Antioxidant and ACE-Inhibition Activities After In Vitro Digestion of a Non-Fermented Dairy Beverage Enriched with Postbiotics of Lactobacillus spp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Postbiotics Obtention
2.3. Beverage Elaboration
2.4. In Vitro Digestion
2.5. Antioxidant Activities
2.6. Angiotensin Converting Enzyme-Inhibitory Activity
2.7. Bioaccessibility Index for AA and Inhibitory ACE Activity
2.8. Antimicrobial Activity
2.9. Statistical Analysis
3. Results and Discussion
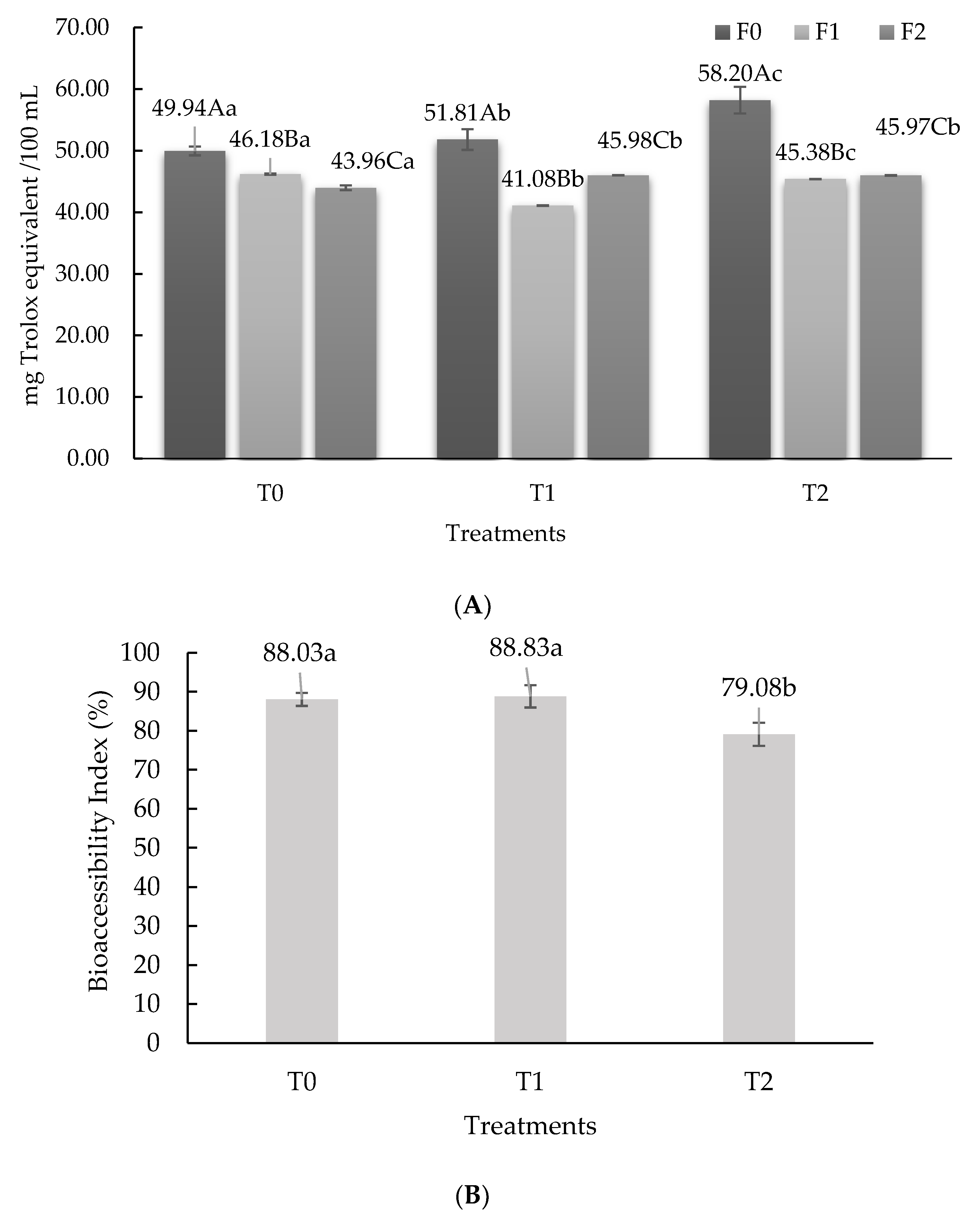
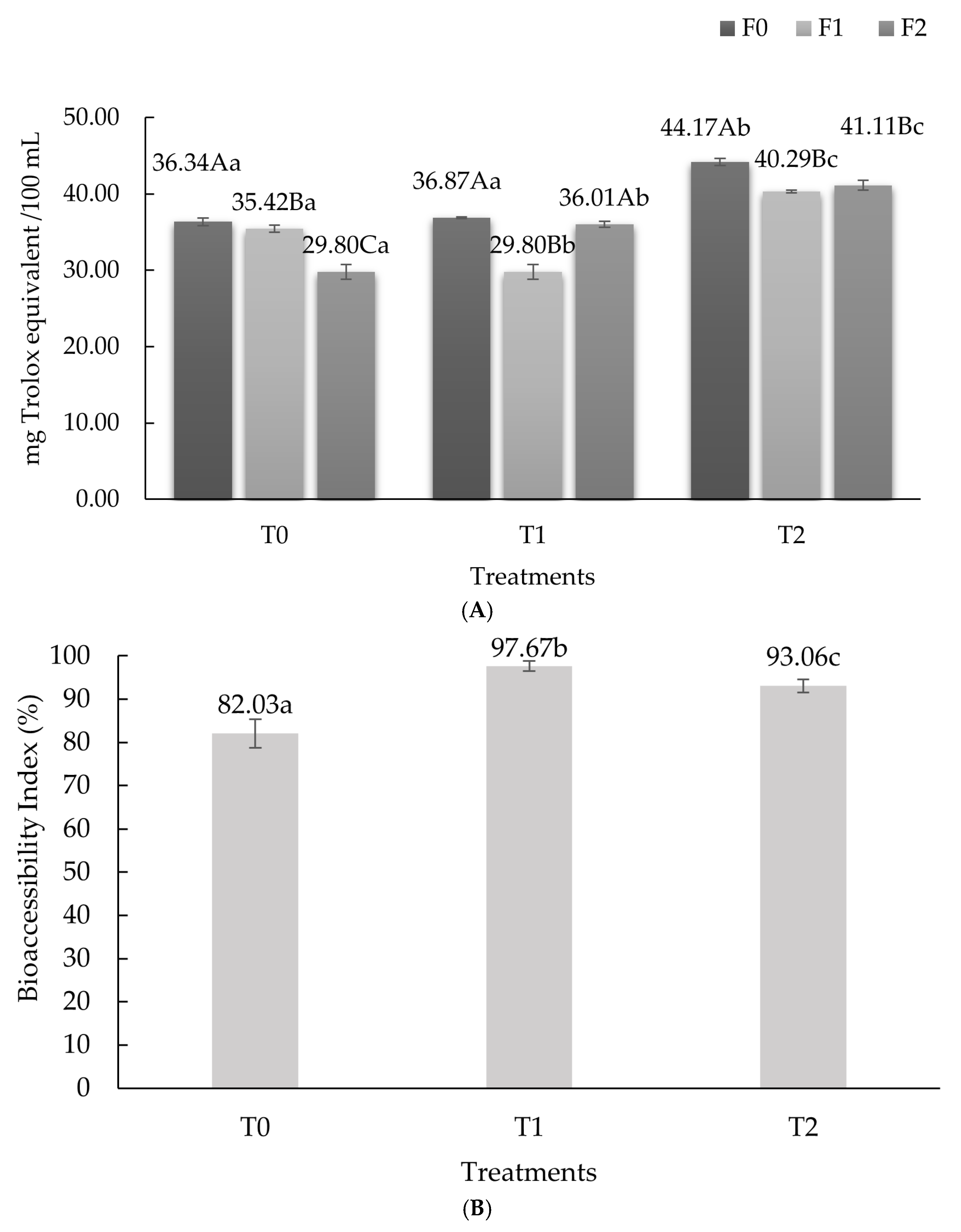
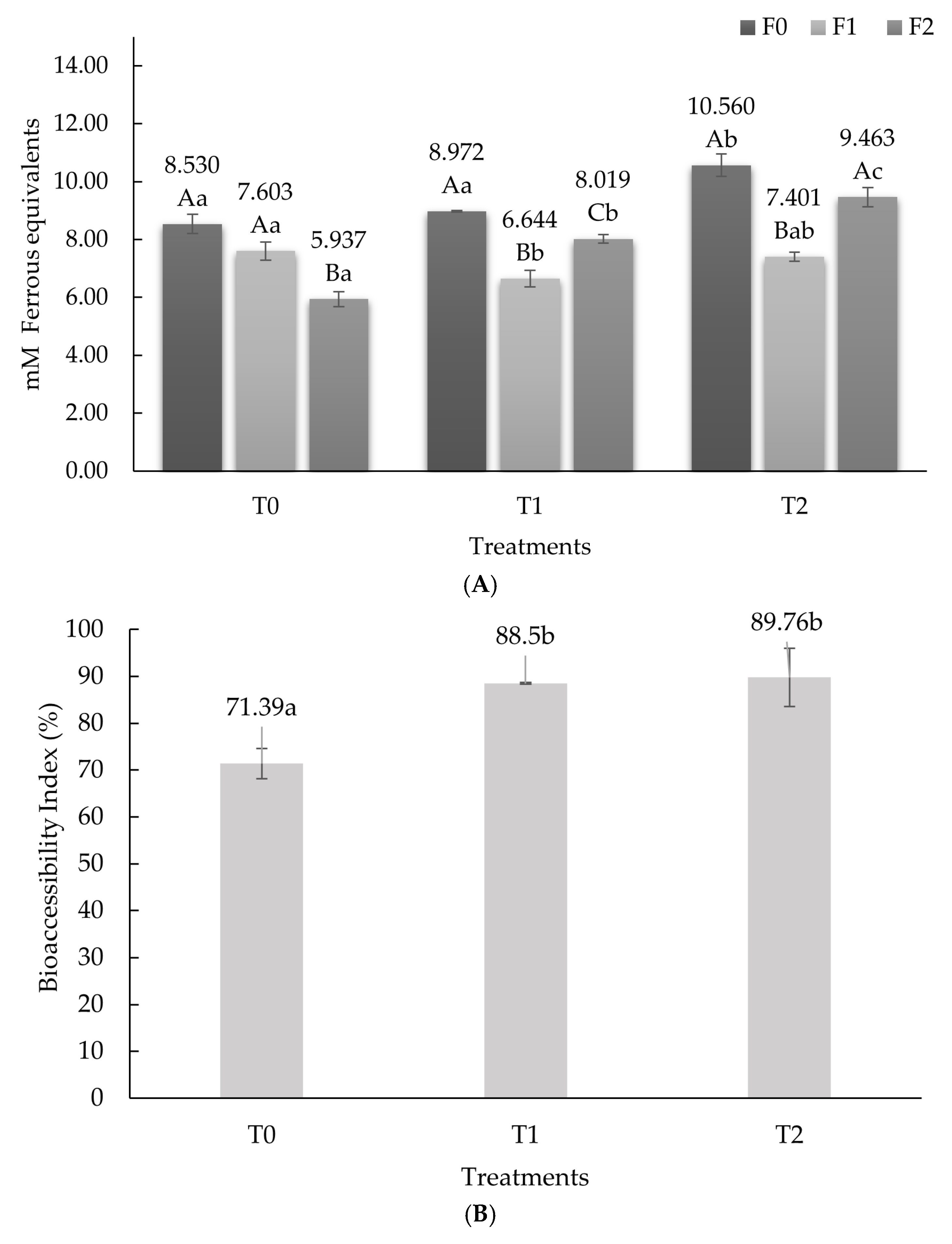
3.1. Antioxidant Activity
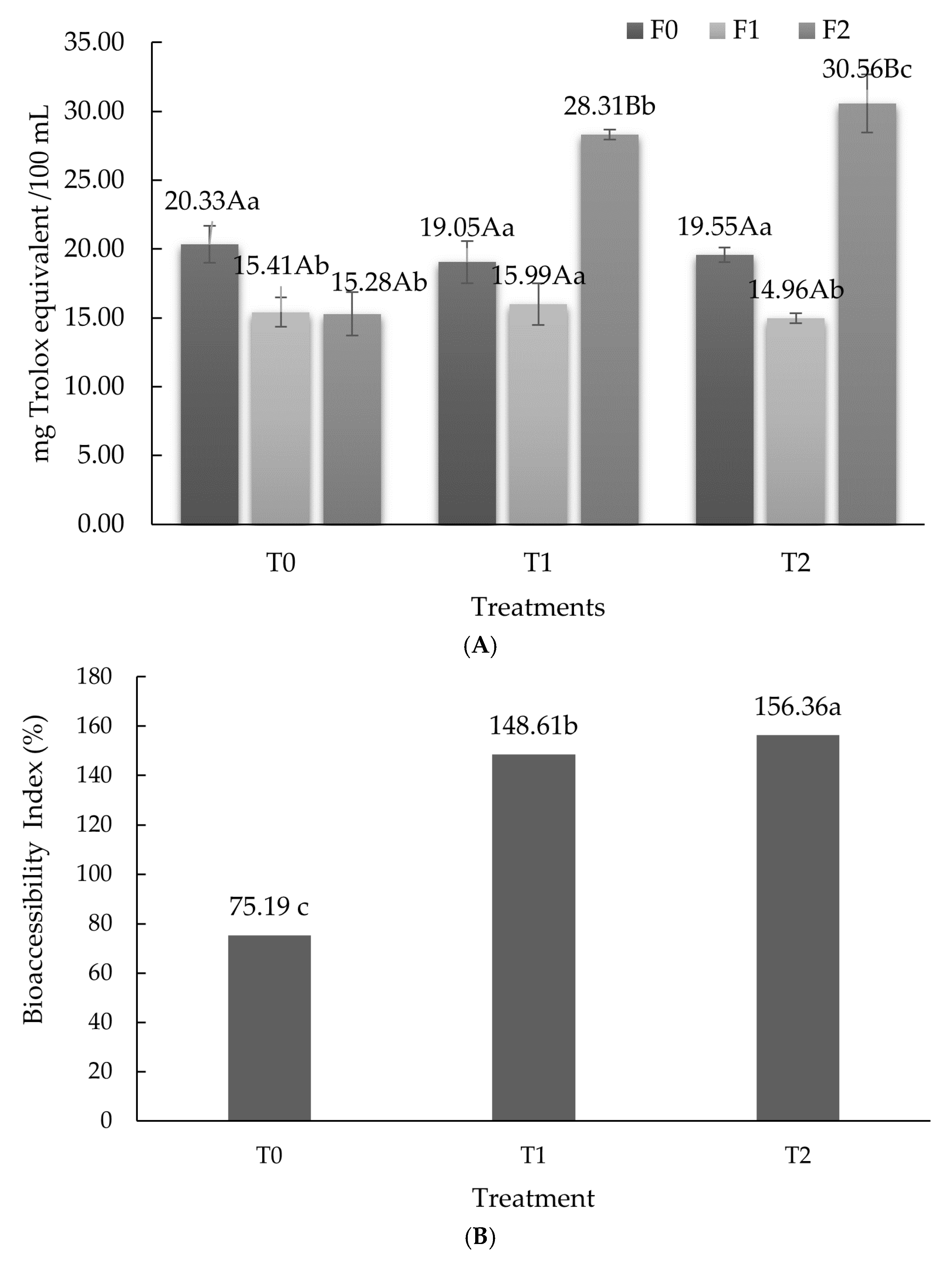
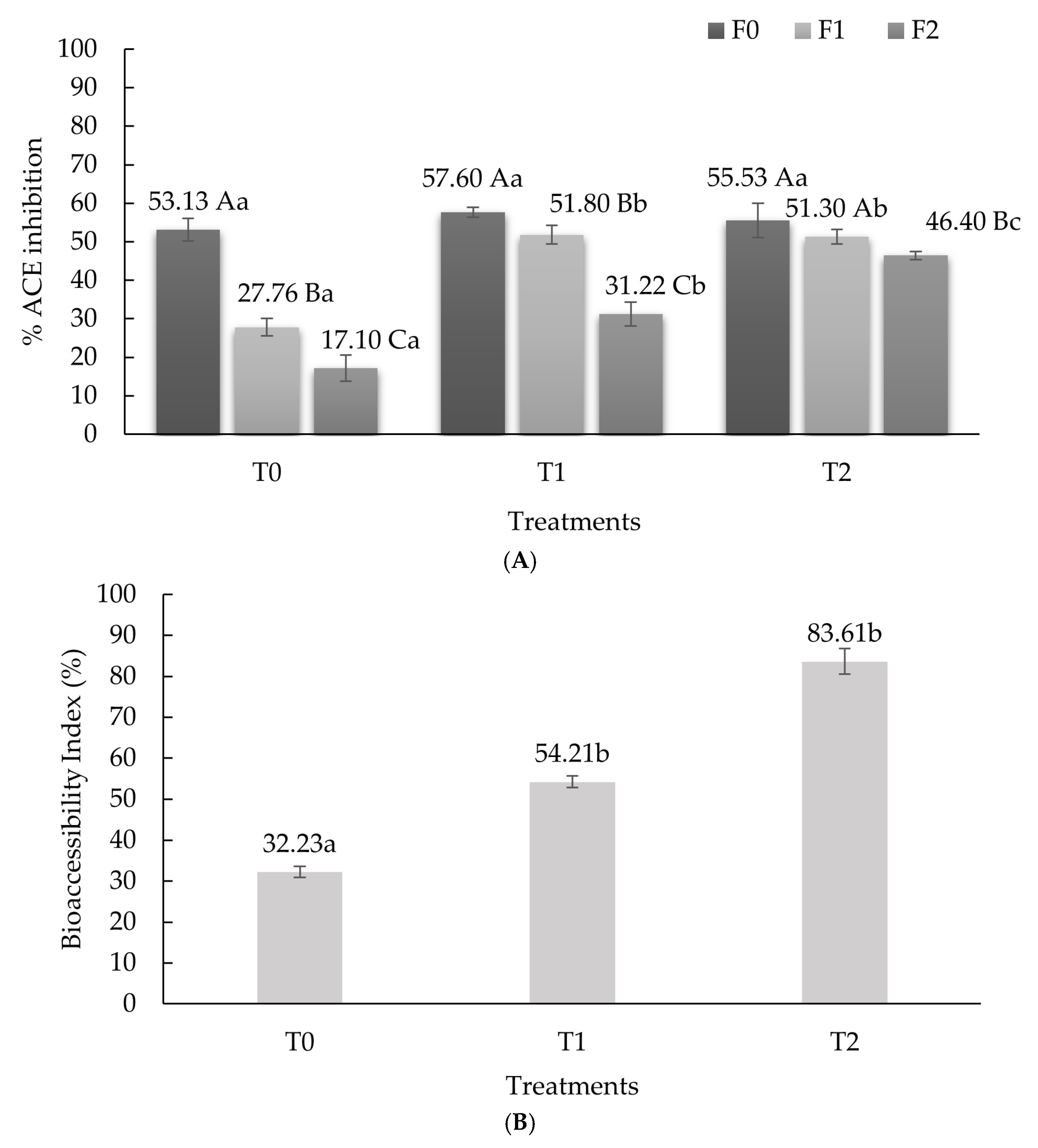
3.2. Angiotensin Converting Enzyme-Inhibitory Activity
3.3. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.Y.; Zhou, D.D.; Gan, R.Y.; Huang, S.Y.; Zhao, C.N.; Shang, A.; Xu, X.Y.; Li, H.B. Effects and mechanisms of probiotics, prebiotics, synbiotics, and postbiotics on metabolic diseases targeting gut microbiota: A narrative review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Hajipour, N.; Hasannezhad, P.; Baghbanzadeh, A.; Aghebati-Maleki, L. Potential in vivo delivery routes of postbiotics. Crit. Rev. Food Sci. Nutr. 2020, 62, 3345–3369. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidi, M.; Koutelidakis, A.E. Functional Foods and Bioactive Compounds: A Review of Its Possible Role on Weight Management and Obesity’s Metabolic Consequences. Medicines 2019, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Miano, T. Functional Food—A Review. Eur. J. Res. 2016, IV, 5695–5702. [Google Scholar]
- Guimarães, J.T.; Balthazar, C.F.; Scudino, H.; Pimentel, T.C.; Esmerino, E.A.; Ashokkumar, M.; Freitas, M.Q.; Cruz, A.G. High-intensity ultrasound: A novel technology for the development of probiotic and prebiotic dairy products. Ultrason. Sonochem. 2019, 57, 12–21. [Google Scholar] [CrossRef]
- Homayouni Rad, A.; Aghebati Maleki, L.; Samadi Kafil, H.; Fathi Zavoshti, H.; Abbasi, A. Postbiotics as novel health-promoting ingredients in functional foods. Health Promot. Perspect. 2020, 10, 3–4. [Google Scholar] [CrossRef]
- Rafique, N.; Jan, S.Y.; Dar, A.H.; Dash, K.K.; Sarkar, A.; Shams, R.; Pandey, V.K.; Khan, S.A.; Amin, Q.A.; Hussain, S.Z. Promising bioactivities of postbiotics: A comprehensive review. J. Agric. Food Res. 2023, 14, 100708. [Google Scholar] [CrossRef]
- Mosca, A.; Abreu YAbreu, A.T.; Gwee, K.A.; Ianiro, G.; Tack, J.; Nguyen, T.V.H.; Hill, C. The clinical evidence for postbiotics as microbial therapeutics. Gut Microbes 2022, 14, 2117508. [Google Scholar] [CrossRef]
- Ma, L.; Tu, H.; Chen, T. Postbiotics in Human Health: A Narrative Review. Nutrients 2023, 15, 291. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020, 19, 1–22. [Google Scholar] [CrossRef]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Noori, S.M.A.; Behfar, A.; Saadat, A.; Ameri, A.; Yazdi, S.S.A.; Saadat, A. Antimicrobial and Antioxidant Properties of Natural Postbiotics Derived from Five Lactic Acid Bacteria. Jundishapur J. Nat. Pharm. Prod. 2023, 18, e130785. [Google Scholar] [CrossRef]
- Khani, N.; Shkouhian, S.M.J.; Kafil, H.S.; Gilani, N.; Abbasi, A.; Rad, A.H. Assessing the growth-inhibitory activity of postbiotics of Lactobacillus spp. against Staphylococcus aureus under in vitro circumstances and food model. Lett. Appl. Microbiol. 2023, 76, ovac056. [Google Scholar] [CrossRef]
- Hosseini, S.; Homayouni-Rad, A.; Samadi Kafil, H.; Dorud, N. Evaluating the antimicrobial effect of postbiotic extract from Lactobacillus casei on Escherichia coli in commercial sterilized milk. J. Food Hyg. Saf. 2022, 8, 42–52. [Google Scholar] [CrossRef]
- Gómez-Guzmán, M.; Toral, M.; Romero, M.; Jiménez, R.; Galindo, P.; Sánchez, M.; Zarzuelo, M.J.; Olivares, M.; Gálvez, J.; Duarte, J. Antihypertensive effects of probiotics Lactobacillus strains in spontaneously hypertensive rats. Mol. Nutr. Food Res. 2015, 59, 2326–2336. [Google Scholar] [CrossRef]
- Robles-Vera, I.; Toral, M.; Romero, M.; Jiménez, R.; Sánchez, M.; Pérez-Vizcaíno, F.; Duarte, J. Antihypertensive Effects of Probiotics. Curr. Hypertens. Rep. 2017, 19, 26. [Google Scholar] [CrossRef]
- Jama, H.A.; Rhys-Jones, D.; Nakai, M.; Yao, C.K.; Climie, R.E.; Sata, Y.; Anderson, D.; Creek, D.J.; Head, G.A.; Kaye, D.M.; et al. Prebiotic intervention with HAMSAB in untreated essential hypertensive patients assessed in a phase II randomized trial. Nat. Cardiovasc. Res. 2023, 2, 35–43. [Google Scholar] [CrossRef]
- Ahmad, H.; Khan, H.; Haque, S.; Ahmad, S.; Srivastava, N.; Khan, A. Angiotensin-Converting Enzyme and Hypertension: A Systemic Analysis of Various ACE Inhibitors, Their Side Effects, and Bioactive Peptides as a Putative Therapy for Hypertension. JRAAS—J. Renin-Angiotensin-Aldosterone Syst. 2023, 2023, 7890188. [Google Scholar] [CrossRef]
- Santana Andrade, J.K.; Chagas Barros, R.G.; Pereira, U.C. Bioaccessibility of bioactive compounds after in vitro gastrointestinal digestion and probiotics fermentation of Brazilian fruits residues with antioxidant and antidiabetic potential. LWT-Food Sci. Technol. 2022, 153, 112469. [Google Scholar] [CrossRef]
- Bolivar-Jacobo, N.A.; Reyes-Villagrana, R.A.; Rentería-Monterrubio, A.L.; Sánchez-Vega, R.; Santellano-Estrada, E.; Tirado-Gallegos, J.M.; Chávez-Martínez, A. Culture Age, Growth Medium, Ultrasound Amplitude, and Time of Exposure Influence the Kinetic Growth of Lactobacillus acidophilus. Fermentation 2023, 9, 63. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. In vitro bioaccessibility of health-related compounds from a blended fruit juice–soymilk beverage: Influence of the food matrix. J. Funct. Foods 2014, 7, 161–169. [Google Scholar] [CrossRef]
- Chávez-Alzaga, G.; Reyes-Villagrana, R.A.; Espino-Solis, G.P.; Arévalos-Sánchez, M.M.; Rentería-Monterrubio, A.L.; Sánchez-Vega, R.; Santellano-Estrada, E.; Bolivar-Jacobo, N.A.; Tirado-Gallegos, J.M.; Chávez-Martínez, A. The Effects of Substrates and Sonication Methods on the Antioxidant Activity of Kefir Postbiotics. Fermentation 2024, 10, 492. [Google Scholar] [CrossRef]
- Číž, M.; Čížová, H.; Denev, P.; Kratchanova, M.; Slavov, A.; Lojek, A. Different Methods for Control and Comparison of the Antioxidant Properties of Vegetables. Food Control 2010, 21, 518–523. [Google Scholar] [CrossRef]
- Donkor, O.N.; Henriksson, A.; Vasiljevic, T.; Shah, N.P. Probiotic Strains as Starter Cultures Improve Angiotensin-converting Enzyme Inhibitory Activity in Soy Yogurt. J. Food Sci. 2005, 70, m375–m381. [Google Scholar] [CrossRef]
- Mushtaq, M.; Gani, A.; Masoodi, F.A. Himalayan cheese (Kalari/Kradi) fermented with different probiotic strains: In vitro investigation of nutraceutical properties. LWT 2019, 104, 53–60. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutiére, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Thennakoon, T.M.E.S.; Abeysinghe1, P.D.; Ranasinghe, P.; Pathirana, R.N.; White, A.; Dilantha-Fernando, W.G.; Abeysinghe, S.; Premakumara, S. Total phenolic content, total flavonoid content and in vitro antioxidant activities measured by the FRAP, ABTS, DPPH and ORAC assays of Sri Lankan black and green tea (Camellia sinensis) infusions. Food Biol. 2022, 11, 1–10. [Google Scholar] [CrossRef]
- Yang, W.; Hao, X.; Zhang, X.; Zhang, G.; Li, X.; Liu, L.; Sun, Y.; Pan, Y. Identification of antioxidant peptides from cheddar cheese made with Lactobacillus helveticus. LWT 2021, 141, 110866. [Google Scholar] [CrossRef]
- Chelladhurai, K.; Ayyash, M.; Turner, M.; Kamal-Eldin, A. Lactobacillus helveticus: Health effects, current applications, and future trends in dairy fermentation. Trends Food Sci. Technol. 2023, 136, 159–168. [Google Scholar] [CrossRef]
- Lin, M.Y.; Yen, C.L. Antioxidative ability of lactic acid bacteria. J. Agric. Food Chem. 1999, 47, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hwang, K.T.; Chung, M.Y.; Cho, D.H.; Park, C.S. Resistance of Lactobacillus casei KCTC 3260 to reactive oxygen species (ROS): Role for a metal ion chelating effect. J. Food Sci. 2005, 70, m388–m391. [Google Scholar] [CrossRef]
- Li, W.; Ji, J.; Chen, X.; Jiang, M.; Rui, X.; Dong, M. Structural elucidation and antioxidant activities of exopolysaccharides from Lactobacillus helveticus MB2-1. Carbohydr. Polym. 2014, 102, 351–359. [Google Scholar] [CrossRef]
- Gagnon, M.; Savard, P.; Rivière, A.; Lapointe, G.; Roy, D. Bioaccessible antioxidants in milk fermented by bifidobacterium longum subsp. longum strains. Biomed. Res. Int. 2015, 2015, 169381. [Google Scholar] [CrossRef]
- Saide, J.A.O.; Gilliland, S.E. Antioxidative activity of lactobacilli measured by oxygen radical absorbance capacity. J. Dairy Sci. 2005, 88, 1352–1357. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Cui, Y.; Wang, L.; Duan, J.; Yang, X.; Liu, X.; Zhang, S.; Sun, B.; Yu, H.; et al. Characteristics of Lactic Acid Bacteria as Potential Probiotic Starters and Their Effects on the Quality of Fermented Sausages. Foods 2024, 13, 198. [Google Scholar] [CrossRef]
- Gu, X.; Wang, H.; Wang, L.; Zhang, K.; Tian, Y.; Wang, X.; Xu, G.; Guo, Z.; Ahmad, S.; Egide, H.; et al. The antioxidant activity and metabolomic analysis of the supernatant of Streptococcus alactolyticus strain FGM. Sci. Rep. 2024, 14, 8413. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspal, M.H. Antioxidant properties of Milk and dairy products: A comprehensive review of the current knowledge. Lipids Health Dis. 2019, 18, 41. [Google Scholar] [CrossRef]
- Stobiecka, M.; Król, J.; Brodziak, A. Antioxidant Activity of Milk and Dairy Products. Animals 2022, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Bielecka, M.; Cichosz, G.; Czeczot, H. Antioxidant, antimicrobial and anticarcinogenic activities of bovine milk proteins and their hydrolysates—A review. Int. Dairy J. 2022, 127, 105208. [Google Scholar] [CrossRef]
- Power, O.; Jakeman, P.; FitzGerald, R.J. Antioxidative peptides: Enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milk-derived antioxidative peptides. Amino Acids 2013, 44, 797e820. [Google Scholar] [CrossRef]
- Katz, D.L.; Doughty, K.; Ali, A. Cocoa and chocolate in human health and disease. Antioxid. Redox Signal. 2011, 15, 2779–2811. [Google Scholar] [CrossRef]
- George, S.; Abrahamse, H. Redox potential of antioxidants in cancer progression and prevention. Antioxidants 2020, 9, 1156. [Google Scholar] [CrossRef]
- Isaac-Bamgboye, F.J.; Mgbechidinma, C.L.; Onyeaka, H.; Isaac-Bamgboye, I.T.; Chukwugozie, D.C. Exploring the Potential of Postbiotics for Food Safety and Human Health Improvement. J. Nutr. Metab. 2024, 2024, 1868161. [Google Scholar] [CrossRef]
- Aggarwal, S.; Sabharwal, V.; Kaushik, P.; Joshi, A.; Aayushi, A.; Suri, M. Probiotics: From emerging concept to application. Front. Sustain. Food Syst. 2022, 6, 887642. [Google Scholar] [CrossRef]
- Moser, A.; Schafroth, K.; Meile, L.; Egger, L.; Badertscher, R.; Irmler, S. Population dynamics of Lactobacillus helveticus in Swiss Gruyère-type cheese manufactured with natural whey cultures. Front. Microbiol. 2018, 9, 637. [Google Scholar] [CrossRef]
- Zhong, Z.; Hu, R.; Zhao, J.; Liu, W.; Kwok, L.Y.; Sun, Z.; Zhang, H.; Chen, Y. Acetate kinase and peptidases are associated with the proteolytic activity of Lactobacillus helveticus isolated from fermented food. Food Microbiol. 2021, 94, 103651. [Google Scholar] [CrossRef]
- Garbowska, M.; Berthold-Pluta, A.; Stasiak-Różańska, L.; Pluta, A. The Impact of the Adjunct Heat-Treated Starter Culture and Lb. helveticus LH-B01 on the Proteolysis and ACE Inhibitory Activity in Dutch-Type Cheese Model during Ripening. Animals 2021, 11, 2699. [Google Scholar] [CrossRef]
- Hati, S.; Patel, N.P.; Pipaliya, R. Bioactivities and production of antihypertensive peptides during fermentation of soy milk by lactic cultures. Rev. Res. Med. Microbiol. 2023, 34, 79–88. [Google Scholar] [CrossRef]
- Ahn, J.; Park, S.; Atwal, A.S.; Gibbs, B.F.; Lee, B. Angiotensin i-converting enzyme (ace) inhibitory peptides from whey fermented by lactobacillus species. J. Food Biochem. 2009, 33, 587–602. [Google Scholar] [CrossRef]
- Minervini, F.; Algaron, F.; Rizzello, C.G.; Fox, P.F.; Monnet, V.; Gobbetti, M. Angiotensin I-converting-enzyme-inhibitory and antibacterial peptides from Lactobacillus helveticus PR4 proteinase-hydrolyzed caseins of milk from six species. Appl. Environ. Microbiol. 2003, 69, 5297–5305. [Google Scholar] [CrossRef]
- Mengzhu, F.; Tingting, G.; Wanru, L.; Jing, C.; Fushuo, L.; Chao, W.; Shi, Y.; Li, D.X.; Shaohui, Z. Isolation and identification of novel casein-derived bioactive peptides and potential functions in fermented casein with Lactobacillus helveticus. Food Sci. Hum. Well. 2019, 8, 156–176. [Google Scholar] [CrossRef]
- Diop, M.B.; Alvarez, V.B.; Guiro, A.T.; Thonart, P. Efficiency of neutralized antibacterial culture supernatant from bacteriocinogenic lactic acid bacteria supplemented with salt in control of microorganisms present in Senegalese artisanally handled fish by immersion preservative technology during Guedj seafood processing at 10 °C and 30 °C. J. Food Microbiol. Saf. Hyg. 2016, 1, 102. [Google Scholar] [CrossRef]
- Abedi, D.; Feizizadeh, S.; Akbari, V.; Jafarian-dehkordi, A. In vitro anti-bacterial and anti-adherence effects of Lactobacillus delbrueckii subsp bulgaricus on Escherichia coli. Res. Pharm. Sci. 2013, 8, 260–268. [Google Scholar]
- Abbasi, A.; Hashemi, M.; Pourjafar, H.; Hosseini, S.M.; Kafil, H.S.; Rad, A.H.; Taghizadeh, M.; Hosseini, H. Chemical Characterization, Cell-Based Safety, and Antioxidant Assessments of Lactobacillus helveticus Postbiotics and Their Potential Antibacterial Effects and Mode of Action Against Food-Borne Multidrug-Resistant Staphylococcus aureus and Enterohaemorrhagic Escherichia coli O157:H7. J. Food Saf. 2024, 44, e13174. [Google Scholar] [CrossRef]
- Kishilova, S.A.; Kolokolova, A.Y.; Rozhkova, I.V. Antimicrobial Activity of Metabolite Complexes of Lactobacillus against Pseudomonas aeruginosa. Biofizika 2024, 69, 324–332. [Google Scholar] [CrossRef]
- Khani, N.; Abedi Soleimani, R.; Homayouni-Rad, A. Characterization and Antimicrobial Activity of Postbiotic from Lactobacillus Acidophilus LA5 on Staphylococcus Aureus in Food Model and In vitro. Curr. Nutr. Food Sci. 2025, 21, e15734013305717. [Google Scholar] [CrossRef]
- Mirnejad, R.; Vahdati, A.R.; Rashidiani, J.; Erfani, M.; Piranfar, V. The antimicrobial effect of Lactobacillus casei culture supernatant against multiple drug-resistant clinical isolates of Shigella sonnei and Shigella flexneri in vitro. Iran. Red Crescent Med. J. 2013, 15, 122–126. [Google Scholar] [CrossRef]
- Gálvez, A.; Abriouel, H.; López, R.L.; Ben Omar, N. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef]

| Microwell | Buffer Solution | Water | Sample | ACE |
|---|---|---|---|---|
| A | 100 μL | 40 μL | ------- | 20 μL |
| B | 100 μL | ------- | 40 μL | ------- |
| C | 100 μL | 20 μL | 40 μL | 20 μL |
| Postbiotics | Microorganism | ||
|---|---|---|---|
| Bacillus spp. | S. aureus | E. coli | |
| Lactobacillus acidophilus | 11.66 ± 0.57 b | 12.33 ± 0.57 a | -- |
| Lactobacillus helveticus | 13.33 ± 0.57 a | 12.33 ± 0.57 a | 11.667 ± 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolivar-Jacobo, N.A.; Reyes-Villagrana, R.A.; Arévalos-Sánchez, M.M.; Rentería-Monterrubio, A.L.; Santellano-Estrada, E.; Salas-Salazar, N.A.; Chávez-Martínez, A. Antioxidant and ACE-Inhibition Activities After In Vitro Digestion of a Non-Fermented Dairy Beverage Enriched with Postbiotics of Lactobacillus spp. Fermentation 2025, 11, 223. https://doi.org/10.3390/fermentation11040223
Bolivar-Jacobo NA, Reyes-Villagrana RA, Arévalos-Sánchez MM, Rentería-Monterrubio AL, Santellano-Estrada E, Salas-Salazar NA, Chávez-Martínez A. Antioxidant and ACE-Inhibition Activities After In Vitro Digestion of a Non-Fermented Dairy Beverage Enriched with Postbiotics of Lactobacillus spp. Fermentation. 2025; 11(4):223. https://doi.org/10.3390/fermentation11040223
Chicago/Turabian StyleBolivar-Jacobo, Norma Angélica, Raúl Alberto Reyes-Villagrana, Martha María Arévalos-Sánchez, Ana Luisa Rentería-Monterrubio, Eduardo Santellano-Estrada, Nora Aidee Salas-Salazar, and América Chávez-Martínez. 2025. "Antioxidant and ACE-Inhibition Activities After In Vitro Digestion of a Non-Fermented Dairy Beverage Enriched with Postbiotics of Lactobacillus spp." Fermentation 11, no. 4: 223. https://doi.org/10.3390/fermentation11040223
APA StyleBolivar-Jacobo, N. A., Reyes-Villagrana, R. A., Arévalos-Sánchez, M. M., Rentería-Monterrubio, A. L., Santellano-Estrada, E., Salas-Salazar, N. A., & Chávez-Martínez, A. (2025). Antioxidant and ACE-Inhibition Activities After In Vitro Digestion of a Non-Fermented Dairy Beverage Enriched with Postbiotics of Lactobacillus spp. Fermentation, 11(4), 223. https://doi.org/10.3390/fermentation11040223







