Microbial Poly-Glutamic Acid: Production, Biosynthesis, Properties, and Their Applications in Food, Environment, and Biomedicals
Abstract
1. Introduction
2. Polyglutamic Acid (PGA)
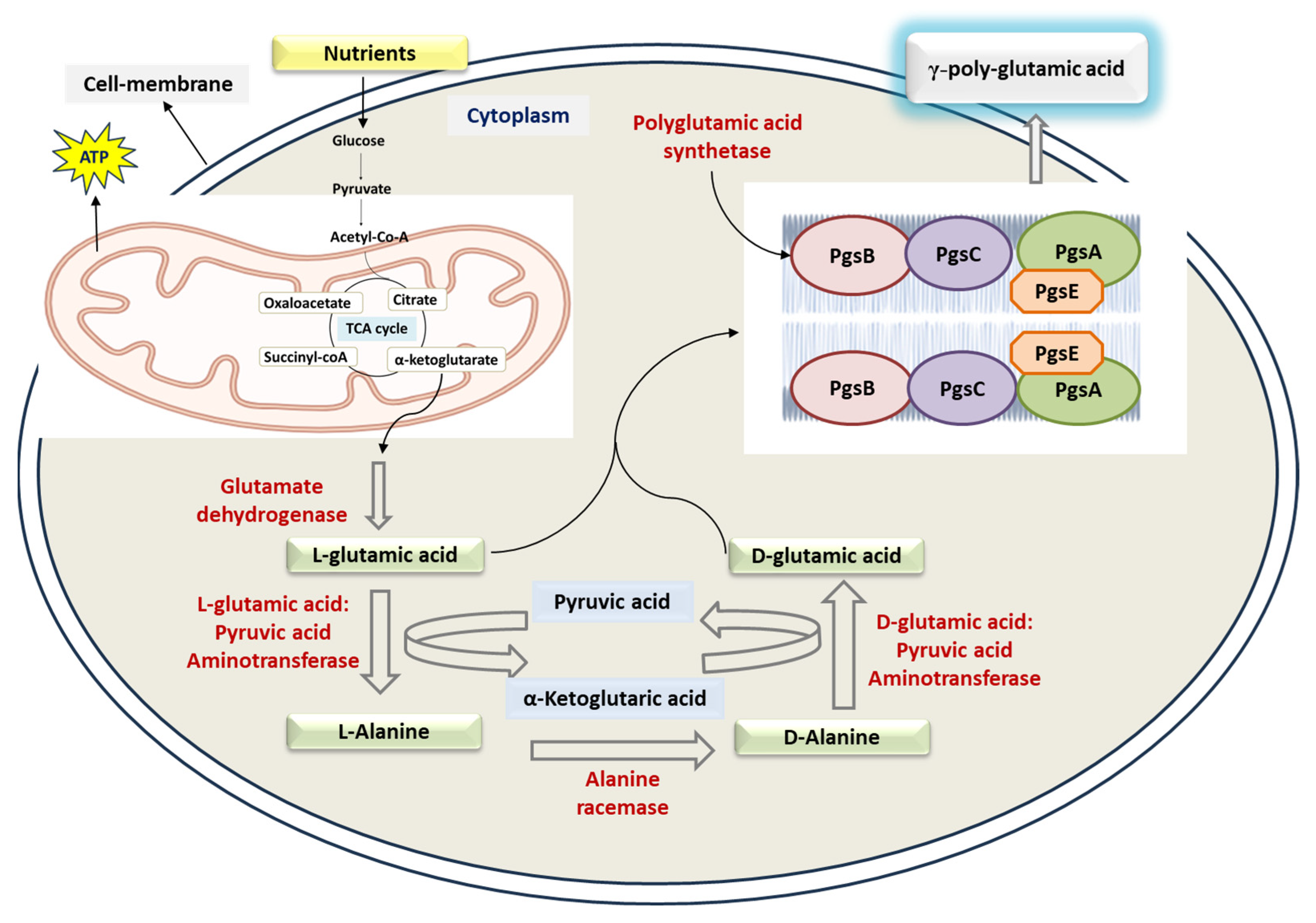
3. Biosynthesis of γ-PGA
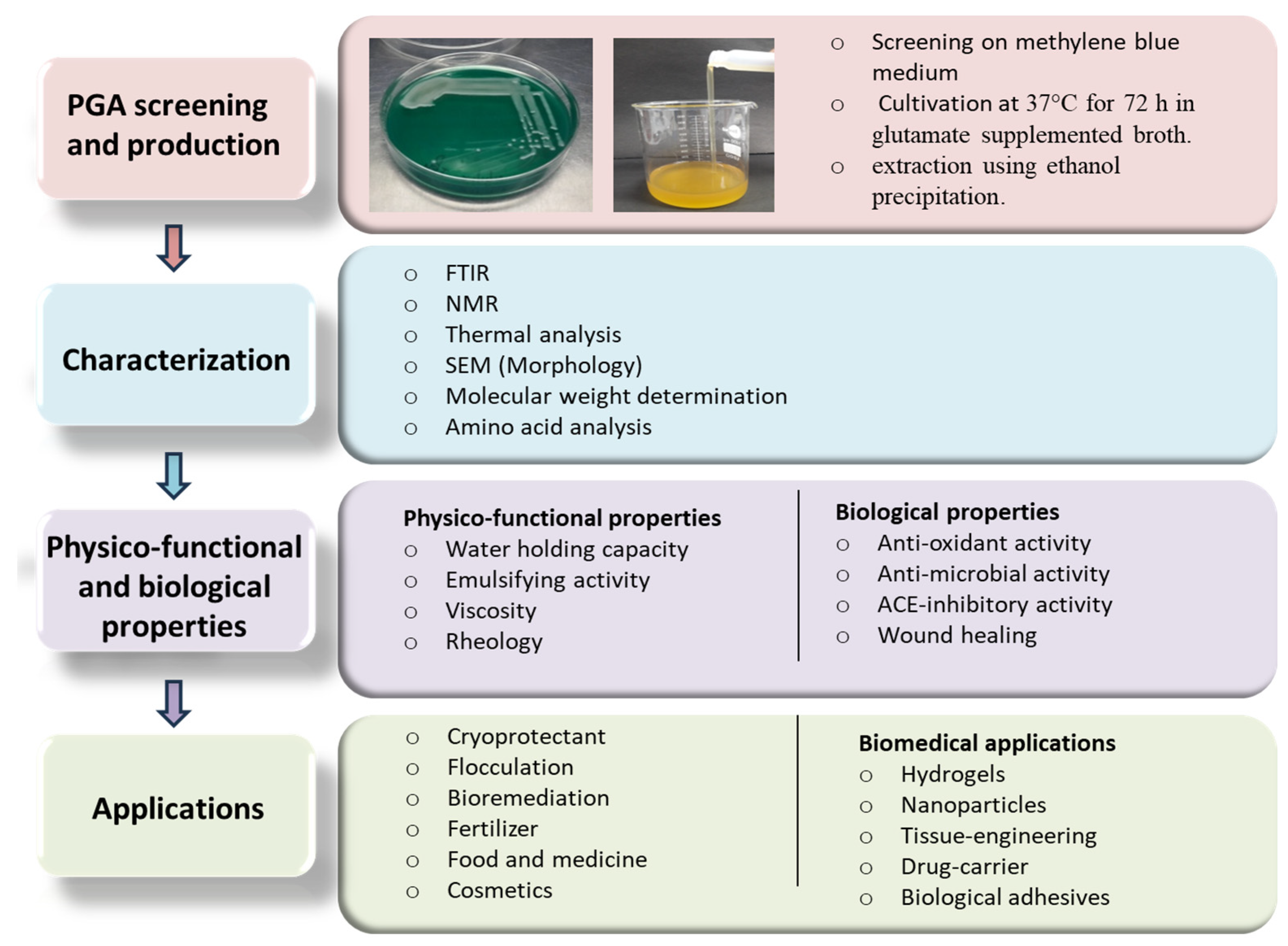
4. Production of γ-PGA
5. γ-PGA from Bacillus spp.
| Sl. No. | Name of Bacteria | Sources | Properties Studied | References |
|---|---|---|---|---|
| 1 | B. subtilis NRRL B-2612 | Devitalized wheat gluten | Solubility in water, molecular mass determination, viscosity | [61] |
| 2 | B. subtilis | Natto | Culture conditions, PGA analysis | [62] |
| 3 | B. subtilis ZJU-17 | Fermented bean curd | Effects of carbon sources and influence of nitrogen source on gamma polyglutamic acid production | [63] |
| 4 | B. subtilis | Natto | Application of γ-polyglutamic acid (Na+ form) in skincare products | [64] |
| 5 | B. subtilis DYU1 | Soil samples from a soy sauce manufacturing site | Flocculating activity and harmlessness to humans and environment | [65] |
| 6 | B. subtilis | Natto | Factors affecting production and agricultural applications | [66] |
| 7 | B. subtilis C10 | Sauce (from a local supermarket, China) | Isolation and characterisation of exogenous glutamic-acid-independent strain | [57] |
| 8 | B. subtilis | Natto | Rheology of biopolymers | [67] |
| 9 | B. subtilis | Nattokinase | High safety, simple production process, drug delivery system, excellent water solubility, biocompatibility, biodegradability | [68] |
| 10 | B. subtilis | Analysis of heavy metal distribution in soil | [69] | |
| 11 | B. subtilis ZC-5 | CICC, China | Solid-state fermentation, low cost substrates, environmental friendly process, reduced energy requirement and waste-water production | [70] |
| 12 | B. subtilis | Soil sample of the electroplating industry | Biodegradability, film-forming property, fibrogenicity, water-holding capacity | [71] |
| 13 | B. subtilis | Natto | Cryoprotective effects of γ-PGA, determination of dynamic rheological properties, Ca2+-ATPase activity, gel strength, salt-soluble protein content | [72] |
| 14 | B. subtilis (CGMCC17326) | Natto | Film forming property, reduced degree of browning in shiitake mushrooms | [73] |
| 15 | B. subtilis W-17 CICC 10260 | CICC | Use of γ-polyglutamic acid waste biomass | [74] |
| 16 | B. licheniformis A35 | Natto | Determination of amino acid | [75] |
| 17 | B. licheniformis | ATCC | Production optimization | [76] |
| 18 | B. licheniformis CCRC 12826 | CCRC, Taiwan | Production of biodegradable and harmless PGA | [41] |
| 19 | B. licheniformis WBL-3 (mutant of 9945) | ATCC | Effect of glycerol on cell growth and g-PGA production | [77] |
| 20 | B. licheniformis NCIM 2324 | NCIM | Molecular mass determination, amino acid analysis, total sugar content | [78] |
| 21 | B. licheniformis 9945 | ATCC | Production and purification and molecular size estimation | [79] |
| 22 | B. licheniformis A13 | Isolated from a tannery effluent | Optimization of PGA production | [80] |
| 23 | B. licheniformis NRC20 | Mine soil | Viscosity measurement, molecular mass determination, amino acid analysis | [25] |
| 24 | B. licheniformis ATCC 9945a | ATCC | Water absorption and solubility, graft content and efficiency, rheological behaviour | [81] |
| 25 | B. licheniformis | Applied Chemistry Research Center (Saltillo, Coahuila, Mexico) | Characterization of nanoparticles, encapsulation assays, bioactivity assays, in vitro release assays | [82] |
| 26 | B. licheniformis NBRC12107 | Fermented locust bean products | Characterization, tensile strength and porosity | [83] |
| 27 | B. licheniformis A14 | Marine sands | Microbially derived biopolymers are renewable in nature | [84] |
| 28 | B. subtilis and B. licheniformis | Reviewing different sources | Biopolymer rheology and viscosity–molecular mass correlation | [85] |
| 29 | B. subtilis and B. licheniformis | Chunkookjang | Chemical and microbial synthesis, application of PGA in medicine as a drug carrier and biological adhesives | [86] |
| 30 | B. subtilis and B. licheniformis | Natto | Biofilm formation, biosynthesis of PGA, genes involed, applications | [12] |
| 31 | B. subtilis ZJU-7 and B. licheniformis 9945a (NCIM 2324) | Reviewing many sources | Molecular mass determination, amino acid analysis, biodegradability, edibility and mmunogenicity | [7] |
| 32 | B. subtilis, B. licheniformis, and B. methylotrophicus | Natto and rhizosphere of pepper, cabbage, sweet corn, fenugreek leaves, barley, tomato, and sugarcane plants | Analysis to differentiate the monomeric and the polymeric forms of glutamic acid | [87] |
| 33 | Bacillus natto 20646 | Natto | PCR analysis | [88] |
| 34 | Bacillus sp. SJ-10 | Chungkookjang | Physicochemical properties and biofunctionality of PGA, molecular mass determination | [76] |
| 35 | Bacillus spp. FBL-2. | Cheonggukjang | Optimization of medium components by central composite design (CCD) | [89] |
| 36 | Natrialba aegyptiaca and N. asiatica | Beach sand (Egypt) | Analysis of the extracellular polymer | [90] |
| 37 | B. amyloliquefaciens C06 | Mesophilic cheese starter | Molecular mass determination, UV scanning and amino acid analysis with paper chromatography | [91] |
5.1. B. licheniformis
5.2. Bacillus subtilis
5.3. Bacillus anthracis and B. thuringiensis
6. Structural and Physico-Chemical Properties of γ-PGA
7. Physico-Functional Properties
8. Biological Properties
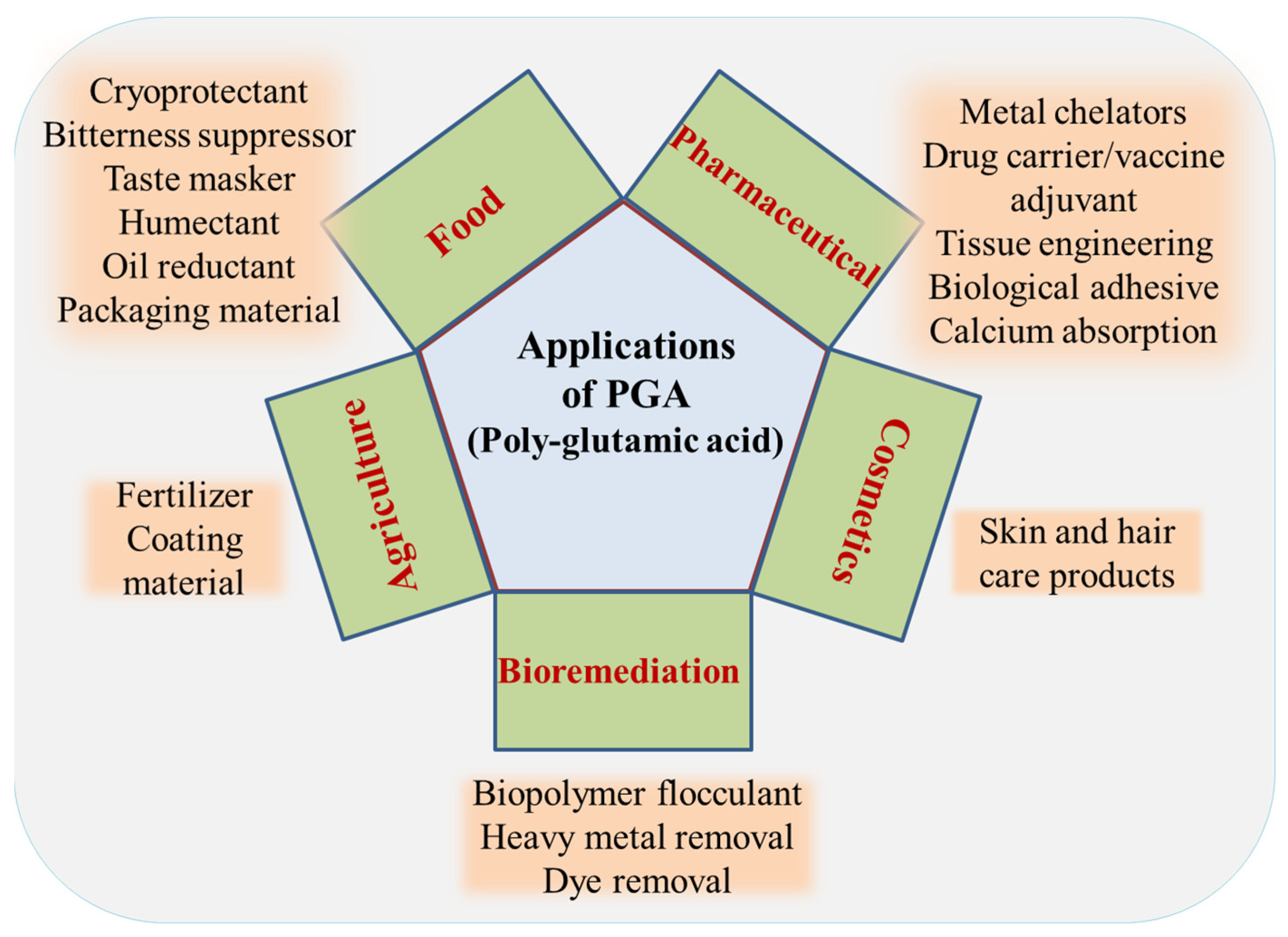
9. Applications of γ-PGA
9.1. Flocculation
9.2. Bioremediation
9.3. Fertilizer
9.4. Cryoprotectant
9.5. In Food and Medicine
9.6. Cosmetics
9.7. Biomedical Applications
9.7.1. Hydrogels
9.7.2. Nanoparticles
9.7.3. Tissue Engineering
9.7.4. Drug Carrier/Deliverer
9.7.5. Metal Chelators
9.7.6. Biological Adhesive
9.7.7. Humectant
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Russo, T.; Fucile, P.; Giacometti, R.; Sannino, F. Sustainable removal of contaminants by biopolymers: A novel approach for wastewater treatment. Current state and future perspectives. Processes 2021, 9, 719. [Google Scholar] [CrossRef]
- Jonnalagadda, S.S.; Harnack, L.; Liu, R.H.; McKeown, N.; Seal, C.; Liu, S.; Fahey, G.C. Putting the whole grain puzzle together: Health benefits associated with whole grains-summary of American Society for Nutrition 2010 satellite symposium. J. Nutr. 2011, 141, 1011S–1022S. [Google Scholar] [CrossRef]
- Ajayeoba, T.A.; Dula, S.; Ijabadeniyi, O.A. Properties of poly-γ-glutamic acid producing-Bacillus species isolated from ogi liquor and lemon-ogi liquor. Front. Microbiol. 2019, 10, 771. [Google Scholar] [CrossRef]
- Obst, M.; Steinbuchel, A. Microbial degradation of poly (amino acid)s. Biomacromolecules 2004, 5, 1166–1176. [Google Scholar] [CrossRef]
- Ivanovics, G.; Bruckner, V. The chemical nature of the immuno-specific capsule substance of anthrax bacillus. Naturwissenschaften 1937, 25, 250. [Google Scholar]
- Bajaj, I.; Singhal, R. Poly (glutamic acid) an emerging biopolymer of commercial interest. Bioresour. Technol. 2011, 102, 5551–5561. [Google Scholar] [CrossRef]
- Ogunleye, A.; Bhat, A.; Irorere, V.U.; Hill, D.; Williams, C.; Radecka, I. Poly-γ-glutamic acid: Production, propertiess and applications. Microbiology 2015, 161, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ashiuchi, M.; Kamei, T.; Baek, D.H.; Shin, S.Y.; Sung, M.H.; Soda, K.; Yagi, T.; Misono, H. Isolation of Bacillus subtilis (chungkookjang), a poly-γ-glutamate producer with high genetic competence. Appl. Microbiol. Biotechnol. 2001, 57, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.B.; Zhan, X.B.; Zhu, L.; Gao, M.J.; Lin, C.C. Optimization of a low-cost hyperosmotic medium and establishing the fermentation kinetics of erythritol production by Yarrowia lipolytica from crude glycerol. Prep. Biochem. Biotechnol. 2016, 46, 376–383. [Google Scholar] [CrossRef]
- Hseu, Z.; Guo, Y.; Liu, J.; Qiu, H.; Zhao, M.; Zou, W.; Li, S. Microbial synthesis of poly-γ-glutamic acid: Current progress, challenges, and future perspectives. Biotechnol. Biofuels 2016, 9, 134. [Google Scholar] [CrossRef]
- Sung, M.H.; Park, C.; Kim, C.J.; Poo, H.; Soda, K.; Ashiuchi, M. Natural and edible biopolymer poly-γ-glutamic acid: Synthesis, production, and applications. Chem. Rec. 2005, 5, 352–366. [Google Scholar] [CrossRef]
- Najar, I.N.; Das, S. Poly-glutamic acid (PGA)-Structure, synthesis, genomic organization and its application: A Review. Int. J. Pharm. Sci. Res. 2015, 6, 2258. [Google Scholar] [CrossRef]
- Wang, L.L.; Chen, J.T.; Wang, L.F.; Wu, S.; Zhang, G.Z.; Yu, H.Q.; Shi, Q.S. Conformations and molecular interactions of poly-γ-glutamic acid as a soluble microbial product in aqueous solutions. Sci. Rep. 2017, 7, 12787. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.B.; Strey, H.H. Osmotically induced helix-coil transition in poly (glutamic acid). Biophys. J. 2008, 94, 4427–4434. [Google Scholar] [CrossRef] [PubMed]
- Gooding, E.A.; Sharma, S.; Petty, S.A.; Fouts, E.A.; Palmer, C.J.; Nolan, B.E.; Volk, M. pH-dependent helix folding dynamics of poly-glutamic acid. Chem. Phys. 2013, 422, 115–123. [Google Scholar] [CrossRef]
- Jose, A.A.; Anusree, G.; Pandey, A.; Binod, P. Production optimization of poly-γ-glutamic acid by Bacillus amyloliquefaciens under solid-state fermentation using soy hull as substrate. Indian. J. Biotechnol. 2018, 17, 44–49. [Google Scholar]
- Oppermann-Sanio, F.B.; Steinbüchel, A. Occurrence, functions and biosynthesis of polyamides in microorganisms and biotechnological production. Naturwissenschaften 2002, 89, 11–22. [Google Scholar] [CrossRef]
- Yao, J.; Jing, J.; Xu, H.; Liang, J.; Wu, Q.; Feng, X.; Ouyang, P. Investigation on enzymatic degradation of γ-polyglutamic acid from Bacillus subtilis NX-2. J. Mol. Catal. B 2009, 56, 158–164. [Google Scholar] [CrossRef]
- Moraes, L.P.; Brito, P.N.; Alegre, R.M. The existing studies on biosynthesis of poly (γ-glutamic acid) by fermentation. Food Public Health 2013, 3, 28–36. [Google Scholar] [CrossRef]
- Candela, T.; Moya, M.; Haustant, M.; Fouet, A. Fusobacterium nucleatum, the first gram-negative bacterium demonstrated to produce polyglutamate. Can. J. Microbiol. 2009, 55, 627–632. [Google Scholar] [CrossRef]
- Cao, M.; Geng, W.; Liu, L.; Song, C.; Xie, H.; Guo, W.; Jin, Y.; Wang, S. Glutamic acid independent production of poly-γ-glutamic acid by Bacillus amyloliquefaciens LL3 and cloning of pgsBCA genes. Bioresour. Technol. 2011, 102, 4251–4257. [Google Scholar] [CrossRef] [PubMed]
- Chettri, R.; Bhutia, M.O.; Tamang, J.P. Poly-γ-glutamic acid (PGA)-producing Bacillus species isolated from Kinema, Indian fermented soybean food. Front. Microbiol. 2016, 7, 971. [Google Scholar] [CrossRef]
- Kambourova, M.; Tangney, M.; Priest, F.G. Regulation of polyglutamic acid synthesis by glutamate in Bacillus licheniformis and Bacillus subtilis. Appl. Environ. Microbiol. 2001, 67, 1004–1007. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meerak, J.; Yukphan, P.; Miyashita, M.; Sato, H.; Nahagawa, Y.; Tahara, Y. Phylogeny of (γ-polyglutamic acid-producing Bacillus strains isolated from a fermented locust bean product. J. General. Appl. Microbiol. 2008, 54, 159–166. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tork, S.E.; Aly, M.M.; Alakilli, S.Y.; Al-Seeni, M.N. Purification and characterization of gamma poly glutamic acid from newly Bacillus licheniformis NRC20. Int. J. Biol. Macromol. 2015, 74, 382–391. [Google Scholar] [CrossRef]
- Buescher, J.M.; Margaritis, A. Microbial biosynthesis of polyglutamic acid biopolymer and applications in the biopharmaceutical, biomedical and food industries. Crit. Rev. Biotechnol. 2007, 27, 1–19. [Google Scholar] [CrossRef]
- Ko, Y.H.; Gross, R.A. Effects of glucose and glycerol on γ-poly(glutamic acid) formation by Bacillus licheniformis ATCC 9945a. Biotechnol. Bioeng. 1998, 57, 430–437. [Google Scholar] [CrossRef]
- Li, D.; Hou, L.; Gao, Y.; Tian, Z.; Fan, B.; Wang, F.; Li, S. Recent advances in microbial synthesis of poly-γ-glutamic acid: A review. Foods 2022, 11, 739. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, H.; Xu, L.; Ouyang, P. Biosynthesis of poly (gamma-glutamic acid) in Bacillus subtilis NX-2: Regulation of stereochemical composition of poly (gamma-glutamic acid). Process Biochem. 2006, 41, 1650–1655. [Google Scholar] [CrossRef]
- Ashiuchi, M. Enzymatic synthesis of high-molecular-mass poly-gamma-glutamate and regulation of its stereochemistry. Appl. Environ. Microbiol. 2004, 70, 4249–4255. [Google Scholar] [CrossRef]
- Candela, T.; Fouet, A. Bacillus anthracis CapD, belonging to the gamma-glutamyl transpeptidase family, is required for the covalent anchoring of capsule to peptidoglycan. Mol. Microbiol. 2005, 57, 717–726. [Google Scholar] [CrossRef]
- Yamashiro, D.; Yoshioka, M.; Ashiuchi, M. Bacillus subtilis pgsE (formerly ywtC) stimulates poly-γ-glutamate production in the presence of Zinc. Biotechnol. Bioeng. 2011, 108, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.S.P.; Nagai, T.; Itoh, Y. Divergent structure of the ComQXPA quorum-sensing components: Molecular basis of strain-specific communication mechanism in Bacillus subtilis. Mol. Microbiol. 2000, 37, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Do, T.H. Mutations suppressing the loss of DegQ function in Bacillus subtilis (natto) poly-γ-glutamate synthesis. Appl. Environ. Microbiol. 2011, 77, 8249–8258. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, T.; Tsukahara, K.; Ogura, M. Bacillus subtilis response regulator DegU is a direct activator of pgsB transcription involved in gamma-poly-glutamic acid synthesis. Biosci. Biotechnol. Biochem. 2009, 73, 2096–2102. [Google Scholar] [CrossRef]
- Stanley, N.R.; Lazazzera, B.A. Defining the genetic differences between wild and domestic strains of Bacillus subtilis that affect poly-gamma-dl-glutamic acid production and biofilm formation. Mol. Microbiol. 2005, 57, 1143–1158. [Google Scholar] [CrossRef]
- King, E.C.; Blacker, A.J.; Bugg, T.D.H. Enzymatic breakdown of poly-gamma-D-glutamic acid in Bacillus licheniformis: Identification of a polyglutamyl gamma-hydrolase enzyme. Biomacromolecules 2000, 1, 75–83. [Google Scholar] [CrossRef]
- Hsueh, Y.H.; Huang, K.Y.; Kunene, S.C.; Lee, T.Y. Poly-γ-glutamic acid synthesis, gene regulation, phylogenetic relationships, and role in fermentation. Int. J. Mol. Sci. 2017, 18, 2644. [Google Scholar] [CrossRef]
- Sanda, F.; Fujiyama, T.; Endo, T. Chemical synthesis of poly-γ-glutamic acid by polycondensation of γ-glutamic acid dimer: Synthesis and reaction of poly-γ-glutamic acid methyl ester. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 732–741. [Google Scholar] [CrossRef]
- Inatsu, Y.; Nakamura, N.; Yuriko, Y.; Fushimi, T.; Watanasiritum, L.; Kawamoto, S. Characterization of Bacillus subtilis strains in Thua nao, a traditional fermented soybean food in northern Thailand. Lett. Appl. Microbiol. 2006, 43, 237–242. [Google Scholar] [CrossRef]
- Shih, I.L.; Van, Y.T.; Yeh, L.C.; Lin, H.G.; Chang, Y.N. Production of a biopolymer flocculant from Bacillus licheniformis and its flocculation properties. Bioresour. Technol. 2001, 78, 267–272. [Google Scholar] [CrossRef]
- Goto, A.; Kunioka, M. Biosynthesis and hydrolysis of poly (γ-glutamic acid) from Bacillus subtilis IFO3335. Biosci. Biotechnol. Biochem. 1992, 56, 1031–1035. [Google Scholar] [CrossRef]
- Kunioka, M. Biosynthesis of poly (γ-glutamic acid) from l-glutamine, citric acid and ammonium sulfate in Bacillus subtilis IFO3335. Appl. Microbiol. Biotechnol. 1995, 44, 501–506. [Google Scholar] [CrossRef]
- Cao, M.; Geng, W.; Zhang, W.; Sun, J.; Wang, S.; Feng, J.; Zheng, P.; Jiang, A.; Song, C. Engineering of recombinant Escherichia coli cells co-expressing poly-γ-glutamic acid (γ-PGA) synthetase and glutamate racemase for differential yielding of γ-PGA. J. Microbiol. Biotechnol. 2013, 6, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, S.; Fu, J.; Xie, J.; Ju, J.; Yu, B.; Wang, L. Efficient molasses utilization for low-molecular-weight poly-γ-glutamic acid production using a novel Bacillus subtilis strain. Microb. Cell Factories 2022, 21, 140. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yan, Q.; Wang, Y.; Li, Y.; Jiang, Z. Efficient production of poly-γ-glutamic acid by Bacillus velezensis via solid-state fermentation and its application. Food Biosci. 2022, 46, 101575. [Google Scholar] [CrossRef]
- Wu, C.; Gou, Y.; Jing, S.; Li, W.; Ge, F.; Li, J.; Ren, Y. Analysis of glutamate-dependent mechanism and optimization of fermentation conditions for poly-gamma-glutamic acid production by Bacillus subtilis SCP017-03. PLoS ONE 2025, 20, e0310556. [Google Scholar] [CrossRef]
- Ashiuchi, M. Biochemical engineering of PGA. Microb. Biotechnol. 2013, 6, 664–674. [Google Scholar] [CrossRef]
- Wei, X.; Yang, L.; Chen, Z.; Xia, W.; Chen, Y.; Cao, M.; He, N. Molecular weight control of poly-γ-glutamic acid reveals novel insights into extracellular polymeric substance synthesis in Bacillus licheniformis. Biotechnol. Biofuels Bioprod. 2024, 17, 60. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, Y.; Shu, L.; Guo, Y.; Wang, L.; Liang, Z. Production of ultra-high-molecular-weight poly-γ-glutamic acid by a newly isolated Bacillus subtilis strain and genomic and transcriptomic analyses. Biotechnol. J. 2024, 19, 2300614. [Google Scholar] [CrossRef]
- Bhat, A.R.; Irorere, V.U.; Bartlett, T.; Hill, D.; Kedia, G.; Morris, M.R.; Charalampopoulos, D.; Radecka, I. Bacillus subtilis natto: A non-toxic source of poly-γ-glutamic acid that could be used as a cryoprotectant for probiotic bacteria. AMB Express 2013, 3, 36. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, H.; Ying, H.; Ouyang, P. Kinetic analysis and pH-shift control strategy for poly (γ-glutamic acid) production with Bacillus subtilis CGMCC 0833. Biochem. Eng. J. 2010, 50, 24–28. [Google Scholar] [CrossRef]
- Cao, M.; Song, C.; Jin, Y.; Liu, L.; Liu, J.; Xie, H.; Guo, W.; Wang, S. Synthesis of poly (γ-glutamic acid) and heterologous expression of pgsBCA genes. J. Mol. Catal. B Enzym. 2010, 67, 111–116. [Google Scholar] [CrossRef]
- Birrer, G.A.; Cromwick, A.M.; Gross, R.A. γ-Poly (glutamic acid) formation by Bacillus licheniformis 9945a: Physiological and biochemical studies. Int. J. Biol. Macromol. 1994, 16, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.L.; Wu, P.J.; Shieh, C.J. Microbial production of a poly (γ-glutamic acid) derivative by Bacillus subtilis. Process Biochem. 2005, 40, 2827–2832. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Zhu, X.; Cai, J.; Zhang, A.; Hong, Y.; Huang, J.; Huang, L.; Xu, Z. High-level exogenous glutamic acid-independent production of poly-(γ-glutamic acid) with organic acid addition in a new isolated Bacillus subtilis C10. Bioresour. Technol. 2012, 116, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Cromwick, A.M.; Birrer, G.A.; Gross, R.A. Effects of pH and aeration on γ-poly (glutamic acid) formation by Bacillus licheniformis in controlled batch fermentor cultures. Biotechnol. Bioeng. 1996, 50, 222–227. [Google Scholar] [CrossRef]
- Kubota, H.; Matsunobu, T.; Uotani, K.; Takebe, H.; Satoh, A.; Tanaka, T.; Taniguchi, M. Production of poly (γ-glutamic acid) by Bacillus subtilis F-2-01. Biosci. Biotechnol. Biochem. 1993, 57, 1212–1213. [Google Scholar] [CrossRef]
- Shih, L.; Van, Y.T. The production of poly-(γ-glutamic acid) from microorganisms and its various applications. Bioresour. Technol. 2001, 79, 207–225. [Google Scholar] [CrossRef]
- Yoon, W.K.; Garcia, C.V.; Kim, C.S.; Lee, S.P. Fortification of mucilage and GABA in Hovenia dulcis extract by co-fermentation with Bacillus subtilis HA and Lactobacillus plantarum EJ2014. Food Sci. Technol. Res. 2018, 24, 265–271. [Google Scholar] [CrossRef]
- Ward, R.M.; Anderson, R.F.; Dean, F.K. Polyglutamic acid production by Bacillus subtilis NRRL B-2612 grown on wheat gluten. Biotechnol. Bioeng. 1963, 5, 41–48. [Google Scholar] [CrossRef]
- Ogawa, Y.; Yamaguchi, F.; Yuasa, K.; Tahara, Y. Efficient production of γ-polyglutamic acid by Bacillus subtilis (natto) in jar fermenters. Biosci. Biotechnol. Biochem. 1997, 61, 1684–1687. [Google Scholar] [CrossRef]
- Shi, F.; Xu, Z.; Cen, P. Optimization of γ-polyglutamic acid production by Bacillus subtilis ZJU-7 using a surface-response methodology. Biotechnol. Bioprocess. Eng. 2006, 11, 251–257. [Google Scholar] [CrossRef]
- Mohanraj, R.; Gnanamangai, B.M.; Ramesh, K.; Priya, P.; Srisunmathi, R.; Poornima, S.; Robinson, J.P. Optimized production of gamma poly glutamic acid (γ-PGA) using sago. Biocatal. Agric. Biotechnol. 2019, 22, 101413. [Google Scholar] [CrossRef]
- Wu, J.Y.; Ye, H.F. Characterization and flocculating properties of an extracellular biopolymer produced from a Bacillus subtilis DYU1 isolate. Process Biochem. 2007, 42, 1114–1123. [Google Scholar] [CrossRef]
- Yu, X.; Wang, M.; Wang, Q.H.; Wang, X.M. Biosynthesis of polyglutamic acid and its application on agriculture. Adv. Mater. Res. 2011, 183, 1219–1223. [Google Scholar] [CrossRef]
- Kreyenschulte, D.; Krull, R.; Margaritis, A. Recent advances in microbial biopolymer production and purification. Crit. Rev. Biotechnol. 2014, 34, 1–15. [Google Scholar] [CrossRef]
- Zhang, S.F.; Gao, C.; Lü, S.; He, J.; Liu, M.; Wu, C.; Liu, Z. Synthesis of PEGylated polyglutamic acid peptide dendrimer and its application in dissolving thrombus. Colloids Surf. B Biointerfaces 2017, 159, 284–292. [Google Scholar] [CrossRef]
- Yang, Z.H.; Dong, C.D.; Chen, C.W.; Sheu, Y.T.; Kao, C.M. Using poly-glutamic acid as soil-washing agent to remediate heavy metal-contaminated soils. Environ. Sci. Pollut. Res. 2018, 25, 5231–5242. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, D.; Ren, H. Economical production of agricultural γ-polyglutamic acid using industrial wastes by Bacillus subtilis. Biochem. Eng. J. 2019, 146, 117–123. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, H.X.; Zhong, C.Q.; Wu, D. Biosorption of Cr (VI) by immobilized waste biomass from polyglutamic acid production. Sci. Rep. 2020, 10, 3705. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Tian, L.; Zhang, X.; Huang, X.; Long, H.; Chang, F.; Li, S. Effects of γ-polyglutamic acid on the physicochemical properties and microstructure of grass carp (Ctenopharyngodon idellus) surimi during frozen storage. LWT 2020, 134, 109960. [Google Scholar] [CrossRef]
- Tao, L.; Long, H.; Zhang, J.; Qi, L.; Zhang, S.; Li, T.; Li, S. Preparation and coating application of γ-polyglutamic acid hydrogel to improve storage life and quality of shiitake mushrooms. Food Control 2021, 130, 108404. [Google Scholar] [CrossRef]
- Zhang, C.; Zhong, C.; Wu, D. Study on the reuse process of hydrolysate from γ-polyglutamic acid fermentation residues. Arab. J. Chem. 2021, 14, 103145. [Google Scholar] [CrossRef]
- Cheng, C.; Asada, Y.; Aida, T. Production of γ-polyglutamic acid by Bacillus licheniformis A35 under denitrifying conditions. Agric. Biol. Chem. 1989, 53, 2369–2375. [Google Scholar] [CrossRef]
- Giannos, S.A.; Shah, D.; Gross, R.A.; Kaplan, D.L.; Mayer, J.M. Poly (glutamic acid) produced by bacterial fermentation. In Novel Biodegradable Microbial Polymers; Springer Netherlands: Dordrecht, The Netherlands, 1990; pp. 457–460. [Google Scholar] [CrossRef]
- Du, G.; Yang, G.; Qu, Y.; Chen, J.; Lun, S. Effects of glycerol on the production of poly (γ-glutamic acid) by Bacillus licheniformis. Process Biochem. 2005, 40, 2143–2147. [Google Scholar] [CrossRef]
- Bajaj, I.B.; Lele, S.S.; Singhal, R.S. A statistical approach to optimization of fermentative production of poly (γ-glutamic acid) from Bacillus licheniformis NCIM 2324. Bioresour. Technol. 2009, 100, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Konglom, N.; Chuensangjun, C.; Pechyen, C.; Sirisansaneeyakul, S. Production of poly- γ-glutamic acid by Bacillus licheniformis: Synthesis and characterization. J. Met. Mater. Miner. 2012, 22, 7–11. [Google Scholar]
- Mabrouk, M.; Abou-Zeid, D.; Sabra, W. Application of Plackett–Burman experimental design to evaluate nutritional requirements for poly (γ-glutamic acid) production in batch fermentation by Bacillus licheniformis A13. Afr. J. Appl. Microbiol. Res. 2012, 2, 6–18. [Google Scholar]
- Xu, J.; Krietemeyer, E.F.; Finkenstadt, V.L.; Solaiman, D.; Ashby, R.D.; Garcia, R.A. Preparation of starch–poly–glutamic acid graft copolymers by microwave irradiation and the characterization of their properties. Carbohydr. Polym. 2016, 140, 233–237. [Google Scholar] [CrossRef]
- Pereira, A.E.S.; Sandoval-Herrera, I.E.; Zavala-Betancourt, S.A.; Oliveira, H.C.; Ledezma-Pérez, A.S.; Romero, J.; Fraceto, L.F. γ-Polyglutamic acid/chitosan nanoparticles for the plant growth regulator gibberellic acid: Characterization and evaluation of biological activity. Carbohydr. Polym. 2017, 157, 1862–1873. [Google Scholar] [CrossRef]
- Yu, K.; Aubin-Tam, M.E. Bacterially grown cellulose/graphene oxide composites infused with γ-Poly (glutamic acid) as biodegradable structural materials with enhanced toughness. ACS Appl. Nano Mater. 2020, 3, 12055–12063. [Google Scholar] [CrossRef]
- Mahaboob Ali, A.A.; Momin, B.; Ghogare, P. Isolation of a novel poly-γ-glutamic acid-producing Bacillus licheniformis A14 strain and optimization of fermentation conditions for high-level production. Prep. Biochem. Biotechnol. 2020, 50, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.; Margaritis, A. Poly (glutamic acid) for biomedical applications. Crit. Rev. Biotechnol. 2001, 21, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.L.; Van, Y.T.; Shen, M.H. Biomedical applications of chemically and microbiologically synthesized poly (glutamic acid) and poly (lysine). Mini Rev. Med. Chem. 2004, 4, 179–188. [Google Scholar] [CrossRef]
- Chatterjee, P.M.; Datta, S.; Tiwari, D.P.; Raval, R.; Dubey, A.K. Selection of an effective indicator for rapid detection of microorganisms producing γ-polyglutamic acid and its biosynthesis under submerged fermentation conditions using Bacillus methylotrophicus. Appl. Biochem. Biotechnol. 2018, 185, 270–288. [Google Scholar] [CrossRef]
- Qi, H.; Na, R.; Xin, J.; Xie, Y.J.; Guo, J.F. Effect of corona electric field on the production of gamma-poly glutamic acid based on Bacillus natto. J. Phys. Conf. Ser. 2013, 418, 012139. [Google Scholar] [CrossRef]
- Min, J.H.; Reddy, L.V.; Dimitris, C.; Kim, Y.M.; Wee, Y.J. Optimized production of poly (γ-glutamic acid) by Bacillus sp. FBL-2 through response surface methodology using central composite design. J. Microbiol. Biotechnol. 2019, 29, 1061–1070. [Google Scholar] [CrossRef]
- Hezayen, F.F.; Rehm, B.H.; Tindall, B.J.; Steinbüchel, A. Transfer of Natrialba asiatica B1T to Natrialba taiwanensis sp. nov. and description of Natrialba aegyptiaca sp. nov.; a novel extremely halophilic, aerobic, non-pigmented member of the Archaea from Egypt that produces extracellular poly (glutamic acid). Int. J. Syst. Evol. Microbiol. 2001, 51, 1133–1142. [Google Scholar] [CrossRef]
- Liu, J.; Ma, X.; Wang, Y.; Liu, F.; Qiao, J.; Li, X.Z.; Zhou, T. Depressed biofilm production in Bacillus amyloliquefaciens C06 causes γ-polyglutamic acid (γ-PGA) overproduction. Curr. Microbiol. 2011, 62, 235–241. [Google Scholar] [CrossRef]
- Bajaj, I.B.; Lele, S.S.; Singhal, R.S. Enhanced production of poly (γ-glutamic acid) from Bacillus licheniformis NCIM 2324 in solid state fermentation. J. Ind. Microbiol. Biotechnol. 2008, 35, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yang, G.; Che, C.; Liu, Y. Heterogenous expression of poly-γ-glutamic acid synthetase complex gene of Bacillus licheniformis WBL-3. Appl. Biochem. Microbiol. 2011, 47, 381–385. [Google Scholar] [CrossRef]
- Ho, G.H.; Ho, T.I.; Hsieh, K.H.; Su, Y.C.; Lin, P.Y.; Yang, J.; Yang, S.C. γ-Polyglutamic acid produced by Bacillus Subtilis (Natto): Structural characteristics, chemical properties and biological functionalities. J. Chin. Chem. Soc. 2006, 53, 1363–1384. [Google Scholar] [CrossRef]
- Bovarnick, M. The formation of extracellular d (-)-glutamic acid polypeptide by Bacillus subtilis. J. Biol. Chem. 1942, 145, 415–424. [Google Scholar] [CrossRef]
- Scoffone, V.; Dondi, D.; Biino, G.; Borghese, G.; Pasini, D.; Galizzi, A.; Calvio, C. Knockout of pgdS and ggt genes improves γ-PGA yield in B. subtilis. Biotechnol. Bioeng. 2013, 110, 2006–2012. [Google Scholar] [CrossRef]
- Shi, F.; Xu, Z.; Cen, P. Efficient production of poly-γ-glutamic acid by Bacillus subtilis ZJU-7. Appl. Biochem. Biotechnol. 2006, 133, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Zwartouw, H.T.; Smith, H. Polyglutamic acid from Bacillus anthracis grown in vivo: Structure and aggression activity. Biochem. J. 1956, 63, 437–442. [Google Scholar] [CrossRef]
- Ezzell, J.W.; Abshire, T.G.; Panchal, R.; Chabot, D.; Bavari, S.; Leffel, E.K.; Purcell, B.; Friedlander, A.M.; Ribot, W.J. Association of Bacillus anthracis capsule with lethal toxin during experimental infection. Infect. Immun. 2009, 77, 749–755. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Chen, B.-H. Inbaraj BS, Chen B-H In vitro removal of toxic heavy metals by poly (γ-glutamic acid)-coated superparamagnetic nanoparticles. Int. J. Nanomed. 2012, 7, 4419. [Google Scholar] [CrossRef][Green Version]
- Lee, J.M.; Kim, J.H.; Kim, K.W.; Lee, B.J.; Kim, D.G.; Kim, Y.O.; Kong, I.S. Physicochemical properties, production, and biological functionality of poly-γ-D-glutamic acid with constant molecular weight from halotolerant Bacillus sp. SJ-10. Int. J. Biol. Macromol. 2018, 108, 598–607. [Google Scholar] [CrossRef]
- Jianbo, Z.; Jun, W.; Xuanlin, W.; Hui, C. Coupling fermentation of glutamic acid and γ-polyglutamic acid and preparation of poly (amino acid) superabsorbent polymers. BMC Biotechnol. 2023, 23, 47. [Google Scholar] [CrossRef]
- Bajestani, M.I.; Mousavi, S.M.; Mousavi, S.B.; Jafari, A.; Shojaosadati, S.A. Purification of extra cellular poly-γ-glutamic acid as an antibacterial agent using anion exchange chromatography. Int. J. Biol. Macromol. 2018, 113, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Shyu, Y.S.; Sung, W.C. Improving the emulsion stability of sponge cake by the addition of γ-polyglutamic acid. J. Mar. Sci. Technol. 2010, 18, 14. [Google Scholar] [CrossRef]
- Johnson, L.C.; Akinmola, A.T.; Scholz, C. Poly (glutamic acid): From natto to drug delivery systems. Biocatal. Agric. Biotechnol. 2022, 40, 102292. [Google Scholar] [CrossRef]
- Shi, W.; Liang, J.; Tao, W.; Tan, S.; Wang, Q. γ-PGA additive decreasing soil water infiltration and improving water holding capacity. Trans. Chin. Soc. Agric. Eng. 2015, 31, 94–100. [Google Scholar]
- Lim, S.; Kim, J.; Shim, J.; Imm, B.; Sung, M.; Imm, J. Effect of poly-γ-glutamic acids (PGA) on oil uptake and sensory quality in doughnuts. Food Sci. Biotechnol. 2012, 21, 247–252. [Google Scholar] [CrossRef]
- Lee, N.R.; Go, T.H.; Lee, S.M.; Jeong, S.Y.; Park, G.T.; Hong, C.O.; Son, H.J. In vitro evaluation of new functional properties of poly-γ-glutamic acid produced by Bacillus subtilis D7. Saudi J. Biol. Sci. 2014, 21, 153–158. [Google Scholar] [CrossRef]
- Mundo, J.L.M.; Zhou, H.; Tan, Y.; Liu, J.; Mc Clements, D.J. Stabilization of soybean oil-in-water emulsions using polypeptide multilayers: Cationic polylysine and anionic polyglutamic acid. Food Res. Int. 2020, 137, 109304. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, S.; Jiang, G.; Gan, L.; Xu, Z.; Tian, Y. Optimization of fermentation conditions, purification and rheological properties of poly (γ-glutamic acid) produced by Bacillus subtilis 1006-3. Prep. Biochem. Biotechnol. 2022, 52, 302–310. [Google Scholar] [CrossRef]
- de Cesaro, A.; da Silva, S.B.; da Silva, V.Z.; Ayub, M.A.Z. Physico-chemical and rheological characterization of poly-gamma-glutamic acid produced by a new strain of Bacillus subtilis. Eur. Polym. J. 2014, 57, 91–98. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, B.; Xu, D.L.; Xu, H.; Liang, L.; Feng, X.H.; Chi, B. Antimicrobial and biocompatible ε-polylysine–γ-poly (glutamic acid)–based hydrogel system for wound healing. J. Bioact. Compat. Polym. 2016, 31, 242–259. [Google Scholar] [CrossRef]
- Pihlanto, A.; Akkanen, S.; Korhonen, H.J. ACE-inhibitory and antioxidant properties of potato (Solanum tuberosum). Food Chem. 2008, 109, 104–112. [Google Scholar] [CrossRef]
- Tsao, C.T.; Chang, C.H.; Lin, Y.Y.; Wu, M.F.; Wang, J.L.; Young, T.H.; Han, J.L.; Hsieh, K.H. Evaluation of chitosan/γ-poly (glutamic acid) polyelectrolyte complex for wound dressing materials. Carbohydr. Polym. 2011, 84, 812–819. [Google Scholar] [CrossRef]
- Ye, H.; Xian, Y.; Li, S.; Zhang, C.; Wu, D. In situ forming injectable γ-poly (glutamic acid)/PEG adhesive hydrogels for hemorrhage control. Biomater. Sci. 2022, 10, 4218–4227. [Google Scholar] [CrossRef]
- Manocha, B.; Margaritis, A. Controlled Release of Doxorubicin from Doxorubicin/γ-Polyglutamic Acid Ionic Complex. J. Nanomater. 2010, 2010, 780171. [Google Scholar] [CrossRef]
- Yazdanparast, S.; Benvidi, A.; Banaei, M.; Nikukar, H.; Tezerjani, M.D.; Azimzadeh, M. Dual-aptamer based electrochemical sandwich biosensor for MCF-7 human breast cancer cells using silver nanoparticle labels and a poly (glutamic acid)/MWNT nanocomposite. Microchim. Acta 2018, 185, 405. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, V.; Bellia, F.; Viale, M.; Maric, I.; Vecchio, G. Linear polymers of β and γ cyclodextrins with a polyglutamic acid backbone as carriers for doxorubicin. Carbohydr. Polym. 2017, 177, 355–360. [Google Scholar] [CrossRef]
- Ko, W.C.; Chang, C.K.; Wang, H.J.; Wang, S.J.; Hsieh, C.W. Process optimization of microencapsulation of curcumin in γ-polyglutamic acid using response surface methodology. Food Chem. 2015, 172, 497–503. [Google Scholar] [CrossRef]
- Li, M.; Zhu, X.; Yang, H.; Xie, X.; Zhu, Y.; Xu, G.; Li, A. Treatment of potato starch wastewater by dual natural flocculants of chitosan and poly-glutamic acid. J. Clean. Prod. 2020, 264, 121641. [Google Scholar] [CrossRef]
- Santos, D.P.; Bergamini, M.F.; Zanoni, M.V.B. Voltammetric sensor for amoxicillin determination in human urine using polyglutamic acid/glutaraldehyde film. Sens. Actuators B Chem. 2008, 133, 398–403. [Google Scholar] [CrossRef]
- Garcia, J.P.D.; Hsieh, M.F.; Doma, B.T., Jr.; Peruelo, D.C.; Chen, I.H.; Lee, H.M. Synthesis of gelatin-γ-polyglutamic acid-based hydrogel for the in vitro controlled release of epigallocatechin gallate (EGCG) from Camellia sinensis. Polymers 2013, 6, 39–58. [Google Scholar] [CrossRef]
- Rethore, G.; Mathew, A.; Naik, H.; Pandit, A. Preparation of chitosan/polyglutamic acid spheres based on the use of polystyrene template as a nonviral gene carrier. Tissue Eng. Part. C Methods 2009, 15, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Bai, R.; Hu, X.; Luo, Q. Characteristics of a bioflocculant produced by Bacillus mucilaginosus and its use in starch wastewater treatment. Appl. Microbiol. Biotechnol. 2003, 60, 588–593. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Y.; Wei, X.; Hu, Z.; Zhu, F.; Xu, L.; Luo, M.; Liu, H. Production of ultra-high molecular weight poly-γ-glutamic acid with Bacillus licheniformis P-104 and characterization of its flocculation properties. Appl. Biochem. Biotechnol. 2013, 170, 562–572. [Google Scholar] [CrossRef]
- Kinnersley, A.; Strom, D.; Meah, R.Y.; Koskan, C.P. Composition and Method for Enhanced Fertilizer Uptake by Plants. WO patent no. 94/09,628, 27 September 1994. [Google Scholar]
- Inbaraj, B.S.; Chiu, C.P.; Ho, G.H.; Yang, J.; Chen, B.H. Removal of cationic dyes from aqueous solution using an anionic poly-γ-glutamic acid-based adsorbent. J. Hazard. Mater. 2006, 137, 226–234. [Google Scholar] [CrossRef]
- Campos, V.; Fernandes, A.R.; Medeiros, T.A.; Andrade, E.L. Physicochemical characterization and evaluation of PGA bioflocculant in coagulation-flocculation and sedimentation processes. J. Environ. Chem. Eng. 2016, 4, 3753–3760. [Google Scholar] [CrossRef]
- Zheng, H.; Gao, Z.; Yin, J.; Tang, X.; Ji, X.; Huang, H. Harvesting of microalgae by flocculation with poly(γ-glutamic acid). Bioresour. Technol. 2012, 112, 212–220. [Google Scholar] [CrossRef]
- Sheu, Y.T.; Tsang, D.C.; Dong, C.D.; Chen, C.W.; Luo, S.G.; Kao, C.M. Enhanced bioremediation of TCE-contaminated groundwater using gamma poly-glutamic acid as the primary substrate. J. Clean. Prod. 2018, 178, 108–118. [Google Scholar] [CrossRef]
- Hajdu, I.; Bodnár, M.; Csikós, Z.; Wei, S.; Daróczi, L.; Kovács, B.; Gyori, Z.; Tamás, J.; Borbély, J. Combined nano-membrane technology for removal of lead ions. J. Membr. Sci. 2012, 409–410, 44–53. [Google Scholar] [CrossRef]
- Xu, Z.; Wan, C.; Xu, X.; Feng, X.; Xu, H. Effect of poly (γ-glutamic acid) on wheat productivity, nitrogen use efficiency and soil microbes. J. Plant Nutr. Soil. Sci. 2013, 13, 744–755. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Y.; Zheng, J.; Yang, C.; Li, L.; Li, R.; Li, Z. Preparation of potential organic fertilizer rich in γ-polyglutamic acid via microbial fermentation using brewer’s spent grain as basic substrate. Bioresour. Technol. 2024, 394, 130216. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, S.; Zhang, J.; Sun, M.; Liu, Z.; Yu, Z. Co-producing lipopeptides and poly-γ-glutamic acid by solid-state fermentation of Bacillus subtilis using soybean and sweet potato residues and its biocontrol and fertilizer synergistic effects. Bioresour. Technol. 2008, 99, 3318–3323. [Google Scholar] [CrossRef]
- Shih, I.L.; Van, Y.T.; Sau, Y.Y. Antifreeze activities of poly (γ-glutamic acid) produced by Bacillus licheniformis. Biotechnol. Lett. 2003, 25, 1709–1712. [Google Scholar] [CrossRef]
- Bhat, A.; Irorere, V.; Bartlett, T.; Hill, D.; Kedia, G.; Charalampopoulos, D.; Nualkaekul, S. Radecka Improving survival of probiotic bacteria using bacterial poly-γ-glutamic acid. Int. J. Food Microbiol. 2015, 196, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, H.; Fox, T.; Eagles, J.; Satoh, H.; Nozawa, H.; Okiyama, A.; Morinaga, Y.; Fairweather-Tait, S.J. Acute effect of poly-γ-glutamic acid on calcium absorption in post-menopausal women. J. Am. Coll. Nutr. 2007, 26, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, N.; Morishima, H.; Uotani, K. Compositions for Prevention of Increase of Blood Pressure. Japanese Patent 2008-255063, 2008. [Google Scholar]
- Tamura, M.; Watanabe, J.; Hori, S.; Inose, A.; Kubo, Y.; Noguchi, T.; Kobori, M. Effects of a high-γ-polyglutamic acid-containing natto diet on liver lipids and cecal microbiota of adult female mice. Biosci. Microbiota Food Health 2021, 40, 176–185. [Google Scholar] [CrossRef]
- Siaterlis, A.; Deepika, G.; Charalampopoulos, D. Effect of culture medium and cryoprotectants on the growth and survival of probiotic lactobacilli during freeze drying. Lett. Appl. Microbiol. 2009, 48, 295–301. [Google Scholar] [CrossRef]
- Jagannath, A.; Raju, P.S.; Bawa, A.S. Comparative evaluation of bacterial cellulose (nata) as a cryoprotectant and carrier support during the freeze drying process of probiotic lactic acid bacteria. LWT–Food Sci. Technol. 2010, 43, 1197–1203. [Google Scholar] [CrossRef]
- Ben-Zur, N.; Goldman, D.M. Polyglutamic Acid: A Novel Peptide for Skin Care. Cosmetics & Toiletries. 2014. Available online: https://www.cosmeticsandtoiletries.com/cosmetic-ingredients/moisturizing/article/21836259/polyglutamic-acid-a-novel-peptide-for-skin-care (accessed on 16 July 2021).
- Elbanna, K.; Alsulami, F.S.; Neyaz, L.A.; Abulreesh, H.H. Poly (γ) glutamic acid: A unique microbial biopolymer with diverse commercial applicability. Front. Microbiol. 2024, 15, 1348411. [Google Scholar] [CrossRef]
- Abd Alsaheb, R.A.; Othman, N.Z.; Abd Malek, R.; Leng, O.M.; Aziz, R.; El Enshasy, H.A. Polyglutamic acid applications in pharmaceutical and biomedical industries. Der Pharm. Lett. 2016, 8, 217–225. [Google Scholar]
- Murakami, S.; Aoki, N.; Matsumura, S. Bio-based biodegradable hydrogels prepared by cross-linking of microbial poly (γ-glutamic acid) with l-lysine in aqueous solution. Polym. J. 2011, 43, 414–420. [Google Scholar] [CrossRef]
- Lin, W.-C.; Yu, D.-G.; Yang, M.-C. Blood compatibility of novel poly (γ-glutamic acid)/polyvinyl alcohol hydrogels. Colloids Surf. B Biointerfaces 2006, 47, 43–49. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Mishra, R.; Rana, D.; Kundu, P.P. Strategies for effective oral insulin delivery with modified chitosan nanoparticles: A review. Progress. Polym. Sci. 2012, 37, 1457–1475. [Google Scholar] [CrossRef]
- Hsieh, C.-Y.; Tsai, S.-P.; Wang, D.M.; Chang, Y.N.; Hsieh, H.J. Preparation of γ-PGA/chitosan composite tissue engineering matrices. Biomaterials 2005, 26, 5617–5623. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Jin, L.; Hu, R.; Yi, Z.; Li, J.; Wu, Y.; Xi, X.; Wu, Z. Poly (γ,l-glutamic acid)–cisplatin conjugate effectively inhibits human breast tumor xenografted in nude mice. Biomaterials 2006, 27, 5958–5965. [Google Scholar] [CrossRef]
- Singer, J.W. Paclitaxel poliglumex (XYOTAX, CT-2103): A macromolecular taxane. J. Control. Release 2005, 109, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Otani, Y.; Tabata, Y.; Ikada, Y. Sealing effect of rapidly curable gelatin-poly (l-glutamic acid) hydrogel glue on lung air leak. Ann. Thorac. Surg. 1999, 67, 922–926. [Google Scholar] [CrossRef]
- Xie, X.; Wu, X.; Shen, Y.; Song, M.; Xu, C.; Zhang, B.; Xu, X. Effect of poly-γ-glutamic acid on hydration and structure of wheat gluten. J. Food Sci. 2020, 85, 3214–3219. [Google Scholar] [CrossRef]
| Sl No. | Source | Properties | Reference |
|---|---|---|---|
| 1 | Natto Biosciences (Montreal, QC, Canada) | Hydrophilicity, biodegradability, biocompatibility, immunogenicity, and ionic nature | [116] |
| 2 | Sigma Aldrich (St. Louis, MO, USA) | Detection of MCF-7 human breast cancer cells and MUC1 biomarker | [117] |
| 3 | VEDAN Co. (Taichung, Taiwan) | Polyelectrolyte complex formation | [114] |
| 4 | IRIS Biotech Gmbh (CAS No 26247-79-0, Marktredwitz, Germany) | Protective agent of protein aggregation, drug delivery, low physical stability | [118] |
| 5 | VEDAN Co. (Taichung, Taiwan) | Water-soluble properties, anti-cancer and antioxidant activties, increased biocompatible and biodegradable abilities, encapsulation efficiency | [119] |
| 6 | Bioshinking Company (Nanjing, China) | Biodegradability, physico-chemical characterization, and evaluation of PGA bioflocculant in coagulation flocculation and sedimentation processes | [120] |
| 7 | Sigma Aldrich | Antibacterial activity, low solubility in organic solvents, high positive potential, low sentivity | [121] |
| 8 | VEDAN Co. (Taichung, Taiwan) | Determination of swelling degree | [122] |
| 9 | New England BioLabs, Hitchin, Hertfordshire, UK | Biodegradable polymer; increased rigidity, porosity, and availailibity; rate of degradation | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manika, V.; Devi, P.B.; Singh, S.P.; Reddy, G.B.; Kavitake, D.; Shetty, P.H. Microbial Poly-Glutamic Acid: Production, Biosynthesis, Properties, and Their Applications in Food, Environment, and Biomedicals. Fermentation 2025, 11, 208. https://doi.org/10.3390/fermentation11040208
Manika V, Devi PB, Singh SP, Reddy GB, Kavitake D, Shetty PH. Microbial Poly-Glutamic Acid: Production, Biosynthesis, Properties, and Their Applications in Food, Environment, and Biomedicals. Fermentation. 2025; 11(4):208. https://doi.org/10.3390/fermentation11040208
Chicago/Turabian StyleManika, Verma, Palanisamy Bruntha Devi, Sanjay Pratap Singh, Geereddy Bhanuprakash Reddy, Digambar Kavitake, and Prathapkumar Halady Shetty. 2025. "Microbial Poly-Glutamic Acid: Production, Biosynthesis, Properties, and Their Applications in Food, Environment, and Biomedicals" Fermentation 11, no. 4: 208. https://doi.org/10.3390/fermentation11040208
APA StyleManika, V., Devi, P. B., Singh, S. P., Reddy, G. B., Kavitake, D., & Shetty, P. H. (2025). Microbial Poly-Glutamic Acid: Production, Biosynthesis, Properties, and Their Applications in Food, Environment, and Biomedicals. Fermentation, 11(4), 208. https://doi.org/10.3390/fermentation11040208






