Biohydrogen and Biobutanol Production from Spent Coffee and Tea Waste Using Clostridium beijerinckii
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Food Wastes Characterization
2.3. Pretreatment and Enzymatic Hydrolysis of Food Wastes
2.4. Bacteria and Inoculum Preparation
2.5. C. beijerinckii Fermentation of Spent Coffee and Biosolids Hydrolysates
2.5.1. ABE Fermentation of Spent Coffee and Biosolids Hydrolysates
2.5.2. Fermentation of Modified Spent Coffee and Biosolids Hydrolysates
2.5.3. Effect of CaCO3 Supplementation of Spent Coffee and Biosolids Hydrolysates
2.6. Fermentation and Gas Analysis
2.7. Other Analytical Methods and Calculations
2.8. Statistical Analysis
3. Results
3.1. Enzymatic Hydrolysis and Compositional Analysis of Spent Coffee Grounds and Biosolids cake Wastes
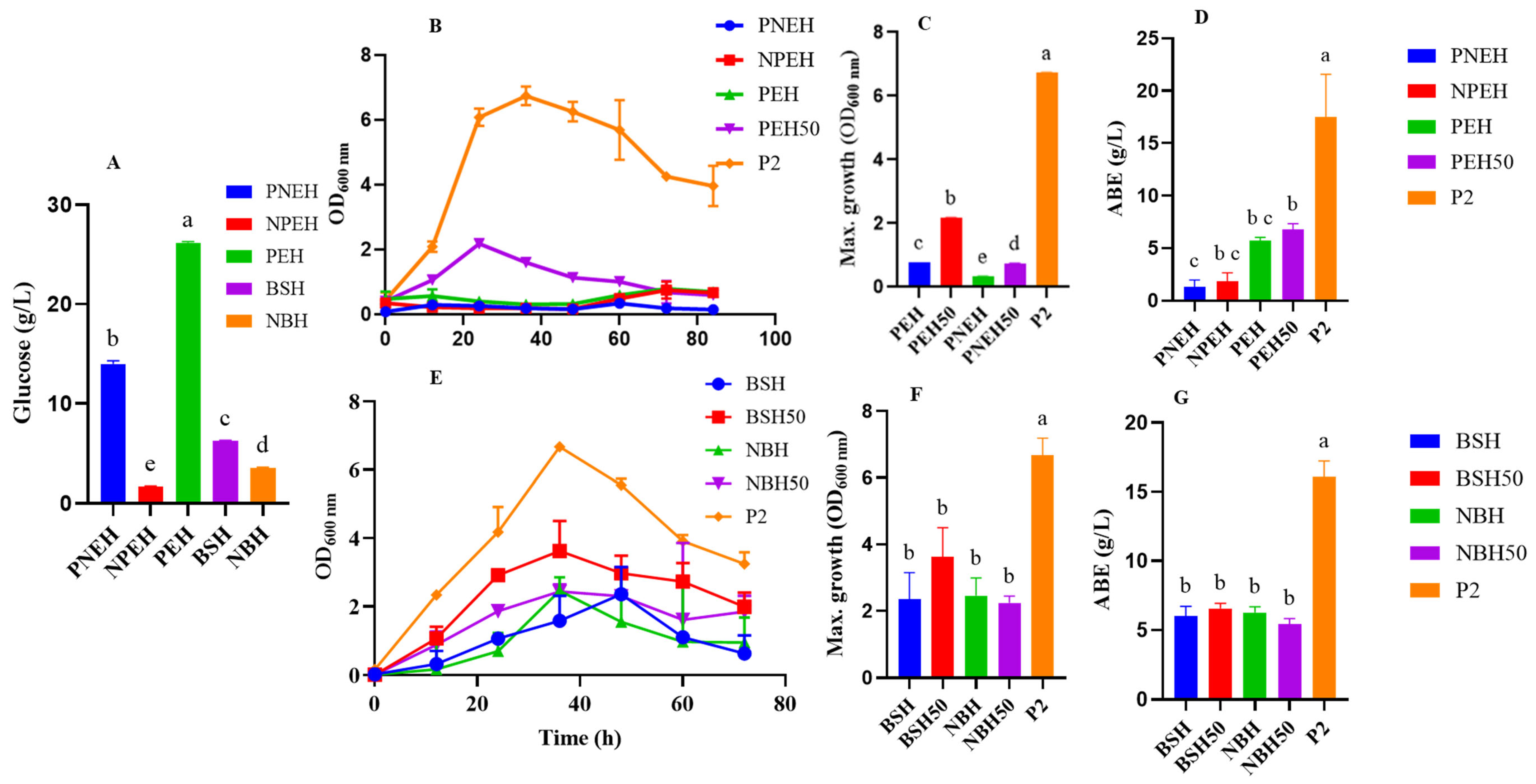
3.2. Batch Fermentation of Non-Detoxified Spent Coffee Grounds and Biosolids Hydrolysate
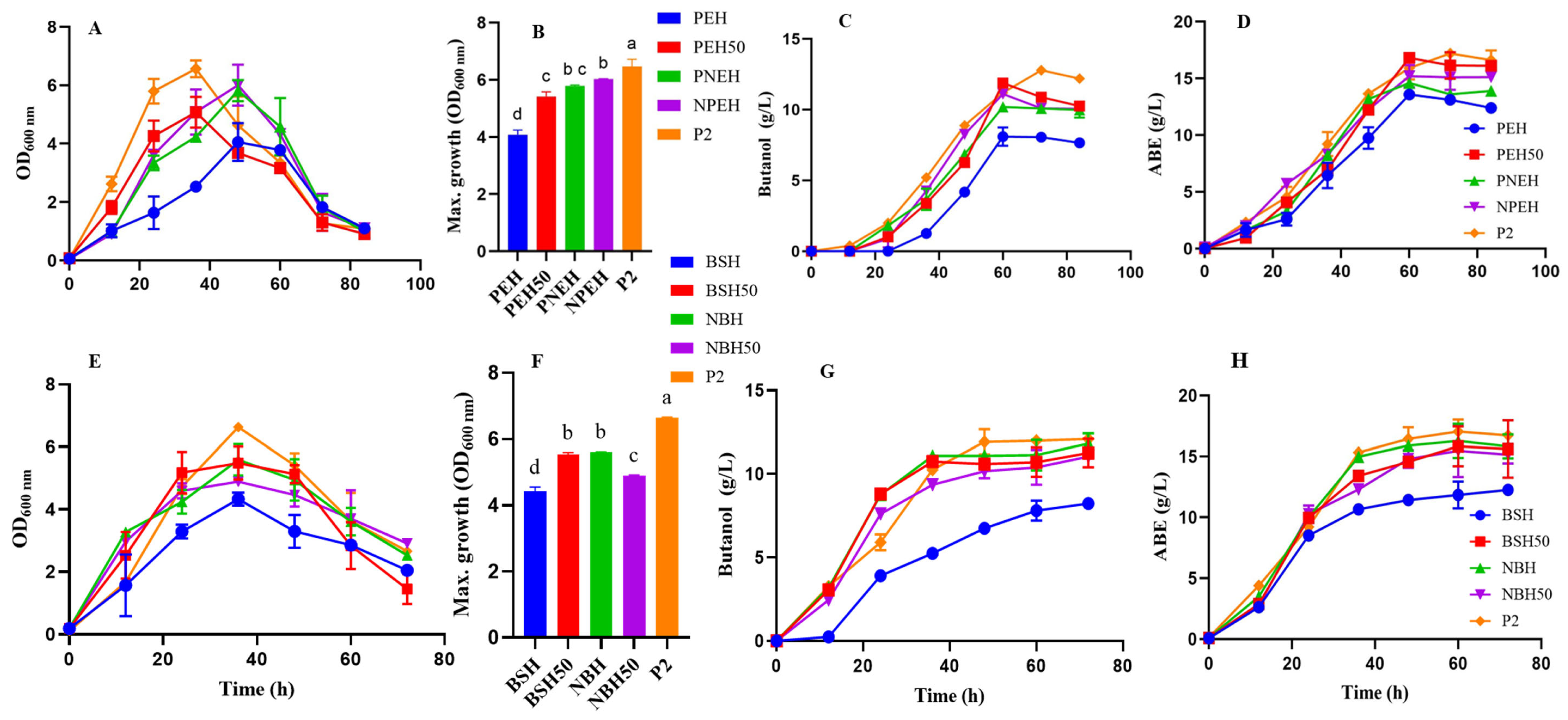
3.3. Fermentation Profile of C. beijerinckii Grown on Glucose Adjusted SC and BS Hydrolysate
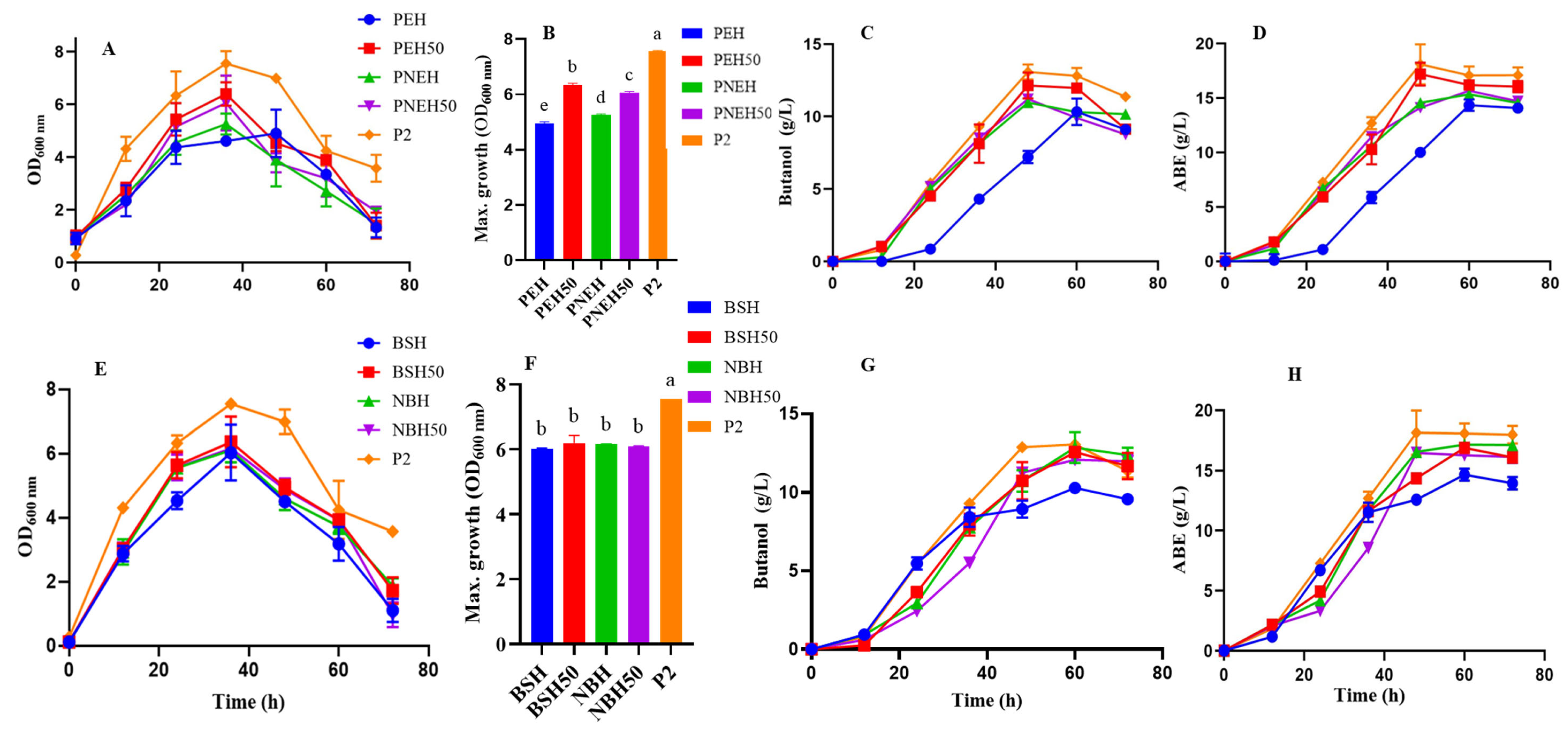
3.4. Fermentation Profile of CaCO3 Modified SC and BS Hydrolysate
3.5. Biohydrogen Production by C. beijerinckii Grown on Spent Coffee Grounds and Biosolids Hydrolysates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LDMICs | Lignocellulosic Derived Microbial Inhibitory Compounds |
| LB | Lignocellulosic Biomass |
| ABE | Acetone–Butanol–Ethanol |
| SC | Spent Coffee Waste |
| BS | Biosolids cake Waste |
| BSH | Enzyme Hydrolyzed Biosolids Hydrolysate |
| NBH | Non-Enzyme Hydrolyzed Hydrolysate |
| PEH | Parr-treated Enzyme Hydrolyzed Spent Coffee Waste |
| PNEH | Parr-treated Non-Enzyme Hydrolyzed Spent Coffee Waste |
| NPEH | Non-Parr-treated and Enzyme-Hydrolyzed Spent Coffee Waste |
| NAD(P)H | Nicotinamide Adenine Dinucleotude Phosphate |
| DDGS | Distiller Dried Grains with Solubles |
| HPLC | High Performance Liquid Chromatography |
| TCD | Thermal Conductivity Detector |
| GC | Gas Chromatography |
| FID | Flame Ionization Detector |
| DNS | 3,5-dinitro Salicylic Acid |
| PDA | Photodiode Array |
| TGY | Tryptone Glucose Yeast extract |
| ICP-OES | Inductively Coupled Plasma Optical Emission Spectroscopy |
References
- Malik, K.; Capareda, S.C.; Kamboj, B.R.; Malik, S.; Singh, K.; Arya, S.; Bishnoi, D.K. Biofuels production: A review on sustainable alternatives to traditional fuels and energy sources. Fuels 2024, 5, 157–175. [Google Scholar] [CrossRef]
- Sikiru, S.; Abioye, K.J.; Adedayo, H.B.; Adebukola, S.Y.; Soleimani, H.; Anar, M. Technology projection in biofuel production using agricultural waste materials as a source of energy sustainability: A comprehensive review. Renew. Sustain. Energy Rev. 2024, 200, 114535. [Google Scholar] [CrossRef]
- Sahu, R.B.; Singh, P. Current status and challenges of biobutanol production from biomass. In Production of Biobutanol from Biomass; Wiley: Hoboken, NJ, USA, 2023; pp. 301–322. [Google Scholar] [CrossRef]
- Tucker, C.M. Coffee Culture: Local Experiences, Global Connections, 2nd ed.; Routledge: Abingdon, UK, 2017; ISBN 9781317392255. [Google Scholar] [CrossRef]
- Karmee, S.K. A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. Waste Manag. 2018, 72, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.A.; Roberto, I.C.; Teixeira, J.A. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydr. Polym. 2011, 83, 368–374. [Google Scholar] [CrossRef]
- Arya, S.S.; Venkatram, R.; More, P.R.; Vijayan, P. The wastes of coffee bean processing for utilization in food: A review. J. Food Sci. Technol. 2022, 59, 429–444. [Google Scholar] [CrossRef]
- Negi, T.; Kumar, Y.; Sirohi, R.; Singh, S.; Tarafdar, A.; Pareek, S.; Awasthi, M.K.; Sagar, N.A. Advances in bioconversion of spent tea leaves to value-added products. Bioresour. Technol. 2022, 346, 126409. [Google Scholar] [CrossRef]
- Wang, Z.; Ahmad, W.; Zhu, A.; Zhao, S.; Ouyang, Q.; Chen, Q. Recent advances review in tea waste: High-value applications, processing technology, and value-added products. Sci. Total Environ. 2024, 946, 174225. [Google Scholar] [CrossRef]
- Kondo, M.; Hirano, Y.; Kita, K.; Jayanegara, A.; Yokota, H.O. Nutritive evaluation of spent green and black tea leaf silages by in vitro gas production characteristics, ruminal degradability and post-ruminal digestibility assessed with inhibitory activity of their tannins. Anim. Sci. J. 2018, 89, 1656–1662. [Google Scholar] [CrossRef]
- Zhang, Y.; Ezeji, T.C. Elucidating and alleviating impacts of lignocellulose-derived microbial inhibitors on Clostridium beijerinckii during fermentation of Miscanthus giganteus to butanol. J. Ind. Microbiol. Biotechnol. 2014, 41, 1505–1516. [Google Scholar] [CrossRef]
- Beluhan, S.; Mihajlovski, K.; Šantek, B.; Ivančić Šantek, M. The Production of Bioethanol from Lignocellulosic Biomass: Pretreatment Methods, Fermentation, and Downstream Processing. Energies 2023, 16, 7003. [Google Scholar] [CrossRef]
- Olorunsogbon, T.; Adesanya, Y.; Atiyeh, H.K.; Okonkwo, C.C.; Ujor, V.C.; Ezeji, T.C. Effects of Clostridium beijerinckii and medium modifications on acetone–butanol–ethanol production from switchgrass. Front. Bioeng. Biotechnol. 2022, 10, 942701. [Google Scholar] [CrossRef] [PubMed]
- DD CEN/TS 14774-2:2004; Solid Biofuels: Methods for the Determination of Moisture Content—Oven Dry Method. Total Moisture—Simplified Method. British Standards Insitute: London, UK, 2004.
- Ujor, V.; Bharathidasan, A.K.; Cornish, K.; Ezeji, T.C. Feasibility of producing butanol from industrial starchy food wastes. Appl. Energy 2014, 136, 590–598. [Google Scholar] [CrossRef]
- US Composting Council. The Test Method for the Examination of Composting and Compost (TMECC); US Composting Council: Bethesda, MD, USA, 2002. [Google Scholar]
- Huzir, N.M.; Aziz, M.M.A.; Ismail, S.B.; Abdullah, B.; Mahmood, N.A.N.; Umor, N.A.; Muhammad, S.A.F.A.S. Agro-industrial waste to biobutanol production: Eco-friendly biofuels for next generation. Renew. Sustain. Energy Rev. 2018, 94, 476–485. [Google Scholar] [CrossRef]
- Panahi, S.H.K.; Dehhaghi, M.; Guillemin, G.J.; Okonkwo, C.C.; Kinder, J.E.; Ezeji, T.C. Biobutanol Production: Scope, Significance, and Applications. In Advances in Pollution Research, Advances and Developments in Biobutanol Production; Segovia-Hernandez, J.G., Behera, S., Sanchez-Ramirez, E., Eds.; Woodhead Publishing: Sawston, UK, 2023; pp. 1–45. ISBN 9780323911788. [Google Scholar] [CrossRef]
- Jain, A.; Jain, R.; Jain, S. Quantitative Analysis of Reducing Sugars by 3,5-Dinitrosalicylic Acid (DNSA Method). In Basic Techniques in Biochemistry, Microbiology and Molecular Biology; Humana: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, B.; Ezeji, T.C. Biotransformation of furfural and 5-hydroxymethyl furfural (HMF) by Clostridium acetobutylicum ATCC 824 during butanol fermentation. New Biotechnol. 2012, 29, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Ujor, V.; Lai, L.B.; Gopalan, V.; Ezeji, T.C. Use of proteomic analysis to elucidate the role of calcium in acetone-butanol-ethanol fermentation by Clostridium beijerinckii NCIMB 8052. Appl. Environ. Microbiol. 2013, 79, 282–293. [Google Scholar] [CrossRef]
- Hernandez-Hosaka, C.; Park, B.R.; Zhao, Y.; Jung, J. Effect of pretreatment and peracetic acid pulping on cellulosic materials converted from spent coffee grounds. J. Food Sci. 2024, 89, 9407–9419. [Google Scholar] [CrossRef]
- Hwang, J.H.; Choi, J.A.; Abou-Shanab, R.A.; Min, B.; Song, H.; Kim, Y.; Lee, E.S.; Jeon, B.H. Feasibility of hydrogen production from ripened fruits by a combined two-stage (dark/dark) fermentation system. Bioresour. Technol. 2011, 102, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Ferchichi, M.; Crabbe, E.; Gil, G.H.; Hintz, W.; Almadidy, A. Influence of initial pH on hydrogen production from cheese whey. J. Biotechnol. 2005, 120, 402–409. [Google Scholar] [CrossRef]
- Van Ginkel, S.W.; Oh, S.E.; Logan, B.E. Biohydrogen gas production from food processing and domestic wastewaters. Int. J. Hydrogen Energy 2005, 30, 1535–1542. [Google Scholar] [CrossRef]
- Karlsson, A.; Vallin, L.; Ejlertsson, J. Effects of temperature, hydraulic retention time and hydrogen extraction rate on hydrogen production from the fermentation of food industry residues and manure. Int. J. Hydrogen Energy 2008, 33, 953–962. [Google Scholar] [CrossRef]
- Zhang, C.; Li, T.; Su, G.; He, J. Enhanced direct fermentation from food waste to butanol and hydrogen by an amylolytic Clostridium. Renew. Energy 2020, 153, 522–529. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, H.; Fang, H.H. Biohydrogen production from starch in wastewater under thermophilic condition. J. Environ. Manag. 2003, 69, 149–156. [Google Scholar] [CrossRef]
- Wu, J.H.; Lin, C.Y. Biohydrogen production by mesophilic fermentation of food wastewater. Water Sci. Technol. 2004, 49, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Voget, C.E.; Mignone, C.F.; Ertola, R.J. Butanol production from apple pomace. Biotechnol. Lett. 1985, 7, 43–46. [Google Scholar] [CrossRef]
- Ezeji, T.; Blaschek, H.P. Fermentation of dried distillers’ grains and solubles (DDGS) hydrolysates to solvents and value-added products by solventogenic clostridia. Bioresour. Technol. 2008, 99, 5232–5242. [Google Scholar] [CrossRef]
- Oh, S.; Logan, B.E. Hydrogen and electricity production from a food processing wastewater using fermentation and microbial fuel cell technologies. Water Res. 2005, 39, 4673–4682. [Google Scholar] [CrossRef]
- Krysenko, S.; Wohlleben, W. Role of Carbon, Nitrogen, Phosphate and Sulfur Metabolism in Secondary Metabolism Precursor Supply in Streptomyces spp. Microorganisms 2024, 12, 1571. [Google Scholar] [CrossRef]
- Stautz, J.; Hellmich, Y.; Fuss, M.F.; Silberberg, J.M.; Devlin, J.R.; Stockbridge, R.B.; Hänelt, I. Molecular mechanisms for bacterial potassium homeostasis. J. Mol. Biol. 2021, 433, 166968. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef]
- Huang, Y.F.; Chiueh, P.T.; Lo, S.L. A review on microwave pyrolysis of lignocellulosic biomass. Sustain. Environ. Res. 2016, 26, 103–109. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Martins, J.R.; Abe, M.M.; Brienzo, M. Chemical Modification Strategies for Developing Functionalized Hemicellulose. In Hemicellulose Biorefinery: A Sustainable Solution for Value Addition to Bio-Based Products and Bioenergy; Springer: Singapore, 2022; pp. 171–205. [Google Scholar] [CrossRef]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Hydrothermal pretreatment of rice straw biomass: A potential and promising method for enhanced methane production. Appl. Energy 2012, 94, 129–140. [Google Scholar] [CrossRef]
- Doan, C.T.; Chen, C.L.; Nguyen, V.B.; Tran, T.N.; Nguyen, A.D.; Wang, S.L. Conversion of pectin-containing by-products to pectinases by Bacillus amyloliquefaciens and its applications on hydrolyzing banana peels for prebiotics production. Polymers 2021, 13, 1483. [Google Scholar] [CrossRef] [PubMed]
- Mankar, A.R.; Pandey, A.; Arindam Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Richmond, C.; Ujor, V.; Ezeji, T.C. Impact of syringaldehyde on the growth of Clostridium beijerinckii NCIMB 8052 and butanol production. 3 Biotech 2012, 2, 159–167. [Google Scholar] [CrossRef][Green Version]
- Ezeji, T.; Qureshi, N.; Blaschek, H.P. Butanol production from agricultural residues: Impact of degradation products on Clostridium beijerinckii growth and butanol fermentation. Biotechnol. Bioeng. 2007, 97, 1460–1469. [Google Scholar] [CrossRef]
- Kumar, S.; Agyeman-Duah, E.; Ujor, V.C. Whole-Genome Sequence and Fermentation Characteristics of Enterobacter hormaechei UW0SKVC1: A Promising Candidate for Detoxification of Lignocellulosic Biomass Hydrolysates and Production of Value-Added Chemicals. Bioengineering 2023, 10, 1090. [Google Scholar] [CrossRef]
- Bei, Q.; Chen, G.; Lu, F.; Wu, S.; Wu, Z. Enzymatic action mechanism of phenolic mobilization in oats (Avena sativa L.) during solid-state fermentation with Monascus anka. Food Chem. 2018, 245, 297–304. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, C.; Lu, M.; Tu, M. Effect of overliming and activated carbon detoxification on inhibitors removal and butanol fermentation of poplar prehydrolysates. Biotechnol. Biofuels 2018, 11, 178. [Google Scholar] [CrossRef]
- Su, Z.; Wang, F.; Xie, Y.; Xie, H.; Mao, G.; Zhang, H.; Song, A.; Zhang, Z. Reassessment of the role of CaCO3 in n-butanol production from pretreated lignocellulosic biomass by Clostridium acetobutylicum. Sci. Rep. 2020, 10, 17956. [Google Scholar] [CrossRef]
- El Bari, H.; Lahboubi, N.; Habchi, S.; Rachidi, S.; Bayssi, O.; Nabil, N.; Mortezaei, Y.; Villa, R. Biohydrogen production from fermentation of organic waste, storage and applications. Clean. Waste Syst. 2022, 3, 100043. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Aranda, B. Microbial growth under limiting conditions-future perspectives. Microorganisms 2023, 11, 1641. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Kim, D.H.; Yun, Y.M.; Lee, M.K.; Moon, C.; Kang, W.S.; Kwak, S.S.; Kim, M.S. Hydrogen fermentation of food waste by alkali-shock pretreatment: Microbial community analysis and limitation of continuous operation. Bioresour. Technol. 2015, 186, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jang, S.; Yun, Y.M.; Lee, M.K.; Moon, C.; Kang, W.S.; Kwak, S.S.; Kim, M.S. Effect of acid-pretreatment on hydrogen fermentation of food waste: Microbial community analysis by next generation sequencing. Int. J. Hydrogen Energy 2014, 39, 16302–16309. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, J.; He, Y.; Yuan, J. Photo-fermentative hydrogen production performance of a newly isolated Rubrivivax gelatinosus YP03 strain with acid tolerance. Int. J. Hydrogen Energy 2022, 47, 20784–20792. [Google Scholar] [CrossRef]
- Akaniro, I.R.; Oladipo, A.A.; Onwujekwe, E.C. Metabolic engineering approaches for scale-up of fermentative biohydrogen production—A review. Int. J. Hydrogen Energy 2024, 52, 240–264. [Google Scholar] [CrossRef]

| Parameters | Spent Coffee Grounds | Biosolids Cake |
|---|---|---|
| pH | 5.99 ± 0.01 | 11.69 ± 0.01 * |
| Total solids (%) | 72.14 ± 0.73 * | 23.04 ± 0.06 |
| Moisture (%) | 27.86 ± 0.73 | 76.96 ± 0.06 * |
| Ash (%) | 1.77 ± 0.10 | 9.29 ± 0.27 * |
| Calorific value (kJ/g) | 21.93 ± 0.08 * | 8.27 ± 0.08 |
| Nitrogen (%) | 2.50 ± 0.10 | 3.92 ± 0.07 * |
| Organic carbon (%) | 52.95 ± 0.49 * | 21.72 ± 0.09 |
| Carbon/Nitrogen ratio | 21.18:1 | 5.5:1 |
| Major Element (µg/g) | ||
| Aluminum (Al) | 69.74 ± 2.00 | 49340 ± 4120 * |
| Boron (B) | 11.10 ± 1.03 * | 7.93 ± 0.01 |
| Calcium (Ca) | 1350.60 ± 15.0 | 97,588 ± 3620 * |
| Copper (Cu) | 17.19 ± 2.10 | 18.49 ± 1.00 |
| Iron (Fe) | 65.70 ± 3.22 | 4543.80 ± 348 * |
| Potassium (K) | 7486 ± 25.0 * | 684.51 ± 19 |
| Magnesium (Mg) | 1591.40 ± 28.3 | 60,465 ± 745 * |
| Manganese (Mn) | 30.13 ± 4.80 | 59.29 ± 5.0 * |
| Molybdenum (Mo) | <1 | 1.10 ± 0.10 * |
| Sodium (Na) | 18.79 ± 1.90 | 803.69 ± 11 * |
| Phosphorus (P) | 1291.60 ± 28.60 * | 9483.30 ± 594 |
| Sulphur (S) | 1555.20 ± 15.04 | 6743.60 ± 309 * |
| Zinc (Zn) | 7.06 ± 0.19 | 374.21 ± 12 * |
| Minor Element (µg/g) | ||
| Arsenic (As) | <1 | <1 |
| Barium (Ba) | 3.32 | 52.58 * |
| Beryllium (Be) | <1 | <1 |
| Cadmium (Cd) | <1 | <1 |
| Cobalt (Co) | <1 | <1 |
| Chromium (Cr) | <1 | 10.16 ± 0.35 * |
| Lithium (Li) | 1.98 ± 0.10 | <1 |
| Nickel (Ni) | 1.98 ± 0.24 | 6.19 ± 0.20 * |
| Lead (Pb) | <1 | 0.79 ± 0.01 |
| Antimony (Sb) | <1 | 3.03 ± 0.06 |
| Selenium (Se) | 2.99 ± 0.30 * | 1.85 ± 0.08 |
| Silicon (Si) | 49.12 ± 2.25 | 1180.95 ± 324 * |
| Strontium (Sr) | 5.22 ± 0.09 | 273.58 ± 18 * |
| Titanium (Tl) | 1 | <1 |
| Vanadium (V) | <1 | 11.59 ± 1.00 |
| Parameters | ABE Yield (g/g) | ABE Productivity (g/L/h) | Butanol Yield (g/g) | Butanol Productivity (g/L/h) | H2 Yield (mmol/g) | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gluc | Glu+CaCO3 | Gluc | Gluc+CaCO3 | Gluc | Glu+CaCO3 | Gluc | Gluc+CaCO3 | Gluc+CaCO3 | ||
| Spent coffee hydrolysates | ||||||||||
| PEH A | 0.24 e | 0.27 e | 0.23 e | 0.24 e | 0.14 e | 0.20 c | 0.14 e | 0.17 e | 0.62 d | This study |
| PEH50 A | 0.3 b | 0.32 b | 0.28 b | 0.36 d | 0.21 b | 0.22 b | 0.20 b | 0.25 b | 0.84 b | This study |
| PNEH A | 0.26 d | 0.29 d | 0.24 d | 0.26 c | 0.18 d | 0.20 c | 0.17 d | 0.18 d | 0.55 e | This study |
| NPEH A | 0.27 c | nd | 0.25 c | nd | 0.20 c | nd | 0.19 c | nd | nd | This study |
| PNEH50 A | nd | 0.30 c | nd | 0.26 c | nd | 0.22 b | nd | 0.19 c | 0.67 c | This study |
| Biosolids hydrolysates | ||||||||||
| BSH A | 0.21 e | 0.28 c | 0.17 e | 0.24 e | 0.14 d | 0.19 c | 0.11 d | 0.17 d | 0.80 e | This study |
| BSH50 A | 0.27 c | 0.32 b | 0.22 c | 0.28 c | 0.19 c | 0.24 b | 0.16 b | 0.21 b | 0.87 d | This study |
| NBH A | 0.28 b | 0.35 a | 0.23 b | 0.29 b | 0.20 b | 0.25 a | 0.16 b | 0.21 b | 0.93 b | This study |
| NBH50 A | 0.26 d | 0.32 b | 0.21 d | 0.26 d | 0.19 c | 0.24 b | 0.15 c | 0.20 c | 0.89 c | This study |
| Control (P2) A | 0.39 a | 0.44 a | 0.29 a | 0.38 a | 0.29 a | 0.32 a | 0.21 a | 0.27 a | 1.06 a | This study |
| Grape waste B | nd | nd | nd | nd | nd | nd | nd | nd | 12.21 | [23] |
| Crude cheese whey C | nd | nd | nd | nd | nd | nd | nd | nd | 0.03 | [24] |
| Potato processing waste D | nd | nd | nd | nd | nd | nd | nd | nd | 0.04 | [25] |
| Food industry residue and manure E | nd | nd | nd | nd | nd | nd | nd | nd | 0.74 | [26] |
| Restaurants food waste F | nd | nd | nd | nd | 0.17 | 0.06 | 0.17 | 0.17 | 0.08 | [27] |
| Starch wastewater G | nd | nd | nd | nd | nd | nd | nd | nd | 4.11 | [28] |
| Molasses wastewater H | nd | nd | nd | nd | nd | nd | nd | nd | 2.99 | [29] |
| Corn starch A | 0.3 | nd | 0.25 | nd | 0.22 | nd | nd | nd | nd | [15] |
| Molasses I | 0.21 | nd | 0.08 | nd | nd | nd | nd | nd | nd | [30] |
| Inedible dough A | 0.37 | nd | 0.24 | nd | 0.19 | nd | nd | nd | nd | [15] |
| Batter liquid A | 0.37 | nd | 0.31 | nd | 0.21 | nd | nd | nd | nd | [15] |
| Glucose medium A | 0.36 | 0.43 | 0.22 | 0.42 | nd | nd | nd | nd | nd | [21] |
| Distiller dried grains with solubles (DDGS) J | 0.32 | nd | 0.18 | nd | nd | nd | nd | nd | nd | [31] |
| Cereal waste water K | nd | nd | nd | nd | nd | nd | nd | nd | 0.79 | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinola, S.A.; Saba, B.; Christy, A.; Cornish, K.; Ezeji, T.C. Biohydrogen and Biobutanol Production from Spent Coffee and Tea Waste Using Clostridium beijerinckii. Fermentation 2025, 11, 177. https://doi.org/10.3390/fermentation11040177
Akinola SA, Saba B, Christy A, Cornish K, Ezeji TC. Biohydrogen and Biobutanol Production from Spent Coffee and Tea Waste Using Clostridium beijerinckii. Fermentation. 2025; 11(4):177. https://doi.org/10.3390/fermentation11040177
Chicago/Turabian StyleAkinola, Stephen Abiola, Beenish Saba, Ann Christy, Katrina Cornish, and Thaddeus Chukwuemeka Ezeji. 2025. "Biohydrogen and Biobutanol Production from Spent Coffee and Tea Waste Using Clostridium beijerinckii" Fermentation 11, no. 4: 177. https://doi.org/10.3390/fermentation11040177
APA StyleAkinola, S. A., Saba, B., Christy, A., Cornish, K., & Ezeji, T. C. (2025). Biohydrogen and Biobutanol Production from Spent Coffee and Tea Waste Using Clostridium beijerinckii. Fermentation, 11(4), 177. https://doi.org/10.3390/fermentation11040177







