Optimizing Silage Efficiency: The Role of Ryegrass Varieties, Harvest Time, and Additives in Enhancing Perennial Ryegrass (Lolium perenne) Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Extraction of Silage Products
2.3. Measurement of Water-Soluble Carbohydrates
2.4. Density of the Extract
2.5. Measurement of Moisture Content
2.6. Calculation of Mass Concentration of Silage Products and Lactic Acid Yield
2.6.1. Dry Mass of the Sample
2.6.2. Mass of Substance in Silage Products
2.6.3. Volume of the Extract
2.6.4. Mass Concentration of Silage Products
2.7. Preparation of Pressed Juice
2.8. pH Measurement of Pressed Juice
2.9. Silage Production
2.9.1. Silage in Vacuum Bags
2.9.2. Ensiling in Bottles
2.10. Analysis of Silage Products
2.11. pH Control and Buffers
2.11.1. Silage at pH 7
2.11.2. Silage at pH 4.5 and pH 6 Using Citrate Buffer
2.11.3. Silage at pH 6 Using Phosphate Buffer
2.12. Experimental Design and Controls
- Silage duration: Six time points (0, 4, 7, 14, 21, and 75 days) were tested under identical conditions using three Lolium perenne varieties.
- pH regulation: Four groups (control, citrate buffer at pH 4.5 and 6, and calcium carbonate at pH 7) were tested using the same biomass batch (Arvicola) to assess the effect of initial pH on silage.
- Buffer comparison: Citrate and phosphate buffers at pH 6 were compared in 10 L glass bottles to assess silage dynamics over the full fermentation period.
- Harvest time and ploidy: Two Lolium perenne varieties (diploid and tetraploid) were harvested at early and late stages to examine genetic and agronomic effects on silage under identical conditions.
3. Results and Discussion
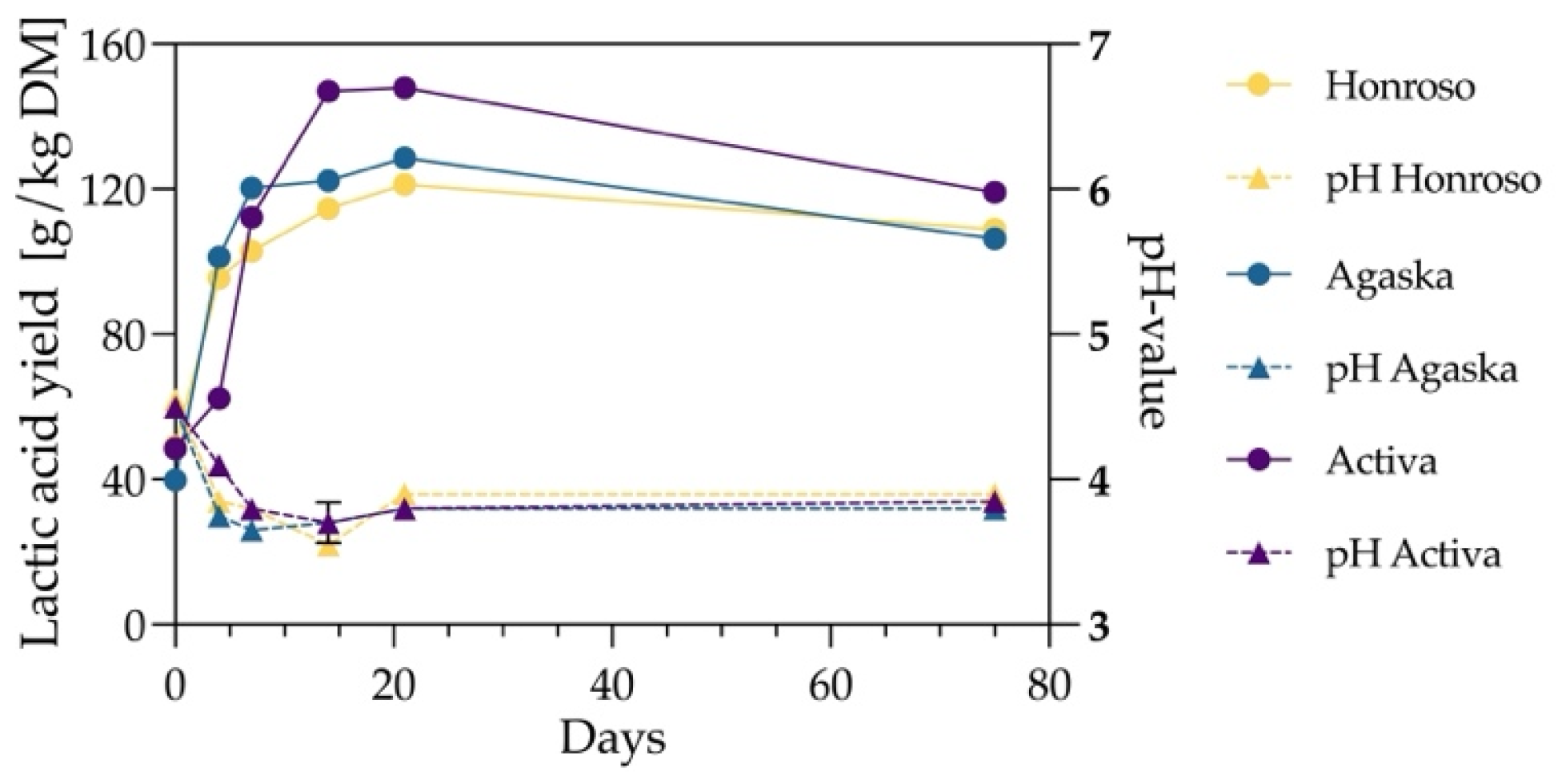
3.1. Validation of Optimal Silage Duration
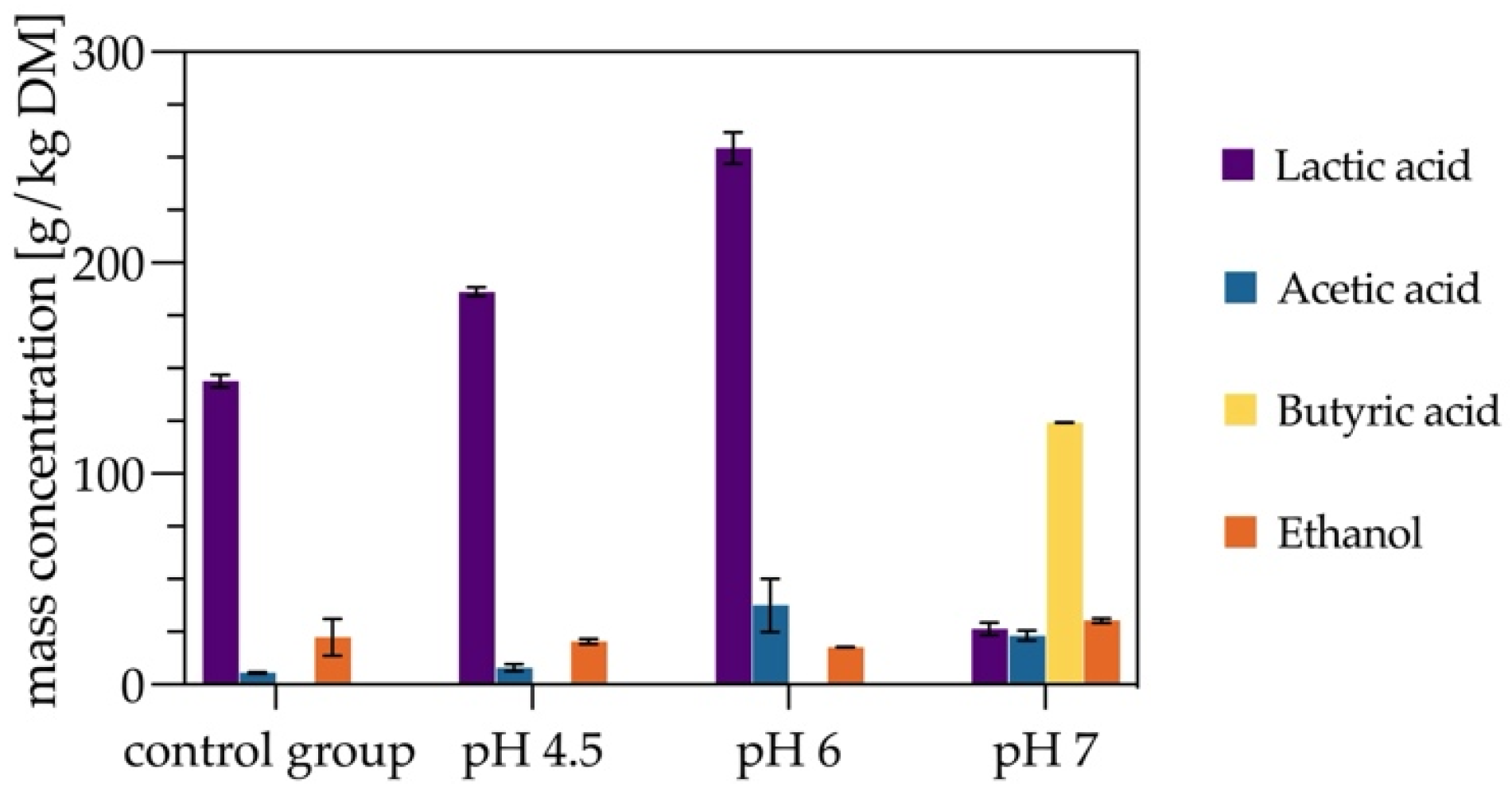
3.2. Evaluating the Effect of pH on Silage Quality
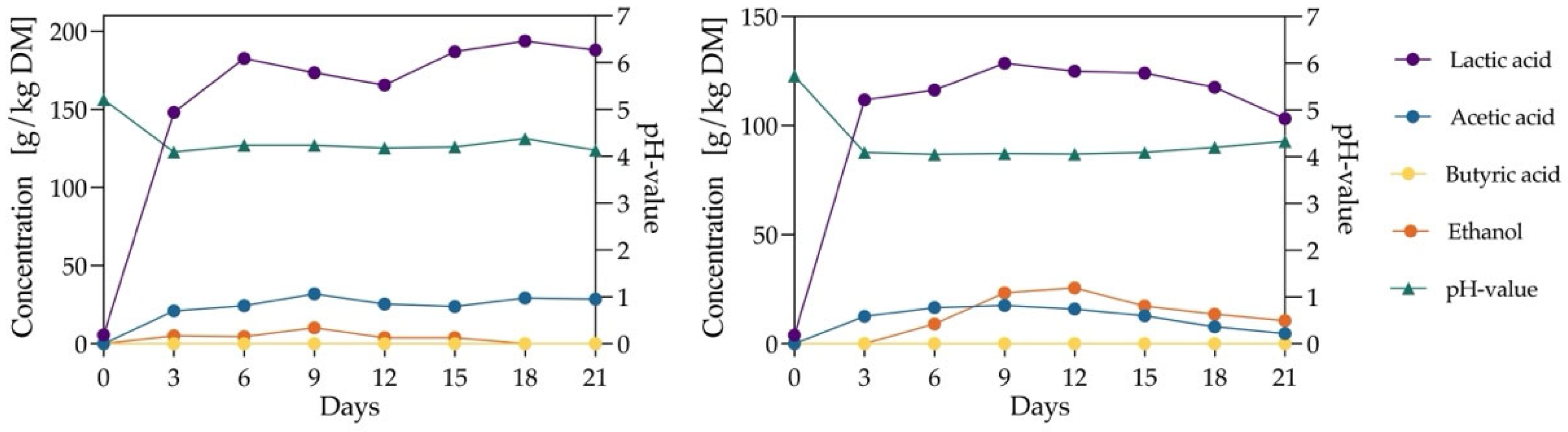
3.3. Silage Dynamics at pH 6 Using Citrate and Phosphate Buffers
3.4. Impact of Lolium perenne Varieties and Harvest Time on Silage Quality
3.5. Evaluation of Field Yield and Harvest Timing Effects on Lactic Acid Production in Different Lolium perenne Varieties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a Sustainable Bioeconomy: An Overview of World Biomass Production and Utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Yadav, V.G.; Yadav, G.D.; Patankar, S.C. The Production of Fuels and Chemicals in the New World: Critical Analysis of the Choice between Crude Oil and Biomass Vis-à-Vis Sustainability and the Environment. Clean Technol. Environ. Policy 2020, 22, 1757–1774. [Google Scholar] [CrossRef] [PubMed]
- Waldron, K.W. (Ed.) Advances in Biorefineries: Biomass and Waste Supply Chain Exploitation; Woodhead Publishing Series in Energy; Elsevier/Woodhead Publishing: Amsterdam, The Netherland, 2014; ISBN 978-0-85709-521-3. [Google Scholar]
- Conaghan, P.; O’Kiely, P.; Howard, H.; O’Mara, F.P.; Halling, M.A. Evaluation of Lolium perenne L. Cv. AberDart and AberDove for Silage Production. Ir. J. Agric. Food Res. 2008, 47, 119–134. [Google Scholar]
- Alves De Oliveira, R.; Komesu, A.; Vaz Rossell, C.E.; Maciel Filho, R. Challenges and Opportunities in Lactic Acid Bioprocess Design—From Economic to Production Aspects. Biochem. Eng. J. 2018, 133, 219–239. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Alexandri, M.; Schneider, R.; Venus, J. A Review on the Current Developments in Continuous Lactic Acid Fermentations and Case Studies Utilising Inexpensive Raw Materials. Process Biochem. 2019, 79, 1–10. [Google Scholar] [CrossRef]
- Varriale, L.; Hengsbach, J.-N.; Guo, T.; Kuka, K.; Tippkötter, N.; Ulber, R. Sustainable Production of Lactic Acid Using a Perennial Ryegrass as Feedstock—A Comparative Study of Fermentation at the Bench- and Reactor-Scale, and Ensiling. Sustainability 2024, 16, 8054. [Google Scholar] [CrossRef]
- Pang, H.; Qin, G.; Tan, Z.; Li, Z.; Wang, Y.; Cai, Y. Natural Populations of Lactic Acid Bacteria Associated with Silage Fermentation as Determined by Phenotype, 16S Ribosomal RNA and recA Gene Analysis. Syst. Appl. Microbiol. 2011, 34, 235–241. [Google Scholar] [CrossRef]
- Zhu, X.-M.; Jiang, D.-D.; Yuan, B.-J.; Ni, K.-K. Effect of Low-Temperature-Tolerant Lactic Acid Bacteria on the Fermentation Quality and Bacterial Community of Oat Silage at 5 °C vs. 15 °C. Fermentation 2022, 8, 158. [Google Scholar] [CrossRef]
- Hetényi, K.; Németh, Á.; Sevella, B. Role of pH-Regulation in Lactic Acid Fermentation: Second Steps in a Process Improvement. Chem. Eng. Process. Process Intensif. 2011, 50, 293–299. [Google Scholar] [CrossRef]
- Bujňák, L.; Maskaľová, I.; Vajda, V. Determination of Buffering Capacity of Selected Fermented Feedstuffs and the Effect of Dietary Acid-Base Status on Ruminal Fluid pH. Acta Vet. Brno 2011, 80, 269–273. [Google Scholar] [CrossRef]
- Grant, R.J.; Mertens, D.R. Development of Buffer Systems for pH Control and Evaluation of pH Effects on Fiber Digestion In Vitro. J. Dairy Sci. 1992, 75, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Moorby, J.M.; Evans, R.T.; Scollan, N.D.; MacRae, J.C.; Theodorou, M.K. Increased Concentration of Water-soluble Carbohydrate in Perennial Ryegrass (Lolium perenne L.). Evaluation in Dairy Cows in Early Lactation. Grass Forage Sci. 2006, 61, 52–59. [Google Scholar] [CrossRef]
- Przemieniecki, S.W.; Purwin, C.; Mastalerz, J.; Borsuk, M.; Lipiński, K.; Kurowski, T. Biostimulating Effect of L-Tryptophan on the Yield and Chemical and Microbiological Quality of Perennial Ryegrass (Lolium perenne) Herbage and Silage for Ruminant. J. Sci. Food Agric. 2021, 101, 3969–3974. [Google Scholar] [CrossRef]
- Keating, T.; O’kiely, P. Comparison of Old Permanent Grassland, Lolium perenne and Lolium multiflorum Swards Grown for Silage: 4. Effects of Varying Harvesting Date. Ir. J. Agric. Food Res. 2000, 39, 55–71. [Google Scholar]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH Monograph; Blackwell Wissenschafts-Verlag: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Beschreibende Sortenliste Futtergräser Esparsette, Klee, Luzerne. 2024. Available online: https://www.bundessortenamt.de/bsa/aktuell/die-beschreibende-sortenliste-futtergraeser-esparsette-klee-luzerne-2024-ist-erschienen (accessed on 1 April 2025).
- Wang, Y.; Zhou, W.; Wang, C.; Yang, F.; Chen, X.; Zhang, Q. Effect on the Ensilage Performance and Microbial Community of Adding Neolamarckia cadamba Leaves to Corn Stalks. Microb. Biotechnol. 2020, 13, 1502–1514. [Google Scholar] [CrossRef]
- Williams, P.C.; Sobering, D.C. Comparison of Commercial near Infrared Transmittance and Reflectance Instruments for Analysis of Whole Grains and Seeds. J. Near Infrared Spectrosc. 1993, 1, 25–32. [Google Scholar] [CrossRef]
- Cherney, D.J.R.; Alessi, M.A.; Cherney, J.H. Influence of Grass Species and Sample Preparation on Ensiling Characteristics. Crop Sci. 2006, 46, 256–263. [Google Scholar] [CrossRef]
- Hoedtke, S.; Zeyner, A. Comparative Evaluation of Laboratory-Scale Silages Using Standard Glass Jar Silages or Vacuum-Packed Model Silages: Evaluation of Silages Using Standard Glass Jar Silages or Vacuum-Packed Model Silages. J. Sci. Food Agric. 2011, 91, 841–849. [Google Scholar] [CrossRef]
- Pieper, B.; Hoedtke, S.; Wensch-Dorendorf, M.; Korn, U.; Wolf, P.; Zeyner, A. Validation of the Rostock Fermentation Test as an in Vitro Method to Estimate Ensilability of Forages Using Glass Jar Model Silages as a Basis for Comparison. Grass Forage Sci. 2017, 72, 568–580. [Google Scholar] [CrossRef]
- Tix, J.; Moll, F.; Krafft, S.; Betsch, M.; Tippkötter, N. Hydrogen Production from Enzymatic Pretreated Organic Waste with Thermotoga Neapolitana. Energies 2024, 17, 2938. [Google Scholar] [CrossRef]
- Canale, A.; Valente, M.E.; Ciotti, A. Determination of Volatile Carboxylic Acids (C1–C5i) and Lactic Acid in Aqueous Acid Extracts of Silage by High Performance Liquid Chromatography. J. Sci. Food Agric. 1984, 35, 1178–1182. [Google Scholar] [CrossRef]
- Pejin, J.; Radosavljević, M.; Mojović, L.; Kocić-Tanackov, S.; Djukić-Vuković, A. The Influence of Calcium-Carbonate and Yeast Extract Addition on Lactic Acid Fermentation of Brewer’s Spent Grain Hydrolysate. Food Res. Int. 2015, 73, 31–37. [Google Scholar] [CrossRef]
- Zi, X.; Li, M.; Chen, Y.; Lv, R.; Zhou, H.; Tang, J. Effects of Citric Acid and Lactobacillus Plantarum on Silage Quality and Bacterial Diversity of King Grass Silage. Front. Microbiol. 2021, 12, 631096. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-B.; Tian, Y.; Wang, Q.; Cong, W. Effect of Different Types of Calcium Carbonate on the Lactic Acid Fermentation Performance of Lactobacillus Lactis. Biochem. Eng. J. 2015, 98, 38–46. [Google Scholar] [CrossRef]
- AAT Bioquest Inc. Citrate Buffer (pH 3.0 to 6.2) Preparation and Recipe. Available online: https://www.aatbio.com/resources/buffer-preparations-and-recipes/citrate-buffer-ph-3-to-6-2 (accessed on 3 January 2025).
- Oude Elferink, S.J.W.H.; Krooneman, J.; Gottschal, J.C.; Spoelstra, S.F.; Faber, F.; Driehuis, F. Anaerobic Conversion of Lactic Acid to Acetic Acid and 1,2-Propanediol by Lactobacillus buchneri. Appl. Environ. Microbiol. 2001, 67, 125–132. [Google Scholar] [CrossRef]
- Xu, D.; Ding, W.; Ke, W.; Li, F.; Zhang, P.; Guo, X. Modulation of Metabolome and Bacterial Community in Whole Crop Corn Silage by Inoculating Homofermentative Lactobacillus Plantarum and Heterofermentative Lactobacillus Buchneri. Front. Microbiol. 2019, 9, 3299. [Google Scholar] [CrossRef]
- Boontawan, P.; Kanchanathawee, S.; Boontawan, A. Extractive Fermentation of L-(+)-Lactic Acid by Pediococcus Pentosaceus Using Electrodeionization (EDI) Technique. Biochem. Eng. J. 2011, 54, 192–199. [Google Scholar] [CrossRef]
- Lindgren, S. Anaerobic?—Lactate Degradation by Lactobacillus Plantarum. FEMS Microbiol. Lett. 1990, 66, 209–213. [Google Scholar] [CrossRef]
- Nannen, N.L.; Hutkins, R.W. Intracellular pH Effects in Lactic Acid Bacteria. J. Dairy Sci. 1991, 74, 741–746. [Google Scholar] [CrossRef]
- Baribo, L.E.; Foster, E.M. Some Characteristics of the Growth Inhibitor Produced by a Lactic Streptococcus. J. Dairy Sci. 1951, 34, 1128–1135. [Google Scholar] [CrossRef]
- Gilliland, T.J.; Barrett, P.D.; Mann, R.L.; Agnew, R.E.; Fearon, A.M. Canopy Morphology and Nutritional Quality Traits as Potential Grazing Value Indicators for Lolium perenne Varieties. J. Agric. Sci. 2002, 139, 257–273. [Google Scholar] [CrossRef]
- Smith, K.F.; Simpson, R.J.; Culvenor, R.A.; Humphreys, M.O.; Prud’Homme, M.P.; Oram, R.N. The Effects of Ploidy and a Phenotype Conferring a High Water-Soluble Carbohydrate Concentration on Carbohydrate Accumulation, Nutritive Value and Morphology of Perennial Ryegrass (Lolium perenne L.). J. Agric. Sci. 2001, 136, 65–74. [Google Scholar] [CrossRef]
- McGrath, S.; Hodkinson, T.R.; Frohlich, A.; Grant, J.; Barth, S. Seasonal and Genetic Variations in Water-Soluble Carbohydrates and Other Quality Traits in Ecotypes and Cultivars of Perennial Ryegrass (Lolium perenne L.). Plant Genet. Res. 2014, 12, 236–247. [Google Scholar] [CrossRef]

| Variety | Ploidy | Breeding Companies (Country) | BBCH | Harvest Time |
|---|---|---|---|---|
| Agaska | 2× | DLF (Denmark) | 51 | 31 May 2021 |
| Honroso | 2× | DSV (Germany) | 45 | 31 May 2021 |
| Arvicola | 4× | Freudenberger (Germany) | 65 | 3 June 2022 |
| Activa | 4× | DSV (Germany) | 55 | 31 May 2021 |
| Variety | Ploidy | Breeding Companies (Country) | BBCH | Harvest Time | |
|---|---|---|---|---|---|
| Honroso | 2× | DSV (Germany) | 31 | Early cut (E) | 17 May 2022 |
| 41 | Late cut (L) | 31 May 2022 | |||
| Explosion | 4× | DSV (Germany) | 31 | Early cut (E) | 17 May 2022 |
| 41 | Late cut (L) | 31 May 2022 | |||
| Harvest Time | Honroso | Explosion |
|---|---|---|
| early cut | HonrosoE | ExplosionE |
| late cut (early cut + 14 Days) | HonrosoL | ExplosionL |
| Sample | Day | pH | WSC | Concentrations of Silage Products [g/kg DM] | ||
|---|---|---|---|---|---|---|
| [%DM] | Lactic Acid | Acetic Acid | Ethanol | |||
| HonrosoE | 0 | 6.01 | 23.5 | - | - | - |
| 21 | 4.12 | n.a. * | 151.2 | 15.8 | 4.6 | |
| HonrosoL | 0 | 6.03 | 21.6 | - | - | - |
| 21 | 4.31 | n.a. * | 107.5 | 26.6 | 4.5 | |
| ExplosionE | 0 | 6.07 | 26.4 | 10.1 | - | - |
| 21 | 4.17 | n.a. * | 174.4 | 29.3 | 5.6 | |
| ExplosionL | 0 | 6.02 | 23.2 | - | - | - |
| 21 | 4.27 | n.a. * | 147.6 | 33.0 | 4.8 | |
| Sample | Field Yield | Lactic Acid Yield | |
|---|---|---|---|
| [t DM/ha] | [g/kg DM] | [kg/ha] | |
| HonrosoE | 3.33 ± 0.2 | 151. 2 | 503 ± 3 |
| HonrosoL | 4.43 ± 0.2 | 107.5 | 476 ± 3 |
| ExplosionE | 4.06 ± 0.5 | 174.4 | 707 ± 8 |
| ExplosionL | 4.85 ± 0.0 | 147.6 | 716 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, T.; Niu, T.; Kuka, K.; Tippkötter, N. Optimizing Silage Efficiency: The Role of Ryegrass Varieties, Harvest Time, and Additives in Enhancing Perennial Ryegrass (Lolium perenne) Fermentation. Fermentation 2025, 11, 192. https://doi.org/10.3390/fermentation11040192
Guo T, Niu T, Kuka K, Tippkötter N. Optimizing Silage Efficiency: The Role of Ryegrass Varieties, Harvest Time, and Additives in Enhancing Perennial Ryegrass (Lolium perenne) Fermentation. Fermentation. 2025; 11(4):192. https://doi.org/10.3390/fermentation11040192
Chicago/Turabian StyleGuo, Tianyi, Tong Niu, Katrin Kuka, and Nils Tippkötter. 2025. "Optimizing Silage Efficiency: The Role of Ryegrass Varieties, Harvest Time, and Additives in Enhancing Perennial Ryegrass (Lolium perenne) Fermentation" Fermentation 11, no. 4: 192. https://doi.org/10.3390/fermentation11040192
APA StyleGuo, T., Niu, T., Kuka, K., & Tippkötter, N. (2025). Optimizing Silage Efficiency: The Role of Ryegrass Varieties, Harvest Time, and Additives in Enhancing Perennial Ryegrass (Lolium perenne) Fermentation. Fermentation, 11(4), 192. https://doi.org/10.3390/fermentation11040192







