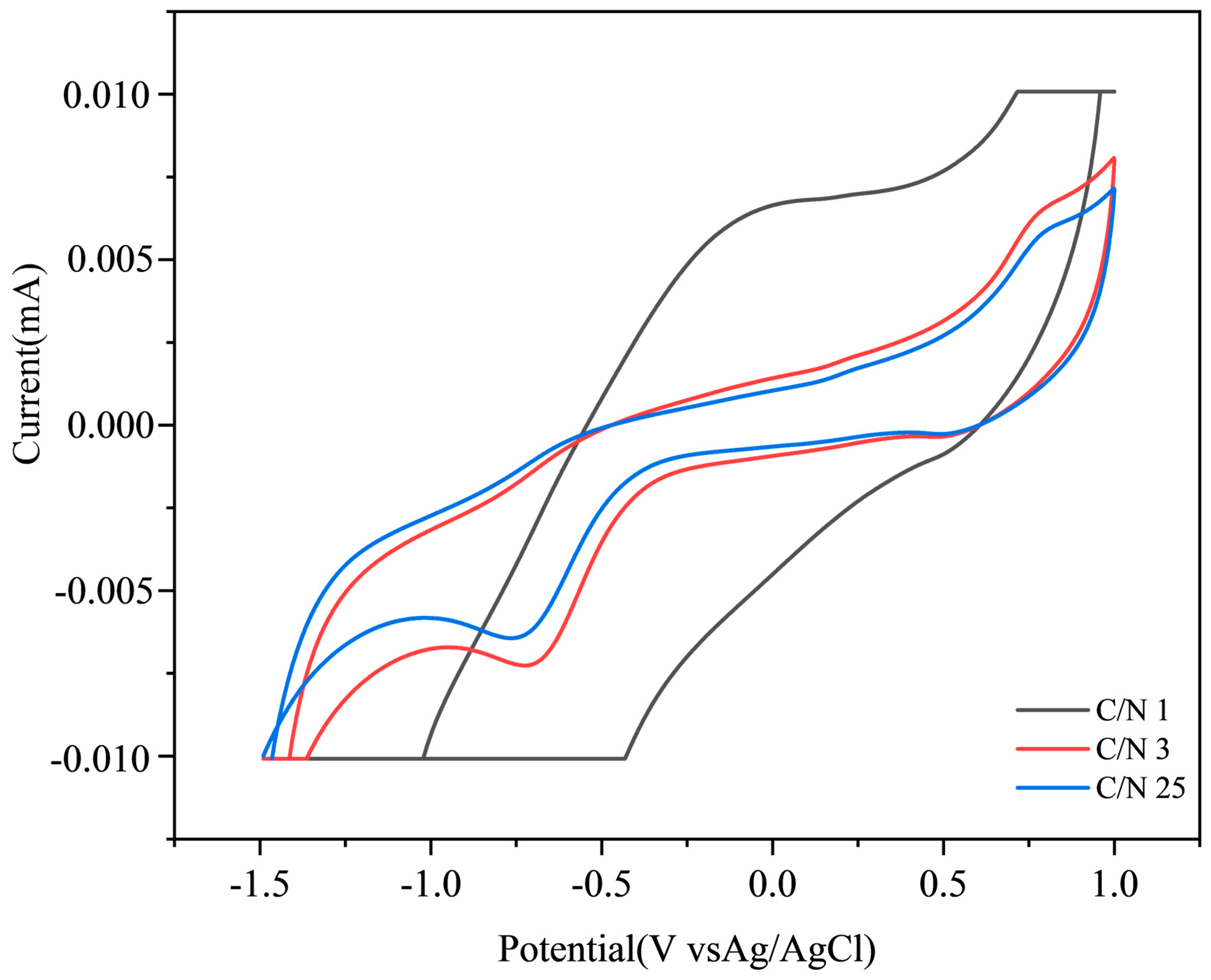
3.4. Electrochemical Analysis
3.5. Microbial Community Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agler, M.T.; Wrenn, B.A.; Zinder, S.H.; Angenent, L.T. Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends Biotechnol. 2011, 29, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-L.; Sun, J.; Chen, X.; Wei, W.; Song, L.; Dai, X.; Ni, B.-J. Unveiling the mechanisms of medium-chain fatty acid production from waste activated sludge alkaline fermentation liquor through physiological, thermodynamic and metagenomic investigations. Water Res. 2020, 169, 115218. [Google Scholar] [CrossRef] [PubMed]
- Agler, M.T.; Spirito, C.M.; Usack, J.G.; Werner, J.J.; Angenent, L.T. Chain extensionwith reactor microbiomes: Upgrading dilute ethanol to medium-chain carboxylates. Energy Environ. Sci. 2012, 5, 8189–8192. [Google Scholar] [CrossRef]
- Fortney, N.W.; Hanson, N.J.; Rosa, P.R.F.; Donohue, T.J.; Noguera, D.R. Diverse Profile of Fermentation Byproducts From Thin Stillage. Front. Bioeng. Biotechnol. 2021, 9, 695306. [Google Scholar] [CrossRef]
- Wu, Q.; Bao, X.; Guo, W.; Wang, B.; Li, Y.; Luo, H.; Wang, H.; Ren, N. Medium chain carboxylic acids production from waste biomass: Current advances and perspectives. Biotechnol. Adv. 2019, 37, 599–615. [Google Scholar] [CrossRef]
- Tang, J.; Hu, Z.; Yang, H.; Pu, Y.; Xiong, Y.; Chen, J.; Liu, R.; Li, T.; Shah, F.; Abomohra, A. Enhancement of caproate production from food waste using biochar produced from agricultural wastes: A microbial perspective. Ind. Crops Prod. 2024, 221, 119401. [Google Scholar] [CrossRef]
- Wu, Q.; Feng, X.; Guo, W.; Bao, X.; Ren, N. Long-term medium chain carboxylic acids production from liquor-making wastewater: Parameters optimization and toxicity mitigation. Chem. Eng. J. 2020, 388, 124218. [Google Scholar] [CrossRef]
- Zan, F.; Hao, T. Sulfate in anaerobic co-digester accelerates methane production from food waste and waste activated sludge. Bioresour. Technol. 2020, 298, 122536. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Bittencourt, P.; Casimir, L.; Jimenez, E.; Wang, M.; Zhang, Q.; Ergas, S.J. Biogas production from high solids anaerobic co-digestion of food waste, yard waste and waste activated sludge. Waste Manag. 2019, 95, 432–439. [Google Scholar] [CrossRef]
- Naran, E.; Toor, U.A.; Kim, D.-J. Effect of pretreatment and anaerobic co-digestion of food waste and waste activated sludge on stabilization and methane production. Int. Biodeterior. Biodegrad. 2016, 113, 17–21. [Google Scholar] [CrossRef]
- Wang, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Optimizing feeding composition and carbon–nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour. Technol. 2012, 120, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Kayhanian, M.; Tchobanoglous, G. Computation of C/N ratios for various organic fractions. BioCycle 1992, 33, 58–60. [Google Scholar]
- Kim, D.H.; Kim, S.H.; Han, S.K.; Shin, H.S. Performance of Pilot-scale H2 fermentor: C/N ratio effect and alkaline shock treatment. J. Environ. Eng. Manag. 2006, 16, 327–331. [Google Scholar]
- Lay, C.Y.L.H. Carbon/nitrogen-ratio effect on fermentative hydrogen production by mixed microflora. Int. J. Hydrogen Energy 2004, 29, 41–45. [Google Scholar]
- Liu, X.; Liu, H.; Chen, Y.; Du, G.; Chen, J. Effects of organic matter and initial carbon–nitrogen ratio on the bioconversion of volatile fatty acids from sewage sludge. J. Chem. Technol. Biotechnol. 2008, 83, 1049–1055. [Google Scholar] [CrossRef]
- Wu, Q.; Guo, W.; Bao, X.; Meng, X.; Yin, R.; Du, J.; Zheng, H.; Feng, X.; Luo, H.; Ren, N. Upgrading liquor-making wastewater into medium chain fatty acid: Insights into co-electron donors, key microflora, and energy harvest. Water Res. 2018, 145, 650–659. [Google Scholar] [CrossRef]
- Kucek, L.A.; Spirito, C.M.; Angenent, L.T. High n-caprylate productivities and specificities from dilute ethanol and acetate: Chain extension with microbiomes to upgrade products from syngas fermentation. Energy Environ. Sci. 2016, 9, 3482–3494. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, H.; Chang, J.; Sun, J.; Tu, W.; Wang, H. Effect of thermal hydrolysis pretreatment on volatile fatty acids production in sludge acidification and subsequent polyhydroxyalkanoates production. Bioresour. Technol. 2019, 279, 92–100. [Google Scholar] [CrossRef]
- Miner, G. Standard Methods for the Examination of Water and Wastewater, 21st Edition. J. Am. Water Work. Assoc. 2006, 98, 130. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, Y.; Huang, L.; Zhang, Q.; Wang, X.; Zhu, J. Effect of pH on volatile fatty acid production and the microbial community during anaerobic digestion of Chinese cabbage waste. Bioresour. Technol. 2021, 336, 125338. [Google Scholar] [CrossRef]
- Chen, R.; Ji, X.; Chen, Z.; Huang, L.; Zhu, J. Regulation of hydraulic retention time on caproic acid production via two-phase anaerobic fermentation of Chinese cabbage waste with autopoietic electron donors. J. Biotechnol. 2024, 381, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Guo, W.; You, S.; Bao, X.; Luo, H.; Wang, H.; Ren, N. Concentrating lactate-carbon flow on medium chain carboxylic acids production by hydrogen supply. Bioresour. Technol. 2019, 291, 121573. [Google Scholar] [CrossRef] [PubMed]
- Rughoonundun, H.; Mohee, R.; Holtzapple, M.T. Influence of carbon-to-nitrogen ratio on the mixed-acid fermentation of wastewater sludge and pretreated bagasse. Bioresour. Technol. 2012, 112, 91–97. [Google Scholar] [CrossRef]
- Spirito, C.M.; Richter, H.; Rabaey, K.; Stams, A.J.M.; Angenent, L.T. Chain extension in anaerobic reactor microbiomes to recover resources from waste. Curr. Opin. Biotechnol. 2014, 27, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.-L.; Yuan, K.-X.; Ren, W.-T.; Deng, L.; Wang, H.-Z.; Feng, X.-C.; Zheng, H.-S.; Ren, N.-Q.; Guo, W.-Q. Unveiling the Mechanism Underlying the Effects of Ammonia on n-Caproate Production: Influenced Pathways, Key Enzymes, and Microbiota Functions. Engineering 2024, 35, 180–190. [Google Scholar] [CrossRef]
- Lou, T.; Yin, Y.; Wang, J. Improved production of medium-chain fatty acids by biochar: Effect of biochar dosage and mechanism. J. Clean. Prod. 2024, 450, 142051. [Google Scholar] [CrossRef]
- Nzeteu, C.O.; Coelho, F.; Trego, A.C.; Abram, F.; Ramiro-Garcia, J.; Paulo, L.; O’Flaherty, V. Development of an enhanced chain elongation process for caproic acid production from waste-derived lactic acid and butyric acid. J. Clean. Prod. 2022, 338, 130655. [Google Scholar] [CrossRef]
- Ji, X.; Chen, Z.; Shen, Y.; Liu, L.; Chen, R.; Zhu, J. Hexanoic acid production and microbial community in anaerobic fermentation: Effects of inorganic carbon addition. Bioresour. Technol. 2024, 403, 130881. [Google Scholar] [CrossRef]
- Wang, X.; Han, J.; Zeng, M.; Chen, Y.; Jiang, F.; Zhang, L.; Zhou, Y. Total ammonia nitrogen inhibits medium-chain fatty acid biosynthesis by disrupting hydrolysis, acidification, chain elongation, substrate transmembrane transport and ATP synthesis processes. Bioresour. Technol. 2024, 409, 131236. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Huang, H.; Duan, X.; Dong, L. Improved anaerobic digestion under ammonia stress by regulating microbiome and enzyme to enhance VFAs bioconversion: The new role of glutathione. Chem. Eng. J. 2022, 433, 134562. [Google Scholar] [CrossRef]
- Tennison-Omovoh, C.A.; Fagbohungbe, M.O.; Bankole, P.O.; Semple, K.T. The effect of different C-N ratios on volatile fatty acid (VFAs) production from acidogenic fermentation of sucrose in continuous-stirred tank reactors. Biomass Convers. Biorefinery 2022, 13, 9339–9351. [Google Scholar] [CrossRef]
- Luo, T.; Xu, Q.; Wei, W.; Sun, J.; Dai, X.; Ni, B.-J. Performance and Mechanism of Fe3O4 Improving Biotransformation of Waste Activated Sludge into Liquid High-Value Products. Environ. Sci. Technol. 2022, 56, 3658–3668. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Song, Y.-C.; Li, J.; Wang, Z.; Wu, Q. Influence of electrostatic field and conductive material on the direct interspecies electron transfer for methane production. Environ. Res. 2020, 188, 109867. [Google Scholar] [CrossRef]
- Sun, M.; Jiang, H.; Zhang, Z.; Lv, M.; Liu, G.; Feng, Y. Coupling direct voltage and granular activated carbon modified nanoscale zero valent iron for enhancing anaerobic methane production. Chemosphere 2022, 286, 131840. [Google Scholar] [CrossRef] [PubMed]
- Grimalt-Alemany, A.; Etler, C.; Asimakopoulos, K.; Skiadas, I.V.; Gavala, H.N. ORP control for boosting ethanol productivity in gas fermentation systems and dynamics of redox cofactor NADH/NAD+ under oxidative stress. J. CO2 Util. 2021, 50, 101589. [Google Scholar] [CrossRef]
- Schievano, A.; Pepé Sciarria, T.; Vanbroekhoven, K.; De Wever, H.; Puig, S.; Andersen, S.J.; Rabaey, K.; Pant, D. Electro-Fermentation—Merging Electrochemistry with Fermentation in Industrial Applications. Trends Biotechnol. 2016, 34, 866–878. [Google Scholar] [CrossRef]
- Guo, H.; Hua, J.; Cheng, J.; Yue, L.; Zhou, J. Microbial electrochemistry enhanced electron transfer in lactic acid anaerobic digestion for methane production. J. Clean. Prod. 2022, 358, 131983. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, F.; Feng, C.; Bai, W.; Zhang, Z.; Cai, Q.; Sun, Z.; Feng, L. Effects of Maize/Peanut Intercropping and Nitrogen Fertilizer Application on Soil Fungal Community Structure. Agronomy 2024, 14, 1053. [Google Scholar] [CrossRef]
- Duan, X.; Wang, X.; Xie, J.; Feng, L.; Yan, Y.; Zhou, Q. Effect of nonylphenol on volatile fatty acids accumulation during anaerobic fermentation of waste activated sludge. Water Res. 2016, 105, 209–217. [Google Scholar] [CrossRef]
- Jang, H.M.; Kim, J.H.; Ha, J.H.; Park, J.M. Bacterial and methanogenic archaeal communities during the single-stage anaerobic digestion of high-strength food wastewater. Bioresour. Technol. 2014, 165, 174–182. [Google Scholar] [CrossRef]
- Yu, J.; Huang, Z.; Wu, P.; Zhao, M.; Miao, H.; Liu, C.; Ruan, W. Performance and microbial characterization of two-stage caproate fermentation from fruit and vegetable waste via anaerobic microbial consortia. Bioresour. Technol. 2019, 284, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Buchenau, B.; Thauer, R.K. Tetrahydrofolate-specific enzymes in Methanosarcina barkeri and growth dependence of this methanogenic archaeon on folic acid or p-aminobenzoic acid. Arch. Microbiol. 2004, 182, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Pompei, A.; Cordisco, L.; Amaretti, A.; Zanoni, S.; Matteuzzi, D.; Rossi, M. Folate Production by Bifidobacteria as a Potential Probiotic Property. Appl. Environ. Microbiol. 2007, 73, 179–185. [Google Scholar] [CrossRef]
- Huang, J.; Gao, K.; Yang, L.; Lu, Y. Successional action of Bacteroidota and Firmicutes in decomposing straw polymers in a paddy soil. Environ. Microbiome 2023, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Zhang, C.; Wang, X.; Wang, N.; Chen, Q.; Cui, G.; Xu, Q. Co-digestion of food waste and hydrothermal liquid digestate: Promotion effect of self-generated hydrochars. Environ. Sci. Ecotechnol. 2023, 15, 100239. [Google Scholar] [CrossRef]
- Guo, X.; Sun, C.; Lin, R.; Xia, A.; Huang, Y.; Zhu, X.; Show, P.-L.; Murphy, J.D. Effects of foam nickel supplementation on anaerobic digestion: Direct interspecies electron transfer. J. Hazard. Mater. 2020, 399, 122830. [Google Scholar] [CrossRef]
- Lou, T.; Yin, Y.; Wang, J. Influence of adding strategy of biochar on medium-chain fatty acids production from sewage sludge. Chemosphere 2024, 354, 141660. [Google Scholar] [CrossRef]
- He, P.; Zhang, H.; Duan, H.; Shao, L.; Lü, F. Continuity of biochar-associated biofilm in anaerobic digestion. Chem. Eng. J. 2020, 390, 124605. [Google Scholar] [CrossRef]
- Chen, S.; Dong, X. Proteiniphilum acetatigenes gen. nov., sp. nov., from a UASB reactor treating brewery wastewater. Int. J. Syst. Evol. Microbiol. 2005, 55, 2257–2261. [Google Scholar] [CrossRef]

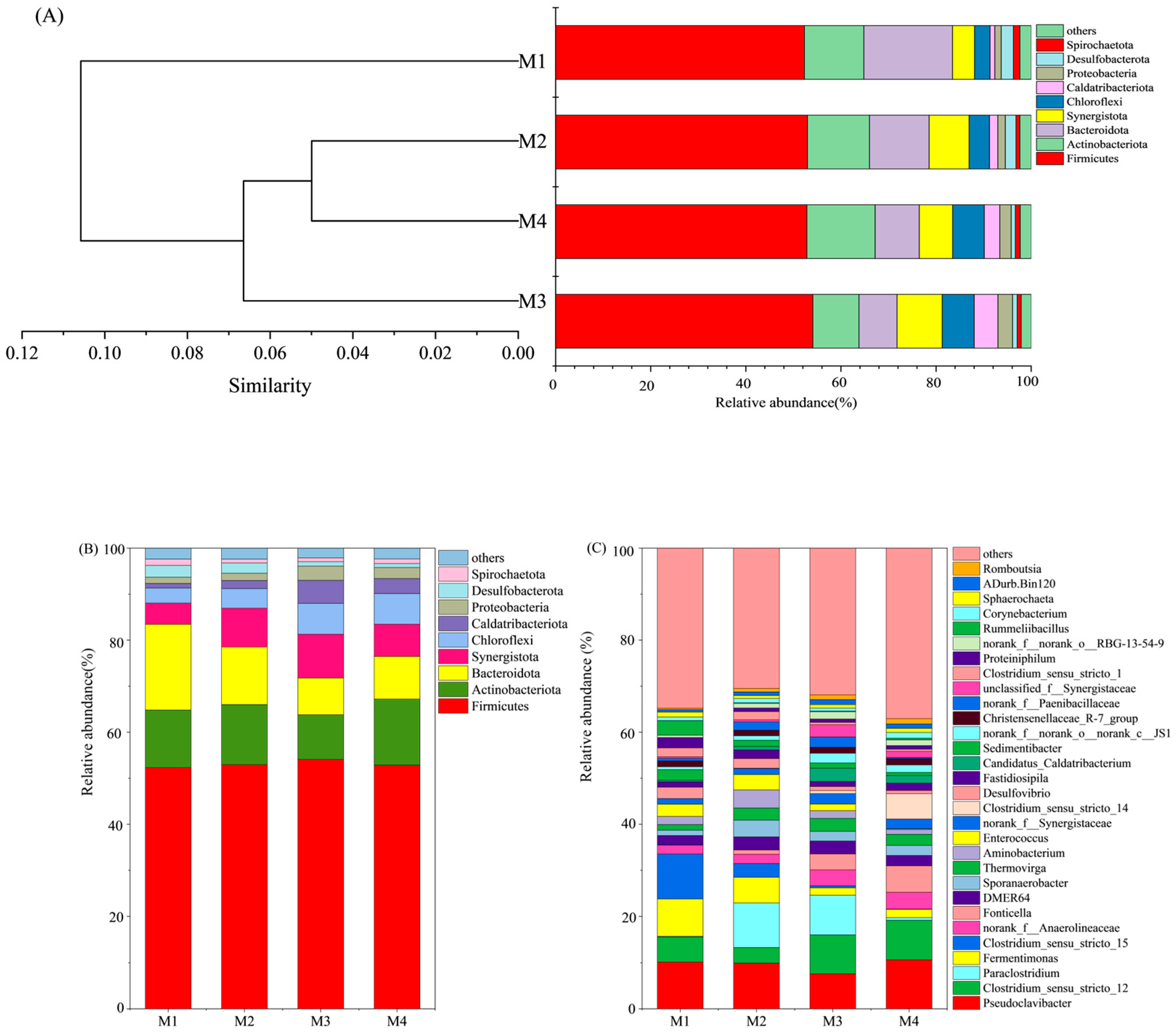
| Sample Group | Sobs | Shannon Index | Simpson Index | Ace Index | Chao Index | Coverage |
|---|---|---|---|---|---|---|
| M1 | 1327 | 4.60 | 0.035 | 1421.40 | 1376.52 | 0.99 |
| M2 | 1323 | 4.73 | 0.030 | 1445.85 | 1410.29 | 0.99 |
| M3 | 1334 | 4.86 | 0.025 | 1447.93 | 1446.19 | 0.99 |
| M4 | 1297 | 4.94 | 0.026 | 1357.79 | 1348.50 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Shen, Y.; Yang, S.; Chen, Z.; Ji, X.; Zhu, J. Optimizing Caproic Acid Biosynthesis in Anaerobic Fermentation of Ethanol and Butanoic Acid: The Effects of C/N Ratio. Fermentation 2025, 11, 316. https://doi.org/10.3390/fermentation11060316
Liu L, Shen Y, Yang S, Chen Z, Ji X, Zhu J. Optimizing Caproic Acid Biosynthesis in Anaerobic Fermentation of Ethanol and Butanoic Acid: The Effects of C/N Ratio. Fermentation. 2025; 11(6):316. https://doi.org/10.3390/fermentation11060316
Chicago/Turabian StyleLiu, Longlong, Yingmeng Shen, Sen Yang, Zhengang Chen, Xiaofeng Ji, and Jiying Zhu. 2025. "Optimizing Caproic Acid Biosynthesis in Anaerobic Fermentation of Ethanol and Butanoic Acid: The Effects of C/N Ratio" Fermentation 11, no. 6: 316. https://doi.org/10.3390/fermentation11060316
APA StyleLiu, L., Shen, Y., Yang, S., Chen, Z., Ji, X., & Zhu, J. (2025). Optimizing Caproic Acid Biosynthesis in Anaerobic Fermentation of Ethanol and Butanoic Acid: The Effects of C/N Ratio. Fermentation, 11(6), 316. https://doi.org/10.3390/fermentation11060316






