Abstract
A 5% salt solution was used to make sponge dough from wheat flour. We devised a new starter (wheat flour saline culture) by adding 5% saline to wheat flour and incubating it for 24 h. The dough’s rise was enhanced by adding wheat flour saline culture to the dough: after two hours, the dough volume increased by 20–30% compared to the control. Furthermore, the specific volume of the bread increased from 2.25 cm3/g in the control to 2.73–3.47 cm3/g when sugar or other auxiliary ingredients were not added to it. Wheat flour saline culture contained a large number of halotolerant bacteria. The addition of wheat flour saline culture increased the air bubble size and specific volume of the bread.
1. Introduction
The sponge dough method for bread is a method in which some or all of the wheat flour used is fermented and ripened before the actual preparation [1]. The liquid dough method is a method in which a dough is made with a portion of flour (30–40%), water, baker’s yeast, etc., and is fermented at a low temperature; the remaining flour and other ingredients are added to this fermented dough to complete the actual dough. The liquid dough method can also be considered a type of sponge dough method [2]. The sponge dough method is said to be suitable for mechanized production because the dough becomes extensible and flexible through fermentation and ripening, making it easier to handle [3,4]. The sponge dough method is usually carried out without the addition of salt or with the addition of about 0.5% salt [3,4,5]. The key factors in making sponge dough and sourdough are the raw materials and fermentation process parameters used (temperature, retarding time, pH, water activity, additives, etc.) [6,7,8,9]. To make bread using only flour and water, we focused on the day-one culture of sourdough [10]. The day-one culture contained more than 109 /g total bacteria, while the number of yeast cells was low at 103 cfu/g. Kosakonia cowanii was frequently isolated from four types of the day-one culture. Bread could be made using Kosakonia cowanii SB and flour without adding sugars or yeast. Kosakonia cowanii is a facultative anaerobic bacterium, commonly found in soil and water, as well as internally in plants, animals, and humans [11,12]. However, there were hesitations about its use in actual bread making. Adding salt is one way to eliminate enterobacteria and microorganisms related to food poisoning. We tried adding salt to flour cultures. The aim of this study was to investigate the effects of 5% saline on the flour microflora and to confirm whether this method can be applied to prepare sponge dough for making bread.
2. Materials and Methods
2.1. Experimental Materials
Strong flour A (Nippn Co., Ltd., Tokyo, Japan; 72% carbohydrates, 12% protein, 1.5% lipids), strong flour B (Nippn Co., Ltd.; 71% carbohydrates, 13% protein, 1.1% lipids), and strong flour C (Nissin Flour Milling Co., Ltd., Tokyo, Japan; 72% carbohydrates, 12.6% protein, 1.4% lipids) were used. Baker’s yeast (Nissin Foods Co., Ltd., Tokyo, Japan, Super Camellia dry yeast) and salt (Japan Salt Co., Ltd., Tokyo, Japan) were used as bread-making materials.
2.2. Noodle Dough Culture (Preliminary Test)
To make the noodle dough, 6 g of wheat flour (three types) and 3 mL of 5% saline were thoroughly mixed and packed into a 50 mL graduated centrifuge tube. This was left at 28 °C to observe the change in volume over time.
2.3. Wheat Flour Cultured in Saline (Wheat Flour Saline Culture)
A total of 6 g of wheat flour (three types) was mixed with 6 ml of 5% saline and incubated at 28 °C for 24 h. Next, 12 g of this wheat flour salt culture, 14 g of wheat flour B, 7 mL of tap water, and 0.1 g of dry yeast were placed in a stainless steel container and kneaded by hand with a spoon for 3 min (at room temperature) to obtain dough. This dough was then placed in a 200 mL measuring cylinder and left at 30 °C to observe the change in volume over time.
When the flour salt culture was scaled up and used for bread making, the mixing ratio was the same. The preparation of sponge dough is shown in Figure 1. No yeast was added initially, unlike in traditional sponge doughs.

Figure 1.
The preparation of sponge dough. No yeast was added at the beginning. All ingredients were added to a mochi pounding machine (Toshiba, Tokyo, Japan, PFC-20FK), and the dough was kneaded at 90 rpm for 3 min and then at 180 rpm for 7 min at a setting of 1 loaf (room temperature). The dough was taken out and stretched. The dough (approximately 90 g) was placed in a paper cup and fermented for 2–3 h in an incubator (MI-100G, Sansho Co., Ltd., Tokyo, Japan) set at 30 °C. The fermented dough was baked in an electric oven at 200 °C for 13 min.
2.4. Measurement of Microbial Count
To measure the microbial count in wheat flour culture, we used PetrifilmTM medium (3M Healthcare, Tokyo, Japan) plates for measuring the rapid mold and yeast count (TM plates), plates for measuring the lactic acid bacteria count (LAB plates), and plates for measuring the viable bacteria count (AC plates). The flour culture was serially diluted 5-fold in sterile 5% saline, and 1 mL of the diluted culture was plated on TM and AC plates and incubated aerobically. The flour culture was also serially diluted 5-fold in sterile water and plated on LAB and AC plates and incubated aerobically. In the case of no 5% salt added, the plates were left to stand at 30 °C for 2 days, and the number of colonies that appeared was counted (triplicate). In the case of salt-tolerant bacteria and salt-tolerant yeast, the plates were left to stand at 30 °C for 1 week.
2.5. Bread with a Simple Composition
The dough was prepared with 196 g of wheat flour, 168 g of wheat flour saline culture, 96 g of distilled water, and 1.4 g of dry yeast. All ingredients were added to a mochi pounding machine (Toshiba, PFC-20FK), and the dough was kneaded at 90 rpm for 3 min and then at 180 rpm for 7 min at a setting of 1 loaf (room temperature: 22 °C; humidity: 72%). The dough was taken out, rolled out, and cut into pieces of about 90 g. It was then placed in a paper cup (13.4 cm in diameter) and fermented for 2–3 h in an incubator (MI-100G, Sansho Co., Ltd.) set at 30 °C. Finally, the fermented dough was baked at 200 °C for 13 min in an electric oven (ER-C7 microwave oven, Toshiba Lifestyle Products Co., Ltd.).
The prototype bread was left to cool naturally for 1.5 h, and after the internal temperature had dropped to almost room temperature, it was placed in a polyethylene bag (Ziploc, Asahi Kasei Home Products Co., Ltd., Tokyo, Japan) [13]. After storing at room temperature for 24 h, the weight and volume of the bread were measured. The volume of bread was calculated using the rapeseed replacement method, and thus, the specific volume of the bread (cm3/g) was determined (n = 3) [14].
The cross-section of the bread was photographed with a digital camera (Cyber-shot WX350, SONY, Tokyo, Japan). Four cross-sectional photographs were enlarged and printed, and the minor diameters of air bubbles were measured with a scale. The occurrence rate (percentage) was calculated from the number of minor diameters.
2.6. Home Bakery
Bread was made according to a program in an automatic home bakery (HBK-100, MK Seiko Co., Ltd., Tokyo, Japan). The recipe included 196 g flour, 168 g flour salt culture, 98 g water, and 1.4 g dry yeast. The control recipe included 280 g flour, 4.2 g salt, 182 g water, and 1.4 g dry yeast.
The prototype bread was stored for 24 h and cut into 2 cm thick pieces, and the physical properties of the crumb were examined using a creep meter (RE2-3300C, Yamaden Co., Ltd., Tokyo, Japan). The stress measurement when the crumb was pressed 40% from the top was considered as the hardness (kPa). The measurement conditions were a disk-shaped plunger (diameter 25 mm), a load cell of 10 kg, and a bite speed of 2 mm/s.
The lightness (L*), redness (a*), and yellowness (b*) of the bread crumbs were measured using a spectrophotometer (CM-7000, Konica Minolta Japan, Inc., Tokyo, Japan). The moisture content was measured at 135 °C for 3 h under normal pressure.
2.7. Statistical Processing
The differences in the mean values of the specific volume of bread were tested using a one-way analysis of variance and Tukey’s multiple comparison test with Excel 2017 (Windows version).
3. Results
3.1. Wheat Flour Cultured in Saline
The preliminary test revealed the following: When wheat flour was mixed with 5% saline to make noodle dough and kept warm, the dough gradually expanded after 20 h. It was thought that the microorganisms in the wheat flour could be active even when 5% saline was added.
The fermentation ability of wheat flour cultured in 5% saline was tested. Figure 2 shows the results of tracking the volume change in dough made from the wheat flour culture. The addition of wheat flour cultured in saline caused the dough to rise faster than when dry yeast alone was used: after two hours, the volume increased by 20–30% compared to the control. An increased dough expansion was observed in all three types of strong wheat flour cultured in saline.
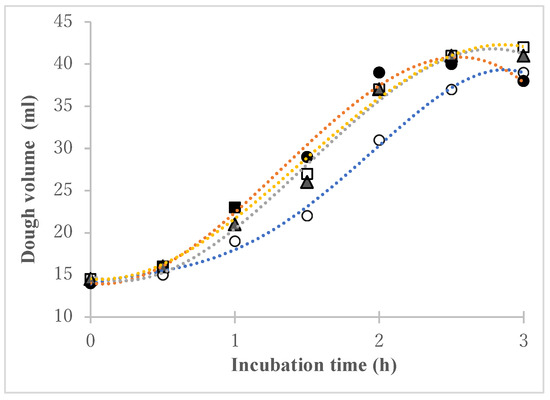
Figure 2.
Volume change in dough made by adding wheat flour cultured in 5% saline. ●: strong flour A; ▲: strong flour B; □: strong flour C; ○: control.
The results of the investigation of the microbiota of wheat flour cultured in saline are shown in Table 1. The concentration of salt-tolerant yeasts was less than 103 cfu/g. The concentration of lactic acid bacteria (LAB) varied depending on the type of flour, reaching 3.3 × 105 cfu/g in strong flour C and 1.1 × 106 cfu/g in strong flour B. This was thought to vary depending on the carbohydrate components of the flour.

Table 1.
Microbial flora of wheat flour saline culture.
The concentration of aerobic bacteria (2.7–4.7 × 108 cfu/g) was almost equal to that of the salt-tolerant bacteria (2.6–4.0 × 108 cfu/g), indicating that wheat flour cultured in 5% saline is mainly composed of salt-tolerant bacteria.
3.2. Bread Made Using Wheat Flour Saline Culture
Wheat flour cultured in saline was added to the dough to produce a prototype bread. The specific volume of this prototype bread is shown in Table 2. For only wheat flour and dry yeast, the specific volume was only 2.25 cm3/g; when wheat flour cultured in saline was added, the specific volume increased to 2.73–3.47 cm3/g. The cross-sections of bread containing wheat flour cultured in saline showed that the air bubbles inside the bread had a tendency to become larger. Measurements from cross-sectional photographs showed that 75% of the control bubbles had a short diameter of 1.3 mm or less. In the bubbles of strong flours A and B, the most common short diameter was 2.0 mm, but larger bubbles measuring 2.7 to 5 mm were also observed. It is believed that adding strong flour cultured in saline to the dough increased the size of the air bubbles and the specific volume of the bread.

Table 2.
Specific volume of bread made from wheat flour saline culture.
The results of the experiment using the home bakery are summarized below. The specific volume of the control was 2.43 cm3/g, but when the wheat flour culture was added, the specific volume increased to 3.23 cm3/g. The hardness of the bread crumbs was 36.3 kPa in the control, but it was significantly reduced to 4.8 kPa when the wheat flour culture was added, and the hardness was significantly reduced. The addition of the wheat flour culture increased the specific volume of the bread by about 33%, which seems to have reduced the hardness of the bread. The redness (a*) of the bread crumbs did not change, the lightness (L*) became slightly lighter, and the yellowness (b*) decreased.
4. Discussion
The key factors in making sponge dough and sourdough are considered to be the raw materials (flour type and quality) and fermentation process parameters used (temperature, pH, water activity, additives, etc.) [6,7,8]. Fujimoto studied the behavior of microorganisms used in sourdough used in Japan [15,16,17]. They found that, when cultured at 28 °C, Gram-negative bacteria attached to raw materials proliferated on the first day, LAB reached their maximum growth on the second day, and the growth of yeast increased from the third day onwards [15,17]. To make bread using only flour and water, we focused on the day-one culture of sourdough [10]. There were more than 109 /g total bacteria in the day-one cultures, whereas the number of yeast cells was low at 103 cfu/g. Kosakonia cowanii was frequently isolated from four types of the day-one culture. Bread could be made using Kosakonia cowanii SB and flour without adding sugars or yeast, and its specific volume was 2.54–2.64 cm3/g. Kosakonia cowanii is a rod-shaped, Gram-negative, facultative anaerobic bacterium, commonly found in soil and water, as well as internally in plants, animals, and humans [11,12]. However, there were hesitations about its use in actual bread making.
Possible methods for eliminating enterobacteria and microorganisms associated with food poisoning include temperature control and the addition of salt. Menezes et al. aimed to determine how temperature changes during the propagation of sourdoughs affect the dynamics of the bacterial ecosystem [18]. At 21 °C, LAB were predominant at the end of the fermentation and transplantation steps. Furthermore, a temperature of 30 °C favored the persistence of atypical bacteria, such as Pseudomonas and Enterobacteriaceae. Therefore, a temperature of 21 °C was more suitable for sourdough propagation.
Adding salt is an also effective way of controlling the microbial concentrations in the food ecosystem. In Japan, salt (5–20%) has long been used to control the fermentation of foods such as miso, soy sauce, and Inaniwa handmade noodles [19,20,21]. The authors investigated the effects of salt concentration on Kosakonia cowanii BS obtained from water cultures of wheat flour and found that it grew well at 0 to 2% salt, but growth was inhibited at 5% or more [10]. A low level of NaCl (up to 0.7%) stimulated LAB growth, but higher levels decreased LAB growth drastically [22]. The growth of L. sanfranciscensis LTH1729 and LTH2581 and the main LAB in sourdough was completely inhibited by 4% NaCl [9].
Inaniwa udon is made by adding 5% salt water to wheat flour, leaving it overnight, and then stretching it by hand. These steps allowed salt-tolerant yeasts to grow in flour [21]. Adding salt-tolerant culture to the dough produced a softer dough that expanded better [23]. Zhu et al. investigated the effects of incorporating Euglena gracilis microalgae powder (MP) on the dough properties, rheology, and quality attributes of Chinese steamed bread (CSB) [24]. Moderate levels of MP (2%) reinforced the gluten network and improved protein structure.
Using the salt-tolerant yeast (Hyphopichia burtonii), a primitive type of bread with a specific volume of 2.42–2.62 cm3/g can be made [25]. In the case of Inaniwa udon, a medium containing 5% salt was more suitable for the growth of salt-tolerant yeasts than a medium containing 10% salt [26]. However, this study found that the number of yeast cells did not increase when wheat flour was cultivated in 5% saline for one day (Table 1). It was hypothesized that the addition of sugar and the extension of the culture time were necessary to increase the number of salt-tolerant yeast cells.
The sponge dough proposed in this study differs from conventional sponge dough in that yeast was not added first, and salt-tolerant bacteria were predominant. Therefore, it is believed that salt-tolerant bacteria play some role in the increase in the specific volume of bread. When strong flour cultured in saline was added to bread dough, the size of air bubbles increased (see Supplementary Materials), which led to an increase in the specific volume of bread. This is thought to be mainly due to gas generation carried out by salt-tolerant bacteria, but the dominant species needs to be identified. Salt-tolerant bacteria such as Bacillus licheniformis and Bacillus amyloliquefaciens are involved in the production of carbon dioxide gas that causes gochujang to rise [27]. The flexibility of the dough, as pointed out by Zhu et al., is also thought to be related to the increase in specific volume. Hori et al. isolated Bacillus methylotrophicus and Bacillus amyloliquefaciens from naan and chapati that grew in a 10% NaCl solution [28]. These strains may be involved in dough flexibility because they have the ability to decompose the allergenic proteins in wheat flour.
We reported that bread with a specific volume of 2.73 to 3.47 cm3/g can be produced with the sponge dough method using 5% saline. This is a primitive type of bread made only with wheat flour, salt, yeast, and water.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation11040206/s1, Figure S1: Volume change of noodle dough made from wheat flour and 5% saline; Figure S2: Cross-section of bread made by adding four saline culture to the dough; Figure S3: Air bubble distribution in bread crumbs made by adding wheat flour saline culture. The columns from right to left show control, strong flour A, and strong flour B. Table S1: Composition used for wheat flour 5% saline culture; Table S2: Bread made in a home bakery using wheat flour saline culture.
Author Contributions
Study conception and design, N.O.; data collection, N.O. and K.E., analysis and interpretation of results, N.O., K.E., and T.K.; draft manuscript preparation, N.O. and T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the paper/Supplementary Materials. Further inquiries should be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yoshino, S. Science of Bread, 1st ed.; Kodansha: Tokyo, Japan, 2018; pp. 149–181. [Google Scholar]
- Tanaka, Y.; Matsumoto, H. Science of Bread-Making Ingredients, 1st ed.; Korin: Tokyo, Japan, 1992; pp. 99–166. [Google Scholar]
- Esaki, O. Easy-to-Understand Bread-Making Techniques, 1st ed.; Shibata Bookstore: Tokyo, Japan, 1996; pp. 9–28. [Google Scholar]
- Hironaka, Y. Effect of fermentation conditions on the quality of white bread produced by sponge dough method. J. Jpn. Soc. Food Sci. Technol. 1984, 31, 360–363. [Google Scholar] [CrossRef]
- Morita, A.; Hayakawa, F. Visualization of aroma by component analysis and color evaluation of bread made with commercially available baker’s yeast. J. Jpn. Soc. Food Sci. Technol. 2022, 55, 105–112. [Google Scholar] [CrossRef]
- Lever, T.; Kelly, A.; Faveri, J.D.; Martin, D.; Sheppard, J.; Quail, K.; Miskelly, D. Australian wheat for the sponge and dough bread making process. Aust. J. Agri. Res. 2005, 56, 1049–1057. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, R.; Yuan, W. Type I sourdough steamed bread made by retarded sponge-dough method. Food Chem. 2020, 311, 126029. [Google Scholar] [CrossRef]
- Salovaara, H.; Valjakka, T. The effect of fermentation temperature, flour type, and starter on the properties of sour wheat bread. Inter. J. Food Sci. Technol. 1987, 22, 591–597. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Ehmann, M.; Hammes, W.P. Modeling of growth of Lactobacillus sanfranciscensis and Candida milleri in response to process parameters of sourdough fermentation. Appl. Environ. Microbiol. 1998, 64, 2616–2623. [Google Scholar] [CrossRef]
- Ohisa, N.; Cho, T.; Komoda, T. Yeast-free bread using Kosakonia cowanii SB isolated from day-one culture of wheat flour. Quest Tradit. Food 2025, 52. in press. [Google Scholar]
- Mosquito, S.; Bertani, I.; Licastro, D.; Compant, S.; Myers, M.P.; Hinarejos, E.; Levy, A.; Venturi, V. In planta colonization and role of T6SS in two rice Kosakonia endophytes. Mol. Plant-Microbe Interact. 2020, 33, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Petrzik, K.; Brázdová, S.; Krawczyk, K. Novel viruses that lyse plant and human strains of Kosakonia cowanii. Viruses 2021, 13, 1418–1432. [Google Scholar] [CrossRef]
- Wang, Y.; Morishima, H.; Seo, Y.; Sagara, Y.; Imou, H. Studies on rheology of white bread (part 2). J. Jap. Soc. Agri. Mach. Food Eng. 1992, 54, 75–82. [Google Scholar] [CrossRef]
- Ohba, K.; Kawabata, A. Cooking Science Experiments, 1st ed.; Gakuken Shoin: Tokyo, Japan, 2003; pp. 12–13. [Google Scholar]
- Fujimoto, A.; Ito, T.; Imura, S. Good taste produced by traditional baker’s yeast and its relation with the bacteria contained in the yeasts. Seibutsu-Kogaku Kaishi 2012, 6, 329–334. [Google Scholar]
- Fujimoto, A.; Ito, K.; Itou, M.; Narushima, N.; Ito, T.; Yamamoto, A.; Hirayama, S.; Furukawa, S.; Morinaga, Y.; Miyamoto, T. Microbial behavior and changes in food constituents during fermentation of Japanese sourdoughs with different rye and wheat starting materials. J. Biosci. Bioeng. 2018, 125, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, A. Fundamental Studies on Leavening Agents for Bread Making. Doctoral Dissertation, Kyushu University, Fukuoa, Japan, 2018; pp. 40–82. Available online: https://hdl.handle.net/2324/2236295 (accessed on 1 March 2025).
- Menezes, L.A.A.; Savo Sardaro, M.L.; Duarte, R.T.D.; Mazzon, R.R.; Neviani, M.; Gatti, M.; Lindner, J.D.D. Sourdough bacterial dynamics revealed by metagenomic analysis in Brazil. Food Microbiol. 2020, 85, 103302–103313. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Do, E. Microorganisms in miso and soy sauce. J. Jpn. Soc. Food Microbiol. 1994, 11, 151–157. [Google Scholar] [CrossRef]
- Ura, T.; Inamori, K.; Uchida, I. Classification and properties of newly isolated halotolerant lactic acid bacteria. J. Brew. Soc. Jpn. 1989, 84, 588–593. [Google Scholar] [CrossRef]
- Ohisa, N.; Suzuki, N.; Miura, M.; Endo, K.; Fujita, Y. Halotolerant yeast participates in the cavity formation of hand-pulled dry noodles. J. Jpn. Soc. Food Sci. Technol. 2012, 59, 442–446. [Google Scholar] [CrossRef][Green Version]
- Simonson, L.; Salovaara, H.; Korhola, M. Response of wheat sourdough parameters to temperature, NaCl and sucrose variations. Food Microbiol. 2003, 20, 193–199. [Google Scholar] [CrossRef]
- Ohisa, N.; Sugawara, Y.; Mohri, S. Breads prepared by using a salt-tolerant yeast enrichment culture and dry yeast. J. Jpn. Soc. Food Sci. Technol. 2020, 67, 514–518. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, Y.; Xu, Y.; Wei, X.; Yang, Z.; Yin, Y.; Wakisaka, M.; Fang, W. Effects of heterotrophic Euglena gracilis powder on dough microstructure, rheological properties, texture, and nutritional composition of steamed bread. Food Chem. X 2024, 23, 101754. [Google Scholar] [CrossRef]
- Ohisa, N.; Hoshi, Y.; Komoda, T. Bread prepared using Hyphopichia burtonii M2 isolated from ‘Inaniwa Udon’. J. Cook. Sci. Jpn. 2019, 52, 109–113. [Google Scholar] [CrossRef]
- Ohisa, N.; Nakamura, K.; Cho, T.; Mohri, S. Bread fermentation seed prepared from Inaniwa udon dough in saline medium. J. Cook. Sci. Jpn. 2022, 55, 295–298. [Google Scholar] [CrossRef]
- Back, J.H.; Suzuki, I.; Takeda, M.; Koizumi, J. Causative microorganisms on swelling incident of Gochujang. Jpn. J. Food Eng. 2018, 19, 105–110. [Google Scholar] [CrossRef]
- Hori, M.; Suzuki, T.; Nagano, H. Influence on wheat proteins of microorganismsin traditional fermented food products. J. Home Econo. Jpn. 2012, 63, 771–780. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).