Microbial Community Succession and Flavor Compound Formation in Sesame-Flavored Baijiu from Zaopei
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Analysis of Flavor Substances
2.3. Measurement of Physical and Chemical Parameters
2.4. Measurement of Amino Acids
2.5. High-Throughput Sequencing
2.6. Data Analysis
3. Results and Discussion
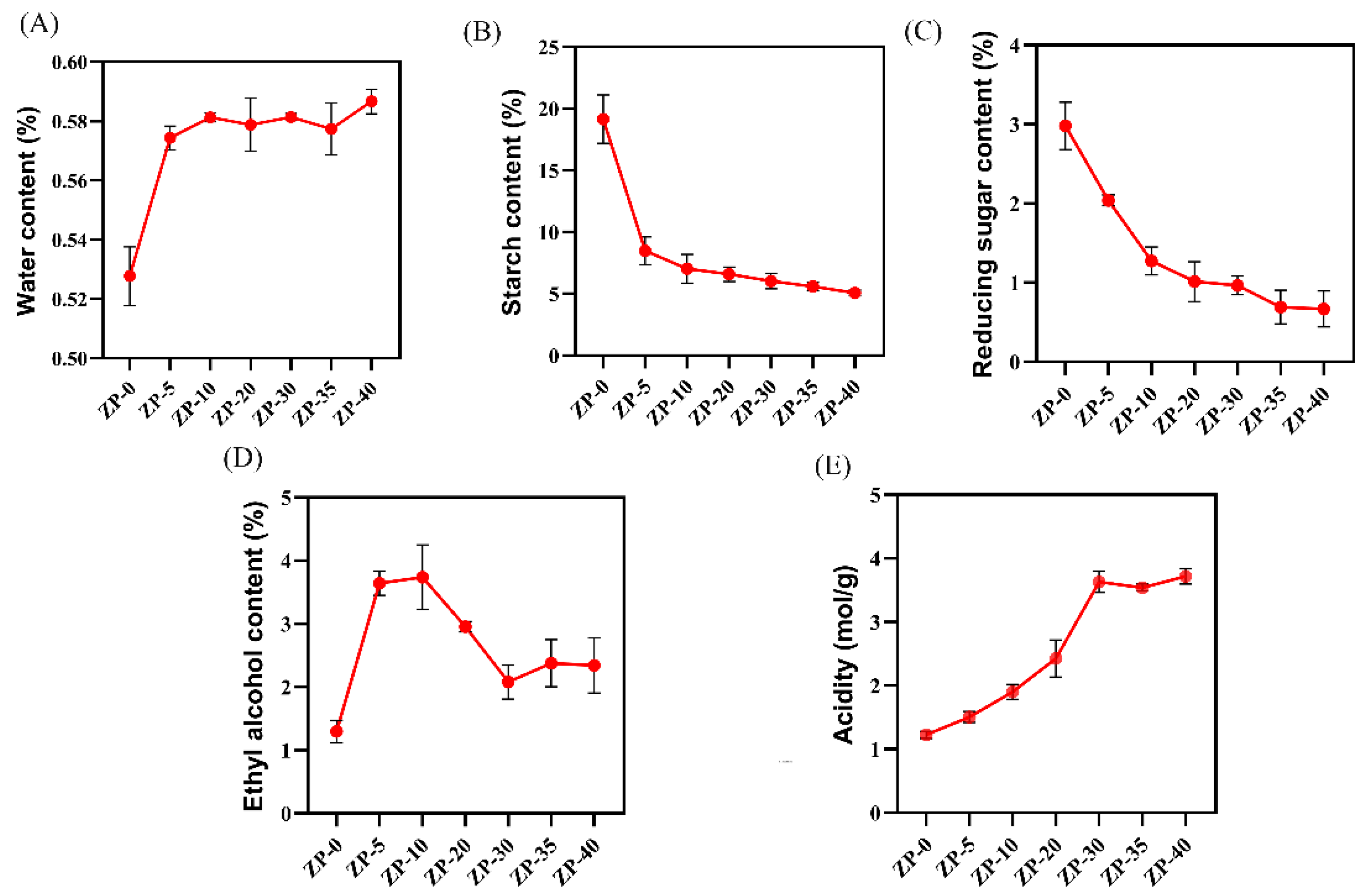
3.1. Changes in Physicochemical Properties During Zaopei Fermentation
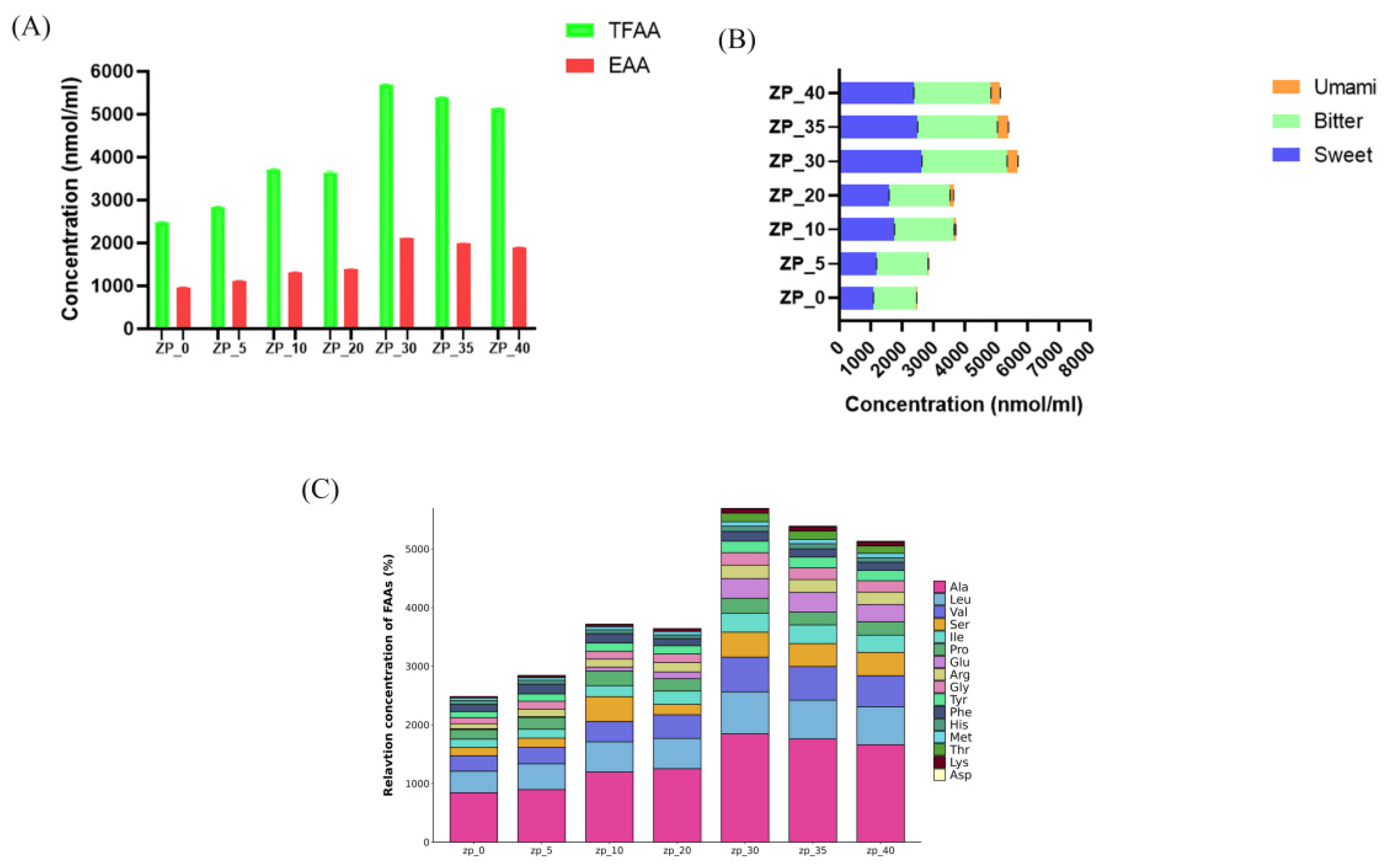
3.2. Flavor Compound Analysis in the Fermentation Process of Sesame-Flavored Zaopei
3.3. Amino Acids in the Fermentation Process of Zaopei
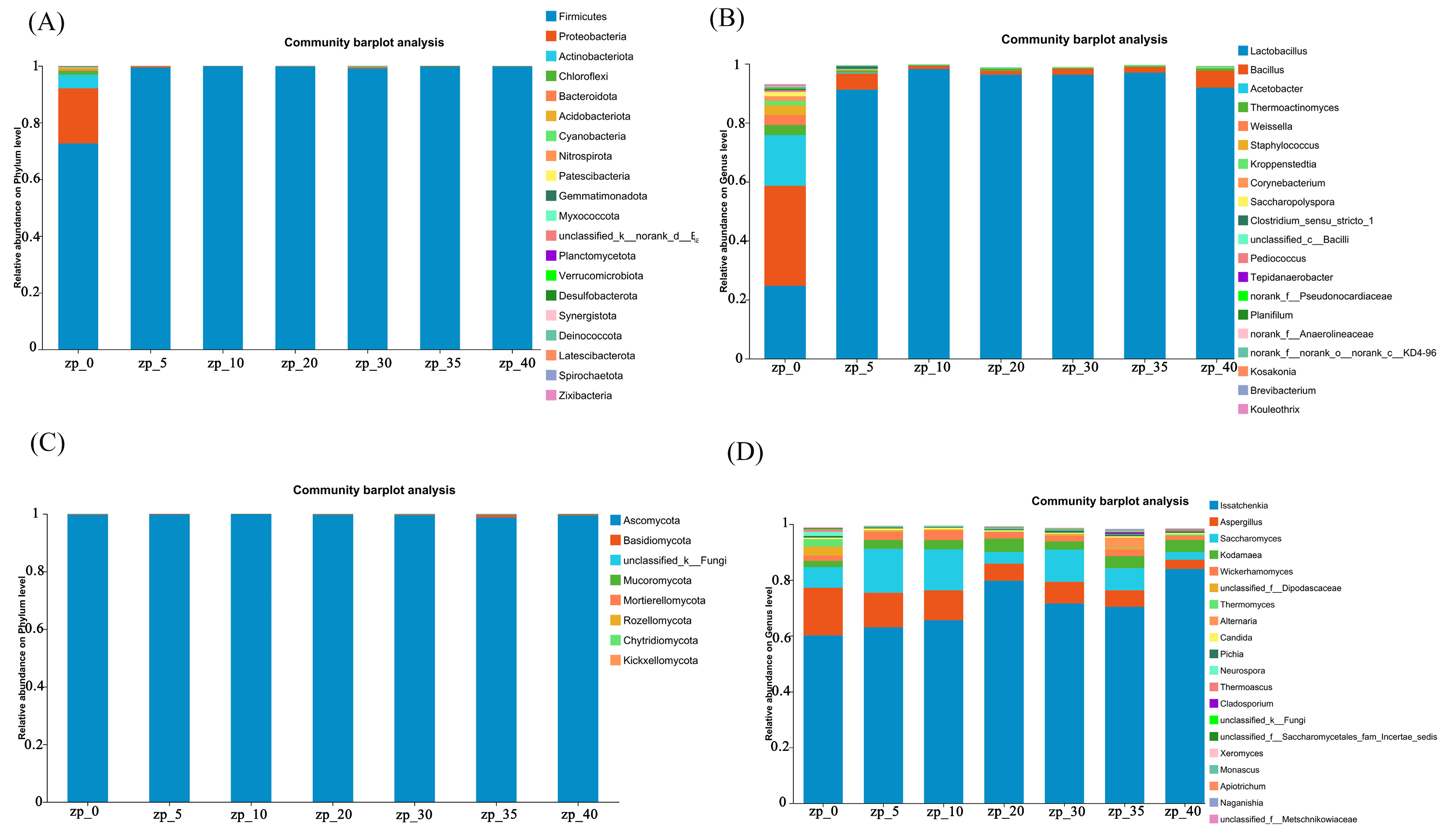
3.4. Microbial Community Composition and Differences in Zaopei Samples
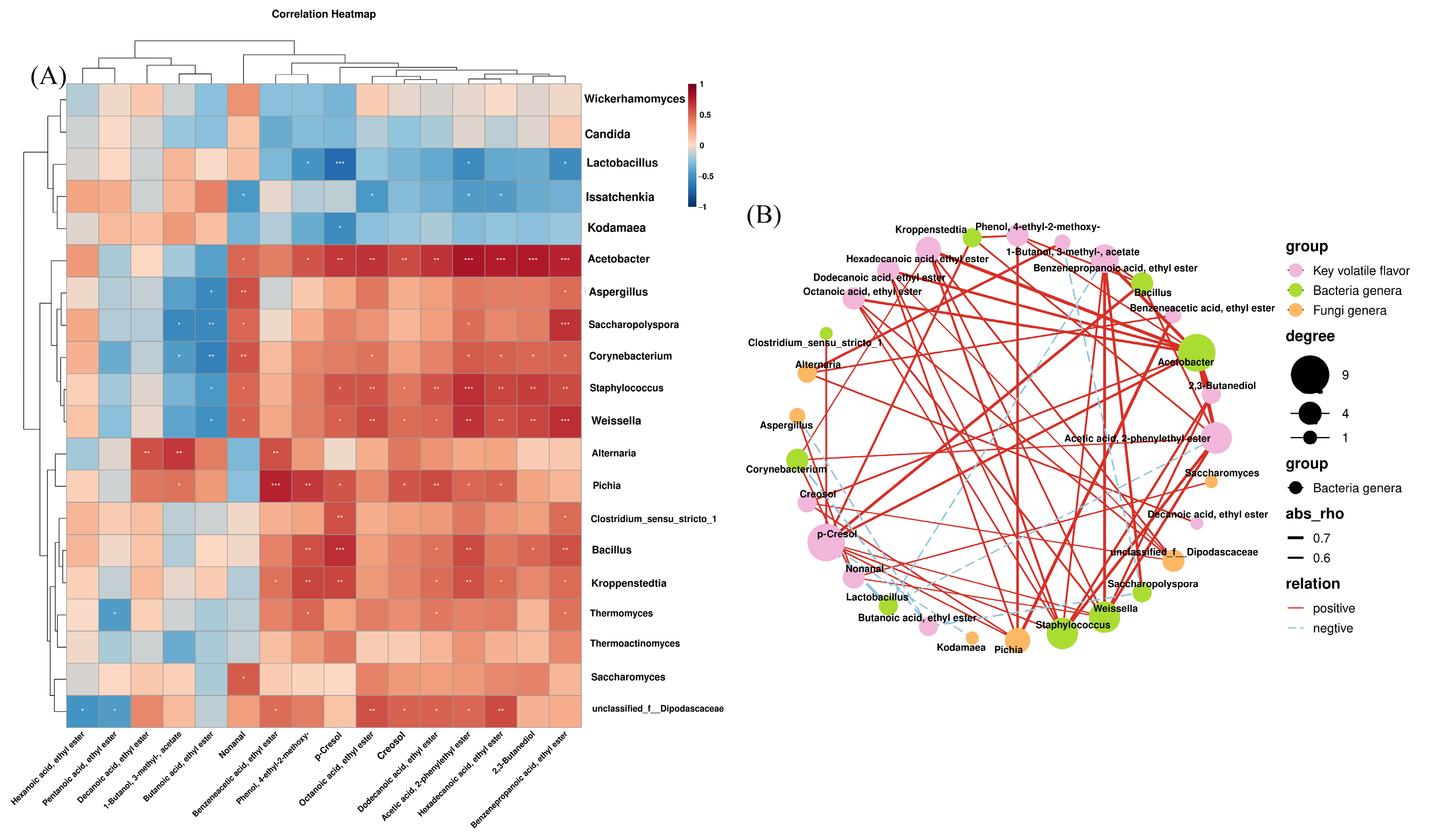
3.5. Correlation Between Microbial Communities, Physicochemical Properties, and Volatile Components
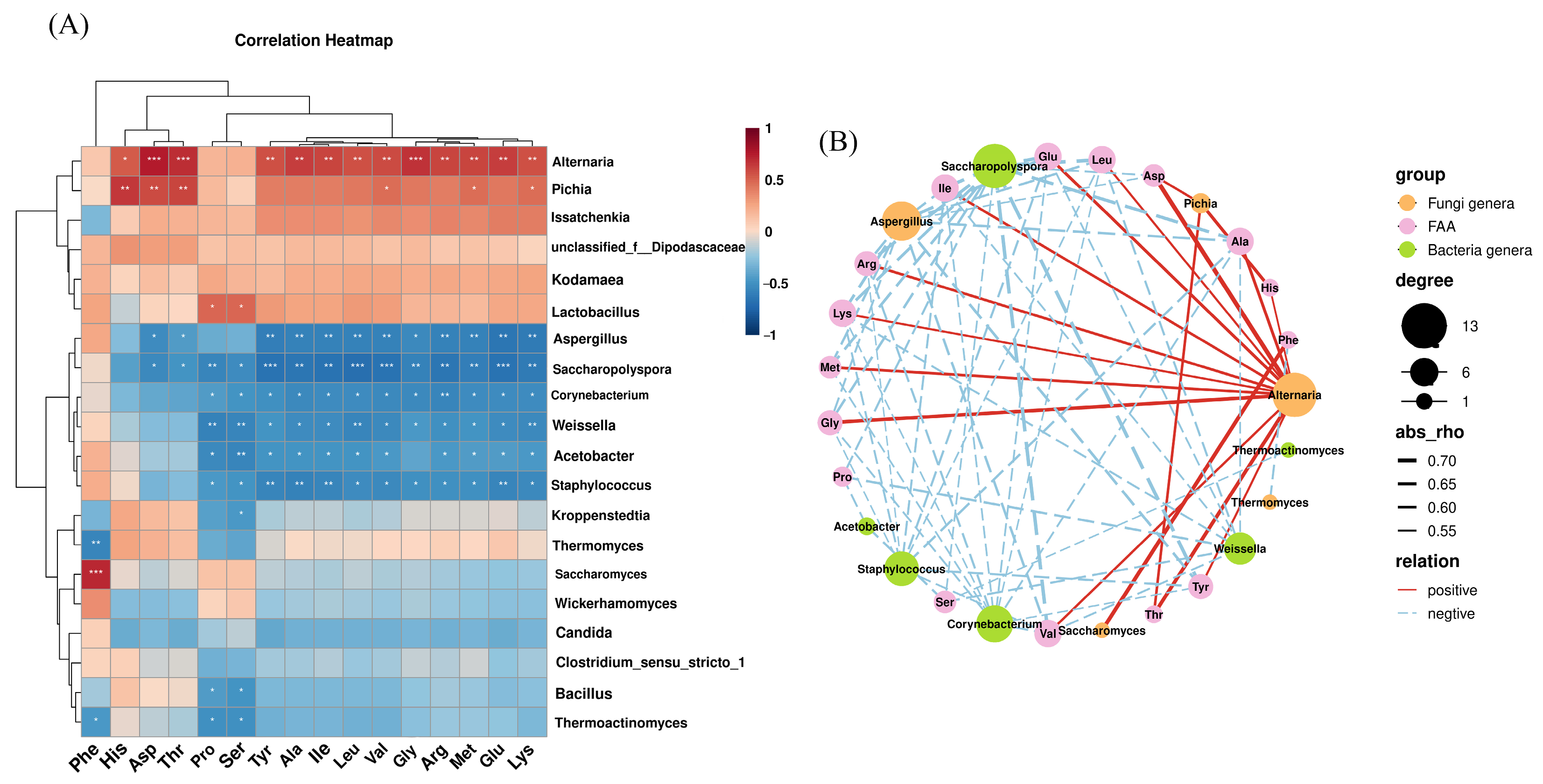
3.6. Correlation Between Bacterial and Fungal Communities and Amino Acids and Volatile Flavor Compounds
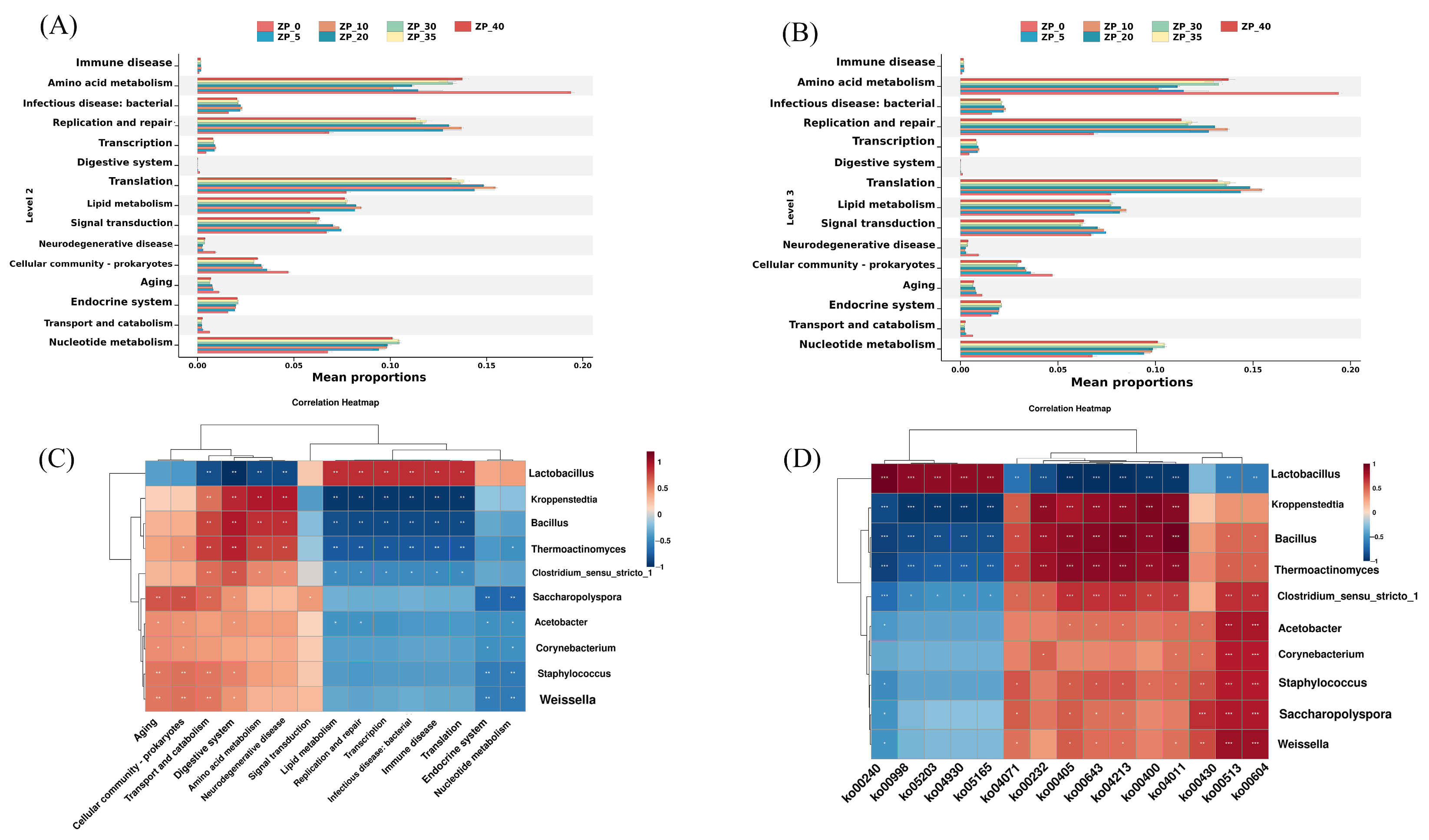
3.7. Functional Prediction of the Bacterial Community in the Fermentation Pit Using PICRUSt2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, G.Y.; Zhu, Y.; Xu, Y. Mystery behind Chinese liquor fermentation. Trends Food Sci. Technol. 2017, 63, 18–28. [Google Scholar] [CrossRef]
- Hong, J.X.; Wang, J.S.; Zhang, C.S.; Zhao, Z.G.; Tian, W.J.; Wu, Y.S.; Chen, H.; Zhao, D.R.; Sun, J.Y. Unraveling variation on the profile aroma compounds of strong aroma type of Baijiu in different regions by molecular matrix analysis and olfactory analysis. RSC Adv. 2021, 11, 34262. [Google Scholar] [CrossRef]
- Sha, S.; Chen, S.; Qian, M.; Wang, C.C.; Xu, Y. Characterization of the Typical Potent Odorants in Chinese Roasted Sesame-like Flavor Type Liquor by Headspace Solid Phase Microextraction-Aroma Extract Dilution Analysis, with Special Emphasis on Sulfur-Containing Odorants. J. Agric. Food Chem. 2017, 65, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Shen, Y.; Yang, S.; Zhang, G.; Wang, D.; Li, H.; Sun, J. Whether the research on ethanol–water microstructure in traditional baijiu should be strengthened? Molecules 2022, 27, 8290. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, G.; Xu, L.; Duan, J.; Li, H.; Sun, J.; Sun, B. Investigation on the interaction between 1,3-dimethyltrisulfide and aroma-active compounds in sesame-flavor baijiu by Feller Additive Model, Odor Activity Value and Partition Coefficient. Food Chem. 2023, 410, 135451. [Google Scholar] [CrossRef]
- Hong, J.; Tian, W.; Zhao, D. Research progress of trace components in sesame-aroma type of baijiu. Food Res. Int. 2020, 137, 109695. [Google Scholar] [CrossRef]
- Dong, W.; Guo, R.; Liu, M.; Shen, C.; Sun, X.; Zhao, M.; Sun, J.; Li, H.; Zheng, F.; Huang, M.; et al. Characterization of key odorants causing the roasted and mud-like aromas in strong-aroma types of base Baijiu. Food Res. Int. 2019, 125, 108546. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Chang, L. Effects of lactic acid bacteria fermentation on the phytochemicals content, taste and aroma of blended edible rose and shiitake beverage. Food Chem. 2023, 405, 134722. [Google Scholar] [CrossRef]
- Xu, S.S.; Zhang, M.Z.; Xu, B.Y.; Liu, L.H.; Sun, W.; Mu, D.D.; Wu, X.F.; Li, X.J. Microbial communities and flavor formation in the fermentation of Chinese strong-flavor Baijiu produced from old and new Zaopei. Food Res. Int. 2022, 156, 111162. [Google Scholar] [CrossRef]
- Guan, Q.Q.; Zheng, W.D.; Mo, J.L.; Huang, T.; Xiao, Y.S.; Liu, Z.G.; Peng, Z.; Xie, M.Y.; Xiong, T. Evaluation and comparison of the microbial communities and volatile profiles in homemade suansun from Guangdong and Yunnan provinces in China. J. Sci. Food Agric. 2020, 100, 5197–5206. [Google Scholar] [CrossRef]
- Guan, T.W.; Lin, Y.J.; Chen, K.B.; Ou, M.Y.; Zhang, J.X. Physicochemical Factors Affecting Microbiota Dynamics During Traditional Solid-State Fermentation of Chinese Strong-Flavor Baijiu. Front. Microbiol. 2020, 11, 02090. [Google Scholar] [CrossRef]
- Song, Z.W.; Du, H.; Zhang, Y.; Xu, Y. Unraveling Core Functional Microbiota in Traditional Solid-State Fermentation by High-Throughput Amplicons and Metatranscriptomics Sequencing. Front. Microbiol. 2017, 8, 01294. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, B.G.; Zhao, M.M.; Zheng, F.P.; Huang, M.Q.; Sun, J.Y.; Sun, X.T.; Li, H.H. Characterization of the Key Odorants in Chinese Zhima Aroma-Type Baijiu by Gas Chromatography-Olfactometry, Quantitative Measurements, Aroma Recombination, and Omission Studies. J. Agric. Food Chem. 2016, 64, 5367–5374. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Qin, D.; Wu, Z.Y.; Sun, B.G.; Sun, X.T.; Huang, M.Q.; Sun, J.Y.; Zheng, F.P. Characterization of key aroma compounds in Chinese Guojing sesame-flavor Baijiu by means of molecular sensory science. Food Chem. 2019, 284, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y.; Qin, D.; Duan, J.W.; Li, H.H.; Sun, J.Y.; Huang, M.Q.; Sun, B.G. Characterization of benzenemethanethiol in sesame-flavour baijiu by high-performance liquid chromatography-mass spectrometry and sensory science. Food Chem. 2021, 364, 8. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Zhang, K.Z.; Cao, X.Z.; Yang, J.G. Effects of Saccharomycopsis fibuligera and Saccharomyces cerevisiae inoculation on small fermentation starters in Sichuan-style Xiaoqu liquor. Food Res. Int. 2020, 137, 12. [Google Scholar] [CrossRef]
- Ji, X.A.; Zhang, L.Y.; Yu, X.W.; Chen, F.J.; Guo, F.X.; Wu, Q.; Xu, Y. Selection of initial microbial community for the alcoholic fermentation of sesame flavor-type baijiu. Food Res. Int. 2023, 172, 8. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Y.; Wang, J.; Hong, J.; Tian, W.; Zhao, D.; Sun, J.; Huang, M.; Li, H.; Zheng, F.; et al. Uncover the Flavor Code of Roasted Sesame for Sesame Flavor Baijiu: Advance on the Revelation of Aroma Compounds in Sesame Flavor Baijiu by Means of Modern Separation Technology and Molecular Sensory Evaluation. Foods 2022, 11, 998. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Wu, X.F.; Mu, D.D.; Xu, B.Y.; Xu, X.H.; Chang, Q.; Li, X.J. Profiling the influence of physicochemical parameters on the microbial community and flavor substances of zaopei. J. Sci. Food Agric. 2021, 101, 6300–6310. [Google Scholar] [CrossRef]
- Ding, X.F.; Wu, C.D.; Huang, J.; Zhou, R.Q. Characterization of interphase volatile compounds in Chinese Luzhou flavor liquor fermentation cellar analyzed by head space-solid phase micro extraction coupled with gas chromatography mass spectrometry (HS-SPME/GC/MS). LWT-Food Sci. Technol. 2016, 66, 124–133. [Google Scholar] [CrossRef]
- Tan, Y.W.; Zhong, H.P.; Zhao, D.; Du, H.; Xu, Y. Succession rate of microbial community causes flavor difference in strong-aroma Baijiu making process. Int. J. Food Microbiol. 2019, 311, 108350. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Khan, A.L.; Waqas, M.; Ullah, I.; Bilal, S.; Kim, Y.H.; Asaf, S.; Kang, S.M.; Lee, I.J. Metabolic and proteomic alteration in phytohormone-producing endophytic Bacillus amyloliquefaciens RWL-1 during methanol utilization. Metabolomics 2019, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Tan, Y.W.; Lv, X.B.; Chen, L.Q.; Yang, F.; Wang, H.Y.; Du, H.; Wang, L.; Xu, Y. Microbial Community Succession and Its Environment Driving Factors During Initial Fermentation of Maotai-Flavor Baijiu. Front. Microbiol. 2021, 12, 8. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, X.; Mu, D.; Yang, W.; Jiang, S.; Sun, W.; Shen, Y.; Cai, J.; Zheng, Z.; Jiang, S.; et al. Profiling the effects of physicochemical indexes on the microbial diversity and its aroma substances in pit mud. Lett. Appl. Microbiol. 2020, 71, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Best, D.J.; Roberts, D.E. The upper tail probabilities of Spearman’s Rho. Appl. Stat. 1975, 24, 377–379. [Google Scholar] [CrossRef]
- Watts, S.C.; Ritchie, S.C.; Inouye, M.; Holt, K.E. FastSpar: Rapid and scalable correlation estimation for compositional data. Bioinformatics 2019, 35, 1064–1066. [Google Scholar] [CrossRef]
- Xie, J.; Zhou, Z.H.; Lu, K.; Chen, L.N.; Zhang, W. Visualization of Biomolecular Networks’ Comparison on Cytoscape. Tsinghua Sci. Technol. 2013, 18, 515–521. [Google Scholar] [CrossRef]
- Zhang, L.L.; Xiong, S.J.; Du, T.H.; Xiao, M.Y.; Peng, Z.; Xie, M.Y.; Guan, Q.Q.; Xiong, T. Effects of microbial succession on the dynamics of flavor metabolites and physicochemical properties during soy sauce koji making. Food Biosci. 2023, 53, 102636. [Google Scholar] [CrossRef]
- Chou, C.C.; Ling, M.Y. Biochemical changes in soy sauce prepared with extruded and traditional raw materials. Food Res. Int. 1998, 31, 487–492. [Google Scholar] [CrossRef]
- Chen, X.; Song, C.; Zhao, J.; Xiong, Z.; Peng, L.; Zou, L.; Liu, B.; Li, Q. Effect of a New Fermentation Strain Combination on the Fermentation Process and Quality of Highland Barley Yellow Wine. Foods 2024, 13, 2193. [Google Scholar] [CrossRef]
- Liu, C.; Gong, X.; Zhao, G.; Htet, M.N.S.; Jia, Z.; Yan, Z.; Liu, L.; Zhai, Q.; Huang, T.; Deng, X. Liquor Flavour Is Associated With the Physicochemical Property and Microbial Diversity of Fermented Grains in Waxy and Non-waxy Sorghum (Sorghum bicolor) During Fermentation. Front. Microbiol. 2021, 12, 618458. [Google Scholar] [CrossRef] [PubMed]
- Longo, R.; Carew, A.; Sawyer, S.; Kemp, B.; Kerslake, F. A review on the aroma composition of Vitis vinifera L. Pinot noir wines: Origins and influencing factors. Crit. Rev. Food Sci. Nutr. 2020, 61, 1589–1604. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Li, J.L.; Rao, Z.M.; Si, G.R.; Zhang, X.; Gao, C.Q.; Ye, M.; Zhou, P. Sesame flavour baijiu: A review. J. Inst. Brew. 2020, 126, 224–232. [Google Scholar] [CrossRef]
- Tu, W.; Cao, X.; Cheng, J.; Li, L.; Zhang, T.; Wu, Q.; Li, Q. Chinese Baijiu: The perfect works of microorganisms. Front. Microbiol. 2022, 13, 919044. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Jiao, G.; Zhang, Z.; Wang, J.; Li, P.; Dong, J.; Wang, R. Relationship between Representative Trace Components and Health Functions of Chinese Baijiu: A Review. Fermentation 2023, 9, 658. [Google Scholar] [CrossRef]
- Youqiang, X.; Mengqin, W.; Dong, Z.; Jia, Z.; Mengqi, D.; Xiuting, L.; Weiwei, L.; Chengnan, Z.; Baoguo, S. Simulated Fermentation of Strong-Flavor Baijiu through Functional Microbial Combination to Realize the Stable Synthesis of Important Flavor Chemicals. Foods 2023, 12, 644. [Google Scholar] [CrossRef]
- Yang, L.; Huang, D.; Li, Z. Study on the function and mechanism of lactic acid bacteria in the brewing process of Baijiu. Int. J. New Dev. Eng. Soc. 2023, 7, 51–59. [Google Scholar]
- Shen, S.M.; Liu, J.L.; Luo, R.Q.; Zhang, J.J.; Zhao, D.; Xue, X.X.; Zheng, J.; Qiao, Z.W.; Zhang, Q.; Feng, Z.; et al. Analysis of the Influence of Microbial Community Structure on Flavor Composition of Jiang-Flavor Liquor in Different Batches of Pre-Pit Fermented Grains. Fermentation 2022, 8, 671. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Zhao, J.R.; Liu, X.; Zhang, C.S.; Zhao, Z.G.; Li, X.T.; Sun, B.G. Flavor mystery of Chinese traditional fermented baijiu: The great contribution of ester compounds. Food Chem. 2022, 369, 13. [Google Scholar] [CrossRef]
- Hu, W.S.; Wang, B.Y.; Ali, M.M.; Chen, X.P.; Zhang, J.S.; Zheng, S.Q.; Chen, F.X. Free Amino Acids Profile and Expression Analysis of Core Genes Involved in Branched-Chain Amino Acids Metabolism during Fruit Development of Longan (Dimocarpus longan Lour.) Cultivars with Different Aroma Types. Biology 2021, 10, 807. [Google Scholar] [CrossRef]
- Ma, S.; Ding, C.; Zhou, C.; Shi, H.; Bi, Y.; Zhang, H.; Xu, X. Peanut oils from roasting operations: An overview of production technologies, flavor compounds, formation mechanisms, and affecting factors. Heliyon 2024, 10, e34678. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Xing, X.; Wang, Y.; Li, X.; Zheng, F.; Liu, C.; Wang, J.; Li, Q. Characterization of color, metabolites and microbial community dynamics of doubanjiang during constant temperature fermentation. Food Res. Int. 2023, 174, 113554. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.J.; Cao, H.L.; Guo, Y.L.; Liu, J.H.; Sun, Y.; Liu, S.R.; Lin, J.K.; Wei, S.; Wu, L.Y. The dynamic change of oolong tea constitutes during enzymatic-catalysed process of manufacturing. Int. J. Food Sci. Technol. 2020, 55, 3604–3612. [Google Scholar] [CrossRef]
- Dermiki, M.; Phanphensophon, N.; Mottram, D.S.; Methven, L. Contributions of non-volatile and volatile compounds to the umami taste and overall flavour of shiitake mushroom extracts and their application as flavour enhancers in cooked minced meat. Food Chem. 2013, 141, 77–83. [Google Scholar] [CrossRef]
- Wei, J.L.; Lu, J.; Nie, Y.; Li, C.W.; Du, H.; Xu, Y. Amino Acids Drive the Deterministic Assembly Process of Fungal Community and Affect the Flavor Metabolites in Baijiu Fermentation. Microbiol. Spectr. 2023, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.D.; Zhang, J.H.; Wang, T.R.; Bai, B.Q.; Hou, Z.X.; Cheng, J.F.; Bo, T.; Fan, S.H. Reveal the microbial communities and functional prediction during the fermentation of Fen-flavor Baijiu via metagenome combining amplicon sequencing. Ann. Microbiol. 2023, 73, 14. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, Y.; Chen, L. Diversity of yeast species during fermentative process contributing to Chinese Maotai-flavour liquor making. Lett. Appl. Microbiol. 2012, 55, 301–307. [Google Scholar] [CrossRef]
- Wang, H.; Sun, C.H.; Yang, S.Z.; Ruan, Y.L.; Lyu, L.; Guo, X.W.; Wu, X.L.; Chen, Y.F. Exploring the impact of initial moisture content on microbial community and flavor generation in Xiaoqu baijiu fermentation. Food Chem. X 2023, 20, 11. [Google Scholar] [CrossRef]
- Wang, J.L.; Lu, C.S.; Xu, Q.; Li, Z.Y.; Song, Y.J.; Zhou, S.; Zhang, T.C.; Luo, X.G. Bacterial Diversity and Lactic Acid Bacteria with High Alcohol Tolerance in the Fermented Grains of Soy Sauce Aroma Type Baijiu in North China. Foods 2022, 11, 1794. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Ye, D.; Duan, L.; Duan, C.; Yan, G. Effect of the addition of branched-chain amino acids to non-limited nitrogen synthetic grape must on volatile compounds and global gene expression during alcoholic fermentation. Aust. J. Grape Wine Res. 2018, 24, 197–205. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, Z.F. Dynamic changes in microbial communities and flavor during different fermentation stages of proso millet Baijiu, a new product from Shanxi light-flavored Baijiu. Front. Microbiol. 2024, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, R.Y.; Liu, C.; Chen, L.Q.; Yang, F.; Wang, L. Spatiotemporal accumulation differences of volatile compounds and bacteria metabolizing pickle like odor compounds during stacking fermentation of Maotai-flavor baijiu. Food Chem. 2023, 426, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Yan, C.Y.; Ma, C.L.; Huang, S.K.; Chang, X.; Li, Z.J.; Chen, X.; Li, X. Effects of two kinds of Bacillus on flavour formation of Baijiu solid-state fermentation with pure mixed bacteria. Int. J. Food Sci. Technol. 2023, 58, 1250–1262. [Google Scholar] [CrossRef]
- Wang, D.Q.; Chen, L.Q.; Yang, F.; Wang, H.Y.; Wang, L. Yeasts and their importance to the flavour of traditional Chinese liquor: A review. J. Inst. Brew. 2019, 125, 214–221. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Yang, L.; Huang, X.D.; Hu, J.F.; Deng, H.; He, J.J.; Zhang, C.L. The spatiotemporal heterogeneity of microbial community assembly during pit fermentation of soy sauce flavor baijiu. Food Biosci. 2024, 61, 9. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Zhou, H.; Cai, J.; Xu, S.; Chen, A.; Mu, D.; Wu, X.; Li, X. Microbial Community Succession and Flavor Compound Formation in Sesame-Flavored Baijiu from Zaopei. Fermentation 2025, 11, 255. https://doi.org/10.3390/fermentation11050255
Liu W, Zhou H, Cai J, Xu S, Chen A, Mu D, Wu X, Li X. Microbial Community Succession and Flavor Compound Formation in Sesame-Flavored Baijiu from Zaopei. Fermentation. 2025; 11(5):255. https://doi.org/10.3390/fermentation11050255
Chicago/Turabian StyleLiu, Wuyang, Hao Zhou, Jing Cai, Shanshan Xu, Anyuan Chen, Dongdong Mu, Xuefeng Wu, and Xingjiang Li. 2025. "Microbial Community Succession and Flavor Compound Formation in Sesame-Flavored Baijiu from Zaopei" Fermentation 11, no. 5: 255. https://doi.org/10.3390/fermentation11050255
APA StyleLiu, W., Zhou, H., Cai, J., Xu, S., Chen, A., Mu, D., Wu, X., & Li, X. (2025). Microbial Community Succession and Flavor Compound Formation in Sesame-Flavored Baijiu from Zaopei. Fermentation, 11(5), 255. https://doi.org/10.3390/fermentation11050255







