Abstract
This study investigated the effects of different doses of thiamine diphosphate (ThDP) on methane reduction and fermentation characteristics of Korean native cow (Hanwoo). In vitro trials used ThDP powder at 240, 360, 480, 600, and 720 ppm of substrate, with each sample incubated at 39 °C for 24 and 48 h. After incubation, each sample was analyzed for total gas, methane production, dry matter digestibility, and rumen fermentation characteristics. Mean comparisons were performed using Tukey’s test, with significant differences declared at p < 0.05. Total gas production, methane ratio, and methane production per digested dry matter had a quadratic pattern (p < 0.001), and the 480 ppm treatment had the lowest (p < 0.05) at 24 and 48 h of incubation. Total volatile fatty acid concentration showed no significant difference at 24 h but differed significantly at 48 h (p > 0.05). The concentration of propionate had a quadratic pattern (p < 0.001), and the 480 ppm treatment showed the highest levels compared to the other treatments (p < 0.05) after 24 and 48 h of incubation. In conclusion, ThDP supplementation had a methane inhibition effect. In particular, the methane inhibition effect was most pronounced when ThDP was supplemented at 480 ppm.
1. Introduction
The escalating challenges of global warming have prompted extensive research across multiple scientific disciplines to develop strategies to reduce greenhouse gas emissions [1]. Among the various contributors, the livestock sector plays a major role in the emission of methane, a potent greenhouse gas with a global warming potential far exceeding that of carbon dioxide [2]. Methane is produced primarily by methanogenic archaea during enteric fermentation in the rumen, a process inherent to the digestion of fibrous feedstuffs by ruminants [3]. As global demand for livestock products continues to rise, the mitigation of methane emissions from ruminants has become a critical focus in addressing climate change. Recent advances in feed additive technology have focused on disrupting enzymatic pathways responsible for methane biosynthesis [4]. Among them, 3-nitrooxypropanol has emerged as one of the most promising methane inhibitors. This compound selectively inhibits methyl coenzyme M reductase (MCR), an enzyme that catalyzes the final step in methane formation, thereby reducing methane output without compromising the stability of the ruminal microbial ecosystem [5]. The efficacy of 3-nitrooxypropanol has been well-documented, with studies reporting reductions in enteric methane emissions of approximately 30% in dairy cattle. Moreover, its favorable safety profile has enabled regulatory approval in multiple regions, including North America and Europe, paving the way for widespread commercial adoption [6,7].
Despite such advancements, the development of alternative or complementary approaches remains an area of active research. A deeper understanding of the key biochemical components involved in methane production by methanogenic archaea has revealed additional targets for intervention. The final step of methane production is facilitated by three critical components: Methyl-S-Coenzyme M, Cofactor F430, and Coenzyme B [8]. Methyl-S-Coenzyme M, a key intermediate in methanogenesis, consists of a methyl group (-CH3) bound to Coenzyme M (CoM, 2-mercaptoethanesulfonate). This intermediate plays a central role in the transfer of the methyl group necessary for methane formation. Cofactor F430, a nickel-containing tetrapyrrole cofactor, is integral to the function of MCR and thus to the overall process of methanogenesis [9]. Coenzyme B, another essential cofactor, donates electrons to Methyl-S-Coenzyme M (CH3-S-CoM), completing the final step in methane production [8].
Interruption of this process, particularly the electron transfer to CH3-S-CoM, has been identified as a viable strategy for inhibiting methane production. Previous studies have suggested that analogs of Coenzyme B could serve as potential inhibitors of methane biosynthesis in methanogenic archaea [10]. Building on this idea, recent research has explored the efficacy of alternative compounds, such as thiamine diphosphate (ThDP), a coenzyme involved in the intermediate metabolism of carbohydrates. ThDP, structurally similar to Coenzyme B, functions as a coenzyme for enzymes such as transketolase, playing roles in both cardiac and neurological systems [11,12].
Chai et al. [11] recently investigated the methane reduction potential of ThDP using an in vitro batch fermentation system. Their findings demonstrated that ThDP effectively reduced methane emissions; however, they also noted a significant decrease in digestibility, suggesting potential trade-offs between methane mitigation and rumen fermentation efficiency. This highlights the need to optimize ThDP application in terms of dosage and its impact on ruminal microbial activity and fermentation characteristics before progressing to in vivo trials. Moreover, exploration of ThDP as a methane inhibitor aligns with broader research trends aimed at identifying feed additives that not only reduce greenhouse gas emissions but also maintain or enhance animal productivity. The balance between environmental sustainability and agricultural efficiency is critical to ensuring the adoption of such technologies by the livestock industry. Therefore, determining the optimal ThDP dose that could minimize methane production without adversely affecting the rumen ecosystem is a key research priority. This study addresses this gap by evaluating the effects of different ThDP dosages on methane emissions and ruminal fermentation characteristics, contributing to the ongoing effort to develop sustainable solutions for the livestock sector.
2. Materials and Methods
Experimental procedures were approved and conducted according to the guidelines of the National Institute of Animal Science Institutional Animal Use and Care Committee of Korea (approval number: NIAS2023-0591). The experiment was conducted using two rumen-cannulated Korean native Hanwoo steers with an average body weight (BW) of 736 ± 15 kg as donor animals. The steers were housed in individual pens equipped with comfortable bedding material and appropriate ventilation to ensure animal welfare. Animals were fed diets comprising 9.4 kg/d of concentrate and 2.4 kg/d of mixed hay (as-fed basis) at 1000 h and 1700 h each day to meet their nutritional requirements and promote stable rumen fermentation. The diet formulation was based on the recommendations for Hanwoo steers, and animals were monitored daily to assess feed intake and overall health. In addition, ad libitum access to clean drinking water and mineral blocks was provided throughout the study period to support physiological needs. The detailed ingredients of the experimental diets are presented in Table 1 for reference.

Table 1.
The ingredients of diets for rumen-cannulated cows.
2.1. Thiamine Diphosphate and Substrate Preparation
Thiamine diphosphate (CAS number: 136-08-3) was obtained from a compound-synthesis company. The substrate for the in vitro experiment was prepared using concentrates (the ingredients were the same as the concentrates in Table 1) and rice straw. Chemical analyses of the substrates were performed using the AOAC method [13]: crude protein (CP), #942.05; ether extract, #920.39; and crude ash, #954.01. The neutral detergent fiber (NDF) and acid detergent fiber (ADF) contents were analyzed using an ANKOM2000 fiber analyzer (ANKOM Technology Corporation, Macedon, NY, USA), as previously described by Van Soest et al. [14]. The CP content was calculated as nitrogen × 6.25. Non-fiber carbohydrate (NFC) was calculated as 100 − (CP + ether extract + crude ash + NDF). Their chemical compositions are listed in Table 2.

Table 2.
The chemical composition of in vitro experimental diets.
2.2. In Vitro Incubation
One hour before morning feeding, ruminal fluid was collected from each of the two rumen-cannulated Hanwoo steers, ensuring equal volumes were obtained from both animals. The collected rumen fluid from both steers was immediately pooled into a sterile flask, thoroughly mixed to ensure homogeneity, and then filtered through four layers of cheesecloth to remove large feed particles and other debris. The flask was tightly sealed to maintain anaerobic conditions during the transfer, and the ruminal fluid was promptly transported to the laboratory in a pre-heated, insulated container to preserve microbial activity and minimize temperature fluctuations.
The substrates used for in vitro incubation were prepared based on the diets fed to the donor animals, consisting of 0.35 g of concentrate and 0.15 g of rice straw on a dry matter (DM) basis. The substrates were selected to simulate the rumen conditions of the Hanwoo steers. The filtered ruminal fluid was then mixed with McDougall buffer [15], a standard solution designed to mimic the inorganic composition of saliva, which included L-cysteine (0.5 g) as a reducing agent to maintain anaerobic conditions. The mixture was prepared at a 1:3 (v/v) ratio of ruminal fluid to buffer, ensuring consistency in the experimental setup. Subsequently, 50 mL of the buffered ruminal fluid mixture was carefully dispensed into each 125 mL serum bottle, which contained pre-weighed substrates of 0.5 ± 0.0003 g. ThDP was added to the bottles at concentrations of 0% (control), 240, 360, 480, 600, and 720 ppm based on the weight of the substrate. Carbon dioxide gas was flushed into the headspace for a few seconds to establish and maintain anaerobic conditions inside the serum bottles before the bottles were securely sealed with rubber stoppers and aluminum caps. The sealed bottles were then placed in a shaking incubator set to 39 °C to mimic the rumen temperature and were incubated for 24 and 48 h. The shaking ensured uniform distribution of nutrients and ThDP throughout the incubation process. Each treatment was replicated five times, with the experimental unit being a single serum bottle. Blanks were set up in triplicate for each incubation treatment.
2.3. Sample Collection and Analysis
After 24 or 48 h of incubation, the volume of produced gas in each serum bottle was measured using a syringe connected to an air pressure detector (TPI656, TPI, Seoul, Republic of Korea), and the sampled gas was collected carefully using a vacuum test tube (Vacutainer, Becton Dickinson, Franklin Lakes, NJ, USA). The collected gas samples were analyzed using a gas chromatograph (GC; NL/450 GC, Bruker, Billerica, MA, USA) equipped with a capillary column (GS-GASPRO 113-4332, 30 m × 0.320 mm, Agilent Technologies Inc., Santa Clara, CA, USA) to determine methane production. The GC system was calibrated with methane standards to ensure accurate and reproducible measurements of methane concentration.
Following gas collection, the serum bottles were unsealed to measure the pH of the incubated rumen fluid using a calibrated pH meter (SevenEasy pH, Mettler-Toledo AG, Schwerzenbach, Switzerland). The pH measurement was performed immediately after opening the bottles to prevent any changes due to exposure to air. Subsequently, the rumen fluid remaining in the serum bottles was transferred to other tubes for the analysis of volatile fatty acids (VFA) and ammonia nitrogen (NH3-N). The samples were treated with specific reagents as described below and stored until analysis.
The concentrations of VFA and NH3-N were analyzed using modified versions of methods described by Erwin et al. [16] and Chaney and Marbach [17]. For VFA analysis, 5 mL of supernatant, obtained by centrifuging the rumen fluid at 14,000× g for 10 min at 4 °C, was mixed with 500 μL of 50% metaphosphoric acid (MPA; Catalog number 239275, Sigma-Aldrich, St. Louis, MO, USA). The mixture was stored at −80 °C until analysis. Before gas chromatographic analysis, the prepared samples were further centrifuged under identical conditions to remove particulates. Subsequently, 1 mL of supernatant was transferred into GC vials and analyzed using a GC system (6890N, Agilent Technologies, Wilmington, DE, USA) equipped with a NukolTM fused silica capillary column (15 m × 0.53 mm × 0.5 µm, Supelco Inc., Bellefonte, PA, USA). A standard curve was generated using the Volatile Free Acid Mix (Catalog number CRM46975; Sigma-Aldrich, St. Louis, MO, USA) to quantify individual VFA.
For NH3-N analysis, 5 mL of the rumen fluid supernatant was mixed with 500 μL of 25% metaphosphoric acid and centrifuged at 14,000× g for 5 min at 4 °C. A 20-µL aliquot of the supernatant was added to a solution containing 1 mL of phenol color reagent (50 g/L phenol and 0.25 g/L nitroferricyanide) and 1 mL of alkali-hypochlorite reagent (25 g/L sodium hydroxide and 16.8 mL/L of 4–6% sodium hypochlorite). The mixture was incubated in a water bath at 37 °C for 15 min to develop color. After incubation, 8 mL of distilled water was added to dilute the solution, and the absorbance was measured at 630 nm using a UV spectrophotometer (Bio-Rad, US/Benchmark Plus, Tokyo, Japan) to determine NH3-N concentrations.
The substrates within the serum bottles were collected and dried in a convection drying oven set at 60 °C for 48 h. The in vitro dry matter digestibility (IVDMD) was calculated by comparing the initial dry weight of the substrate with the residual weight after incubation and drying.
2.4. Statistical Analysis
All data from the experiments are presented as least-squares means, and significance was declared at p < 0.05. The model was Yij = µ + Ti + eij, where Yij = response variable, µ = overall mean, Ti = the effect of ThDP i, and eij = error term. All data were analyzed with polynomial contrasts using the PROG GLM of SAS Enterprise Guide®, Version 7.1 (SAS Institute Inc., Cary, NC, USA) to examine the linear, quadratic, and cubic effects of increasing ThDP levels. Orthogonal coefficients for linear, quadratic, and cubic contrasts were adjusted to account for the unequal spacing of the ThDP levels used in this experiment (0, 240, 360, 480, 600, and 720 ppm) using the Interactive Matrix Programming Language (PROC IML) procedure in SAS. Mean separation was performed using Tukey’s test, and significant differences were considered at p < 0.05.
3. Results
3.1. In Vitro Digestibility and Gas Production
Figure 1 shows the effects of varying concentrations of ThDP on in vitro digestibility and gas production yields after a 24-h incubation period. The in vitro digestibility followed a quadratic pattern (p = 0.001), with notable decreases in digestibility occurring in the 360, 480, and 600 ppm ThDP treatments, which were significantly lower than that in the control group (p < 0.05). The total gas, methane ratio, and methane production per digested dry matter exhibited a quadratic pattern (p < 0.001), and the 480 ppm treatment had the lowest values (p < 0.05).
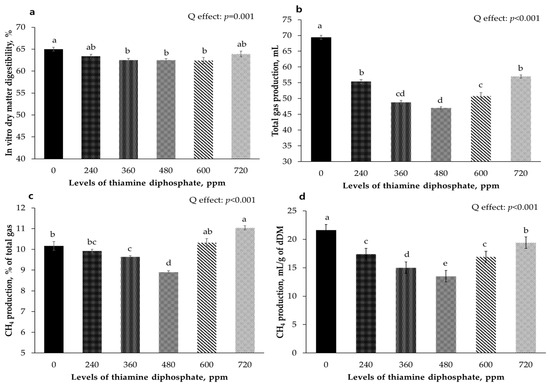
Figure 1.
Effects of different levels of thiamine diphosphate on in vitro digestibility and gas production yields incubated for 24 h. (a) In vitro dry matter digestibility; (b) Total gas production yield (mL); (c) Methane rate of total gas; (d) Methane production yield (mL/g of dry matter). a–e Means without a common superscript letter significantly differ, p < 0.05.
Figure 2 shows the effects of varying ThDP concentrations on in vitro digestibility and gas production yield after a 48-h incubation period. The in vitro digestibility followed a cubic pattern (p = 0.002), with a notable decrease observed at the 600 ppm treatment, which was significantly lower than that in the control group (p < 0.05). The total gas, methane ratio, and methane production per digested dry matter exhibited quadratic patterns (p < 0.001), and the 480 ppm treatment had the lowest values (p < 0.05).
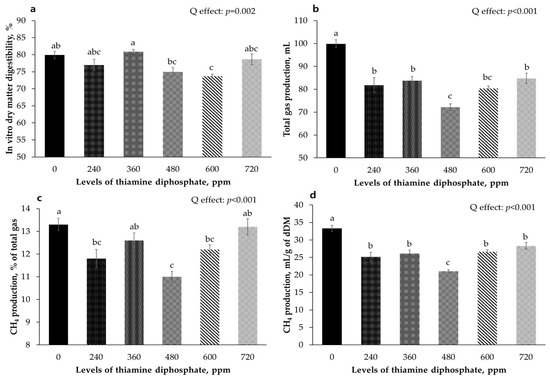
Figure 2.
Effects of different levels of thiamine diphosphate on in vitro digestibility and gas production yields incubated for 48 h. (a) In vitro dry matter digestibility; (b) Total gas production yield (mL); (c) Methane rate of total gas; (d) Methane production yield (mL/g of dry matter). a–c Means without a common superscript letter significantly differ, p < 0.05.
3.2. Ruminal Fermentation Characteristics
Table 3 shows the effects of different ThDP levels on ruminal fermentation characteristics after a 24-h incubation period. Ruminal pH showed a quadratic pattern (p < 0.001). Acetate concentration showed a quadratic pattern (p = 0.044). The concentration of propionate had a quadratic pattern (p < 0.001), and the 480 ppm treatment had the highest values across all treatments (p < 0.05). Quadratic patterns were also observed for the concentrations of butyrate (p < 0.001), valerate (p = 0.006), and the acetate-to-propionate ratio (p = 0.032). The concentrations of butyrate and valerate were relatively low in the 480 ppm treatment (p < 0.05). The concentrations of ammonia nitrogen, total VFA, iso-butyrate, and iso-valerate did not differ significantly.

Table 3.
Effects of different levels of thiamine diphosphate on in vitro ruminal fermentation characteristics incubated for 24 h.
Table 4 shows the effects of different ThDP concentrations on ruminal fermentation characteristics after 48 h of incubation. Rumen pH showed a linear pattern (p < 0.001) with an increase in ThDP supplementation level. NH3-N concentration exhibited a quadratic pattern (p < 0.001), with the 480 ppm treatment showing the lowest concentration (p < 0.05). Linear patterns were observed for total VFA (p < 0.001), acetate (p < 0.001), and iso-valerate (p < 0.001) concentrations. In addition, quadratic patterns were observed for the concentrations of propionate (p < 0.001), butyrate (p = 0.005), ), iso-butyrate (p < 0.001), valerate (p < 0.001), and the acetate-to-propionate ratio (p < 0.001).

Table 4.
Effects of different levels of thiamine diphosphate on in vitro ruminal fermentation characteristics incubated for 48 h.
4. Discussion
The key factors driving the activity of the methane-producing enzyme MCR in methanogens are Coenzyme B and CoFactor F430 [18,19]. CoFactor F430 is essential for the catalytic process in which it facilitates the combination of the methyl group with hydrogen to form methane, whereas Coenzyme B serves as an electron donor to HS-CoM, ultimately driving methane production [8]. The two factors are indispensable for methanogenesis, and when either of them is inhibited, methane production can be suppressed significantly or even halted [20]. Building on this mechanism, a previous study highlighted that a Coenzyme B analog has the potential to act as an effective methane inhibitor [10]. In another study, thiamine triphosphate was identified as a promising Coenzyme B analog, demonstrating its ability to reduce methane production due to its strong binding affinity (−132.39 kcal/mol) and high Fit score (4.6) [21]. However, thiamine triphosphate is structurally unstable and difficult to maintain in its form compared to thiamine diphosphate [22]. Thiamine diphosphate has one less phosphate group than thiamine triphosphate and has been confirmed to have a methane reduction effect similar to that of thiamine triphosphate [11]. Therefore, in vitro tests were conducted using diphosphate instead of triphosphate in the present study. In the present study, methane emissions and the proportion of methane in the total gas exhibited a quadratic pattern, decreasing initially with an increase in ThDP and then increasing as the ThDP concentration increased beyond 480 ppm. Similarly, some studies have shown that methane reduction rates decrease quadratically with increasing 3-nitrooxypropanol levels [23,24]. Romero-Pérez et al. [25] reported that propionate-producing bacteria become active when methanogens are inhibited and that methane reduction can decrease owing to changes in hydrogen accumulation and utilization pathways. Similarly, in the present study, the decrease in methane reduction capacity at higher ThDP concentrations could be attributed to changes in the hydrogen accumulation and utilization pathways, which mirror the findings from earlier studies.
A previous study reported that rumen microbial composition can be influenced by the level of methane inhibitor added, specifically those targeting MCR [5]. This is significant because changes in the rumen microbial composition have various effects, including alterations in feed digestibility, VFA production, and overall rumen fermentation efficiency [26]. Gou et al. [27] emphasized that inhibition of cellulolysis, hydrogen accumulation, and shifts in rumen microbial composition collectively contribute to a decrease in digestibility and reduction in VFA production. The findings are consistent with the results of the present study, which showed decreased digestibility at certain ThDP supplementation levels. The decrease in digestibility is likely a consequence of the impact of ThDP on the rumen microbial environment, altering microbial activity and fermentation processes. Nitrogen compounds [24,25,28,29] and halogen compounds [30,31] that inhibit the methanogenic pathway reduce rumen acetate and butyrate, but increase propionate levels. Under normal conditions, methanogens utilize hydrogen to reduce carbon dioxide to methane and maintain redox balance [29]. However, when methane production is inhibited, excess hydrogen disrupts this balance, necessitating alternative electron sinks [28]. The concentration of propionate increases because propionate acts as an effective hydrogen sink, facilitating NADH (a coenzyme that carries electrons) oxidation and restoring redox homeostasis [32]. In the present study, propionate concentration increased in the ThDP treatment because the increased hydrogen accumulation due to the suppression of methane production activated propionic acid production. The activation of the propionate production pathway serves several important functions within the rumen. It enhances the microbial energy supply by providing an efficient mechanism for utilizing hydrogen, which in turn supports microbial growth and boosts protein synthesis [33]. As the rumen microbes increasingly rely on propionate production, there is a greater demand for NH3-N, which is essential for microbial protein synthesis. Consequently, the increased microbial protein synthesis leads to greater utilization of NH3-N, resulting in a reduction of its concentration within the rumen [34]. The present study showed a negative relationship between NH3-N and propionate, indicating that they can have a positive effect on ruminal microorganisms and protein synthesis. Schilde et al. [35] reported that when microbial protein synthesis decreases, the utilization of branched-chain fatty acids, such as iso-butyrate, may also decrease, resulting in a relative increase in concentration. In other words, when protein synthesis increases, the use of branched-chain fatty acids may increase, and their concentrations may decrease. Similar results were observed in the present study, with iso-butyrate increasing as propionic acid increased and NH3-N decreased. However, iso-valerate increased linearly with ThDP, in contrast to iso-butyrate (Table 4). The observed decrease in iso-butyrate and concurrent increase in iso-valerate concentrations following ThDP supplementation may reflect differences in microbial utilization efficiency.
Iso-butyrate, derived from valine, is rapidly reused in microbial cell protein and valine biosynthesis pathways [36]. Therefore, iso-valerate may appear to be increased relatively.
5. Conclusions
Thiamine diphosphate supplementation was demonstrated to inhibit methane production. In particular, the methane inhibition effect was the highest when thiamine diphosphate was supplemented at 480 ppm. Further studies should be conducted to determine the effects of thiamine diphosphate supplementation on methane emissions and growth performance characteristics in ruminants. In addition, further studies on the toxicity and residue evaluation of ruminants must be conducted on this compound before it can be used as a methane inhibitor agent.
Author Contributions
Conceptualization, S.-S.L., S.-U.J. and H.-S.K.; methodology, H.-S.K.; software, S.-U.J.; validation, S.-D.L., Y.-K.L. and J.-S.W.; formal analysis, H.-S.K.; data curation, S.-S.L.; writing—original draft preparation, S.-S.L. writing—review and editing, H.-S.K., S.-U.J., J.-S.W., Y.-K.L., S.-D.L. and S.-S.L.; visualization, S.-U.J.; supervision, S.-S.L.; project administration, S.-S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01720402)” of the Rural Development Administration and by the 2025 RDA Fellowship Program of the National Institute of Animal Science, Republic of Korea.
Institutional Review Board Statement
The experiments conformed to the guidelines of the Institutional Animal Care and Use Committee of the National Institute of Animal Science, Rural Development Administration, Republic of Korea (approval number: NIAS2023-0591; 21 February 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the first author. The data are not publicly available because of the restrictions imposed by the research group.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Masson-Delmotte, V.; Zhai, A.P.; Pirani, S.L.; Connors, C.; Péan, S.; Berger, N.; Caud, Y.; Chen, L.; Goldfarb, M.I. (Eds.) IPCC, 2021: Summary for Policymakers. In Climate Change 2021: The Physical Science Basis; Cambridge University Press: New York, NY, USA, 2021. [Google Scholar]
- Glasson, C.R.; Kinley, R.D.; de Nys, R.; King, N.; Adams, S.L.; Packer, M.A.; Svenson, J.; Eason, C.T.; Magnusson, M. Benefits and risks of including the bromoform containing seaweed Asparagopsis in feed for the reduction of methane production from ruminants. Algal Res. 2022, 64, 102673. [Google Scholar] [CrossRef]
- Misiukiewicz, A.; Gao, M.; Filipiak, W.; Cieslak, A.; Patra, A.K.; Szumacher-Strabel, M. Methanogens and methane production in the digestive systems of nonruminant farm animals. Animal 2021, 15, 100060. [Google Scholar] [CrossRef]
- Haque, M.N. Dietary manipulation: A sustainable way to mitigate methane emissions from ruminants. J. Anim. Sci. Technol. 2018, 60, 1–10. [Google Scholar] [CrossRef]
- Xuan, T.; Zheng, T.; Li, T.; Wu, B.; Li, T.; Bao, W.; Qin, W. The Effects of Different Doses of 3-NOP on Ruminal Fermentation Parameters, Methane Production, and the Microbiota of Lambs In Vitro. Fermentation 2024, 10, 440. [Google Scholar] [CrossRef]
- Duin, E.C.; Wagner, T.; Shima, S.; Prakash, D.; Cronin, B.; Yáñez-Ruiz, D.R.; Rümbeli, R.; Stemmler, R.T.; Thauer, R.K.; Kindermann, M. Mode of action uncovered for the specific reduction of methane emissions from ruminants by the small molecule 3-nitrooxypropanol. Proc. Natl. Acad. Sci. USA 2016, 113, 6172–6177. [Google Scholar] [CrossRef]
- Luke, J.R.; Tonsor, G.T. The enteric methane emission conundrum: US beef cattle producer adoption of climate-focused technology. Sustain. Prod. Consum. 2024, 50, 364–375. [Google Scholar] [CrossRef]
- Alfano, M.; Cavazza, C. Structure, function, and biosynthesis of nickel-dependent enzymes. Protein Sci. 2020, 29, 1071–1089. [Google Scholar] [CrossRef]
- Thauer, R.K. Methyl (alkyl)-coenzyme M reductases: Nickel F-430-containing enzymes involved in anaerobic methane formation and in anaerobic oxidation of methane or of short chain alkanes. Biochemistry 2019, 58, 5198–5220. [Google Scholar] [CrossRef]
- Cedervall, P.E.; Dey, M.; Pearson, A.R.; Ragsdale, S.W.; Wilmot, C.M. Structural insight into methyl-coenzyme M reductase chemistry using coenzyme B analogues. Biochemistry 2010, 49, 7683–7693. [Google Scholar] [CrossRef]
- Chai, H.; Park, W.; Lim, D.; Seong, P.N.; Wi, J.; Lee, S.; Lee, Y. Composition for Reducing Methane Emission from Ruminants Containing Thiamine Diphosphate. KR 1020230176706, 7 December 2023. [Google Scholar]
- Horecker, B.L.; Smyrniotis, P.Z. The coenzyme function of thiamine pyrophosphate in pentose phosphate metabolism. J. Am. Chem. Soc. 1953, 75, 1009–1010. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- McDougall, E.I. Studies on ruminant saliva. 1. The composition and output of sheep’s saliva. Biochem. J. 1948, 43, 99. [Google Scholar] [CrossRef]
- Erwin, E.S.; Marco, G.J.; Emery, E.M. Volatile fatty acid analyses of blood and rumen fluid by gas chromatography. J. Dairy Sci. 1961, 44, 1768–1771. [Google Scholar] [CrossRef]
- Chaney, A.L.; Marbach, E.P. Modified reagents for determination of urea and ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Horng, Y.C.; Ragsdale, S.W. Rapid ligand exchange in the MCRred1 form of methyl-coenzyme m reductase. J. Am. Chem. Soc. 2003, 125, 2436–2443. [Google Scholar] [CrossRef]
- Yang, N.; Reiher, M.; Wang, M.; Harmer, J.; Duin, E.C. Formation of a nickel− methyl species in methyl-coenzyme M reductase, an enzyme catalyzing methane formation. J. Am. Chem. Soc. 2007, 129, 11028–11029. [Google Scholar] [CrossRef]
- Dey, M.; Li, X.; Kunz, R.C.; Ragsdale, S.W. Detection of organometallic and radical intermediates in the catalytic mechanism of methyl-coenzyme M reductase using the natural substrate methyl-coenzyme M and a coenzyme B substrate analogue. Biochemistry 2010, 49, 10902–10911. [Google Scholar] [CrossRef]
- Chai, H.; Seong, P.N.; Lim, D.; Kim, T.; Park, W.; Wi, J.; Lee, S.; Lee, Y. Composition for Reducing Methane Emission from Ruminants Containing Thiamine Triphosphate. WO2024106663, 22 June 2023. [Google Scholar]
- Friedemann, R.; Uslar, W. Quantum chemical and molecular mechanics calculations on the thiamine pyrophosphate system. J. Mol. Struct. Theochem. 1988, 181, 401–410. [Google Scholar] [CrossRef]
- Melgar, A.; Welter, K.C.; Nedelkov, K.; Martins, C.M.M.R.; Harper, M.T.; Oh, J.; Räisänen, S.E.; Chen, X.; Cueva, S.F.; Duval, S.; et al. Dose-response effect of 3-nitrooxypropanol on enteric methane emissions in dairy cows. J. Dairy Sci. 2020, 103, 6145–6156. [Google Scholar] [CrossRef]
- Romero-Pérez, A.; Okine, E.K.; Guan, L.L.; Duval, S.M.; Kindermann, M.; Beauchemin, K.A. Effects of 3-nitrooxypropanol on methane production using the rumen simulation technique (Rusitec). Anim. Feed Sci. Technol. 2015, 209, 98–109. [Google Scholar] [CrossRef]
- Romero-Perez, A.; Okine, E.K.; McGinn, S.M.; Guan, L.L.; Oba, M.; Duval, S.M.; Kindermann, M.; Beauchemin, K.A. The potential of 3-nitrooxypropanol to lower enteric methane emissions from beef cattle. Anim. Sci. J. 2014, 92, 4682–4693. [Google Scholar] [CrossRef] [PubMed]
- Jami, E.; Israel, A.; Kotser, A.; Mizrahi, I. Exploring the bovine rumen bacterial community from birth to adulthood. ISME J. 2013, 7, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, W.; Zhang, Y.; Zhou, M. Effect of 3-Nitropropionic Acid at Different Doses on In Vitro Rumen Fermentation, Digestibility, and Methane Emissions of Grazing Yak and Cattle. Animals 2024, 14, 1804. [Google Scholar] [CrossRef] [PubMed]
- Haisan, J.; Sun, Y.; Guan, L.L.; Beauchemin, K.A.; Iwaasa, A.; Duval, S.; Barreda, D.R.; Oba, M. The effects of feeding 3-nitrooxypropanol on methane emissions and productivity of Holstein cows in mid lactation. J. Dairy Sci. 2014, 97, 3110–3119. [Google Scholar] [CrossRef]
- Martínez-Fernández, G.; Abecia, L.; Arco, A.; Cantalapiedra-Hijar, G.; Martín-García, A.I.; Molina-Alcaide, E.; Kindermann, M.; Duval, S.; Yáñez-Ruiz, D.R. Effects of ethyl-3-nitrooxy propionate and 3-nitrooxypropanol on ruminal fermentation, microbial abundance, and methane emissions in sheep. J. Dairy Sci. 2014, 97, 3790–3799. [Google Scholar] [CrossRef]
- Cristobal-Carballo, O.; McCoard, S.A.; Cookson, A.L.; Ganesh, S.; Lowe, K.; Laven, R.A.; Muetzel, S. Effect of methane inhibitors on ruminal microbiota during early life and its relationship with ruminal metabolism and growth in calves. Front. Microbiol. 2021, 12, 710914. [Google Scholar] [CrossRef]
- Mitsumori, M.; Shinkai, T.; Takenaka, A.; Enishi, O.; Higuchi, K.; Kobayashi, Y.; Nonaka, I.; Asanuma, N.; Denman, S.E.; McSweeney, C.S. Responses in digestion, rumen fermentation and microbial populations to inhibition of methane formation by a halogenated methane analogue. Br. J. Nutr. 2012, 108, 482–491. [Google Scholar] [CrossRef]
- McAllister, T.A.; Newbold, C.J. Redirecting rumen fermentation to reduce methanogenesis. Aust. J. Exp. Agric. 2008, 48, 7–13. [Google Scholar] [CrossRef]
- Jo, Y.H.; Kim, W.S.; Kim, Y.R.; Ju, M.S.; Nejad, J.G.; Lee, H.G. Impacts of Protein and Energy Levels on Rumen Fermentation and Microbial Activity Under Different Incubation Temperatures. Animals 2024, 14, 3093. [Google Scholar] [CrossRef]
- Lopes, J.C.; De Matos, L.F.; Harper, M.T.; Giallongo, F.; Oh, J.; Gruen, D.; Ono, S.; Kindermann, M.; Duval, S.; Hristov, A.N. Effect of 3-nitrooxypropanol on methane and hydrogen emissions, methane isotopic signature, and ruminal fermentation in dairy cows. J. Dairy Sci. 2016, 99, 5335–5344. [Google Scholar] [CrossRef]
- Schilde, M.; von Soosten, D.; Hüther, L.; Kersten, S.; Meyer, U.; Zeyner, A.; Dänicke, S. Dose–response effects of 3-nitrooxypropanol combined with low-and high-concentrate feed proportions in the dairy cow ration on fermentation parameters in a rumen simulation technique. Animals 2021, 11, 1784. [Google Scholar] [CrossRef] [PubMed]
- Allison, M.J.; Peel, J.L. The biosynthesis of valine from isobutyrate by Peptostreptococcus elsdenii and Bacteroides ruminicola. Biochem. J. 1971, 121, 431–437. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).