Comparative Study of the Consumer Acceptance and Health-Promoting Properties of Yogurts Containing Coffee and Wine-Making Byproduct Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Byproduct Extracts
2.1.1. Laboratory Prepared Extracts
2.1.2. Commercial Extracts
2.2. Food Samples
2.2.1. Yogurt Samples
2.2.2. Yogurt Digests
2.2.3. Sample Preparation
2.3. Chemical Characterization of Byproduct Extracts and Yogurts
2.3.1. Total Phenolic Content (TPC)
2.3.2. Total Anthocyanin Content
2.3.3. Capillary Zone Electrophoresis (CZE)
2.4. Health-Promoting Properties of Byproduct Extracts and Yogurts
2.4.1. Antioxidant Properties
- 1,1-Diphenyl-2-picryl-hydrazyl (DPPH) assay
- 2,2′-azinobis-(3-ethylbenzothiazoline 6-sulfonic acid) (ABTS) assay
- Oxygen Radical Absorbance Capacity (ORAC) assay
- Physiological intracellular reactive oxygen species (ROS)
2.4.2. Antidiabetic Properties
2.4.3. Anti-Inflammatory Properties
2.5. Consumer Acceptance Analysis
2.6. Statistical Analysis
3. Results
3.1. Chemical Characterization of Yogurts
3.2. Health-Promoting Properties of Yogurts
3.2.1. Antioxidant Properties
3.2.2. Antidiabetic Properties
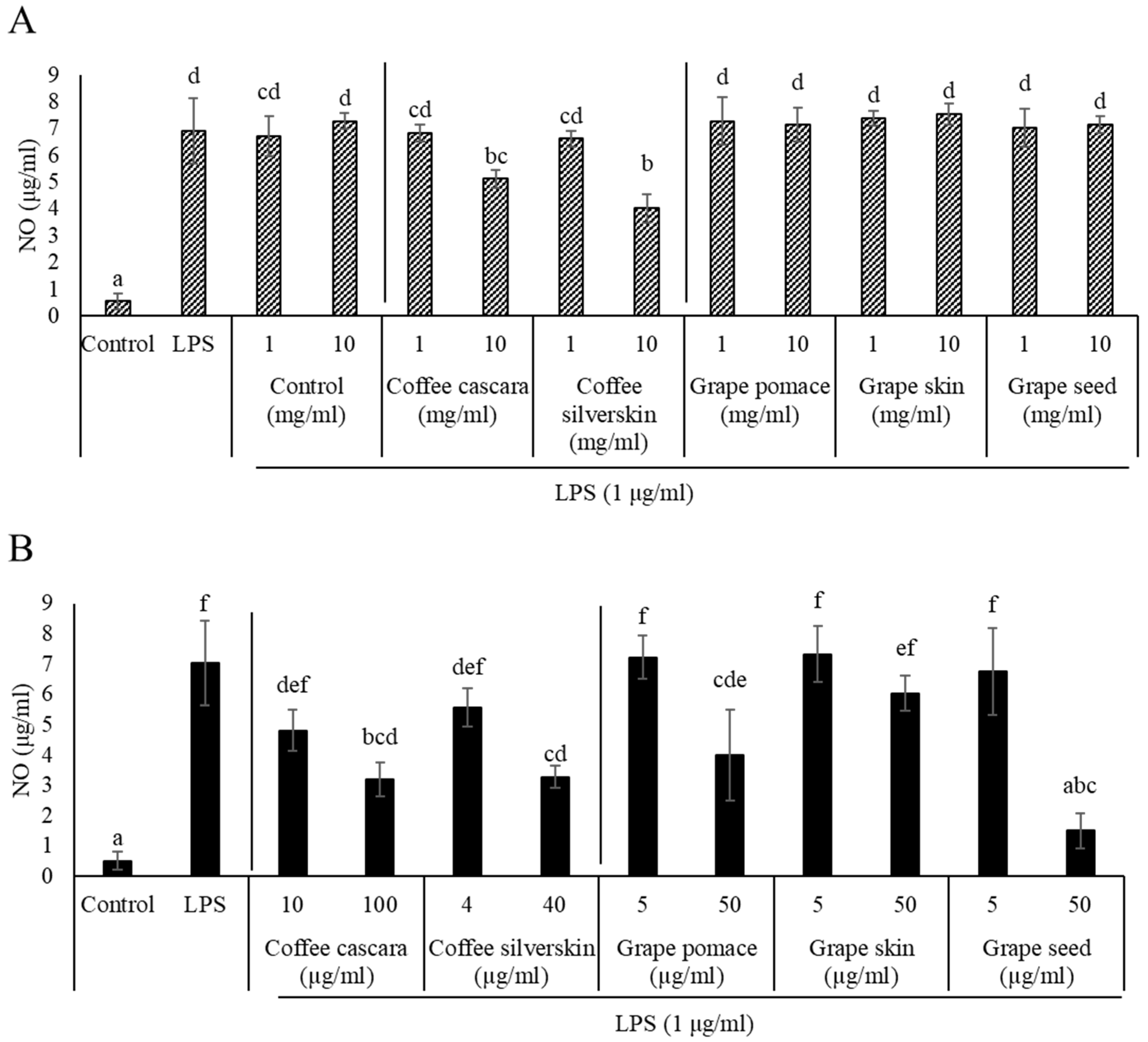
3.2.3. Anti-Inflammatory Properties
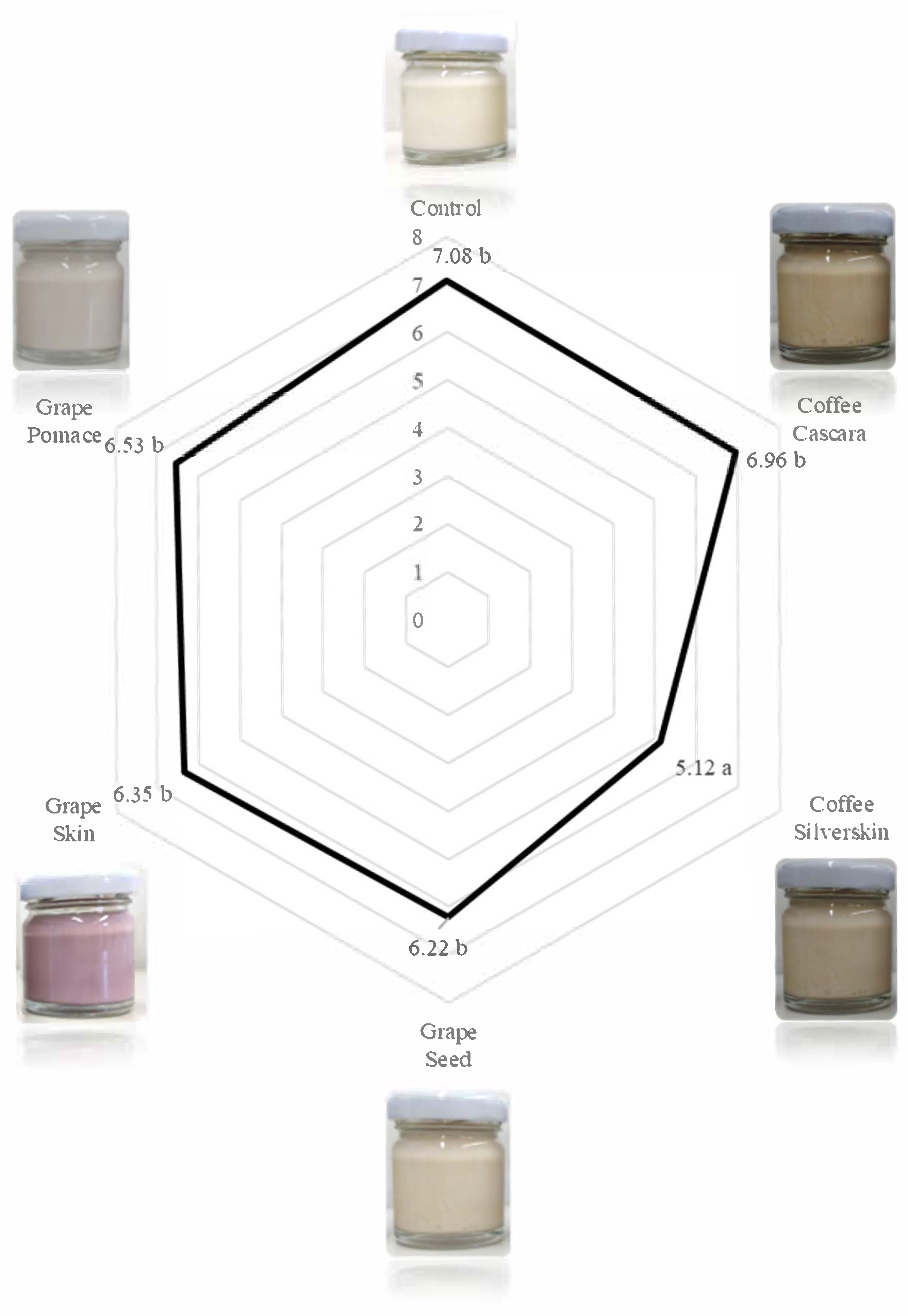
3.3. Consumer Analysis of Yogurts
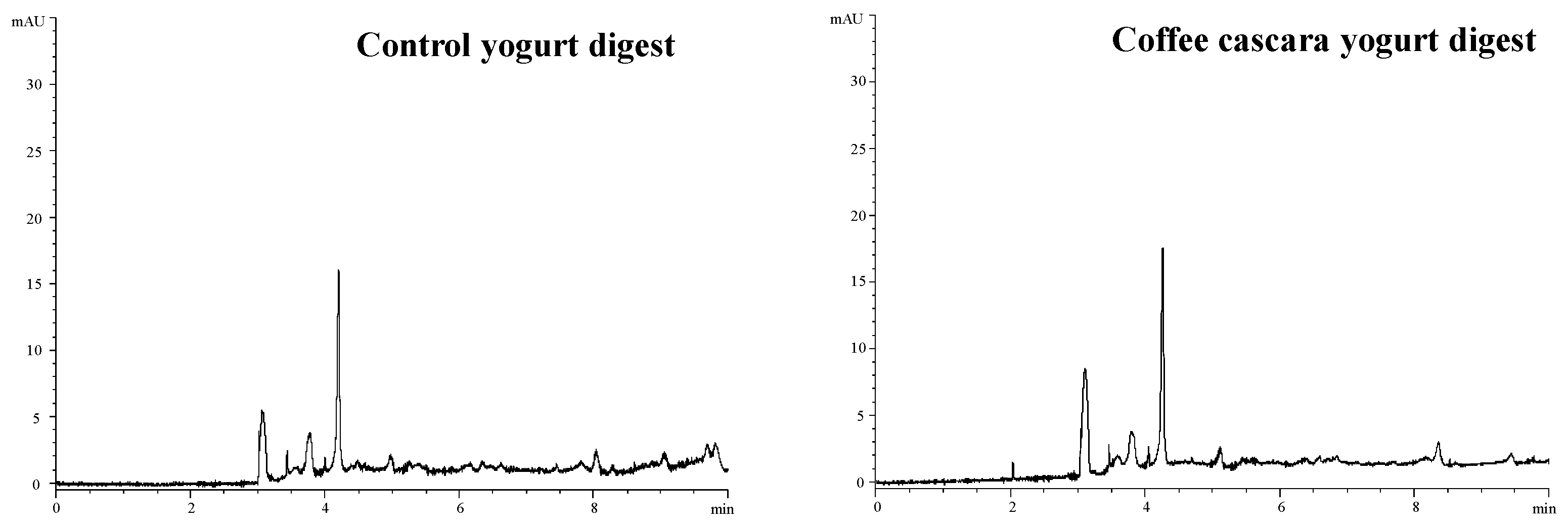
3.4. In Vitro Oral Gastrointestinal Digestion of Coffee Cascara Yogurt
3.4.1. Bioaccessible Compounds in Cascara Yogurt Digests
3.4.2. Antioxidant, Antidiabetic, and Anti-Inflammatory Properties of Bioaccessible Compounds in Cascara Yogurt Digests
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, I.; Hao, M.; Li, Y.; Zhang, J.; Ding, Y.; Lyu, F. Fortification of Yogurt with Bioactive Functional Foods and Ingredients and Associated Challenges—A Review. Trends Food Sci. Technol. 2022, 129, 558–580. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations: FAOSTAT. Available online: http://www.fao.org/faostat (accessed on 25 February 2025).
- Iriondo-DeHond, A.; Aparicio García, N.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Velázquez Escobar, F.; Blanch, G.P.; San Andres, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Validation of Coffee By-Products as Novel Food Ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef]
- Dwyer, K.; Hosseinian, F.; Rod, M. The Market Potential of Grape Waste Alternatives. J. Food Res. 2014, 3, 91. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2022/47 of 13 January 2022 Authorising the Placing on the Market of Coffea arabica L. and/or Coffea canephora Pierre Ex A. Froehner Dried Cherry Pulp and Its Infusion as a Traditional Food from a Third Country under Regulation (EU) 2015/2283 of the European Parliament and of the Council and Amending Commission Implementing Regulation (EU) 2017/2470. Off. J. Eur. Union 2022, 9, 29–32. [Google Scholar]
- EFSA Technical Report on the Notification of Dried Cherry Pulp from Coffea arabica L. and Coffea canephora Pierre Ex A. Froehner as a Traditional Food from a Third Country Pursuant to Article 14 of Regulation (EU) 2015/2283. EFSA Support. Publ. 2021, 18, 6808E. [CrossRef]
- López-Parra, M.B.; Gómez-Domínguez, I.; Iriondo-DeHond, M.; Villamediana Merino, E.; Sánchez-Martín, V.; Mendiola, J.A.; Iriondo-DeHond, A.; del Castillo, M.D. The Impact of the Drying Process on the Antioxidant and Anti-Inflammatory Potential of Dried Ripe Coffee Cherry Pulp Soluble Powder. Foods 2024, 13, 1114. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Iriondo-DeHond, M.; del Castillo, M.D. Applications of Compounds from Coffee Processing By-Products. Biomolecules 2020, 10, 1219. [Google Scholar] [CrossRef]
- Iriondo-DeHond, M.; Iriondo-DeHond, A.; Herrera, T.; Fernández-Fernández, A.M.; Sorzano, C.O.S.; Miguel, E.; del Castillo, M.D. Sensory Acceptance, Appetite Control and Gastrointestinal Tolerance of Yogurts Containing Coffee-Cascara Extract and Inulin. Nutrients 2020, 12, 627. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Rios, M.B.; Herrera, T.; Rodriguez-Bertos, A.; Nuñez, F.; San Andres, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Coffee Silverskin Extract: Nutritional Value, Safety and Effect on Key Biological Functions. Nutrients 2019, 11, 2693. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Haza, A.I.; Ávalos, A.; del Castillo, M.D.; Morales, P. Validation of Coffee Silverskin Extract as a Food Ingredient by the Analysis of Cytotoxicity and Genotoxicity. Food Res. Int. 2017, 100, 791–797. [Google Scholar] [CrossRef]
- Bertolino, M.; Barbosa-Pereira, L.; Ghirardello, D.; Botta, C.; Rolle, L.; Guglielmetti, A.; Borotto Dalla Vecchia, S.; Zeppa, G. Coffee Silverskin as Nutraceutical Ingredient in Yogurt: Its Effect on Functional Properties and Its Bioaccessibility. J. Sci. Food Agric. 2019, 99, 4267–4275. [Google Scholar] [CrossRef] [PubMed]
- Muzaifa, M.; Rahmi, F. Syarifudin Utilization of Coffee By-Products as Profitable Foods—A Mini Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 672, 012077. [Google Scholar] [CrossRef]
- Osorio-Arias, J.; Pérez-Martínez, A.; Vega-Castro, O.; Martínez-Monteagudo, S.I. Rheological, Texture, Structural, and Functional Properties of Greek-Style Yogurt Fortified with Cheese Whey-Spent Coffee Ground Powder. LWT 2020, 129, 109523. [Google Scholar] [CrossRef]
- Chomphoosee, T.; Seesuriyachan, P.; Wattanutchariya, W.; Tipbunjong, C.; Therdtatha, P.; Techapun, C.; Insomphun, C.; Panti, N.; Moukamnerd, C. A Novel Beverage of Coffee Cherry (Cascara) Water Kefir Rich in Antioxidants, Bioactive Compounds, and Exhibiting Promising Antibacterial and Sensory Qualities. LWT 2025, 219, 117539. [Google Scholar] [CrossRef]
- Arya, S.S.; Venkatram, R.; More, P. The Wastes of Coffee Bean Processing for Utilization in Food: A Review. J. Food Sci. Technol. 2022, 59, 429–444. [Google Scholar] [CrossRef]
- Hu, S.; Gil-Ramírez, A.; Martín-Trueba, M.; Benitez, V.; Aguilera-Gutiérrez, Y.; Martín-Cabrejas, M.A. Valorization of Coffee Pulp as Bioactive Food Ingredient by Sustainable Extraction Methodologies. Curr. Res. Food Sci. 2023, 6, 100475. [Google Scholar] [CrossRef]
- Iriondo-DeHond, M.; Blázquez-Duff, J.M.; del Castillo, M.D.; Miguel, E. Nutritional Quality, Sensory Analysis and Shelf Life Stability of Yogurts Containing Inulin-Type Fructans and Winery Byproducts for Sustainable Health. Foods 2020, 9, 1199. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Silva, P. The Wine Industry By-Products: Applications for Food Industry and Health Benefits. Antioxidants 2022, 11, 2025. [Google Scholar] [CrossRef]
- Almanza-Oliveros, A.; Bautista-Hernández, I.; Castro-López, C.; Aguilar-Zárate, P.; Meza-Carranco, Z.; Rojas, R.; Michel, M.R.; Martínez-Ávila, G.C.G. Grape Pomace—Advances in Its Bioactivity, Health Benefits, and Food Applications. Foods 2024, 13, 580. [Google Scholar] [CrossRef]
- Kurćubić, V.S.; Stanišić, N.; Stajić, S.B.; Dmitrić, M.; Živković, S.; Kurćubić, L.V.; Živković, V.; Jakovljević, V.; Mašković, P.Z.; Mašković, J. Valorizing Grape Pomace: A Review of Applications, Nutritional Benefits, and Potential in Functional Food Development. Foods 2024, 13, 4169. [Google Scholar] [CrossRef]
- Castillo Bilbao, M.D.; Ibáñez Ezequiel, M.E.; Amigo Benavent, M.; Herrero Calleja, M.; Plaza, M.; Ullate, M. Application of Products of Coffee Silverskin in Anti-Ageing Cosmetics and Functional Food. WO2013/004873, 10 January 2013. [Google Scholar]
- Hollebeeck, S.; Borlon, F.; Schneider, Y.-J.; Larondelle, Y.; Rogez, H. Development of a Standardised Human in Vitro Digestion Protocol Based on Macronutrient Digestion Using Response Surface Methodology. Food Chem. 2013, 138, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- Marchiani, R.; Bertolino, M.; Belviso, S.; Giordano, M.; Ghirardello, D.; Torri, L.; Piochi, M.; Zeppa, G. Yogurt Enrichment with Grape Pomace: Effect of Grape Cultivar on Physicochemical, Microbiological and Sensory Properties. J. Food Qual. 2016, 39, 77–89. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Del Castillo, M.D.; Ames, J.M.; Gordon, M.H. Effect of Roasting on the Antioxidant Activity of Coffee Brews. J. Agric. Food Chem. 2002, 50, 3698–3703. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oki, T.; Nagai, S.; Yoshinaga, M.; Nishiba, Y.; Suda, I. Contribution of B-Carotene to Radical Scavenging Capacity Varies among Orange-Fleshed Sweet Potato Cultivars. Food Sci. Technol. Res. 2006, 12, 156–160. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-woodill, M.; Prior, R.L.; Laboratories, B.; Lane, T. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Dávalos, A.; Bartolomé, B.; Gómez-Cordovés, C. Antioxidant Properties of Commercial Grape Juices and Vinegars. Food Chem. 2005, 93, 325–330. [Google Scholar] [CrossRef]
- Bakondi, E. Role of Intracellular Calcium Mobilization and Cell-Density- Dependent Signaling in Oxidative-Stress-Induced Cytotoxicity in HaCaT Keratinocytes. J. Investig. Dermatol. 2003, 121, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, K.; Delmotte, F.M. Purification and Characterization of an Alpha-Glucosidase from Rhizobium Sp. (Robinia pseudoacacia L.) Strain USDA 4280. Appl. Environ. Microbiol. 1999, 65, 2907–2911. [Google Scholar] [CrossRef]
- Geddes, R.; Taylor, J.A. Lysosomal Glycogen Storage Induced by Acarbose, a 1,4-Alpha-Glucosidase Inhibitor. Biochem. J. 1985, 228, 319–324. [Google Scholar] [CrossRef]
- Benayad, Z.; Martinez-Villaluenga, C.; Frias, J.; Gomez-Cordoves, C.; Es-Safi, N.E. Phenolic Composition, Antioxidant and Anti-Inflammatory Activities of Extracts from Moroccan Opuntia Ficus-Indica Flowers Obtained by Different Extraction Methods. Ind. Crops Prod. 2014, 62, 412–420. [Google Scholar] [CrossRef]
- Helal, A.; Cattivelli, A.; Conte, A.; Tagliazucchi, D. In Vitro Bioaccessibility and Antioxidant Activity of Phenolic Compounds in Coffee-Fortified Yogurt. Molecules 2022, 27, 6843. [Google Scholar] [CrossRef]
- Karastergiou, A.; Gancel, A.-L.; Jourdes, M.; Teissedre, P.-L. Valorization of Grape Pomace: A Review of Phenolic Composition, Bioactivity, and Therapeutic Potential. Antioxidants 2024, 13, 1131. [Google Scholar] [CrossRef]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food Phenolics and Lactic Acid Bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef]
- Ozturk, T.; Ávila-Gálvez, M.Á.; Mercier, S.; Vallejo, F.; Bred, A.; Fraisse, D.; Morand, C.; Pelvan, E.; Monfoulet, L.-E.; González-Sarrías, A. Impact of Lactic Acid Bacteria Fermentation on (Poly)Phenolic Profile and In Vitro Antioxidant and Anti-Inflammatory Properties of Herbal Infusions. Antioxidants 2024, 13, 562. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 9456. [Google Scholar] [CrossRef]
- Gaur, G.; Gänzle, M.G. Conversion of (Poly)Phenolic Compounds in Food Fermentations by Lactic Acid Bacteria: Novel Insights into Metabolic Pathways and Functional Metabolites. Curr. Res. Food Sci. 2023, 6, 100448. [Google Scholar] [CrossRef]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Pintado, M. Stability of Polyphenols and Carotenoids in Strawberry and Peach Yoghurt throughout in Vitro Gastrointestinal Digestion. Food Funct. 2015, 6, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Tosif, M.M.; Najda, A.; Bains, A.; Krishna, T.C.; Chawla, P.; Dyduch-Siemińska, M.; Klepacka, J.; Kaushik, R. A Comprehensive Review on the Interaction of Milk Protein Concentrates with Plant-Based Polyphenolics. Int. J. Mol. Sci. 2021, 22, 13548. [Google Scholar] [CrossRef]
- van de Langerijt, T.M.; O’Mahony, J.A.; Crowley, S.V. Structural, Binding and Functional Properties of Milk Protein-Polyphenol Systems: A Review. Molecules 2023, 28, 2288. [Google Scholar] [CrossRef]
- Mao, T.; Akshit, F.; Matiwalage, I.; Sasidharan, S.; Alvarez, C.M.; Wescombe, P.; Mohan, M.S. Preferential Binding of Polyphenols in Blackcurrant Extracts with Milk Proteins and the Effects on the Bioaccessibility and Antioxidant Activity of Polyphenols. Foods 2024, 13, 515. [Google Scholar] [CrossRef]
- Dubeau, S.; Samson, G.; Tajmir-Riahi, H.-A. Dual Effect of Milk on the Antioxidant Capacity of Green, Darjeeling, and English Breakfast Teas. Food Chem. 2010, 122, 539–545. [Google Scholar] [CrossRef]
- Oliveira, A.; Alexandre, E.M.C.; Coelho, M.; Lopes, C.; Almeida, D.P.F.; Pintado, M. Incorporation of Strawberries Preparation in Yoghurt: Impact on Phytochemicals and Milk Proteins. Food Chem. 2015, 171, 370–378. [Google Scholar] [CrossRef]
- Belščak, A.; Komes, D.; Horžić, D.; Ganić, K.K.; Karlović, D. Comparative Study of Commercially Available Cocoa Products in Terms of Their Bioactive Composition. Food Res. Int. 2009, 42, 707–716. [Google Scholar] [CrossRef]
- Zhang, L.; Hogan, S.; Li, J.; Sun, S.; Canning, C.; Zheng, S.J.; Zhou, K. Grape Skin Extract Inhibits Mammalian Intestinal α-Glucosidase Activity and Suppresses Postprandial Glycemic Response in Streptozocin-Treated Mice. Food Chem. 2011, 126, 466–471. [Google Scholar] [CrossRef]
- Shori, A.B.; Baba, A.S. Cinnamomum Verum Improved the Functional Properties of Bioyogurts Made from Camel and Cow Milks. J. Saudi Soc. Agric. Sci. 2011, 10, 101–107. [Google Scholar] [CrossRef]
- Shori, A.B.; Baba, A.S. Comparative Antioxidant Activity, Proteolysis and in Vitro α-Amylase and α-Glucosidase Inhibition of Allium Sativum-Yogurts Made from Cow and Camel Milk. J. Saudi Chem. Soc. 2014, 18, 456–463. [Google Scholar] [CrossRef]
- Shori, A.B.; Rashid, F.; Baba, A.S. Effect of the Addition of Phytomix-3+ Mangosteen on Antioxidant Activity, Viability of Lactic Acid Bacteria, Type 2 Diabetes Key-Enzymes, and Sensory Evaluation of Yogurt. LWT 2018, 94, 33–39. [Google Scholar] [CrossRef]
- del Castillo, M.D.; Fernandez-Gomez, B.; Ullate, M.; Mesa, M.D. Uso de Productos de La Cascarilla de Café Para La Prevención y Tratamiento de Las Patologías Que Conforman El Síndrome Metabólico y de Sus Factores de Riesgo. WO/2016/097450, 23 June 2016. [Google Scholar]
- Ma, C.-M.; Hattori, M.; Daneshtalab, M.; Wang, L. Chlorogenic Acid Derivatives with Alkyl Chains of Different Lengths and Orientations: Potent α-Glucosidase Inhibitors. J. Med. Chem. 2008, 51, 6188–6194. [Google Scholar] [CrossRef]
- Rasouli, H.; Hosseini-Ghazvini, S.M.-B.; Adibi, H.; Khodarahmi, R. Differential α-Amylase/α-Glucosidase Inhibitory Activities of Plant-Derived Phenolic Compounds: A Virtual Screening Perspective for the Treatment of Obesity and Diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef]
- Garrido, J.; Borges, F. Wine and Grape Polyphenols—A Chemical Perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Magoni, C.; Bruni, I.; Guzzetti, L.; Dell’Agli, M.; Sangiovanni, E.; Piazza, S.; Regonesi, M.E.; Maldini, M.; Spezzano, R.; Caruso, D.; et al. Valorizing Coffee Pulp By-Products as Anti-Inflammatory Ingredient of Food Supplements Acting on IL-8 Release. Food Res. Int. 2018, 112, 129–135. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-Inflammatory Effects of Chlorogenic Acid in Lipopolysaccharide-Stimulated RAW 264.7 Cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Kim, K.-J.; Ryu, S.-J.; Lee, B.-Y. Caffeine Prevents LPS-Induced Inflammatory Responses in RAW264.7 Cells and Zebrafish. Chem. Biol. Interact. 2016, 248, 1–7. [Google Scholar] [CrossRef]
- Rodríguez-Morgado, B.; Candiracci, M.; Santa-María, C.; Revilla, E.; Gordillo, B.; Parrado, J.; Castaño, A. Obtaining from Grape Pomace an Enzymatic Extract with Anti-Inflammatory Properties. Plant Foods Hum. Nutr. 2015, 70, 42–49. [Google Scholar] [CrossRef]
- Bak, M.-J.; Truong, V.L.; Kang, H.-S.; Jun, M.; Jeong, W.-S. Anti-Inflammatory Effect of Procyanidins from Wild Grape ( Vitis Amurensis ) Seeds in LPS-Induced RAW 264.7 Cells. Oxid. Med. Cell. Longev. 2013, 2013, 409321. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.; Ruiz del Castillo, M.L.; Gil, C.; Blanch, G.P.; Flores, G. Supercritical Fluid Extraction of Grape Seeds: Extract Chemical Composition, Antioxidant Activity and Inhibition of Nitrite Production in LPS-Stimulated Raw 264.7 Cells. Food Funct. 2015, 6, 2607–2613. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Helal, A.; Verzelloni, E.; Bellesia, A.; Conte, A. Composition and Properties of Peptides That Survive Standardised in Vitro Gastro-Pancreatic Digestion of Bovine Milk. Int. Dairy J. 2016, 61, 196–204. [Google Scholar] [CrossRef]
- Helal, A.; Tagliazucchi, D. Impact of In-Vitro Gastro-Pancreatic Digestion on Polyphenols and Cinnamaldehyde Bioaccessibility and Antioxidant Activity in Stirred Cinnamon-Fortified Yogurt. LWT 2018, 89, 164–170. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 432/2012 Establishing a List of Permitted Health Claims Made on Foods, Other than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. Off. J. Eur. Union 2012, 7, 162. [Google Scholar]
- Meyer, D.; Bayarri, S.; Tárrega, A.; Costell, E. Inulin as Texture Modifier in Dairy Products. Food Hydrocoll. 2011, 25, 1881–1890. [Google Scholar] [CrossRef]
- Aryana, K.J.; McGrew, P. Quality Attributes of Yogurt with Lactobacillus Casei and Various Prebiotics. LWT—Food Sci. Technol. 2007, 40, 1808–1814. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Eisner, P. How Does the Phenol Structure Influence the Results of the Folin-Ciocalteu Assay? Antioxidants 2021, 10, 811. [Google Scholar] [CrossRef]
- Cañas, S.; Rebollo-Hernanz, M.; Braojos, C.; Benítez, V.; Ferreras-Charro, R.; Dueñas, M.; Aguilera, Y.; Martín-Cabrejas, M.A. Understanding the Gastrointestinal Behavior of the Coffee Pulp Phenolic Compounds under Simulated Conditions. Antioxidants 2022, 11, 1818. [Google Scholar] [CrossRef]
- EFSA Scientific Opinion on the Safety of Caffeine. EFSA J. 2015, 13, 4102. [CrossRef]
- Sereshti, H.; Samadi, S. A Rapid and Simple Determination of Caffeine in Teas, Coffees and Eight Beverages. Food Chem. 2014, 158, 8–13. [Google Scholar] [CrossRef]
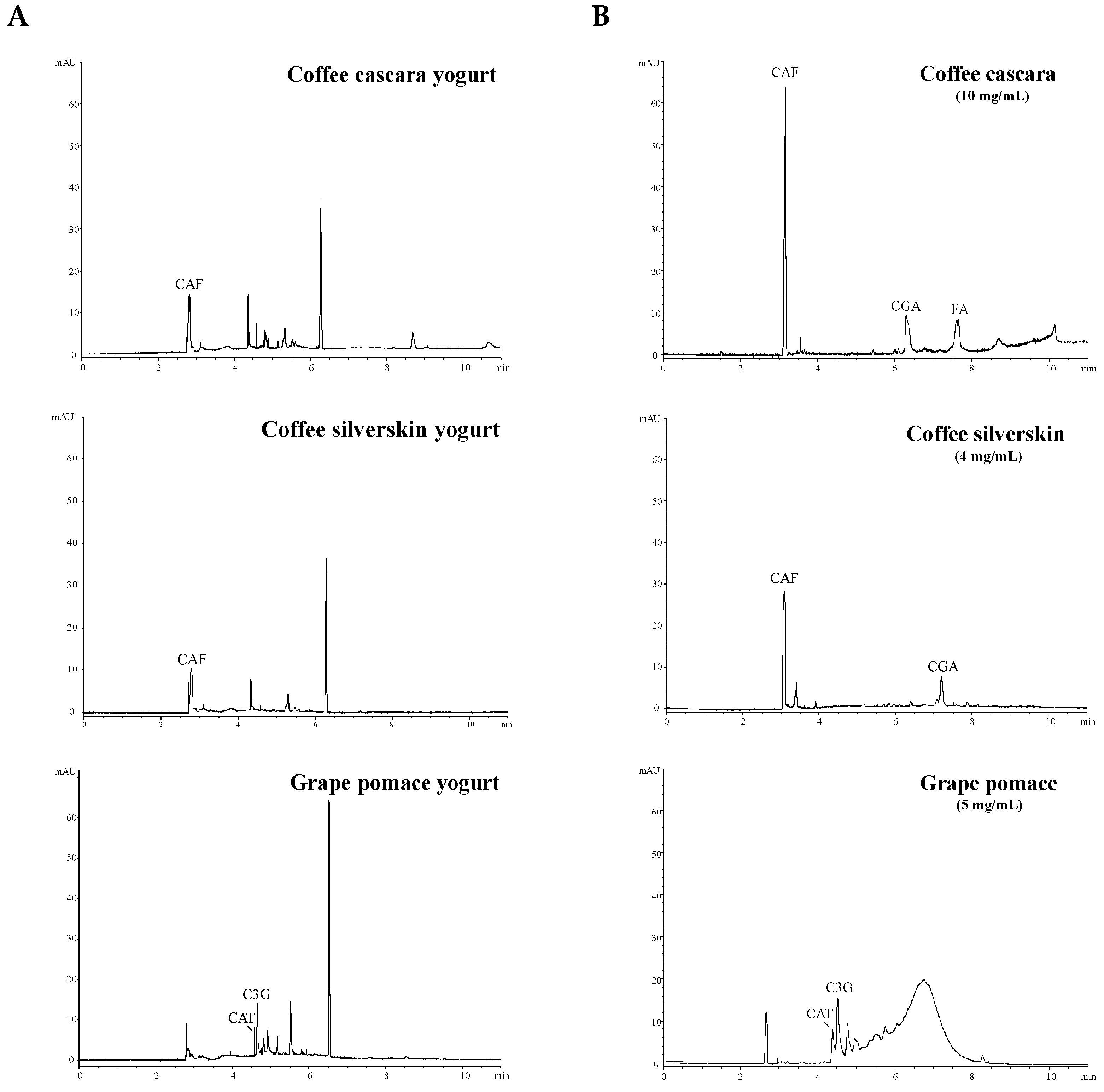
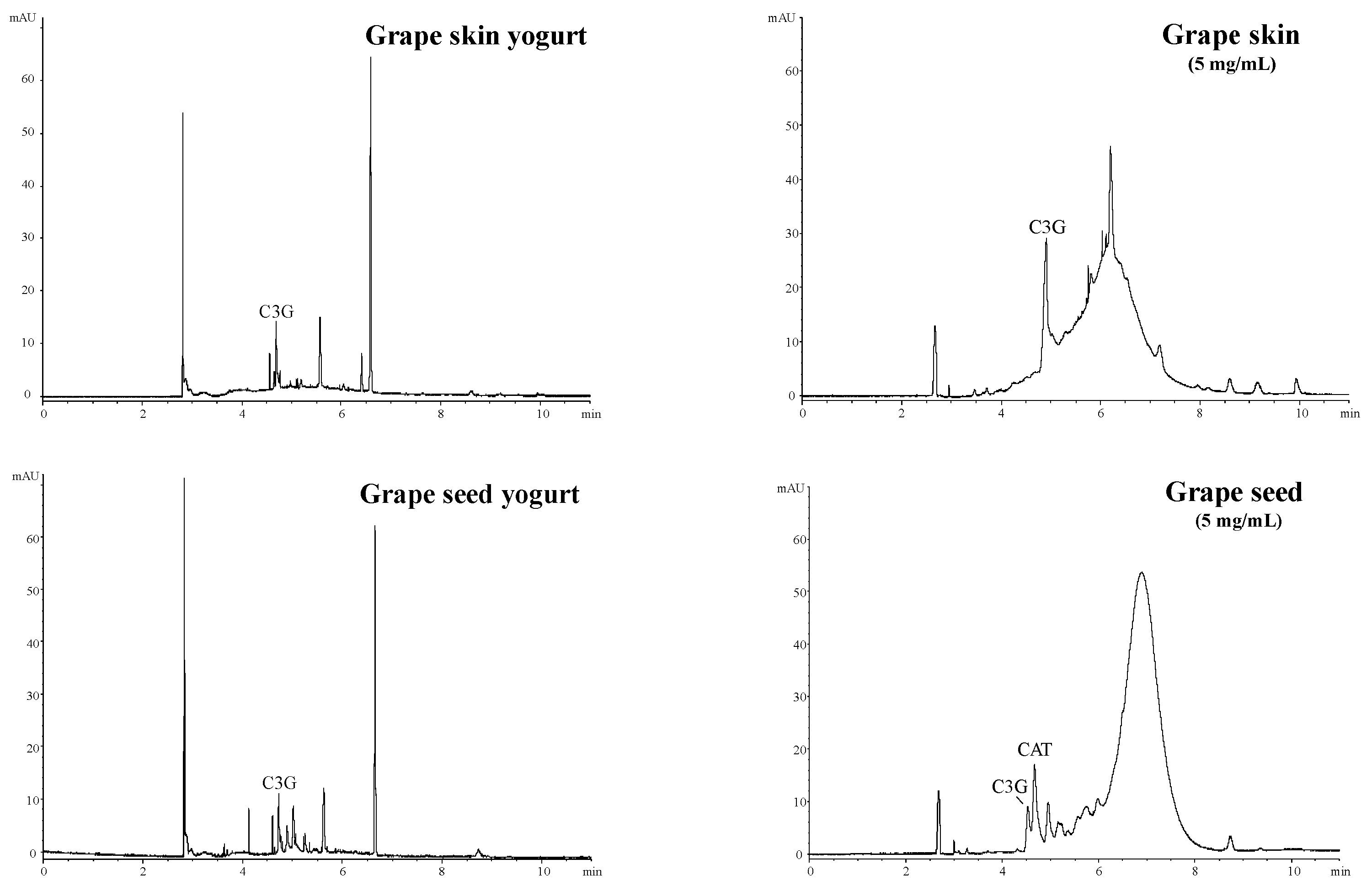
| Type of Yogurt | Ingredients * (%) | Total Phenolic Content Incorporated (mg GAE/100 mL) |
|---|---|---|
| Yogurt control | Milk (85) + SC + inulin (6) + water (10) | |
| Yogurt coffee cascara | Milk (85) + SC + inulin (6) + coffee cascara extract (100 mg/mL) (10) | 760.2 |
| Yogurt coffee silverskin | Milk (85) + SC + inulin (6) + coffee silverskin extract (40 mg/mL) (10) | 151.76 |
| Yogurt grape pomace | Milk (85) + SC + inulin (6) + grape pomace extract (50 mg/mL) (10) | 1237.55 |
| Yogurt grape skin | Milk (85) + SC + inulin (6) + grape skin extract (50 mg/mL) (10) | 1589.45 |
| Yogurt grape seed | Milk (85) + SC + inulin (6) + grape seed extract (50 mg/mL) (10) | 1964.55 |
| Coffee Byproducts | Wine-Making Byproducts | |||||
|---|---|---|---|---|---|---|
| Control | Cascara | Silverskin | Grape Pomace | Skin | Seed | |
| Chemical characterization | ||||||
| TPC (mg GAE/g yogurt) | 0.04 ± 0.01 a | 0.13 ± 0.02 c | 0.05 ± 0.00 ab | 0.10 ± 0.01 bc | 0.09 ± 0.03 bc | 0.09 ± 0.02 bc |
| Anthocyanins (C3G eq./g yogurt) | n.d. | n.d. | n.d. | n.d. | 0.04 ± 0.01 | n.d. |
| CAF (mg/g) | n.d. | 0.11 ± 0.03 a | 0.06 ± 0.01 a | n.d. | n.d. | n.d. |
| CGA (mg/g) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| FA (mg/g) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| C3G (mg/g) | n.d. | n.d. | n.d. | + | + | + |
| CAT (mg/g) | n.d. | n.d. | n.d. | + | n.d. | n.d. |
| Antioxidant capacity | ||||||
| ORAC (µmol TE/g yogurt) | 1.79 ± 0.23 a | 5.03 ± 0.11 b | 2.82 ± 0.19 a | 6.15 ± 0.36 bc | 5.24 ± 1.07 bc | 7.26 ± 1.32 c |
| ABTS (µmol TE/g yogurt) | 0.42 ± 0.04 a | 3.26 ± 0.13 c | 1.01 ± 0.29 ab | 1.63 ± 0.05 b | 1.59 ± 0.36 b | 1.67 ± 0.38 b |
| DPPH (µmol TE/g yogurt) | 0.00 ± 0.00 a | 0.75 ± 0.17 c | 0.22 ± 0.07 ab | 0.73 ± 0.12 c | 0.47 ± 0.07 b | 0.90 ± 0.09 c |
| Antidiabetic properties * | ||||||
| α-Glucosidase inhibition (%) | 41.47 ± 5.72 a | 82.87 ± 2.14 c | 64.54 ± 4.05 b | 54.11 ± 3.96 ab | 63.81 ± 5.62 b | 53.10 ± 5.16 ab |
| Appearance | Smell | Taste | Texture | |
|---|---|---|---|---|
| Control | 7.69 ± 1.26 c | 6.82 ± 1.71 b | 7.38 ± 1.44 c | 7.67 ± 1.26 a |
| Coffee cascara | 6.95 ± 1.83 abc | 6.87 ± 1.49 b | 6.82 ± 1.55 bc | 6.82 ± 1.47 a |
| Coffee silverskin | 6.59 ± 1.60 ab | 6.49 ± 1.62 ab | 4.36 ± 2.08 a | 6.72 ± 1.81 a |
| Grape seed | 5.93 ± 1.49 a | 5.44 ± 1.25 a | 6.48 ± 1.63 bc | 6.74 ± 1.43 a |
| Grape skin | 7.30 ± 1.26 bc | 5.78 ± 1.40 ab | 5.89 ± 1.99 b | 6.85 ± 1.35 a |
| Grape pomace | 5.96 ± 1.34 a | 5.96 ± 1.31 ab | 6.52 ± 1.60 bc | 7.04 ± 1.43 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iriondo-DeHond, M.; Iriondo-DeHond, A.; Herrera, T.; Miguel, E.; del Castillo, M.D. Comparative Study of the Consumer Acceptance and Health-Promoting Properties of Yogurts Containing Coffee and Wine-Making Byproduct Extracts. Fermentation 2025, 11, 291. https://doi.org/10.3390/fermentation11050291
Iriondo-DeHond M, Iriondo-DeHond A, Herrera T, Miguel E, del Castillo MD. Comparative Study of the Consumer Acceptance and Health-Promoting Properties of Yogurts Containing Coffee and Wine-Making Byproduct Extracts. Fermentation. 2025; 11(5):291. https://doi.org/10.3390/fermentation11050291
Chicago/Turabian StyleIriondo-DeHond, Maite, Amaia Iriondo-DeHond, Teresa Herrera, Eugenio Miguel, and María Dolores del Castillo. 2025. "Comparative Study of the Consumer Acceptance and Health-Promoting Properties of Yogurts Containing Coffee and Wine-Making Byproduct Extracts" Fermentation 11, no. 5: 291. https://doi.org/10.3390/fermentation11050291
APA StyleIriondo-DeHond, M., Iriondo-DeHond, A., Herrera, T., Miguel, E., & del Castillo, M. D. (2025). Comparative Study of the Consumer Acceptance and Health-Promoting Properties of Yogurts Containing Coffee and Wine-Making Byproduct Extracts. Fermentation, 11(5), 291. https://doi.org/10.3390/fermentation11050291








