Amylase Production by the New Strains of Kocuria rosea and Micrococcus endophyticus Isolated from Soil in the Guassa Community Conservation Area
Abstract
:1. Introduction
2. Materials and Methods
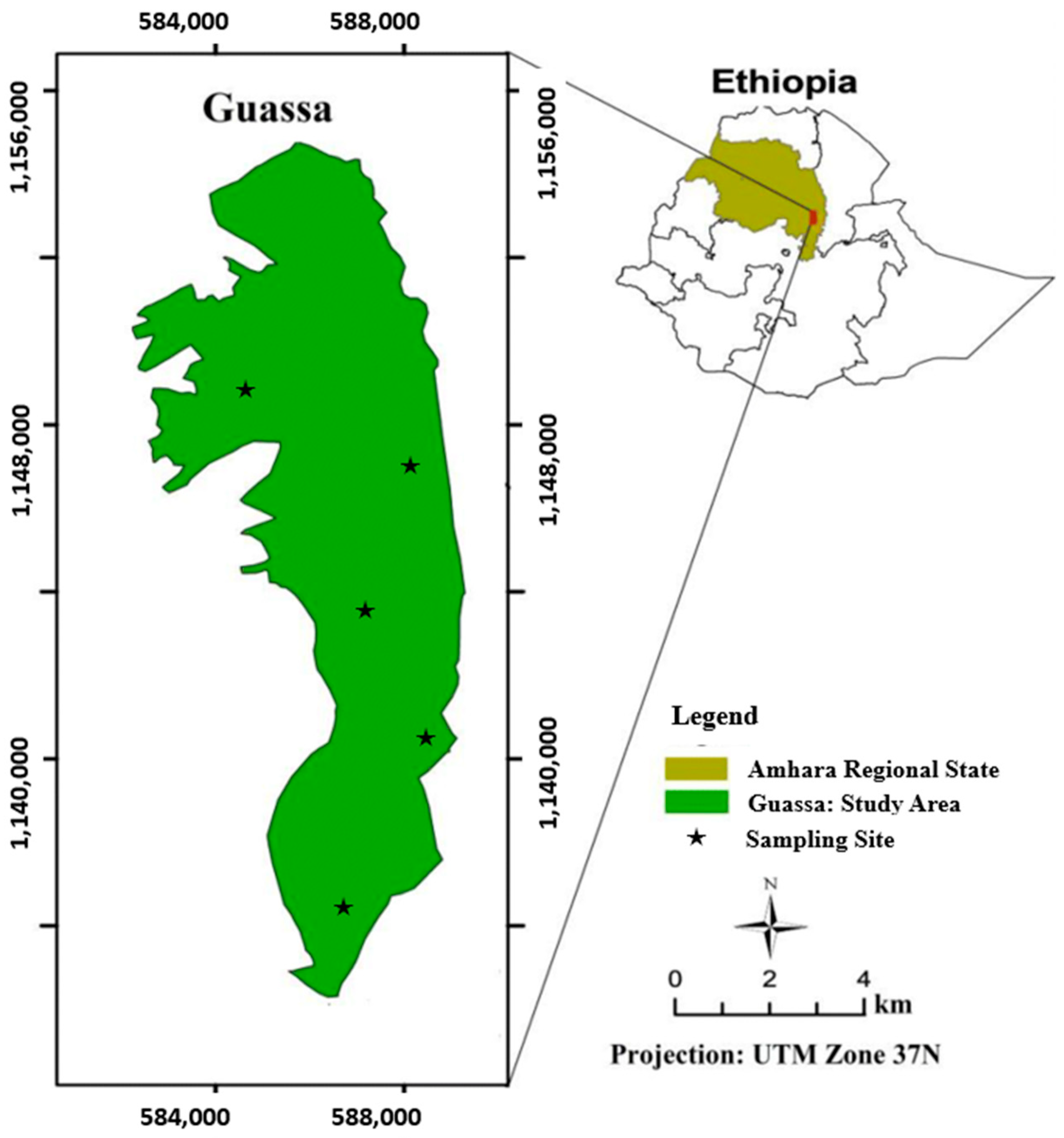
2.1. Description of the Sample Area
2.2. Sample Collection
2.3. Medium Preparation and Isolation of Amylase-Producing Bacteria
2.4. Primary Screening of Strains
2.5. Secondary Screening of Isolates Using Submerged Fermentation
2.6. Utilizing MALDI-TOF for the Identification of Isolates
2.7. Crude Amylase Separation and Extraction
2.8. Amylase Activity Assay
2.9. Biochemical Characterization of Bacteria
2.9.1. Motility Test
2.9.2. Gram Staining Test
2.9.3. Catalase Test
2.9.4. Citrate Utilization Test
2.10. Effect of Nitrogen and Carbon Sources
2.11. Process Optimization for Amylase Activity
2.11.1. Effect of Metal Ions
2.11.2. Effects of Various Buffer Solutions on Enzyme Activity
2.11.3. Effect of Temperature on Amylase Activity
2.12. Application of Amylase in Dough Leavening
2.13. Data Analysis
3. Results
3.1. Isolation and Purification of Amylase Enzyme-Producing Bacteria
3.2. Morphological and Biochemical Characteristics of Isolates
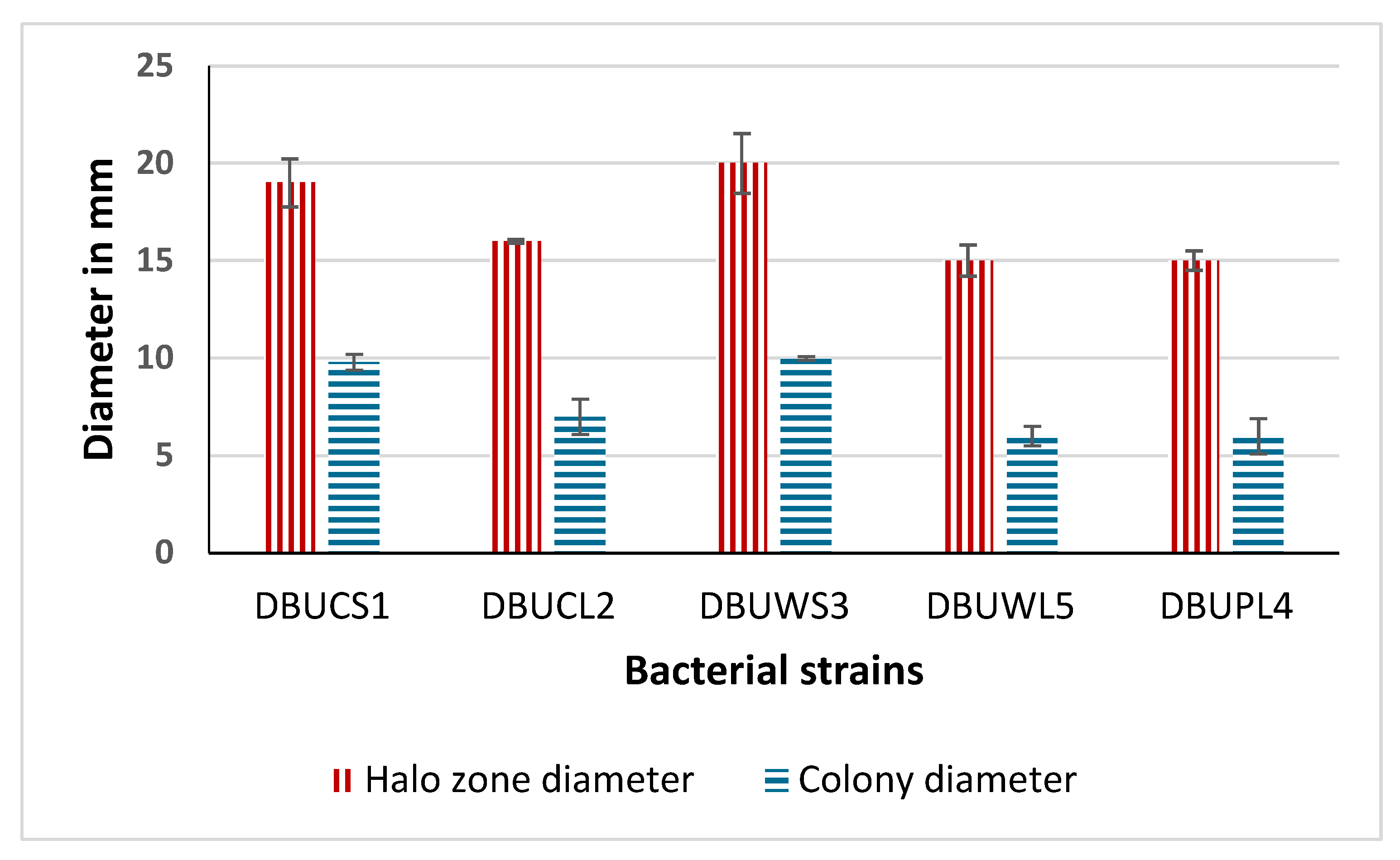
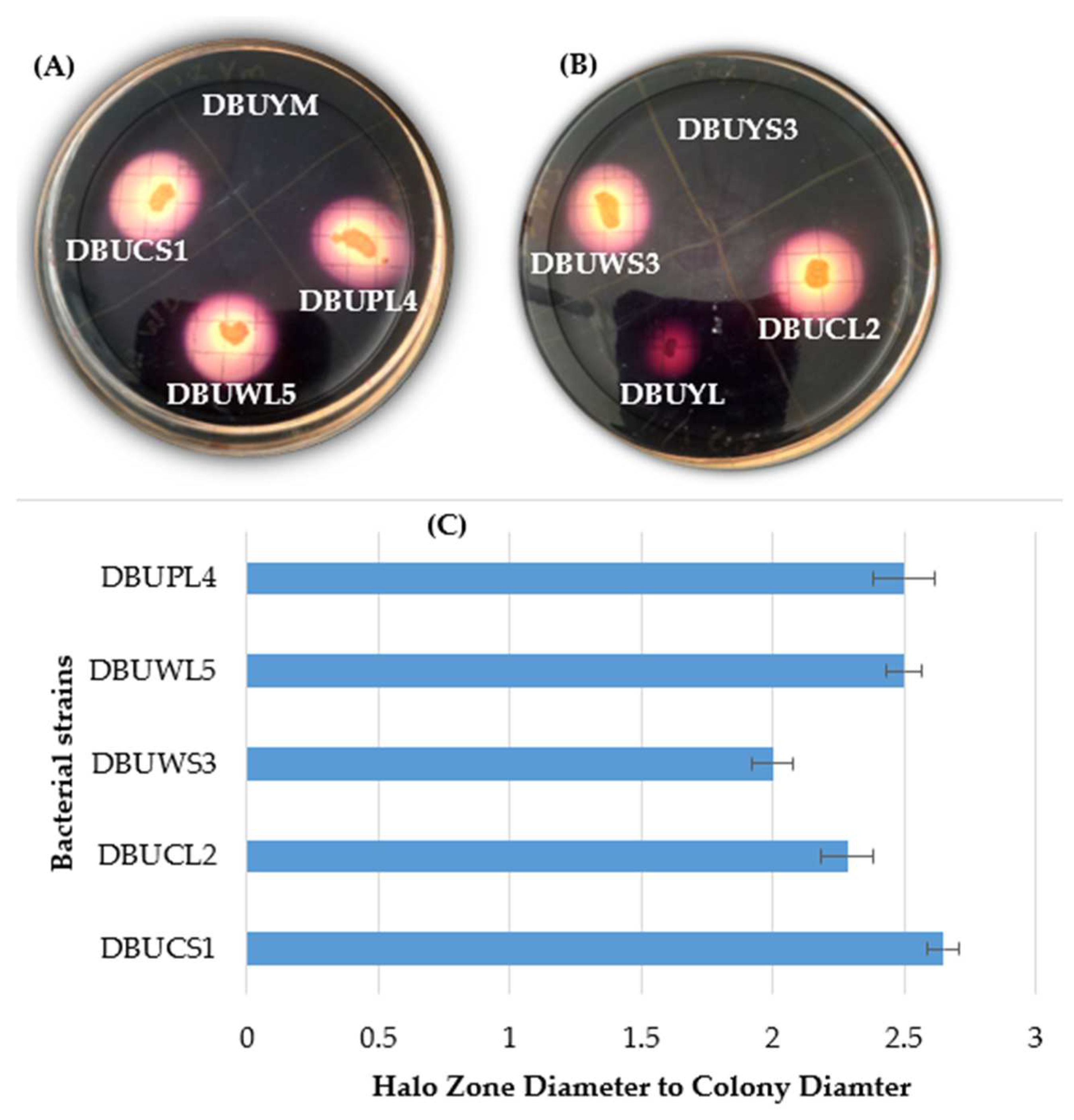
3.3. Primary Screening for Amylase Production
3.4. Bacterial Identification
3.5. Optimization of Growth of Bacteria
3.5.1. Effect of Incubation Period on Growth of Isolates
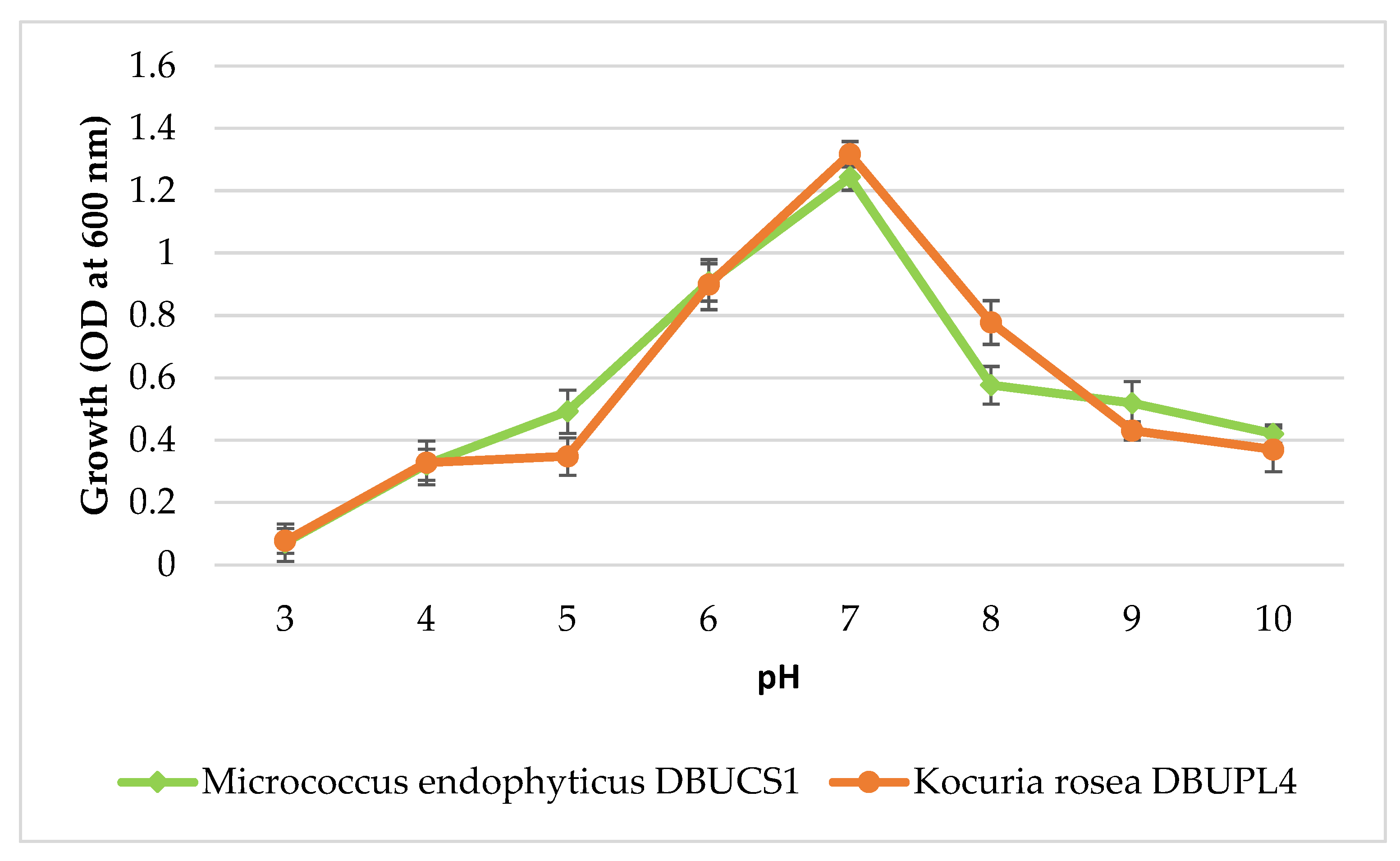
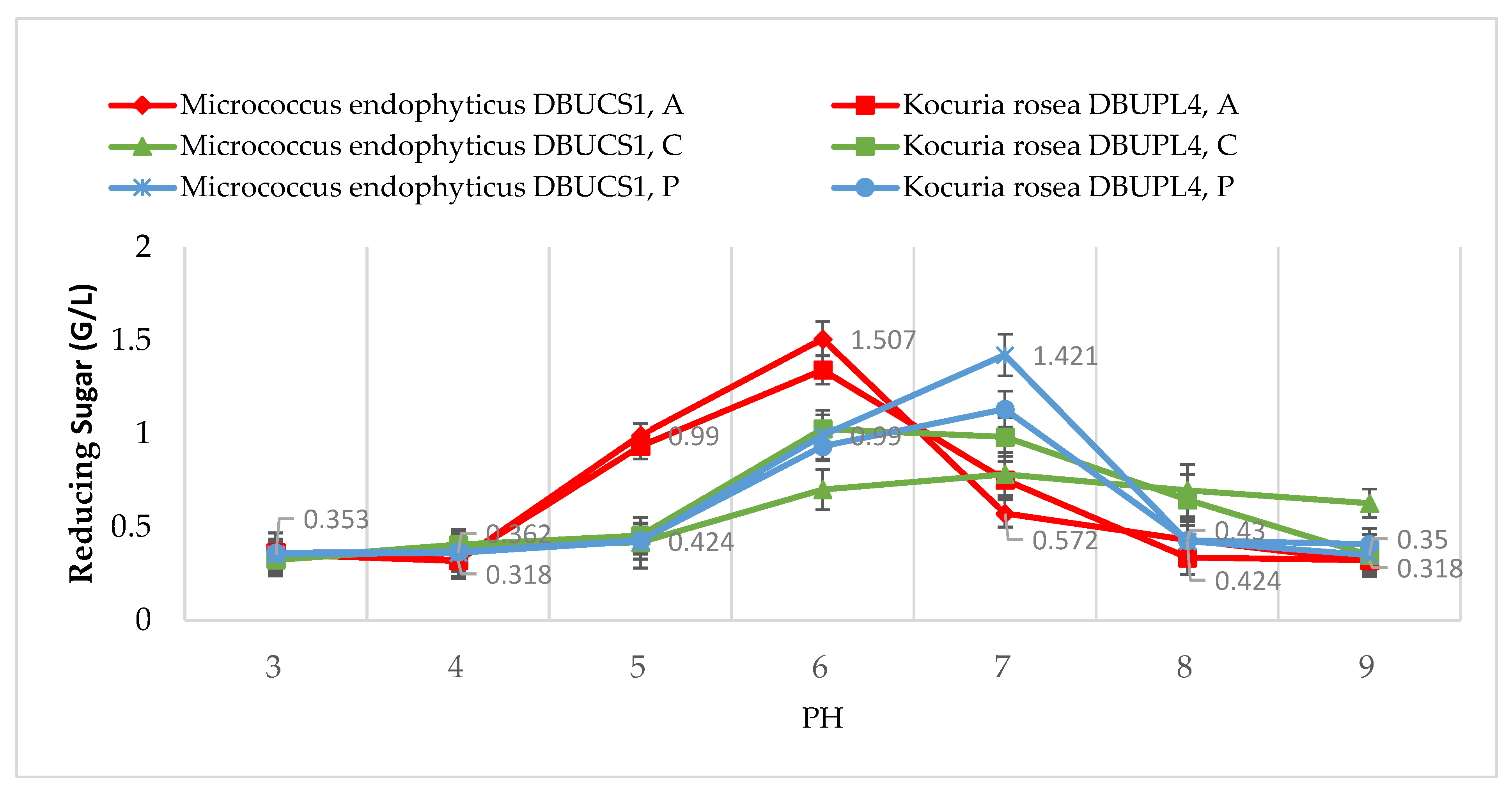
3.5.2. Effect of pH on Isolate Growth
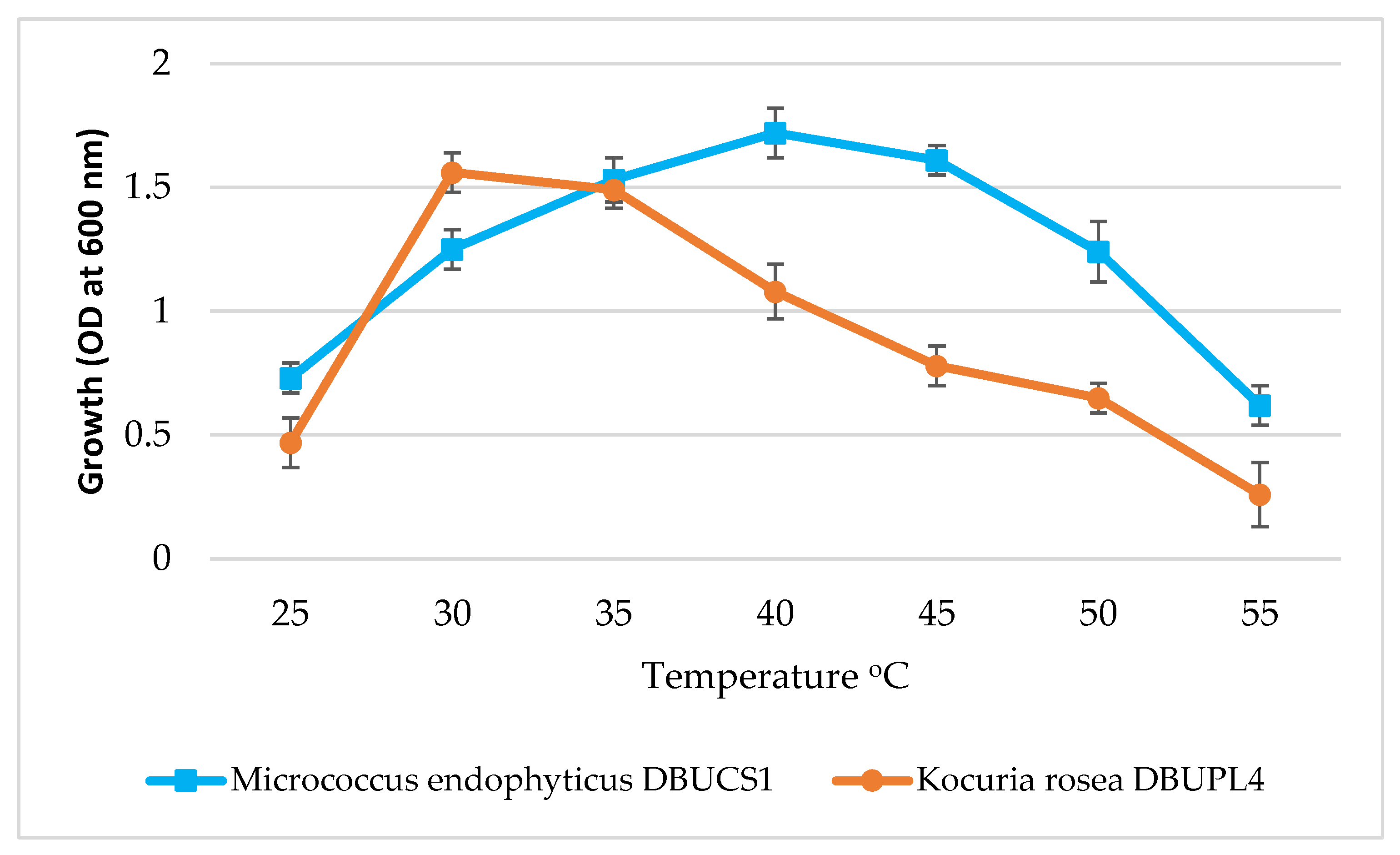
3.5.3. Effect of Temperature on Isolate Growth
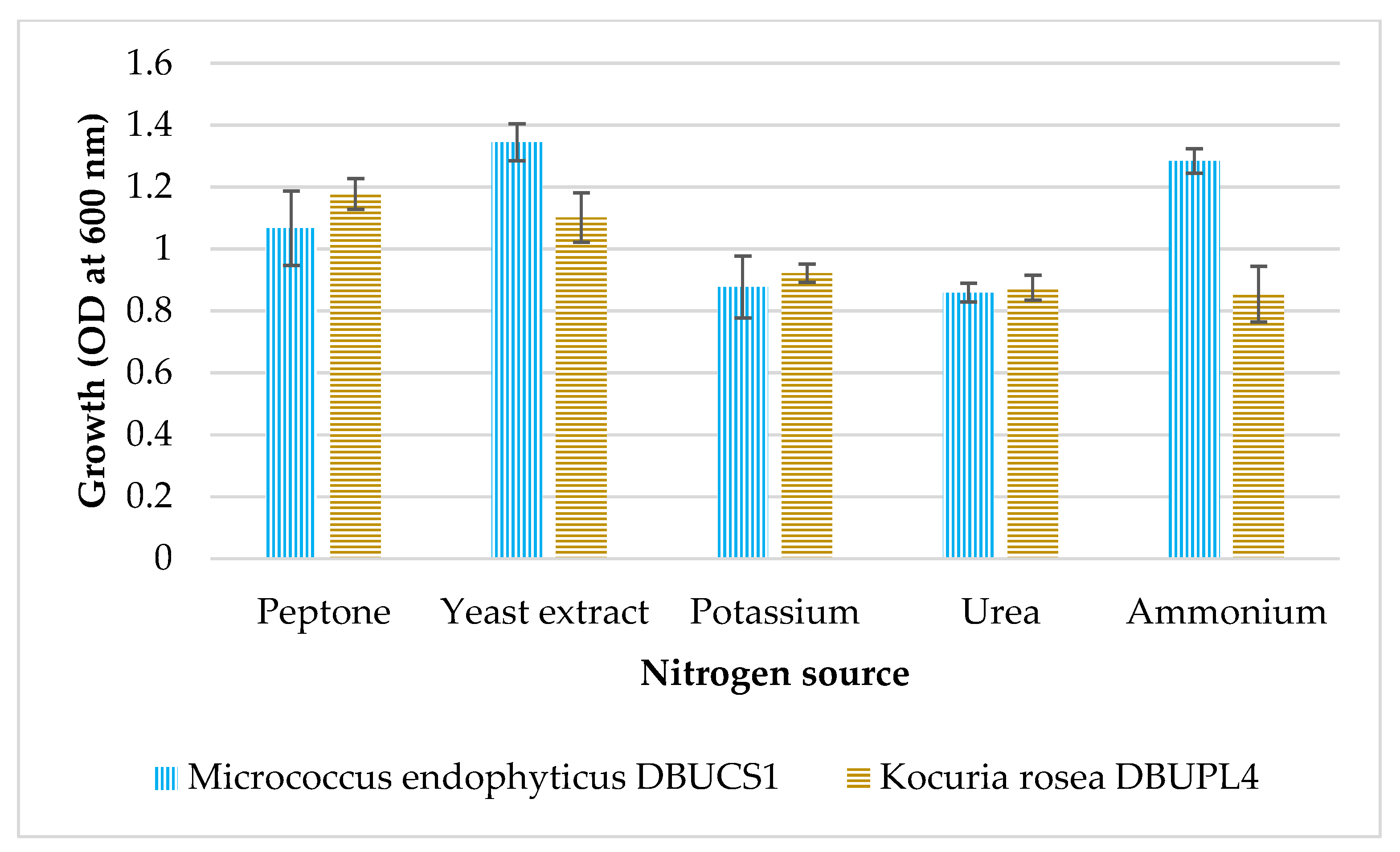
3.5.4. Effect of Nitrogen Source on Growth of Isolates
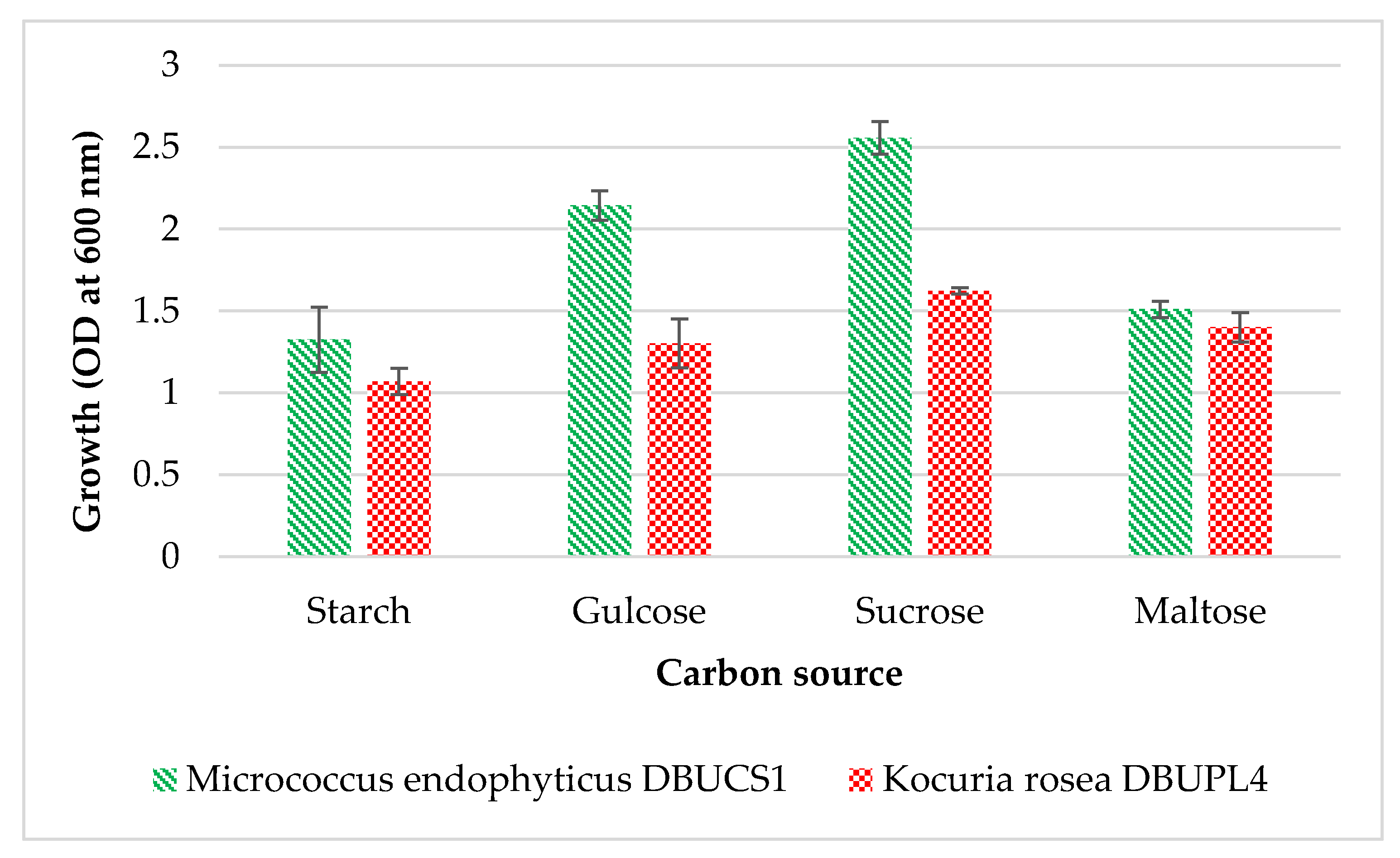
3.5.5. Effect of Carbon Source on Growth of Isolates
3.6. Process Optimization for Amylase Activities
3.6.1. Effect of Temperature on Amylase Activity
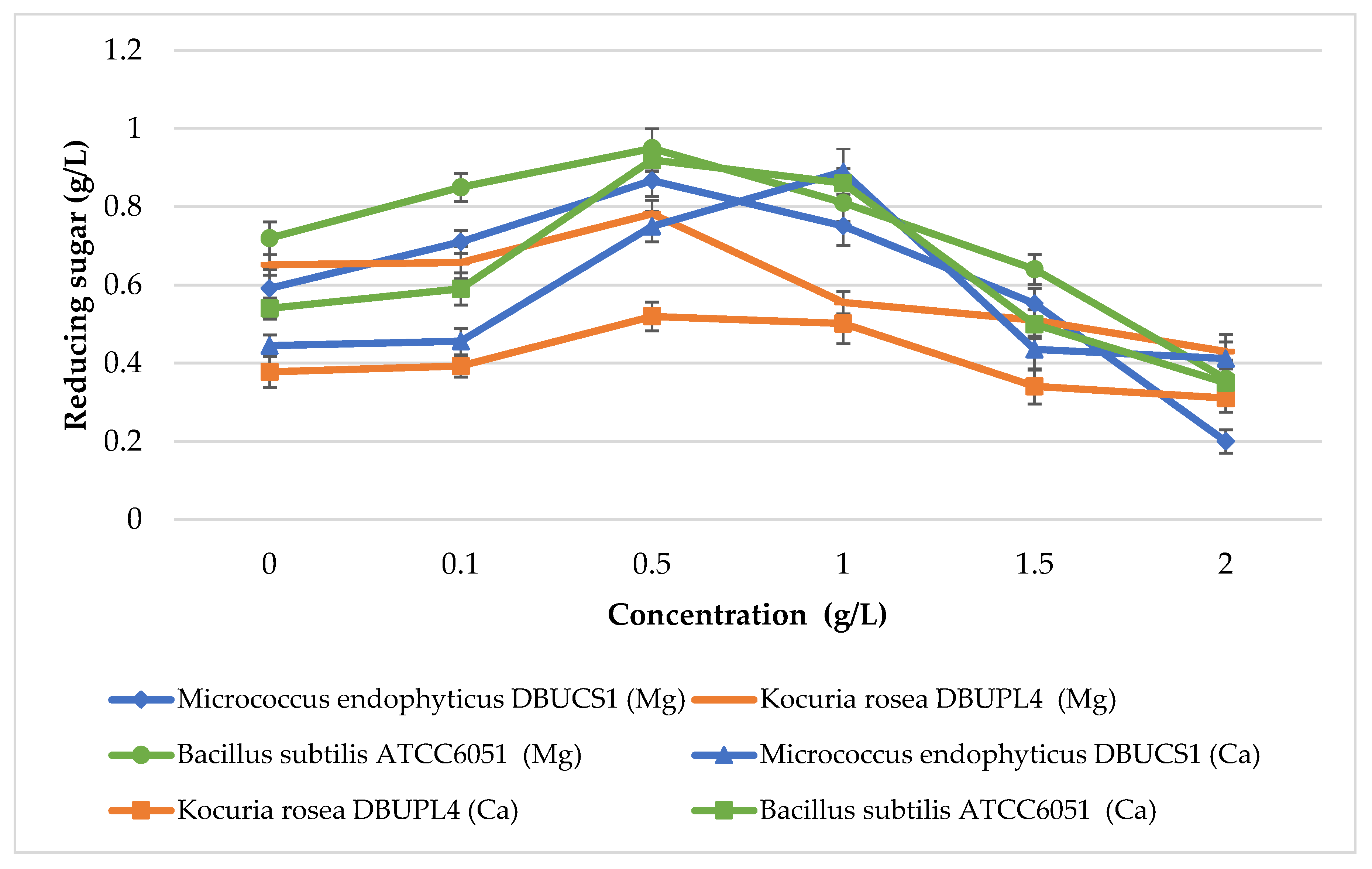
3.6.2. Effect of Metal Ions on Amylase Activity
3.6.3. Effect of Buffers on Amylase Activity
3.7. Application of Amylase in Wheat Flour Dough Leavening Process
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farooq, M.A.; Ali, S.; Hassan, A.; Tahir, H.M.; Mumtaz, S.; Mumtaz, S. Biosynthesis and industrial applications of α-amylase: A review. Arch. Microbiol. 2021, 203, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Morbia, M.; Pandey, A.; Mahla, P.; Gohil, S. Isolation of α-Amylase Producing Microorganisms from Soil of Kachchh, Gujarat. J. Pure Appl. Microbiol. 2024, 18, 9218. [Google Scholar] [CrossRef]
- Lim, S.J.; Oslan, S.N. Native to designed: Microbial-amylases for industrial applications. PeerJ 2021, 9, e11315. [Google Scholar] [CrossRef] [PubMed]
- Yassin, S.N.; Jiru, T.M.; Indracanti, M. Screening and Characterization of Thermostable Amylase-Producing Bacteria Isolated from Soil Samples of Afdera, Afar Region, and Molecular Detection of Amylase-Coding Gene. Int. J. Microbiol. 2021, 2021, 5592885. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Q.; Yan, P.; Dong, C.; Shao, Z. Isolation and Optimization of Aflatoxin B(1) Degradation by Uniform Design and Complete Genome Sequencing of Novel Deep-Sea Kocuria rosea Strain 13. Toxins 2023, 15, 520. [Google Scholar] [CrossRef] [PubMed]
- Parshetti, G.K.; Parshetti, S.; Kalyani, D.C.; Doong, R.-a.; Govindwar, S.P. Industrial dye decolorizing lignin peroxidase from Kocuria rosea MTCC 1532. Ann. Microbiol. 2012, 62, 217–223. [Google Scholar] [CrossRef]
- Gu, D.; Jiao, Y.; Wu, J.; Liu, Z.; Chen, Q. Optimization of EPS Production and Characterization by a Halophilic Bacterium, Kocuria rosea ZJUQH from Chaka Salt Lake with Response Surface Methodology. Molecules 2017, 22, 814. [Google Scholar] [CrossRef] [PubMed]
- Bernal, C.; Cairó, J.; Coello, N. Purification and characterization of a novel exocellular keratinase from Kocuria rosea. Enzym. Microb. Technol. 2006, 38, 49–54. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Telke, A.A.; Kalyani, D.C.; Govindwar, S.P. Decolorization and detoxification of sulfonated azo dye methyl orange by Kocuria rosea MTCC 1532. J. Hazard. Mater. 2010, 176, 503–509. [Google Scholar] [CrossRef]
- Elamary, R.; Salem, W.M. Optimizing and purifying extracellular amylase from soil bacteria to inhibit clinical biofilm-forming bacteria. PeerJ 2020, 8, e10288. [Google Scholar] [CrossRef]
- Chen, H.-H.; Zhao, G.-Z.; Park, D.-J.; Zhang, Y.-Q.; Xu, L.-H.; Lee, J.-C.; Kim, C.-J.; Li, W.-J. Micrococcus endophyticus sp. nov., isolated from surface-sterilized Aquilaria sinensis roots. Int. J. Syst. Evol. Microbiol. 2009, 59, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Pandit, B.; Moin, A.; Mondal, A.; Banik, A.; Alam, M. Characterization of a biofilm-forming, amylase-producing, and heavy-metal-bioremediating strain Micrococcus sp. BirBP01 isolated from oligotrophic subsurface lateritic soil. Arch. Microbiol. 2023, 205, 351. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, S.M.; Dhakephalkar, P. Diversity, cold active enzymes and adaptation strategies of bacteria inhabiting glacier cryoconite holes of High Arctic. Extremophiles 2014, 18, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Dashti, N.; Khanafer, M.; Al-Awadhi, H.; Radwan, S. Bioremediation of soils saturated with spilled crude oil. Sci. Rep. 2020, 10, 1116. [Google Scholar] [CrossRef]
- Rustamova, N.; Wubulikasimu, A.; Mukhamedov, N.; Gao, Y.; Egamberdieva, D.; Yili, A. Endophytic Bacteria Associated with Medicinal Plant Vernonia anthelmintica: Diversity and Characterization. Curr. Microbiol. 2020, 77, 1457–1465. [Google Scholar] [CrossRef]
- Klinfoong, R.; Thummakasorn, C.; Ungwiwatkul, S.; Boontanom, P.; Chantarasiri, A. Diversity and activity of amylase-producing bacteria isolated from mangrove soil in Thailand. Biodiversitas J. Biol. Divers. 2022, 23, 5519–5531. [Google Scholar] [CrossRef]
- Choi, H.J.; Hong, J.B.; Park, J.J.; Chi, W.-J.; Kim, M.-C.; Chang, Y.-K.; Hong, S.-K. Production of agarase from a novel Micrococcus sp. GNUM-08124 strain isolated from the East Sea of Korea. Biotechnol. Bioprocess Eng. 2011, 16, 81–88. [Google Scholar] [CrossRef]
- Ariaeenejad, S.; Zolfaghari, B.; Sadeghian Motahar, S.F.; Kavousi, K.; Maleki, M.; Roy, S.; Hosseini Salekdeh, G. Highly Efficient Computationally Derived Novel Metagenome α-Amylase With Robust Stability Under Extreme Denaturing Conditions. Front. Microbiol. 2021, 12, 713125. [Google Scholar] [CrossRef]
- Ju, L.; Pan, Z.; Zhang, H.; Li, Q.; Liang, J.; Deng, G.; Yu, M.; Long, H. New insights into the origin and evolution of α-amylase genes in green plants. Sci. Rep. 2019, 9, 4929. [Google Scholar] [CrossRef]
- Far, B.E.; Ahmadi, Y.; Khosroshahi, A.Y.; Dilmaghani, A. Microbial alpha-amylase production: Progress, challenges and perspectives. Adv. Pharm. Bull. 2020, 10, 350. [Google Scholar]
- Gupta, R.; Gigras, P.; Mohapatra, H.; Goswami, V.K.; Chauhan, B. Microbial α-amylases: A biotechnological perspective. Process Biochem. 2003, 38, 1599–1616. [Google Scholar] [CrossRef]
- Souza, P.M.d. Application of microbial α-amylase in industry-A review. Braz. J. Microbiol. 2010, 41, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Hallol, M.; Helmy, O.; Shawky, A.-E.; El-Batal, A.; Ramadan, M. Optimization of Alpha-Amylase Production by a Local Bacillus paramycoides Isolate and Immobilization on Chitosan-Loaded Barium Ferrite Nanoparticles. Fermentation 2022, 8, 241. [Google Scholar] [CrossRef]
- Shad, M.; Hussain, N.; Usman, M.; Akhtar, M.; Sajjad, M. Exploration of computational approaches to predict the structural features and recent trends in α-amylase production for industrial applications. Biotechnol. Bioeng. 2023, 120, 2092–2116. [Google Scholar] [CrossRef]
- Nigussie, G.; Fekadu, M.; Demissew, S.; Warkineh, B. Impact of conservation management on land change: A case study in Guassa Community Conservation Area for the last 31 years (1986–2015). Model. Earth Syst. Environ. 2019, 5, 1495–1504. [Google Scholar] [CrossRef]
- Tadesse, S.; Teketay, D. Contribution of the Guassa Community Eco-Lodge to the Conservation of Wild Mammals, Birds and Woody Plants in Menz-Gera Midir District, North Shewa Administrative Zone, Ethiopia. Int J Avian Wildl. Biol 2017, 2, 00029. [Google Scholar] [CrossRef]
- Abd-Elhalem, B.T.; El-Sawy, M.; Gamal, R.F.; Abou-Taleb, K.A. Production of amylases from Bacillus amyloliquefaciens under submerged fermentation using some agro-industrial by-products. Ann. Agric. Sci. 2015, 60, 193–202. [Google Scholar] [CrossRef]
- Rakaz, M.A.; Hussien, M.O.; Ibrahim, H.M. Isolation, extraction, purification, and molecular characterization for thermostable α-amylase from locally isolated bacillus species in Sudan. Biochem. Res. Int. 2021, 2021, 6670380. [Google Scholar] [CrossRef]
- Çevik, Y.N.; Ogutcu, H. Identification of Bacteria in Soil by MALDI-TOF MS and Analysis of Bacillus spp., Paenibacillus spp. and Pseudomonas spp. with PCA. Anal. Chem. Lett. 2020, 10, 784–797. [Google Scholar] [CrossRef]
- Archna Karel, A.K.; Anshu Karel, A.K.; Jat, B. Studies on vesicular-arbuscular mycorrhiza of trigonella foenum-graecum. Trends Biosci. 2017, 10, 5808–5817. [Google Scholar]
- Tyagi, A.; Kumar, V.; Tomar, A. Isolation, identification, biochemical and antibiotic sensitivity characterization of Rhizobium strains from Vigna mungo (L.) Hepper, Cicer arietinum (L.) and Vigna radiata (L.) Wilczek in Muzaffarnagar, Uttar Pradesh India. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2024–2035. [Google Scholar] [CrossRef]
- Padma, S.; Pallavi, K. Isolation and characterization of amylase producing Bacillus spp. from selected soil sample. Int. J. Res. Biosci. 2016, 5, 24–29. [Google Scholar]
- Lal, N.; Jyoti, J.; Sachan, P. Optimization of nitrogen source (s) for the growth and amylase production from Bacillus licheniformis JAR-26 under submerged fermentation. Indian J. Biol. 2016, 3, 127–132. [Google Scholar] [CrossRef]
- Lakew, E. Characterization of wild yeasts isolated from selected fruits for their bread leavening capacity. Master Dissertation, Addis Ababa University, Addis Ababa, Ethiopia, 2017. Available online: http://etd.aau.edu.et/handle/12345678/1091 (accessed on 10 January 2025).
- Alemayhu, F. Isolating Thermostable Amylase Producing Bacteria from Hot Spring Water in Senbete, Ethiopia and Optimization of Its Production. Master Dissertation, Debre Berhaan University, Debre Berhan, Ethiopia, 2019. [Google Scholar]
- Patel, G. Isolation and characterization of starch degrading bacteria from garden soil, Ganpat University, Gujarat, India. Indian J. Microbiol. Res. 2015, 2, 111–114. [Google Scholar]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T. Biotechnological potential of agro-industrial residues. I: Sugarcane bagasse. Bioresour. Technol. 2000, 74, 69–80. [Google Scholar] [CrossRef]
- Xu, X.; Cheng, Y.; Fang, Z.; Yin, J.; Shen, H.; Ma, D. Identification and utilization of a new Bacillus amyloliquefaciens XY-1 against Fusarium head blight. Front. Plant Sci. 2022, 13, 1055213. [Google Scholar] [CrossRef]
- Gholami, M.; Etemadifar, Z.; Bouzari, M. Isolation a new strain of Kocuria rosea capable of tolerating extreme conditions. J. Environ. Radioact. 2015, 144, 113–119. [Google Scholar] [CrossRef]
- Aarti, C.; Khusro, A.; Agastian, P.; Darwish, N.M.; Al Farraj, D.A. Molecular diversity and hydrolytic enzymes production abilities of soil bacteria. Saudi J. Biol. Sci. 2020, 27, 3235–3248. [Google Scholar] [CrossRef] [PubMed]
- Munawar, A.; Shaheen, M.; Ramzan, S.; Masih, S.A.; Jabeen, F.; Younis, T.; Aslam, M. DIVERISTY and enzymatic potential of indigenous bacteria from unexplored contaminted soils in Faisalabad. Heliyon 2023, 9, e15256. [Google Scholar] [CrossRef]
- Darie-Ion, L.; Whitham, D.; Jayathirtha, M.; Rai, Y.; Neagu, A.-N.; Darie, C.C.; Petre, B.A. Applications of MALDI-MS/MS-based proteomics in biomedical research. Molecules 2022, 27, 6196. [Google Scholar] [CrossRef]
- Sun, J.; Shi, H.; Xue, Y.; Cheng, W.; Yu, M.; Ding, C.; Xu, F.; Yu, S. Releasing bacteria from functional magnetic beads is beneficial to MALDI-TOF MS based identification. Talanta 2021, 225, 121968. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Chen, N.; Li, H.; Li, Q.-F. Kocuria rosea HN01, a newly alkaliphilic humus-reducing bacterium isolated from cassava dreg compost. J. Soils Sediments 2014, 14, 423–431. [Google Scholar] [CrossRef]
- Akbari, E.; Rasekh, B.; Maal, K.B.; Karbasiun, F.; Yazdian, F.; Emami-Karvani, Z.; Peighami, R. A novel biosurfactant producing Kocuria rosea ABR6 as potential strain in oil sludge recovery and lubrication. AMB Express 2021, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Inan Bektas, K.; Nalcaoğlu, A.; Ceylan, E.; Colak, D.N.; Caglar, P.; Agirman, S.; Sivri, N.S.; Gunes, S.; Kaya, A.; Canakci, S.; et al. Isolation and characterization of detergent-compatible amylase-, protease-, lipase-, and cellulase-producing bacteria. Braz. J. Microbiol. 2023, 54, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Khalil, A.T.; Ayaz, M.; Ahmad, I.; Nethi, S.K.; Mukherjee, S. Biosynthesis of metal nanoparticles via microbial enzymes: A mechanistic approach. Int. J. Mol. Sci. 2018, 19, 4100. [Google Scholar] [CrossRef]
- Umar Dahot, M.; Saboury, A.A.; Moosavi-Movahedi, A.A. Inhibition of β-Amylase Activity by Calcium, Magnesium and Zinc Ions Determined by Spectrophotometry and Isothermal Titration Calorimetry. J. Enzym. Inhib. Med. Chem. 2004, 19, 157–160. [Google Scholar] [CrossRef]
- Sundarram, A.; Murthy, T.P.K. α-amylase production and applications: A review. J. Appl. Environ. Microbiol. 2014, 2, 166–175. [Google Scholar]
- Pandey, A.; Nigam, P.; Soccol, C.R.; Soccol, V.T.; Singh, D.; Mohan, R. Advances in microbial amylases. Biotechnol. Appl. Biochem. 2000, 31, 135–152. [Google Scholar] [CrossRef]
- Armenta, S.; Sánchez-Cuapio, Z.; Munguia, M.E.; Pulido, N.O.; Farrés, A.; Manoutcharian, K.; Hernandez-Santoyo, A.; Moreno-Mendieta, S.; Sánchez, S.; Rodríguez-Sanoja, R. The role of conserved non-aromatic residues in the Lactobacillus amylovorus α-amylase CBM26-starch interaction. Int. J. Biol. Macromol. 2019, 121, 829–838. [Google Scholar] [CrossRef]
- Mietton, L.; Samson, M.-F.; Marlin, T.; Godet, T.; Nolleau, V.; Guezenec, S.; Segond, D.; Nidelet, T.; Desclaux, D.; Sicard, D. Impact of Leavening Agent and Wheat Variety on Bread Organoleptic and Nutritional Quality. Microorganisms 2022, 10, 1416. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, S.; Parameswaran, B.; Ummalyma, S.B.; Abraham, A.; Mathew, A.K.; Madhavan, A.; Rebello, S.; Pandey, A. Applications of microbial enzymes in food industry. Food Technol. Biotechnol. 2018, 56, 16. [Google Scholar] [CrossRef] [PubMed]



| Isolate | Colony Morphology | Biochemical Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Shape | Color | Size | Margin | Surface | Shape | Motility | Gram | Citrate e Test | Catalase | |
| DBUCS1 | Round | Cream | Small | Entire | Smooth | Coccus | - | + | _ | + |
| DBUCL2 | Round | Cream | Large | Entire | Smooth | Rod | + | + | _ | + |
| DBUWS3 | Round | White | Small | Entire | Smooth | Rod | - | + | + | + |
| DBUPL4 | Round | Pink | Large | Entire | Smooth | Coccus | - | + | + | + |
| DBUWL5 | Round | White | Large | Entire | Smooth | Rod | - | + | + | + |
| Strain Code | Score | Suggested Probable Species |
|---|---|---|
| DBUCS1 | 2.13 | Micrococcus endophyticus |
| DBUCL2 | 1.69 | Cutibacterium granulosum |
| DBUWS3 | 1.46 | Pasteurella canis |
| DBUPL4 | 2.48 | Kocuria rosea |
| DBUWL5 | 1.43 | Cutibacterium granulosum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Kim, W.-J.; Ryu, J.; Yerefu, Y.; Tesfaw, A. Amylase Production by the New Strains of Kocuria rosea and Micrococcus endophyticus Isolated from Soil in the Guassa Community Conservation Area. Fermentation 2025, 11, 211. https://doi.org/10.3390/fermentation11040211
Kim S-H, Kim W-J, Ryu J, Yerefu Y, Tesfaw A. Amylase Production by the New Strains of Kocuria rosea and Micrococcus endophyticus Isolated from Soil in the Guassa Community Conservation Area. Fermentation. 2025; 11(4):211. https://doi.org/10.3390/fermentation11040211
Chicago/Turabian StyleKim, Seong-Hoon, Woon-Ji Kim, Jaihyunk Ryu, Yeshareg Yerefu, and Asmamaw Tesfaw. 2025. "Amylase Production by the New Strains of Kocuria rosea and Micrococcus endophyticus Isolated from Soil in the Guassa Community Conservation Area" Fermentation 11, no. 4: 211. https://doi.org/10.3390/fermentation11040211
APA StyleKim, S.-H., Kim, W.-J., Ryu, J., Yerefu, Y., & Tesfaw, A. (2025). Amylase Production by the New Strains of Kocuria rosea and Micrococcus endophyticus Isolated from Soil in the Guassa Community Conservation Area. Fermentation, 11(4), 211. https://doi.org/10.3390/fermentation11040211








