Assessment of Alternative Media Viability for Cell Growth Phase in the Lab-Scale Xanthan Pruni Production—Part I
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rice Parboiling Wastewater Characterization
2.2. Inoculum Preparation
2.3. Xanthan Pruni Production
2.4. Nitrogen
2.5. Viscosity
2.6. Minerals
2.7. Statistics
3. Results and Discussion
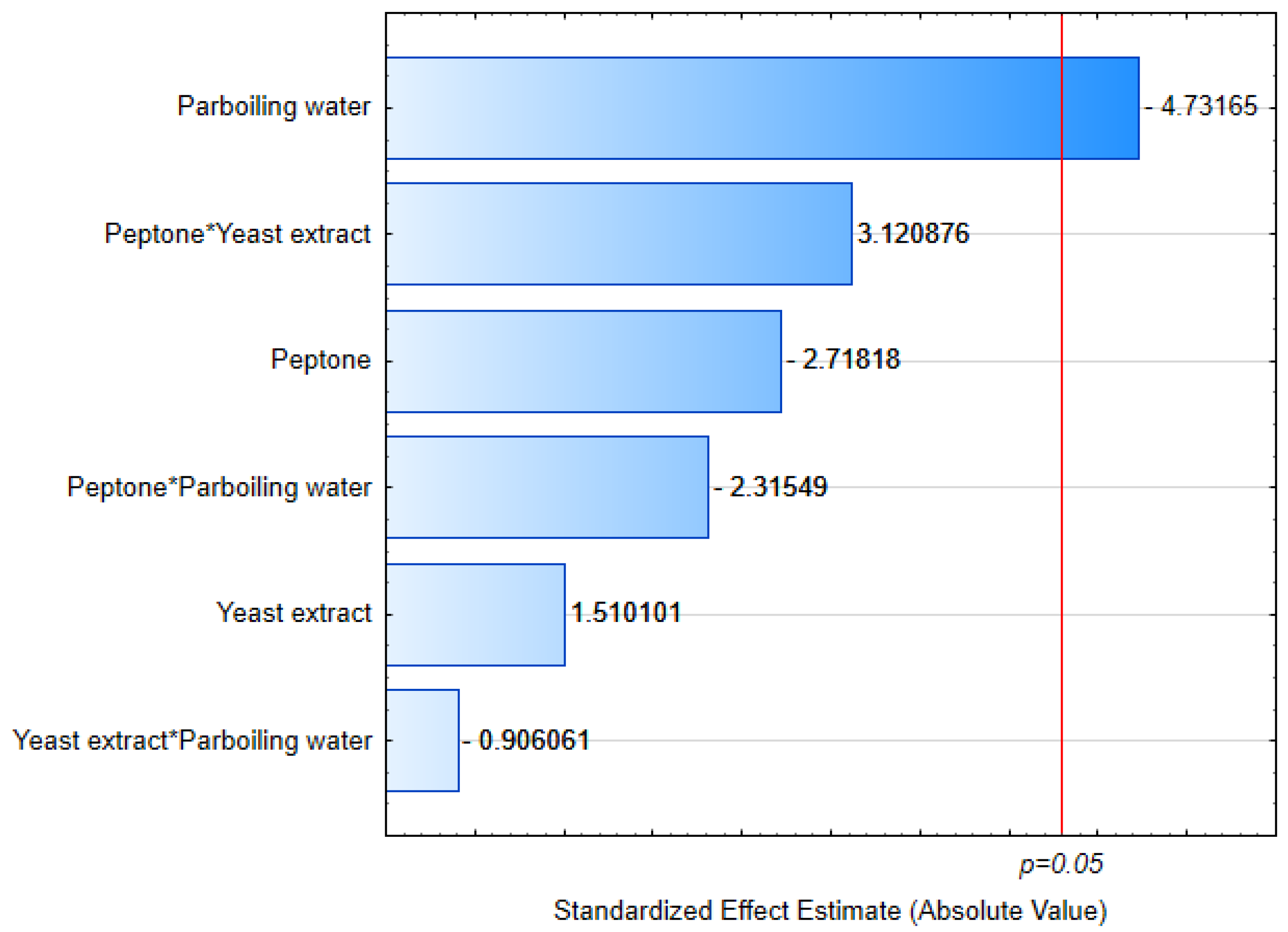
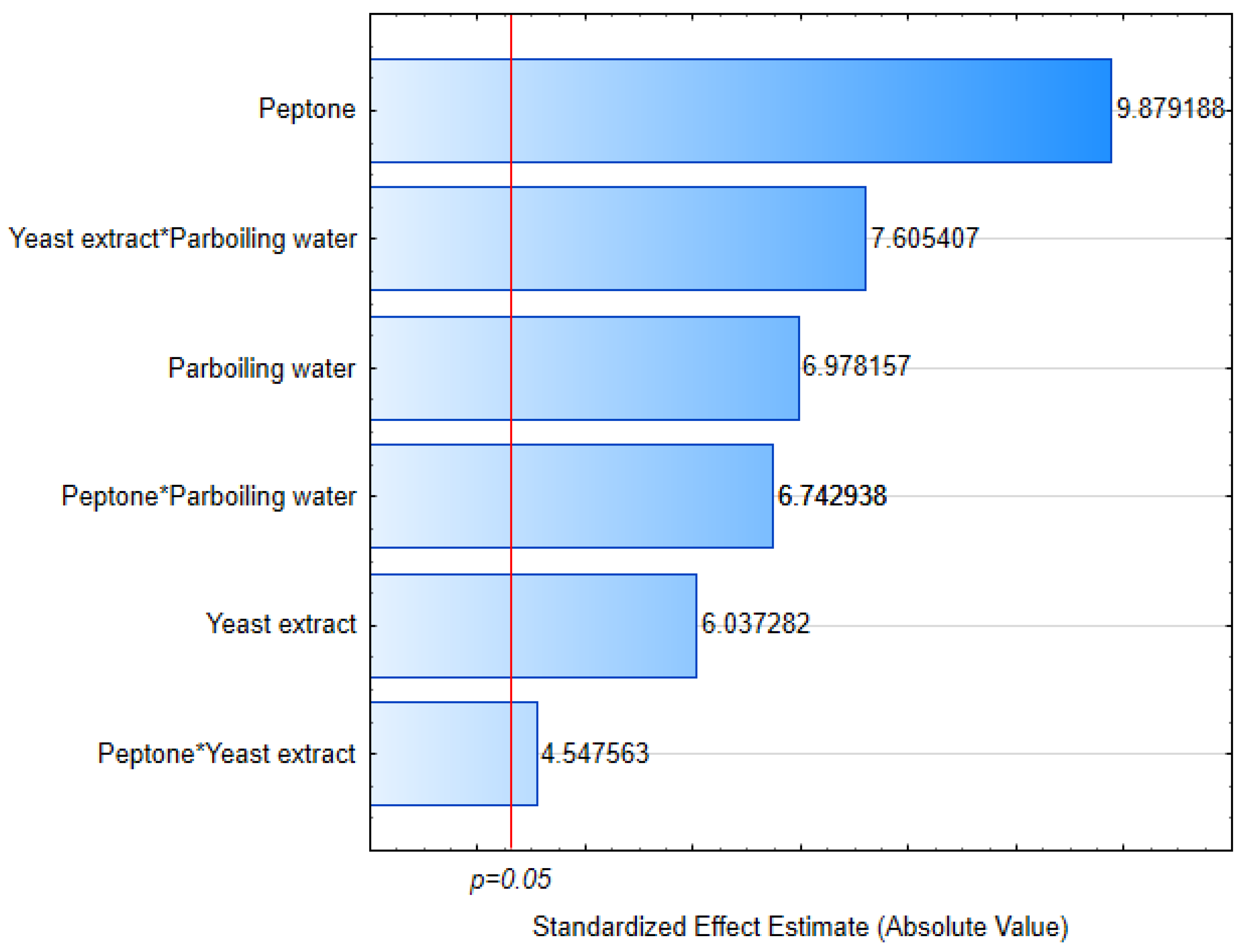
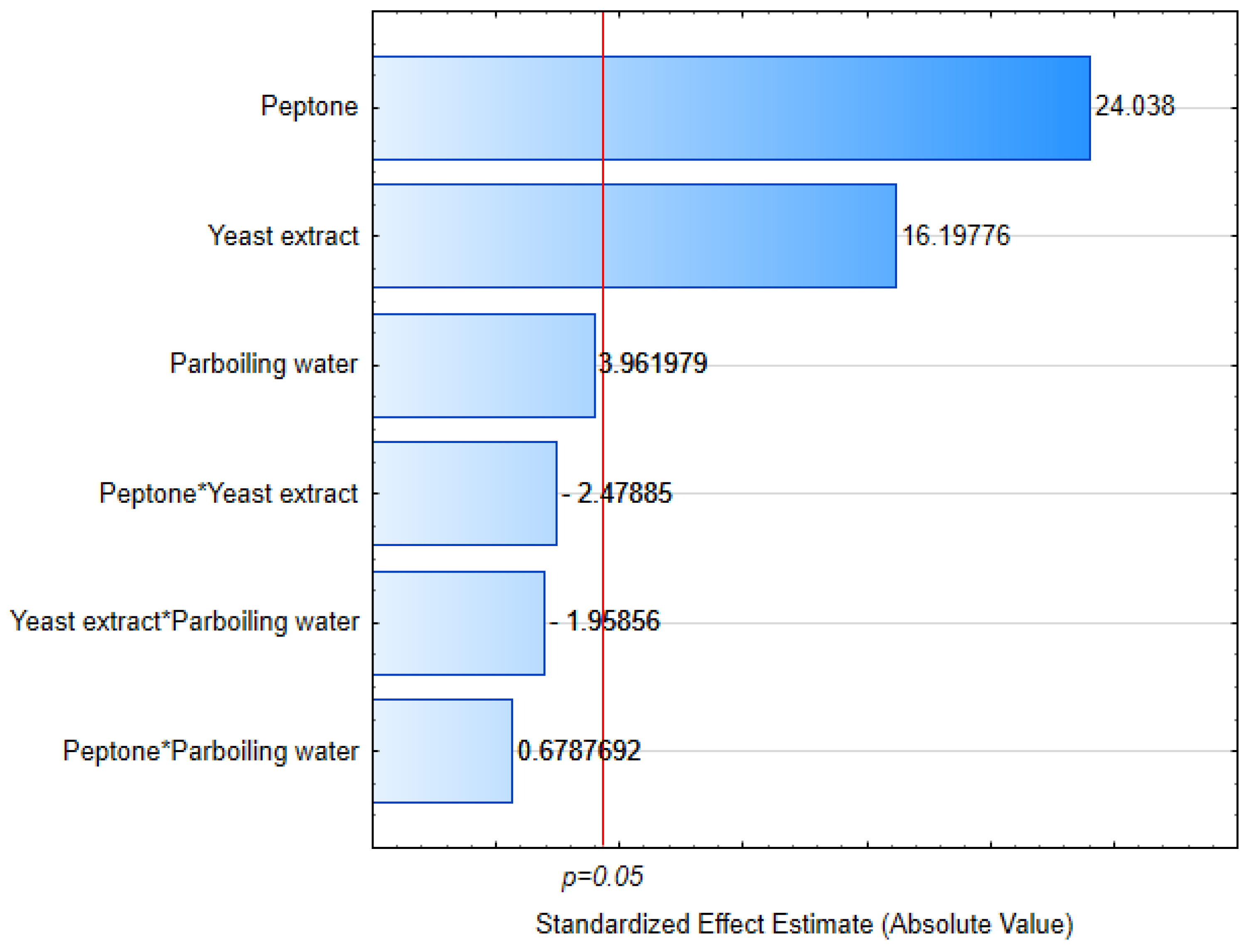
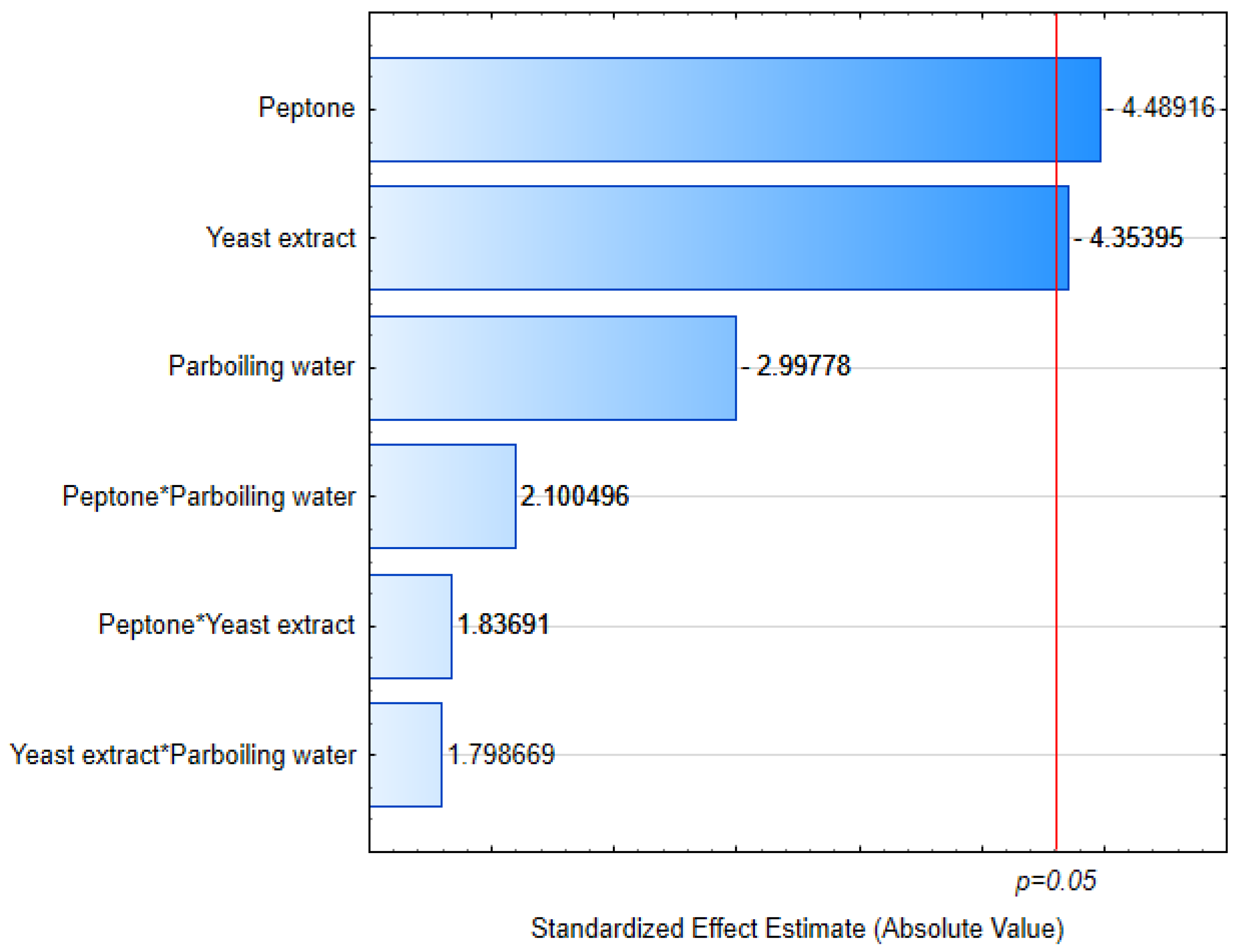
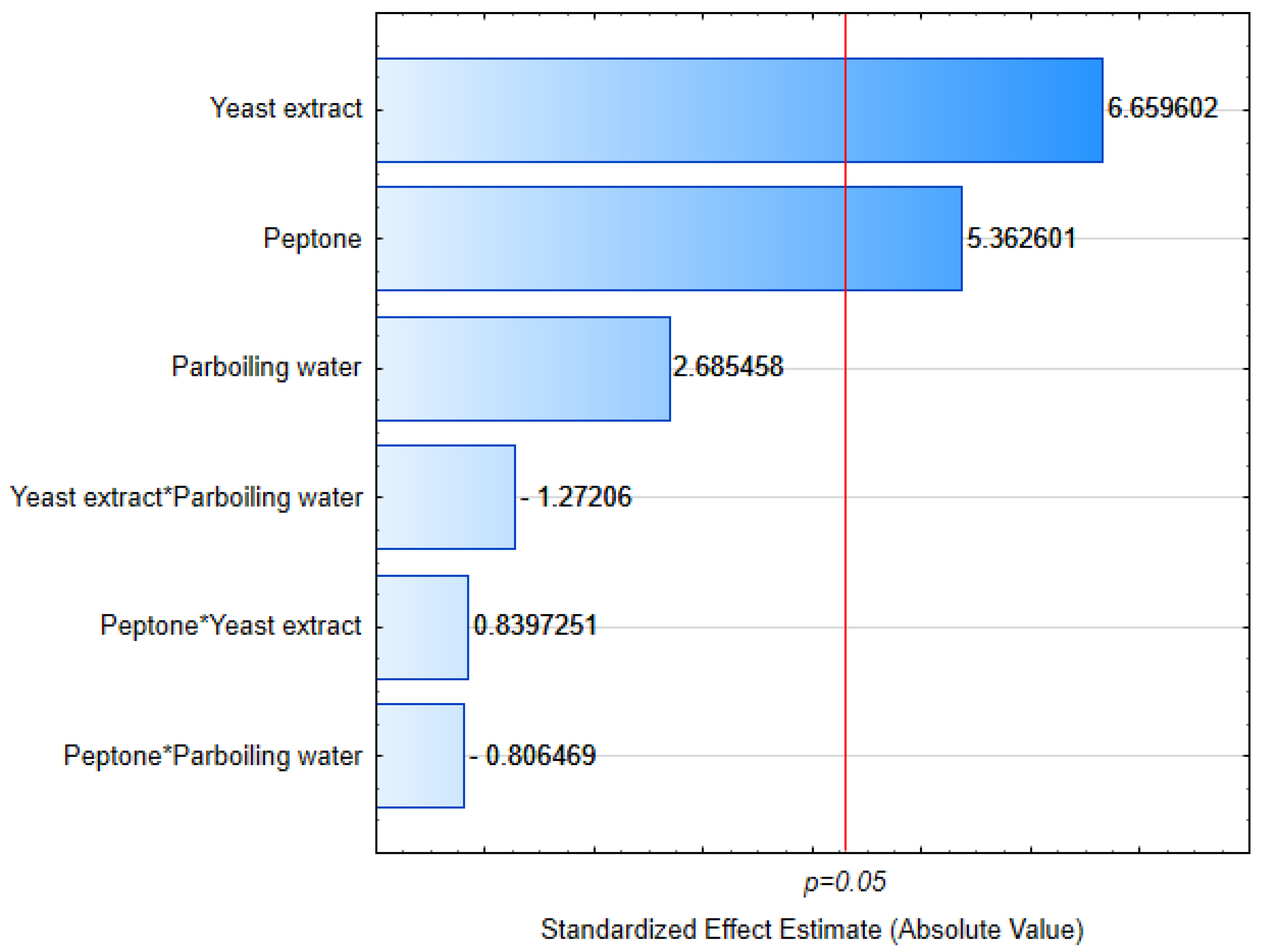
3.1. Cell Growth
3.2. Yield
3.3. Residual Nitrogen
3.4. Viscosity
3.5. Minerals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RPW | rice parboiling water |
References
- Cancella, M.J.; Cerqueira, A.F.L.W.; da Costa Teodoro, L.; Pereira, J.R.; da Costa Ludwig, Z.M.; de Carvalho Anjos, V.; Rodarte, M.P. Xanthan gum produced from milk permeate and deproteinized cheese whey: A comparative analysis with commercial xanthan gums. Biocatal. Agric. Biotechnol. 2024, 56, 103053. [Google Scholar]
- Global Market Insights. Xanthan Gum Market Size, Industry Analysis Report, Regional Outlook, Growth Potential, Price Trends, Competitive Market Share & Forecast. 2024. Available online: https://www.gminsights.com/industry-analysis/xanthan-gum-market (accessed on 26 February 2025).
- Mordor Intelligence. Xanthan Gum Market Size & Share Analysis—Growth Trends & Forecasts (2025–2030). Available online: https://www.mordorintelligence.com/industry-reports/xanthan-gum-market (accessed on 26 February 2025).
- Li, P.; Li, T.; Zeng, Y.; Li, X.; Jiang, X.; Wang, Y.; Xie, T.; Zhang, Y. Biosynthesis of xanthan gum by Xanthomonas campestris LRELP-1 using kitchen waste as the sole substrate. Carbohydr. Polym. 2016, 151, 684–691. [Google Scholar] [PubMed]
- Ramos, B.F.M.; de Almeida, P.F.; Chinalia, F.A. Bacterial xanthan and ramnolipid simultaneous production using industrial oil produced water. Environ. Technol. 2022, 43, 983–990. [Google Scholar]
- Rashidi, A.R.; Dailin, D.J.; Ramli, S.; Hanapi, S.Z.; Ibrahim, S.F.; El Enshasy, H. Variable nitrogen sources effect on Xanthomonas campestris ATCC 13915 ability for xanthan production in culture supplemented with pineapple waste. Biosci. Res. 2023, 20, 7–12. [Google Scholar]
- Vidhyalakshmi, R.; Vallinachiyar, C.; Radhika, R. Production of xanthan from agro-industrial waste. J. Adv. Sci. Res. 2012, 3, 56–59. [Google Scholar]
- Obidah, J.S.; Owuama, C.I. Xanthan yield and conversion efficiency of pre-treated rice husk, sweet potato and cassava flours from Xanthomonas campestris fermentation. Int. J. Eng. Trends Technol. 2017, 49, 477–481. [Google Scholar]
- Ozdal, M.; Kurbanoglu, E.B. Citric acid production by Aspergillus niger from agro-industrial by-products: Molasses and chicken feather peptone. Waste Biomass Valorization 2019, 10, 631–640. [Google Scholar]
- Demirci, A.S.; Palabiyik, I.; Apaydın, D.; Mirik, M.; Gumus, T. Xanthan gum biosynthesis using Xanthomonas isolates from waste bread: Process optimization and fermentation kinetics. LWT 2019, 101, 40–47. [Google Scholar] [CrossRef]
- Mohsin, A.; Zhang, K.; Hu, J.; Rehman, S.U.; Tariq, M.; Zaman, W.Q.; Khan, I.M.; Zhuang, Y.; Guo, M. Optimized biosynthesis of xanthan via effective valorization of orange peels using response surface methodology: A kinetic model approach. Carbohydr. Polym. 2018, 181, 793–800. [Google Scholar]
- Trivunović, Z.; Zahović, I.; Vlajkov, V.; Grahovac, M.; Grahovac, J.; Dodić, J. Xanthan production using wastewaters from rose wine industry: Screening of Xanthomonas euvesicatoria isolates. Period. Polytech. Chem. Eng. 2024, 68, 428–436. [Google Scholar] [CrossRef]
- Zohoun, E.V.; Tang, E.N.; Soumanou, M.M.; Manful, J.; Akissoe, N.H.; Bigoga, J.; Futakuchi, K.; Ndindeng, S.A. Physicochemical and nutritional properties of rice as affected by parboiling steaming time at atmospheric pressure and variety. Food Sci. Nutr. 2018, 6, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, T.B.; Das, B.; Kumar, A.; Kumar, G.; Banerjee, J.; Gain, H.; Adhikari, A.A.; Chattopadhyay, K. Impact of cooking, parboiling and fermentation on nutritional components, predicted glycemic index and pasting properties of rice. J. Cereal Sci. 2023, 114, 103763. [Google Scholar]
- Yang, J.; Li, X.; Zhao, S.; Yuan, W.; Zhou, Q.; Zhang, Y.; Qiu, J.; Wang, J.; Zhu, Q.; Yang, X.; et al. Light calcium carbonate improves pullulan biosynthesis by Aureobasidium pullulans under high concentration of sugar. Food Chem. 2023, 415, 135760. [Google Scholar] [PubMed]
- Zhang, Y.; Wang, X.; Hu, Z.Q.; Xiao, Q.Q.; Wu, Y. Capturing and recovering phosphorus in water via composite material: Research progress, future directions, and challenges. Sep. Purif. Technol. 2025, 353, 128453. [Google Scholar]
- Vendruscolo, C.T.; Vendruscolo, J.L.S.; da Moreira, A.S. Meio de cultura para crescimento de Xanthomonas. Brazilian patent BR1220140300158, 5 November 2004. [Google Scholar]
- Moreira, A.D.S.; Vendruscolo, J.L.S.; Gil-Turnes, C.; Vendruscolo, C.T. Screening among 18 novel strains of Xanthomonas campestris pv pruni. Food Hydrocoll. 2001, 15, 469–474. [Google Scholar] [CrossRef]
- Perez, I.A.; Macagnan, K.L.; Costa, E.D.S.M.; de Oliveira, G.D.; Ames, C.W.; Rossi, D.; da Silveira Moreira, A. Efeitos de novos extratos de levedura no crescimento celular, produção e viscosidade da xantana pruni por Xanthomonas arboricola pv pruni cepa 106. Braz. J. Dev. 2020, 6, 21543–21552. [Google Scholar]
- Moreira, A.S.; Foresti, A.P.; Rodrigues, A.A.; Macagnam, J.L.; Alves, M.I.; Vendruscolo, C.T. Bioconversion of Agri-Food Wastes into Biopolymers: A step towards circular bioeconomy. In Microbial Bioprocessing of Agri-Food Wastes, 1st ed.; CRC Press: Boca Raton, FL, USA, 2023; Volume 1, pp. 81–124. [Google Scholar]
- Instituto Adolfo Lutz. Normas Analíticas do Instituto Adolfo Lutz, 3rd ed.; IMESP: São Paulo, Brazil, 1985; Volume 1, p. 27. [Google Scholar]
- Yu, S.; Olsen, C.E.; Marcussen, J. Methods for the assay of 1,5-anhydro-D-fructose and α-1,4-glucan lyase. Carbohydr. Res. 1997, 305, 73–82. [Google Scholar] [CrossRef]
- Junior, R.A.C.; Chagas, A.V.; Felix, C.S.; Souza, R.C.; Silva, L.A.; Lemos, V.A.; Ferreira, S.L. A closed inline system for sample digestion using 70% hydrogen peroxide and UV radiation. Determination of lead in wine employing ETAAS. Talanta 2019, 191, 479–484. [Google Scholar]
- Hayward, A.C. Bacteriophage sensitivity and biochemical group in Xanthomonas malvacearum. Microbiology 1964, 35, 287–298. [Google Scholar]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Da Rosa, M.B.; Oreste, E.Q.; Bonemann, D.H.; Rodrigues, A.A.; Vendruscolo, C.T.; Moreira, A.S.; Ribeiro, A.S.; Nunes, A.M. Evaluation of the use of a reflux system for sample preparation of xanthan gum and subsequent determination of Ca, Cu, K, Mg, Na and Zn by atomic spectrometry techniques. J. Braz. Chem. Soc. 2016, 27, 919–924. [Google Scholar]
- Murad, H.A.; Mohamed, S.H.; Abu-El-Khair, A.G. Impact of amino acids, nitrogen source, and buffering system on xanthan yield produced on hydrolyzed whey lactose. Biotechnology 2017, 16, 69–76. [Google Scholar] [CrossRef]
- Kurbanoglu, E.B.; Kurbanoglu, N.I. Ram horn hydrolysate as enhancer of xanthan production in batch culture of Xanthomonas campestris EBK-4 isolate. Process Biochem. 2007, 42, 1146–1149. [Google Scholar] [CrossRef]
- Carignatto, C.R.R.; Oliveira, K.S.M.; de Lima, V.M.G.; de Oliva Neto, P. New culture medium to xanthan production by Xanthomonas campestris pv. campestris. Indian J. Microbiol. 2011, 51, 283–288. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.A.; Cardoso, L.G.; de Jesus Assis, D.; Gomes, G.V.P.; Oliveira, M.B.P.P.; de Souza, C.O.; Druzian, J.I. Xanthan gum production by Xanthomonas campestris pv. campestris IBSBF 1866 and 1867 from lignocellulosic agroindustrial wastes. Appl. Biochem. Biotechnol. 2018, 186, 750–763. [Google Scholar] [CrossRef]
- Macagnan, K.L.; Perez, I.A.; Costa, E.D.S.M.; Ames, C.W.; Soares, J.C.M.; Sourabié, A.; da Silveira Moreira, A. Influência de diferentes extratos de levedura (insumos) no crescimento celular de Xanthomonas arboricola pv. pruni cepa 101. Braz. J. Dev. 2021, 7, 15816–15824. [Google Scholar] [CrossRef]
- Ouyabe, M.; Kikuno, H.; Tanaka, N.; Babil, P.; Shiwachi, H. Isolation and identification of nitrogen-fixing bacteria associated with Dioscorea alata L. and Dioscorea esculenta L. Microb. Resour. Syst. 2019, 35, 3–11. [Google Scholar]
- Crugeira, P.J.L.; Almeida, H.H.S.; Marcet, I.; Rendueles, M.; Pires, M.G.; Rafael, H.M.; Rodrigues, A.I.G.; Santamaria-Echart, A.; Barreiro, M.F. Biosynthesis of antioxidant xanthan gum by Xanthomonas campestris using substrates added with moist olive pomace. Food Bioprod. Process. 2023, 141, 210–218. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Santos, V.E.; Casas, J.A.; Gomez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO); World Health Organization (WHO). Xanthan Gum. Compendium of Food Additive Specifications. In Proceedings of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 82nd Meeting 2016, FAO JECFA Monographs 19. Geneva, Switzerland, 7–16 June 2016. [Google Scholar]
- Burdock, G.A. Food Chemicals Codex, 4th ed.; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Torres, L.G.; Brito, E.; Galindo, E.; Choplin, L. Viscous behaviour of xanthan aqueous solutions from a variant strain of Xanthomonas campestris. J. Ferment. Bioeng. 1993, 75, 58–64. [Google Scholar] [CrossRef]
- Borges, C.D.; Bastos, C.P.; Vendruscolo, C.T. Avaliação das características físicas e químicas de gomas xantanas. Ciênc. Exatas Technol. 2007, 28, 107–114. [Google Scholar] [CrossRef]
- Klaic, P.M.A.; Nunes, A.M.; da Silveira Moreira, A.; Vendruscolo, C.T.; Ribeiro, A.S. Determination of Na, K, Ca and Mg in xanthan gum: Sample treatment by acid digestion. Carbohydr. Polym. 2011, 83, 1895–1900. [Google Scholar]
- Galván, Z.R.N.; Soares, L.D.S.; Medeiros, E.A.A.; Soares, N.D.F.F.; Ramos, A.M.; Coimbra, J.S.D.R.; de Oliveira, E.B. Rheological properties of aqueous dispersions of xanthan gum containing different chloride salts are impacted by both sizes and net electric charges of the cations. Food Biophys. 2018, 13, 186–197. [Google Scholar] [CrossRef]
- Luporani, S.; Bretas, R. Rheological characterization of xanthan gum: Influence from univalent and trivalent metallic ions and from the temperature in dynamic experiments. Polímeros 2011, 21, 188–194. [Google Scholar]
| Parameters | |
|---|---|
| pH | 4.6 ± 0.2 |
| Reducing Sugars | 3.40 ± 0.16 |
| Nitrogen (mg/dL) | 140.00 ± 1.12 |
| Phosphorus (mg/L) | 304.96 ± 9.90 |
| Zinc (mg/L) | 1.53 ± 0.05 |
| Iron (mg/L) | 9.36 ± 0.33 |
| Silicon (mg/L) | 39.43 ± 10.26 |
| Calcium (mg/L) | 32.80 ± 0.41 |
| Potassium (mg/L) | 4411.96 ± 196.21 |
| Magnesium (mg/L) | 169.23 ± 8.37 |
| Manganese (mg/L) | 43.17 ± 1.37 |
| Sodium (mg/L) | 21.86 ± 1.88 |
| Treat. | Codified Levels | Real Levels | ||||
|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x1 | x2 | x3 | |
| 1 | −1 | −1 | −1 | 1 | 1 | 20 |
| 2 | +1 | −1 | −1 | 5 | 1 | 20 |
| 3 | −1 | +1 | −1 | 1 | 5 | 20 |
| 4 | +1 | +1 | −1 | 5 | 5 | 20 |
| 5 | −1 | −1 | +1 | 1 | 1 | 80 |
| 6 | +1 | −1 | +1 | 5 | 1 | 80 |
| 7 | −1 | +1 | +1 | 1 | 5 | 80 |
| 8 | +1 | +1 | +1 | 5 | 5 | 80 |
| 9 | 0 | 0 | 0 | 3 | 3 | 50 |
| 10 | 0 | 0 | 0 | 3 | 3 | 50 |
| 11 | 0 | 0 | 0 | 3 | 3 | 50 |
| Treat. | x1 | x2 | x3 | Cell Growth (UFC/mL) |
|---|---|---|---|---|
| 1 | −1 (1) | −1 (1) | −1 (20) | 9.93 ± 0.01 * |
| 2 | +1 (5) | −1 (1) | −1 (20) | 9.87 ± 0.04 * |
| 3 | −1 (1) | +1 (5) | −1 (20) | 9.94 ± 0.04 * |
| 4 | +1 (5) | +1 (5) | −1 (20) | 9.98 ± 0.04 * |
| 5 | −1 (1) | −1 (1) | +1 (80) | 9.92 ± 0.04 * |
| 6 | +1 (5) | −1 (1) | +1 (80) | 9.69 ± 0.01 * |
| 7 | −1 (1) | +1 (5) | +1 (80) | 9.83 ± 0.01 * |
| 8 | +1 (5) | +1 (5) | +1 (80) | 9.81 ± 0.01 * |
| 9 | 0 (3) | 0 (3) | 0 (50) | 9.85 ± 0.03 * |
| 10 | 0 (3) | 0 (3) | 0 (50) | 9.78 ± 0.01 * |
| 11 | 0 (3) | 0 (3) | 0 (50) | 9.82 ± 0.01 * |
| SPA | 5 | - | - | 10.04 ± 0.01 |
| Treat. | x1 | x2 | x3 | Yield (g/L) |
|---|---|---|---|---|
| 1 | −1 (1) | −1 (1) | −1(20) | 7.233 ± 0.20 * |
| 2 | +1 (5) | −1 (1) | −1 (20) | 7.393 ± 0.42 * |
| 3 | −1 (1) | +1 (5) | −1 (20) | 7.123 ± 0.51 * |
| 4 | +1 (5) | +1 (5) | −1 (20) | 7.333 ± 0.22 * |
| 5 | −1 (1) | −1 (1) | +1 (80) | 7.020 ± 0.32 |
| 6 | +1 (5) | −1 (1) | +1 (80) | 7.533 ± 0.26 * |
| 7 | −1 (1) | +1 (5) | +1 (80) | 7.343 ± 0.35 * |
| 8 | +1 (5) | +1 (5) | +1 (80) | 8.943 ± 0.63 * |
| 9 | 0 (3) | 0 (3) | 0 (50) | 7.610 ± 0.46 * |
| 10 | 0 (3) | 0 (3) | 0 (50) | 7.683 ± 0.40 * |
| 11 | 0 (3) | 0 (3) | 0 (50) | 7.393 ± 0.73 * |
| SPA | 5 | - | - | 6.770 ± 0.65 |
| Treat. | x1 | x2 | x3 | Residual Nitrogen 0 h (mg/L) | Residual Nitrogen 24 h (mg/L) |
|---|---|---|---|---|---|
| 1 | −1 (1) | −1 (1) | −1(20) | 45.2 ± 1.3 * | 102.8 ± 8.6 * |
| 2 | +1 (5) | −1 (1) | −1 (20) | 78.2 ± 1.9 * | 280.4 ± 4.5 * |
| 3 | −1 (1) | +1 (5) | −1 (20) | 81.3 ± 3.2 | 236.9 ± 6.1 * |
| 4 | +1 (5) | +1 (5) | −1 (20) | 94.4 ± 2.8 * | 449.9 ± 4.6 * |
| 5 | −1 (1) | −1 (1) | +1 (80) | 48.1 ± 2.5 * | 108.2 ± 8.5 * |
| 6 | +1 (5) | −1 (1) | +1 (80) | 85.7 ± 1.9 * | 374.0 ± 6.1 |
| 7 | −1 (1) | +1 (5) | +1 (80) | 82.1 ± 2.5 * | 286.4 ± 7.5 * |
| 8 | +1 (5) | +1 (5) | +1 (80) | 122.1 ± 9.0 * | 433.9 ± 8.6 * |
| 9 | 0 (3) | 0 (3) | 0 (50) | 82.9 ± 1.6 | 349.4 ± 3.2 * |
| 10 | 0 (3) | 0 (3) | 0 (50) | 90.5 ± 1.9 * | 336.3 ± 5.5 * |
| 11 | 0 (3) | 0 (3) | 0 (50) | 89.7 ± 0.9 | 359.9 ± 9.3 * |
| SPA | 5 | - | - | 91.8 ± 0.6 | 376.4 ± 1.8 |
| Treat. | x1 | x2 | x3 | Consistency (K) | Flow behavior (ɳ) |
|---|---|---|---|---|---|
| 1 | −1 (1) | −1 (1) | −1(20) | 4.874 ± 0.051 * | 0.181 ± 0.004 * |
| 2 | +1 (5) | −1 (1) | −1 (20) | 1.549 ± 0.018 * | 0.308 ± 0.006 |
| 3 | −1 (1) | +1 (5) | −1 (20) | 1.702 ± 0.011 * | 0.361 ± 0.000 * |
| 4 | +1 (5) | +1 (5) | −1 (20) | 0.205 ± 0.002 * | 0.605 ± 0.000 * |
| 5 | −1 (1) | −1 (1) | +1 (80) | 2.102 ± 0.099 * | 0.291 ± 0.013 |
| 6 | +1 (5) | −1 (1) | +1 (80) | 0.798 ± 0.024 * | 0.436 ± 0.004 * |
| 7 | −1 (1) | +1 (5) | +1 (80) | 0.737 ± 0.004 * | 0.461 ± 0.005 * |
| 8 | +1 (5) | +1 (5) | +1 (80) | 0.290 ± 0.002 * | 0.590 ± 0.019 * |
| 9 | 0 (3) | 0 (3) | 0 (50) | 0.853 ± 0.018 * | 0.417 ± 0.000 * |
| 10 | 0 (3) | 0 (3) | 0 (50) | 1.816 ± 0.027 * | 0.337 ± 0.009 * |
| 11 | 0 (3) | 0 (3) | 0 (50) | 0.989 ± 0.071 * | 0.402 ± 0.015 * |
| SPA | 5 | - | - | 2.399 ± 0.066 | 0.298 ± 0.007 |
| Treat. | P (mg/g) | Zn (mg/g) | Fe (mg/g) | Si (mg/g) | Ca (mg/g) | K (mg/g) | Mg (mg/g) | Mn (mg/g) | Na (mg/g) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 13.72 ± 0.53 * | 0.03 ± 0.01 | 0.03 ± 0.004 | 0.05 ± 0.003 | 1.84 ± 0.02 | 21.53 ± 0.39 * | 3.51 ± 0.12 | 0.05 ± 0.001 * | 2.85 ± 0.04 |
| 2 | 13.54 ± 0.29 * | 0.03 ± 0.01 | 0.03 ± 0.004 | 0.05 ± 0.01 | 1.98 ± 0.05 * | 25.33 ± 0.96 | 3.52 ± 0.28 | 0.05 ± 0.005 * | 2.53 ± 0.01 * |
| 3 | 15.42 ± 0.32 * | 0.02 ± 0.004 | 0.06 ± 0.005 * | 0.04 ± 0.005 | 1.67 ± 0.16 | 22.63 ± 0.35 * | 3.75 ± 0.09 * | 0.09 ± 0.14 * | 2.67 ± 0.08 * |
| 4 | 16.08 ± 0.19 * | 0.05 ± 0.02 | 0.07 ± 0.006 * | 0.10 ± 0.01 * | 1.87 ± 0.01 | 26.36 ± 1.35 | 4.06 ± 0.10 * | 0.05 ± 0.001 * | 2.92 ± 0.08 |
| 5 | 31.49 ± 1.73 * | 0.04 ± 0.005 | 0.05 ± 0.006 * | 0.23 ± 0.01 * | 2.41 ± 0.08 * | 22.28 ± 0.15 * | 4.12 ± 0.07 * | 0.18 ± 0.01 | 2.95 ± 0.09 |
| 6 | 25.66 ± 2.03 * | 0.06 ± 0.01 | 0.08 ± 0.01 * | 0.16 ± 0.001 * | 2.76 ± 0.30 * | 26.06 ± 0.78 | 4.33 ± 0.25 * | 0.19 ± 0.02 | 2.41 ± 0.13 * |
| 7 | 29.21 ± 1.37 * | 0.02 ± 0.008 | 0.05 ± 0.003 * | 0.14 ± 0.01 * | 2.33 ± 0.11 * | 24.95 ± 0.10 | 4.34 ± 0.28 * | 0.15 ± 0.004 * | 2.40 ± 0.11 * |
| 8 | 27.10 ± 0.05 * | 0.02 ± 0.001 | 0.05 ± 0.008 * | 0.15 ± 0.02 * | 3.73 ± 0.07 * | 26.11 ± 0.03 | 4.61 ± 0.40 * | 0.11 ± 0.005 * | 2.59 ± 0.08 * |
| 9 | 16.18 ± 0.33 * | 0.06 ± 0.001 | 0.05 ± 0.003 * | 0.06 ± 0.01 | 2.21 ± 0.04 * | 25.66 ± 0.16 | 3.87 ± 0.07 * | 0.11 ± 0.004 * | 2.59 ± 0.05 * |
| 10 | 17.10 ± 0.05 * | 0.06 ± 0.01 | 0.06 ± 0.01 * | 0.08 ± 0.01 * | 1.86 ± 0.003 | 22.28 ± 0.15 * | 4.24 ± 0.10 * | 0.09 ± 0.005 * | 2.48 ± 0.11 * |
| 11 | 18.33 ± 0.50 * | 0.02 ± 0.001 | 0.07 ± 0.004 * | 0.12 ± 0.01 * | 2.24 ± 0.04 | 24.14 ± 0.85 | 3.57 ± 0.02 * | 0.13 ± 0.006 * | 3.12 ± 0.08 * |
| SPA | 12.81 ± 0.03 | 0.03 ± 0.001 | 0.03 ± 0.004 | 0.05 ± 0.001 | 1.59 ± 0.13 | 25.80 ± 1.09 | 3.43 ± 0.05 | 0.19 ± 0.11 | 2.93 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedone, I.S.; Aquino, F.I.; Costa, E.d.S.M.; Macagnan, K.L.; Porto, J.d.R.; Ribeiro, A.S.; Alves, M.I.; Vendruscolo, C.T.; Moreira, A.d.S. Assessment of Alternative Media Viability for Cell Growth Phase in the Lab-Scale Xanthan Pruni Production—Part I. Fermentation 2025, 11, 191. https://doi.org/10.3390/fermentation11040191
Pedone IS, Aquino FI, Costa EdSM, Macagnan KL, Porto JdR, Ribeiro AS, Alves MI, Vendruscolo CT, Moreira AdS. Assessment of Alternative Media Viability for Cell Growth Phase in the Lab-Scale Xanthan Pruni Production—Part I. Fermentation. 2025; 11(4):191. https://doi.org/10.3390/fermentation11040191
Chicago/Turabian StylePedone, Isabel Santos, Fabíola Insaurriaga Aquino, Eduardo dos Santos Macedo Costa, Karine Laste Macagnan, Jéssica da Rosa Porto, Anderson Schwingel Ribeiro, Mariane Igansi Alves, Claire Tondo Vendruscolo, and Angelita da Silveira Moreira. 2025. "Assessment of Alternative Media Viability for Cell Growth Phase in the Lab-Scale Xanthan Pruni Production—Part I" Fermentation 11, no. 4: 191. https://doi.org/10.3390/fermentation11040191
APA StylePedone, I. S., Aquino, F. I., Costa, E. d. S. M., Macagnan, K. L., Porto, J. d. R., Ribeiro, A. S., Alves, M. I., Vendruscolo, C. T., & Moreira, A. d. S. (2025). Assessment of Alternative Media Viability for Cell Growth Phase in the Lab-Scale Xanthan Pruni Production—Part I. Fermentation, 11(4), 191. https://doi.org/10.3390/fermentation11040191





