Effects of Chestnut Tannin on Nutrient Digestibility, Ruminal Protease Enzymes, and Ruminal Microbial Community Composition of Sheep
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
2.2. Sample Collection and Determination Indicators
2.2.1. Growth Performance
2.2.2. Nutrient Digestibility and Nitrogen Balance
2.2.3. Determination of Rumen Protease Enzymes
2.2.4. In Situ CP Digestibility
2.2.5. DNA Extraction, Quantitative Reverse Transcription PCR (qRT-PCR) Analysis, and MiSeq Sequencing
2.3. Statistical Analysis
3. Results
3.1. Effects of CHT on Growth Performance and Nutrient Digestibility of Sheep
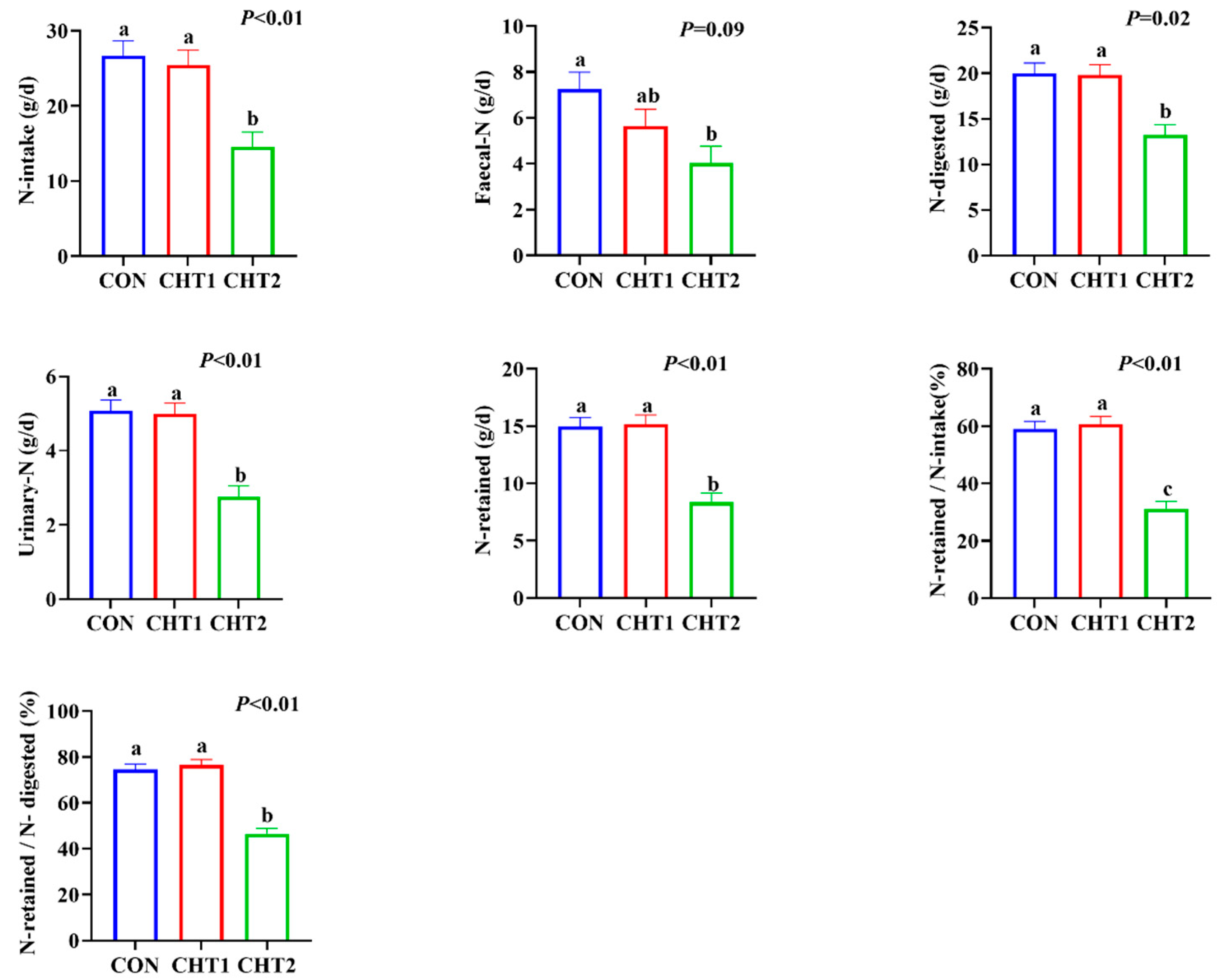
3.2. Effects of CHTs on Nitrogen Metabolism of Sheep
3.3. Effects of CHT In Situ on the CP Digestibility of Sheep
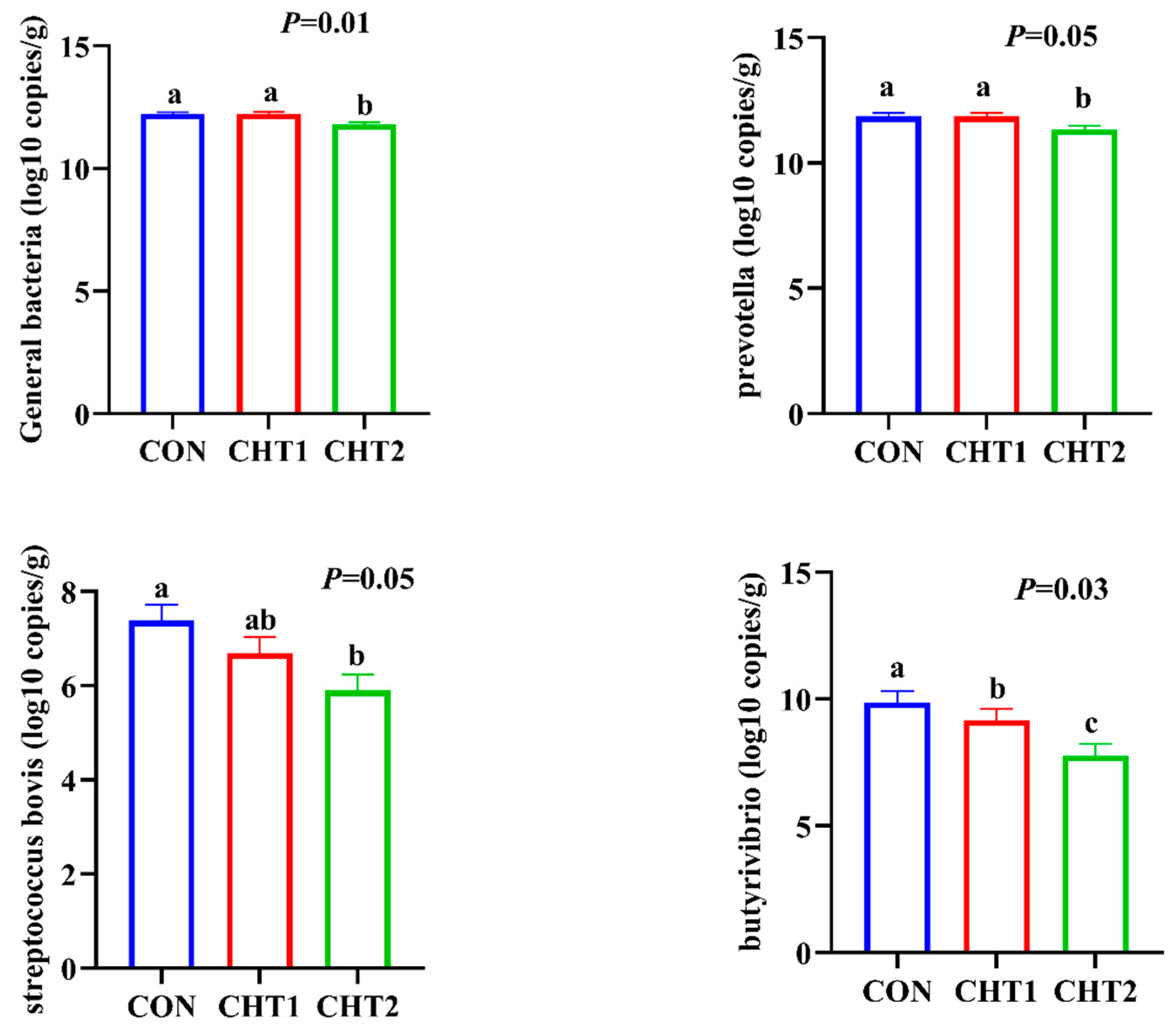
3.4. Effects of CHTs on Rumen Bacterial Abundance in Sheep
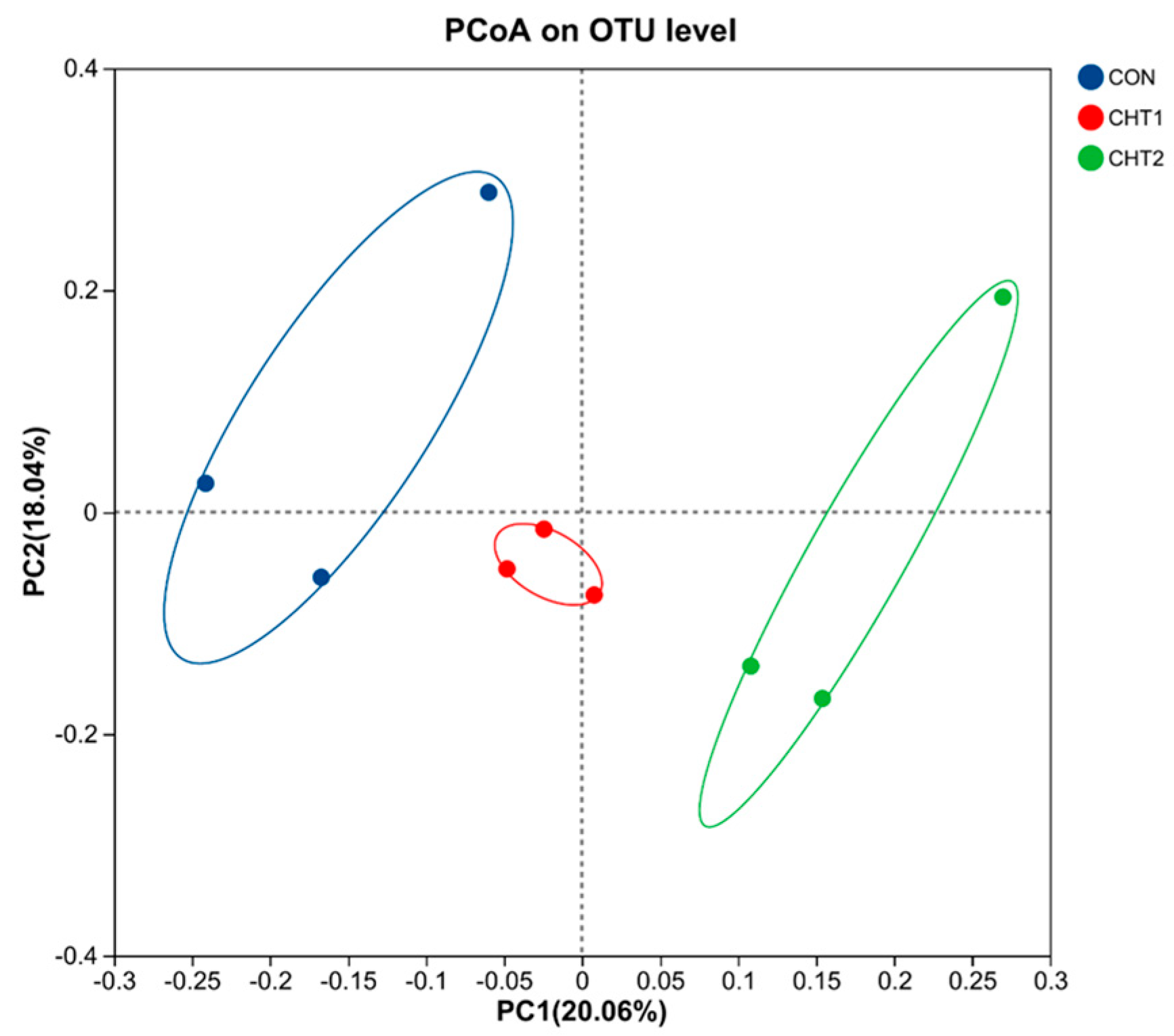
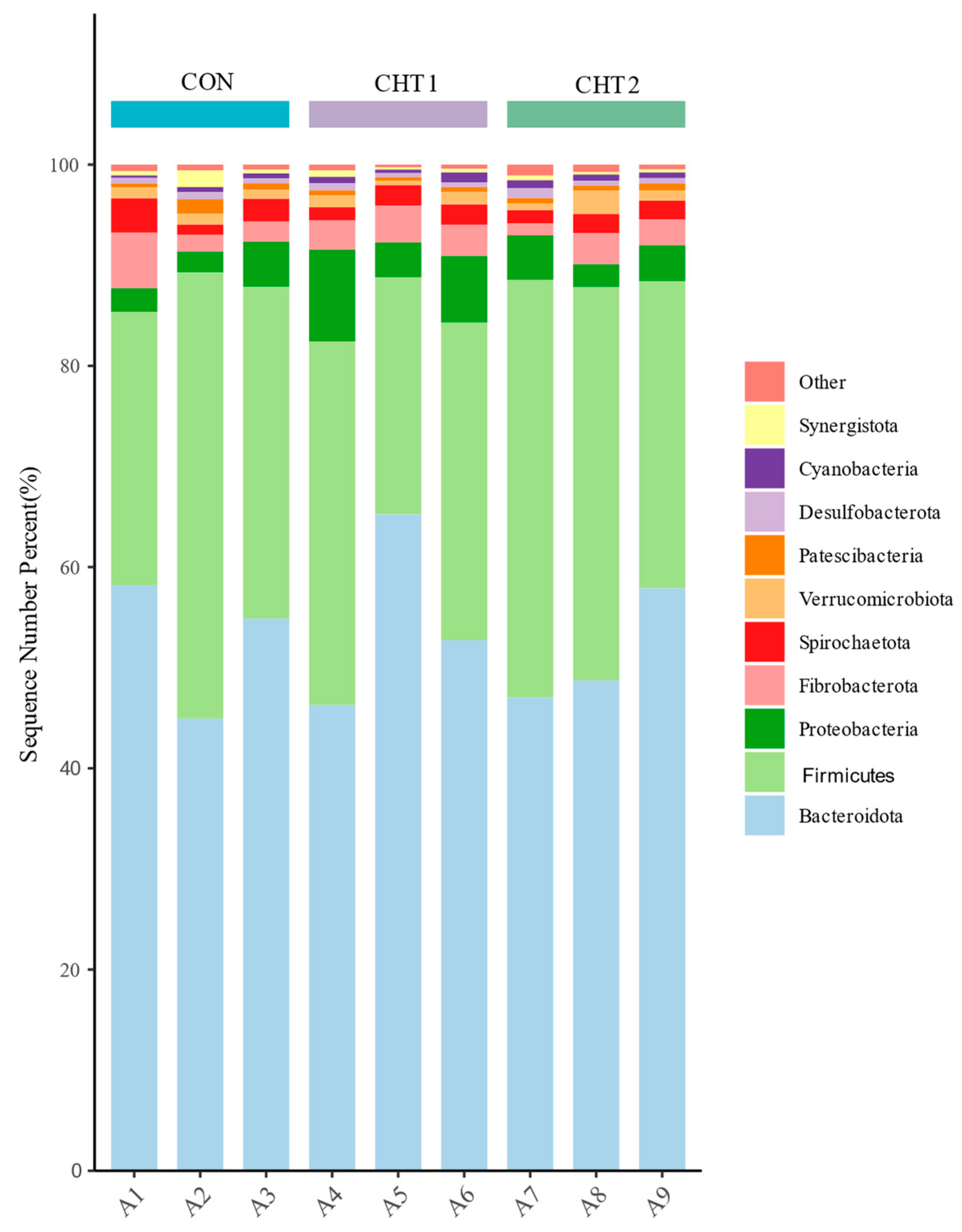
3.5. Effects of CHTs on Overall Structural Change in Gut Microbiota
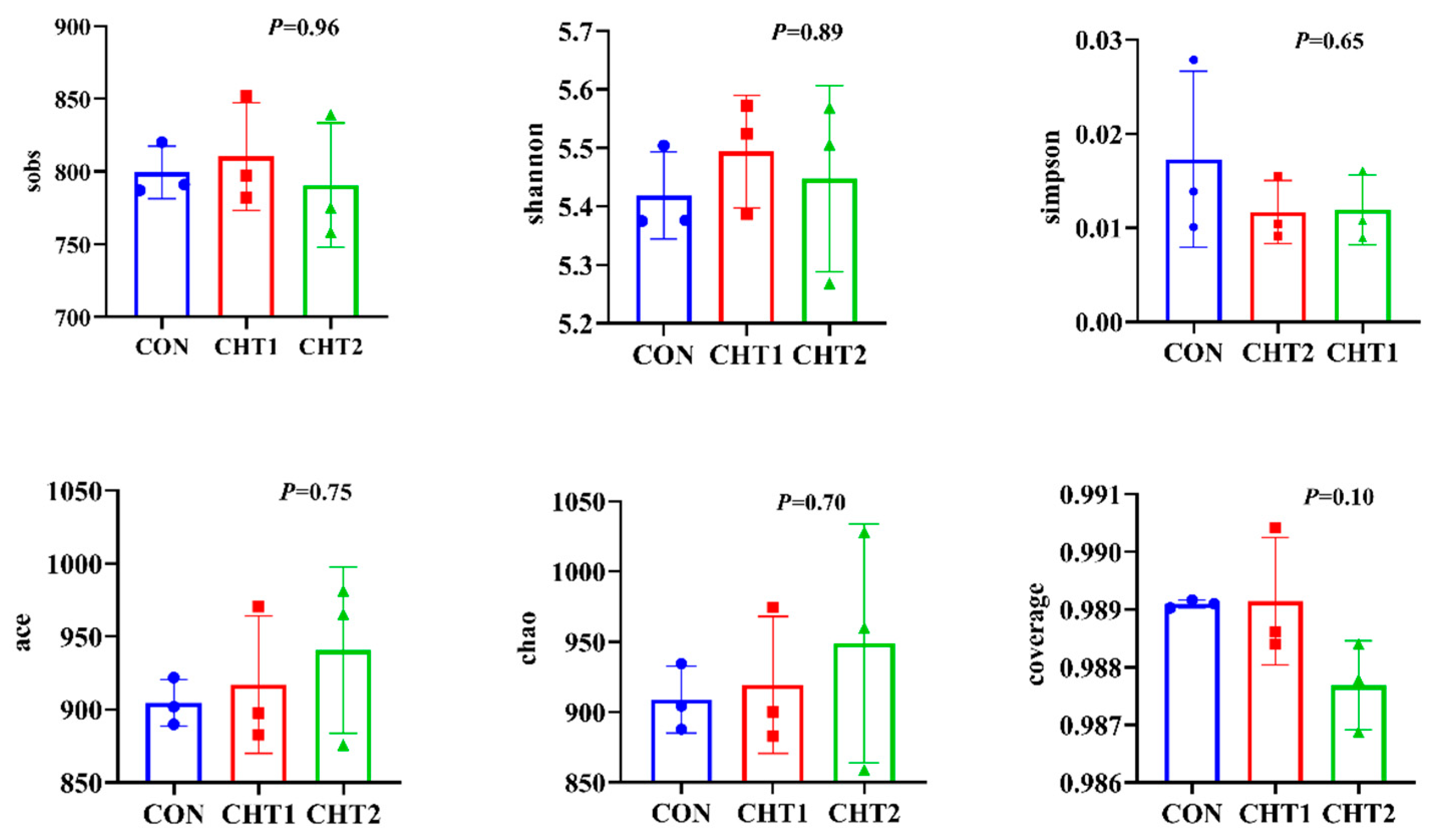
3.5.1. Sequencing Coverage and Bacterial Diversity
3.5.2. Changes in Bacterial Composition After 2% CHT and 6% CHT Supplementation
4. Discussion
4.1. CHTs Affected the Growth Performance and the In Situ CP Digestibility of Sheep
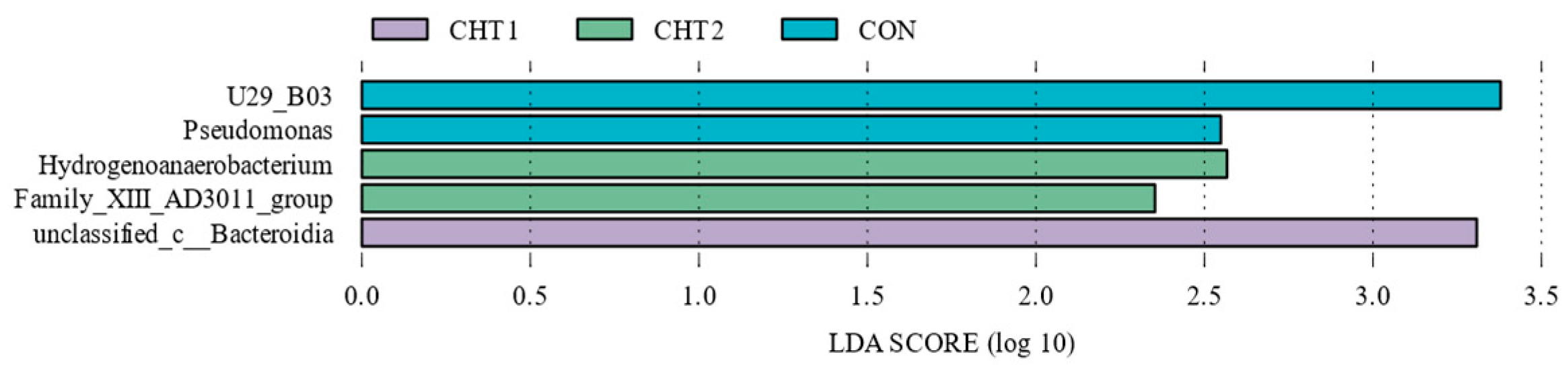
4.2. CHT Changes the Ruminal Microbial Community Composition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADG | average daily gain |
| ADFI | average day feed intake |
| BW | body weight |
| CHT | chestnut tannins |
| DM | direct mail advertising |
| ERD | effective degradability |
References
- Niderkorn, V.; Barbier, E.; Macheboeuf, D.; Torrent, A.; Mueller-Harvey, I.; Hoste, H. In vitro rumen fermentation of diets with different types of condensed tannins derived from sainfoin (Onobrychis viciifolia scop.) pellets and hazelnut (Corylus avellana L.) pericarps. Anim. Feed Sci. Technol. 2020, 259, 114357. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Oba, M.; Koenig, K.M.; Zhao, G.Y.; Beauchemin, K.A. Use of gallic acid and hydrolyzable tannins to reduce methane emission and nitrogen excretion in beef cattle fed a diet containing alfalfa silage1,2. J. Anim. Sci. 2019, 97, 2230–2244. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Bao, Y.; Zhao, G. Effects of dietary crude protein and tannic acid on rumen fermentation, rumen microbiota and nutrient digestion in beef cattle. Arch. Anim. Nutr. 2019, 73, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Jakhesara, S.J.; Koringa, P.G.; Ramani, U.V.; Ahir, V.; Tripathi, A.; Soni, P.; Singh, K.; Bhatt, V.; Patel, J.; Patel, M.; et al. Comparative study of tannin challenged rumen microbiome in goat using high throughput sequencing technology. Dev. Microbiol. Mol. Biol. 2010, 1, 95–106. [Google Scholar]
- de Sant’Ana, A.S.; Ribeiro Silva, A.P.; Oliveira do Nascimento, S.P. Tannin as a modulator of rumen microbial profile, apparent digestibility and ingestive behavior of lactating goats: A preliminary metagenomic view of goats adaptability to tannin. Res. Vet. Sci. 2022, 145, 159–168. [Google Scholar] [CrossRef]
- Min, B.R.; Wang, W.; Pitta, D.W.; Nagaraju, I.; Patra, A.K.; Wang, H.H.; Frank, A.; Mariline, H.; Ryszard, P. Characterization of the ruminal microbiota in sheep and goats fed different levels of tannin-rich sericea lespedeza hay. J. Anim. Sci. 2024, 102, skae198. [Google Scholar] [CrossRef]
- Nelson, K.; Pell, A.; Schofield, P.; Zinder, S.H. Isolation and characterization of an anaerobic ruminal bacterium capable of degrading hydrolyzable tannins. Appl Env. Microbiol. 1995, 61, 3293–3298. [Google Scholar] [CrossRef]
- Min, B.R.; Attwood, G.T.; McNabb, W.C.; Molan, A.L.; Barry, T.N. The effect of condensed tannins from lotus corniculatus on the proteolytic activities and growth of rumen bacteria. Anim. Feed. Sci. Technol. 2005, 121, 45–58. [Google Scholar] [CrossRef]
- Caprarulo, V.; Giromini, C.; Rossi, L. Review: Chestnut and quebracho tannins in pig nutrition: The effects on performance and intestinal health. Anim. Int. J. Anim. Biosci. 2021, 15, 100064. [Google Scholar] [CrossRef]
- Khatib, M.; Campo, M.; Bellumori, M.; Cecchi, L.; Vignolini, P.; Innocenti, M.; Mulinacci, N. Tannins from different parts of the chestnut trunk (Castanea sativa mill.): A green and effective extraction method and their profiling by high-performance liquid chromatography-diode array detector-mass spectrometry. ACS Food Sci. Technol. 2023, 3, 1903–1912. [Google Scholar] [CrossRef]
- Du, L.; Li, J.; Zhang, X. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of MAPKs activation. J. Funct. Foods 2018, 43, 62–69. [Google Scholar] [CrossRef]
- Reggi, S.; Giromini, C.; Dell’anno, M.; Baldi, A.; Rebucci, R.; Rossi, L. In vitro digestion of chestnut and quebracho tannin extracts: Antibacterial effect, anti-oxidant capacity and cytomodulatory activity in swine Intestinal Ipec-j2 cells. Animals. 2020, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Buccioni, A.; Pallara, G.; Pastorelli, R.; Antongiovanni, M.; Mele, M. Effect of Dietary Chestnut or Quebracho Tannin Supplementation on Microbial Community and Fatty Acid Profile in the Rumen of Dairy Ewes. Biomed Res. Int. 2017, 2017, 4969076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qi, S.; Xue, X. Understanding the gastrointestinal protective effects of polyphenols using foodomics-based approaches. Front. Immunol. 2021, 12, 671150. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Oba, M.; Castillo, A.; Koenig, K.M.; Iwaasa, A.D.; Beauchemin, K.A. Effects of hydrolyzable tannin with or without condensed tannin on methane emissions, nitrogen use, and performance of beef cattle fed a high-forage diet. Anim Sci. 2018, 96, 5276–5286. [Google Scholar] [CrossRef]
- Avila, S.; Kozloski, G.; Orlandi, T. Impact of a tannin extract on digestibility, ruminal fermentation and duodenal flow of amino acids in steers fed maize silage and concentrate containing soybean meal or canola meal as protein source. J. Agric. Sci. 2015, 153, 943–953. [Google Scholar] [CrossRef]
- Dentinho, M.T.P.; Belo, A.T.; Bessa, R.J.B. Digestion, ruminal fermentation and bacterial nitrogen supply in sheep fed soybean meal treated with Cistus ladanifer L. tannins. Small Rumin. Res. 2014, 119, 57–64. [Google Scholar] [CrossRef]
- Battelli, M.; Colombini, S.; Parma, P.; Galassi, G.; Crovetto, G.M.; Spanghero, M.; Pravettoni, D.; Zanzani, S.A.; Manfredi, M.T.; Rapetti, L. In vitro effects of different levels of quebracho and chestnut tannins on rumen me-thane production, fermentation parameters, and microbiota. Front. Vet. Sci. 2023, 10, 1178288. [Google Scholar] [CrossRef]
- Fitri, A.; Yanza, Y.R.; Jayanegara, A.; Ridwan, R.; Astuti, W.D.; Sarwono, K.A.; Fidriyanto, R.; Rohmatussolihat, R.; Widyastuti, Y.; Obitsu, T. Divergence effects between dietary acacia and quebracho tannin extracts on nutrient utilization, performance, and methane emission of ruminants: A meta-analysis. Anim. Sci. J. 2022, 93, e13765. [Google Scholar] [CrossRef]
- NYT816-2021; Feeding Standard of Meat-Producing Sheep and Goats. Agricultural Industry Standard of the People’s Republic of China: Beijing, China, 2021.
- ISO14088; Leather—Chemical Tests—Quantitative Analysis of Tanning Agents by Filter Method. ISO: Geneva, Switzerland, 2020.
- Association of Official Analytical Chemists. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Allen, M.S. Relationship between fermentation acid production in the rumen and the requirement for physically effective fiber. Dairy Sci. 1997, 80, 1447–1462. [Google Scholar] [CrossRef]
- Krieg, J.; Koenzen, E.; Seifried, N.; Steingass, H.; Schenkel, H.; Rodehutscord, M. Prediction of CP and starch concentrations in ruminal in situ studies and ruminal degradation of cereal grains using NIRS. Anim. Int. J. Anim. Biosci. 2018, 12, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Raffrenato, E.; Badenhorst, M.J.; Shipandeni, M.N.T.; van Zyl, W.H. Rumen fluid handling affects measurements of its enzymatic activity and in vitro digestibility. Anim. Feed. Sci. Technol. 2021, 280, 115060. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, T.; Tu, Y. Effects of Circadian Rhythm and Feeding Modes on Rumen Fermentation and Microorganisms in Hu Sheep. Microorganisms 2022, 10, 2308. [Google Scholar] [CrossRef]
- Liu, C.; Li, D.; Chen, W.; Li, Y.; Wu, H.; Meng, Q.; Zhou, Z. Estimating ruminal crude protein degradation from beef cattle feedstuff. Sci. Rep. 2019, 9, 11368. [Google Scholar] [CrossRef]
- Bürgmann, H.; Pesaro, M.; Widmer, F. A strategy for optimizing quality and quantity of DNA extracted from soil. J. Microbiol. Methods 2001, 45, 7–20. [Google Scholar] [CrossRef]
- Navidshad, B.; Liang, J.B.; Jahromi, M.F. Correlation coefficients between different methods of expressing bacterial quantification using real time PCR. Int. J. Mol. Sci. 2012, 13, 2119–2132. [Google Scholar] [CrossRef]
- Wischer, G.; Greiling, A.M.; Boguhn, J.; Steingass, H.; Schollenberger, M.; Hartung, K.; Rodehutscord, M. Effects of long-term supplementation of chestnut and valonea extracts on methane release, digestibility and nitrogen excretion in sheep. Anim. Int. J. Anim. Biosci. 2014, 8, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef]
- Aguerre, M.J.; Duval, B.; Powell, J.M.; Vadas, P.A.; Wattiaux, M.A. Effects of feeding a quebracho–chestnut tannin extract on lactating cow performance and nitrogen utilization efficiency. J. Dairy Sci. 2020, 103, 2264–2271. [Google Scholar] [CrossRef]
- Brutti, D.D.; Canozzi, M.E.A.; Sartori, E.D.; Colombatto, D.; Barcellos, J.O.J. Effects of the use of tannins on the ruminal fermentation of cattle: A me-ta-analysis and meta-regression. Anim. Feed Sci. Technol. 2023, 306, 115806. [Google Scholar] [CrossRef]
- Ahnert, S.; Dickhoefer, U.; Schulz, F. Influence of ruminal quebracho tannin extract infusion on apparent nutrient digestibility, nitrogen balance, and urinary purine derivatives excretion in heifers. Livest. Sci. 2015, 177, 63–70. [Google Scholar] [CrossRef]
- Min, B.R.; Solaiman, S.; Terrill, T. The effects of tannins-containing ground pine bark diet upon nutrient digestion, nitrogen balance, and mineral retention in meat goats. J. Anim. Sci. Biotechnol. 2015, 6, 25. [Google Scholar] [CrossRef]
- Ku-Vera, J.C.; Jiménez-Ocampo, R.; Valencia-Salazar, S.S. Role of secondary plant metabolites on enteric methane mitigation in ruminants. Front. Vet. Sci. 2020, 7, 584. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef]
- Qi, M.; Wen, H.; Zhou, Y.; Shi, W.; Wang, L.; Han, L.; Wu, G. Effects of chestnut tannin on Growth Performance, Meat Quality and fatty acid Composition of Muscle of Tan Sheep. Chin. J. Anim. Nutr. 2022, 34, 3857–3866. (In Chinese) [Google Scholar]
- González-Barrio, R.; Truchado, P.; García-Villalba, R.; Hervás, G.; Frutos, P.; Espín, J.C.; Tomás-Barberán, F.A. Metabolism of oak leaf ellagitannins and urolithin production in beef cattle. J. Agric. Food Chem. 2012, 60, 3068–3077. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.F.; Cao, Z.J.; Azarfar, A.; Li, S. Effect of different tannin sources on nutrient intake, digestibility, performance, nitrogen utilization, and blood parameters in dairy cows. Animals 2019, 9, 507. [Google Scholar] [CrossRef]
- Tseu, R.J.; Junior, P.; Flavio; Acácio Sene, G.; Tropaldi, C.B.; Peres, A.H.; Rodrigues, P.H.M. Effect of tannins and monensin on feeding behaviour, feed intake, digestive parameters and bacterial efficiency of nellore cows. Ital. J. Anim. Sci. 2020, 19, 262–273. [Google Scholar] [CrossRef]
- Coblentz, W.K.; Grabber, J.H. In situ protein degradation of alfalfa and birdsfoot trefoil hays and silages as influenced by condensed tannin concentration. J. Dairy Sci. 2013, 96, 3120–3137. [Google Scholar] [CrossRef]
- Chen, L.; Bao, X.Y.; Guo, G.; Huo, W.; Xu, Q.; Wang, C.; Liu, Q. Effects of hydrolysable tannin with or without condensed tannin on alfalfa silage fermentation characteristics and in vitro ruminal methane production, fermentation patterns, and microbiota. Animals 2021, 11, 1967. [Google Scholar] [CrossRef]
- Rira, M.; Morgavi, D.P.; Archimède, H.; Marie-Magdeleine, C.; Popova, M.; Bousseboua, H.; Doreau, M. Potential of tannin-rich plants for modulating ruminal microbes and ruminal fer-mentation in sheep. J. Anim. Sci. 2015, 93, 334–347. [Google Scholar] [CrossRef]
- Carrasco, J.M.D.; Cabral, C.; Redondo, L.M.; Colombatto, D.; Farber, M.D.; Miyakawa, M.E.F. Impact of chestnut and quebracho tannins on rumen microbiota of bovines. BioMed Res. Int. 2017, 2017, 9610810. [Google Scholar] [CrossRef]
- Okuda, T.; Ito, H. Tannins of constant structure in medicinal and food plants-hydrolyzable tannins and polyphenols related to tannins. Molecules 2011, 16, 2191–2217. [Google Scholar] [CrossRef]
- Yang, K.; Wei, C.; Zhao, G.Y.; Xu, Z.W.; Zhao, S.X. Effects of dietary supplementing tannic acid in the ration of beef cattle on rumen fermentation, methane emission, bacterial flora and nutrient digestibility. Anim. Physiol. Anim. Nutr. 2016, 101, 302–310. [Google Scholar] [CrossRef]
- Ge, T.; Yang, C.; Li, B.; Huang, X.; Zhao, L.; Zhang, X.; Tian, L.; Zhang, E. High-energy diet modify rumen bacterial composition and bacterial energy metabolism pattern in fattening sheep. BMC Vet. Res. 2023, 19, 32. [Google Scholar] [CrossRef]
- Huws, S.A.; Edwards, J.E.; Creevey, C.J.; Stevens, P.R.; Lin, W.; Girdwood, S.E.; Pachebat, J.A.; Kingston-Smith, A.H. Temporal dynamics of the metabolically active rumen bacteria colonizing fresh perennial ryegrass. FEMS Microbiol. Ecol. 2016, 92, fiv137. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Zhang, X.X.; Li, F.D.; Li, C.; Li, G.Z.; Zhang, D.Y.; Song, Q.Z.; Li, X.L.; Zhao, Y.; Wang, W.M. Characterization of the rumen microbiota and its relationship with residual feed intake in sheep. Anim. Int. J. Anim. Biosci. 2021, 15, 100161. [Google Scholar] [CrossRef]


| Ingredients | Content (%) | Nutrient Levels | Level, % |
|---|---|---|---|
| Oat grass | 31.00 | Metabolizable energy, (MJ/kg) (2) | 7.86 |
| Alfalfa | 29.00 | Crude protein (%) | 15.47 |
| Sunflower husk | 10.00 | Neutral detergent fiber (%) | 46.90 |
| Corn | 15.00 | Acid detergent fiber (%) | 28.57 |
| Corn husk | 2.00 | Calcium (%) | 0.89 |
| Soybean meal | 7.50 | Available phosphorus (%) | 0.71 |
| Canola meal | 3.00 | ||
| Limestone | 0.00 | ||
| CaHPO4 | 1.00 | ||
| NaCl | 0.50 | ||
| Premix (1) | 1.00 | ||
| Total | 100.00 |
| Primers Name | Primer Sequence (5→3) | Tm (°C) | Product Size (bp) |
|---|---|---|---|
| Prevotella | F: CGGTAAACGATGGATGTAC | 58 | 133 |
| R: ATGTTCTACCGTATGTGC | |||
| Streptococcus bovis | F: ATTTATAGAGATAGGGTTTTATAT | 60 | 134 |
| R: ACTATATGATGGCAATAAACAATA | |||
| Butyrivibrio | F: CGCATGATGCAGTGTGAAAAGTAC | 56 | 125 |
| R: CTACCCGACACTAATTATTCATCG | |||
| General bacteria | F: CGGCAACGAGCGCAACCC | 60 | 130 |
| R: CCATTGTAGCACGTGTGTAGCC |
| Item | Treatment Group (%) 1 | SEM | p-Value 2 | ||
|---|---|---|---|---|---|
| CON | CHT1 | CHT2 | |||
| Growth performance | |||||
| Dry matter intake, g/d | 1342.33 a | 1097.62 b | 504.93 c | 58.40 | <0.01 |
| Average daily gain, g/d | 128.44 a | 119.92 a | −13.83 b | 9.03 | <0.01 |
| Feed efficiency, g/g | 0.10 a | 0.11 a | −0.03 b | 0.005 | <0.01 |
| Nutrients apparent digestibility | |||||
| DM | 66.32 | 69.1 | 70.21 | 0.02 | 0.49 |
| OM | 69.29 | 71.61 | 66.98 | 0.02 | 0.23 |
| CP | 77.53 | 79 | 65.83 | 0.02 | 0.46 |
| NDF | 70.03 | 72.26 | 76.27 | 0.02 | 0.19 |
| ADF | 57.98 | 60.97 | 59.34 | 0.04 | 0.61 |
| Item | Treatment Group (%) | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | CHT1 | CHT2 | |||
| In situ CP digestibility | |||||
| a | 5.80 a | 3.21 b | 2.63 b | 0.24 | <0.01 |
| b | 47.66 a | 41.31 a | 12.12 b | 2.87 | <0.01 |
| c (%/h) | 2.55 b | 2.18 b | 6.35 a | 0.58 | 0.02 |
| Kp (%/h) | 4.25 | 4.25 | 4.25 | ||
| ERD (%) | 22.72 a | 16.76 b | 9.54 c | 0.35 | <0.01 |
| enzyme activity (U·g−1·min−1) | |||||
| Protease enzyme | 2.86 ab | 2.32 b | 2.67 a | 0.52 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Liu, P.; Xing, Y.; Zhang, M.; Yu, Y.; Wang, W.; Li, D. Effects of Chestnut Tannin on Nutrient Digestibility, Ruminal Protease Enzymes, and Ruminal Microbial Community Composition of Sheep. Fermentation 2025, 11, 302. https://doi.org/10.3390/fermentation11060302
Sun M, Liu P, Xing Y, Zhang M, Yu Y, Wang W, Li D. Effects of Chestnut Tannin on Nutrient Digestibility, Ruminal Protease Enzymes, and Ruminal Microbial Community Composition of Sheep. Fermentation. 2025; 11(6):302. https://doi.org/10.3390/fermentation11060302
Chicago/Turabian StyleSun, Mei, Peinan Liu, Yuanyuan Xing, Meimei Zhang, Yongqiang Yu, Weiyun Wang, and Dabiao Li. 2025. "Effects of Chestnut Tannin on Nutrient Digestibility, Ruminal Protease Enzymes, and Ruminal Microbial Community Composition of Sheep" Fermentation 11, no. 6: 302. https://doi.org/10.3390/fermentation11060302
APA StyleSun, M., Liu, P., Xing, Y., Zhang, M., Yu, Y., Wang, W., & Li, D. (2025). Effects of Chestnut Tannin on Nutrient Digestibility, Ruminal Protease Enzymes, and Ruminal Microbial Community Composition of Sheep. Fermentation, 11(6), 302. https://doi.org/10.3390/fermentation11060302





