Volatile Fatty Acid Production vs. Methane and Hydrogen in Anaerobic Digestion
Abstract
1. Introduction
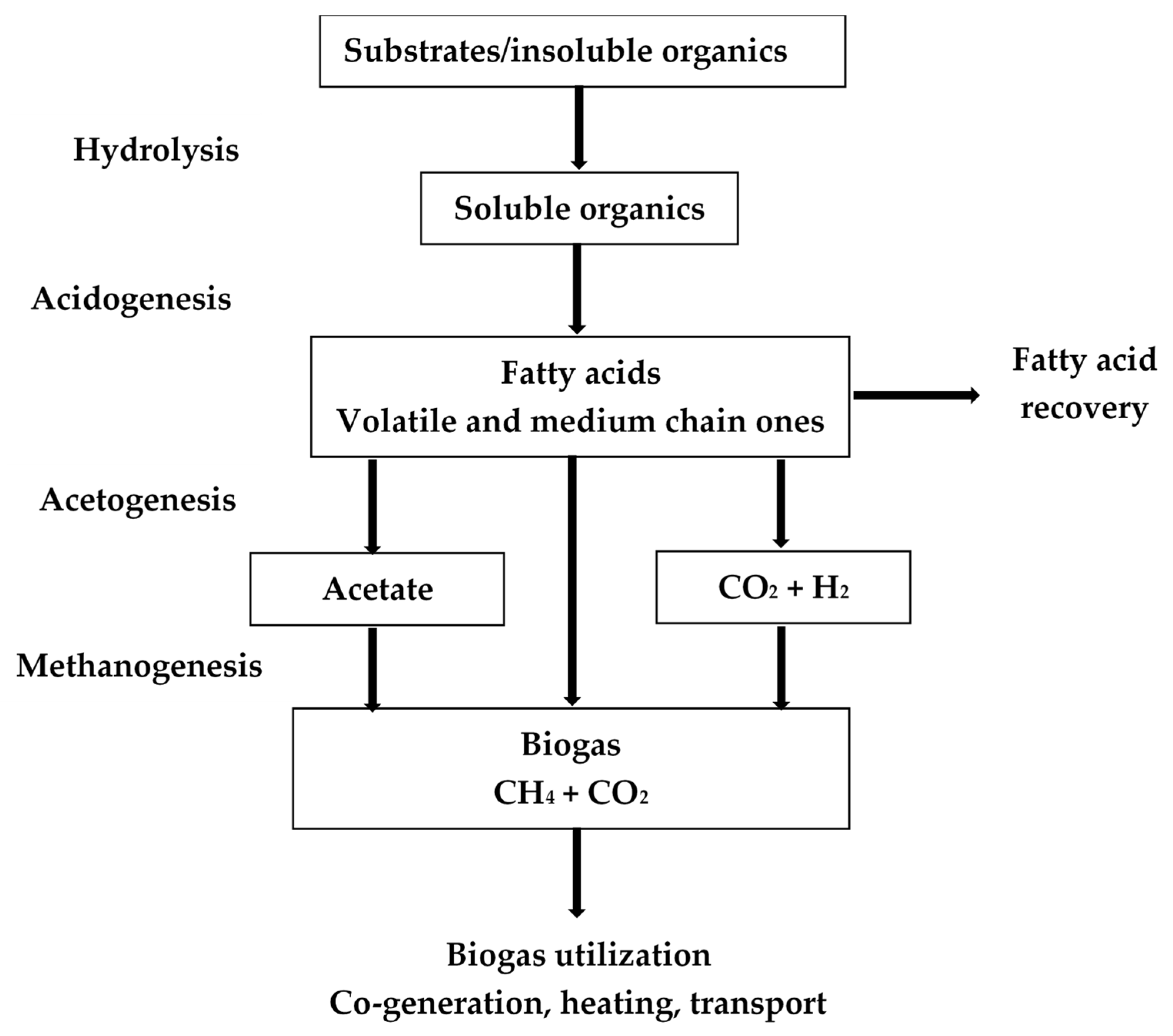
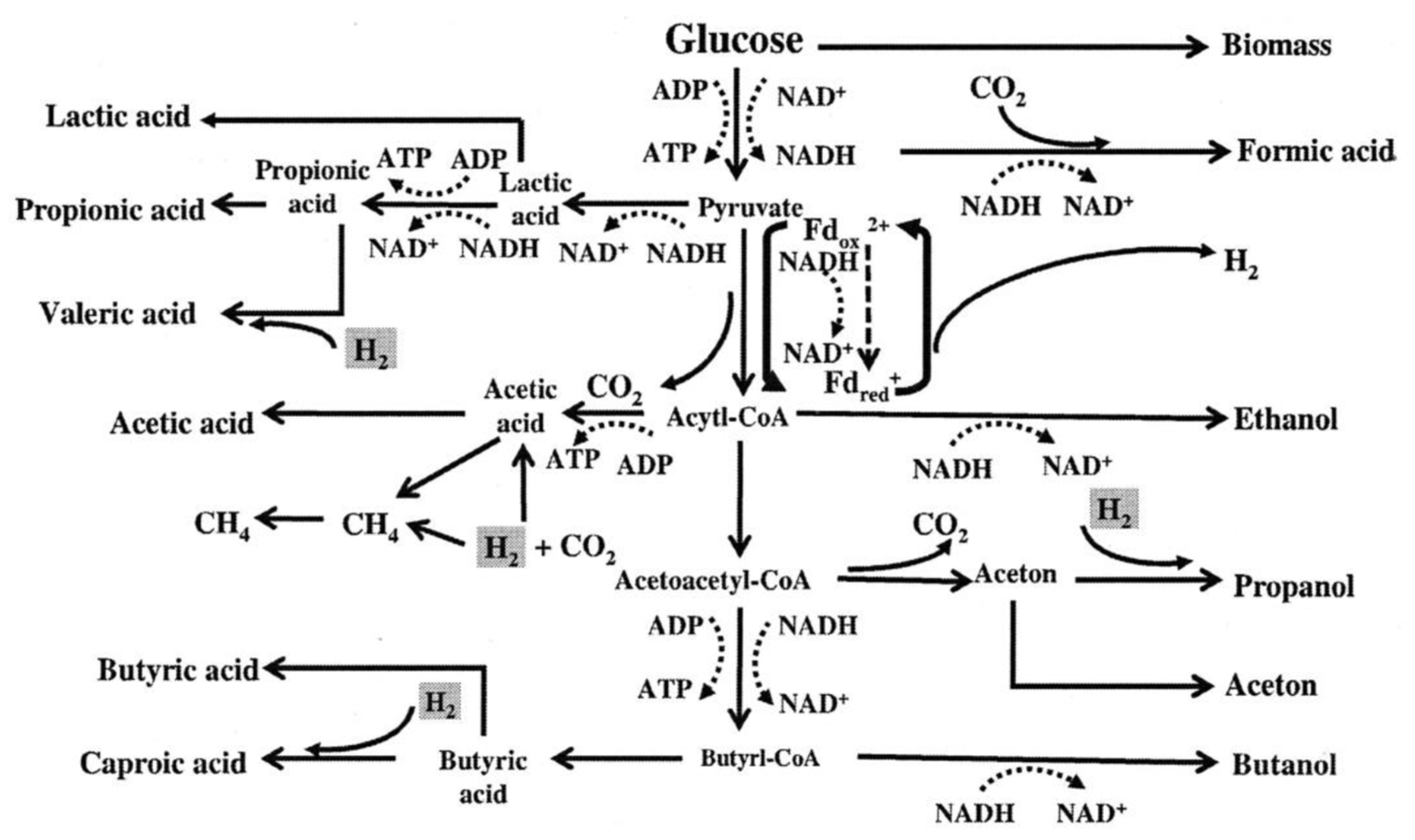
2. Volatile Fatty Acids
2.1. Strategies to Enhance the Production of Volatile Fatty Acids
2.2. Methane vs. VFA Formation
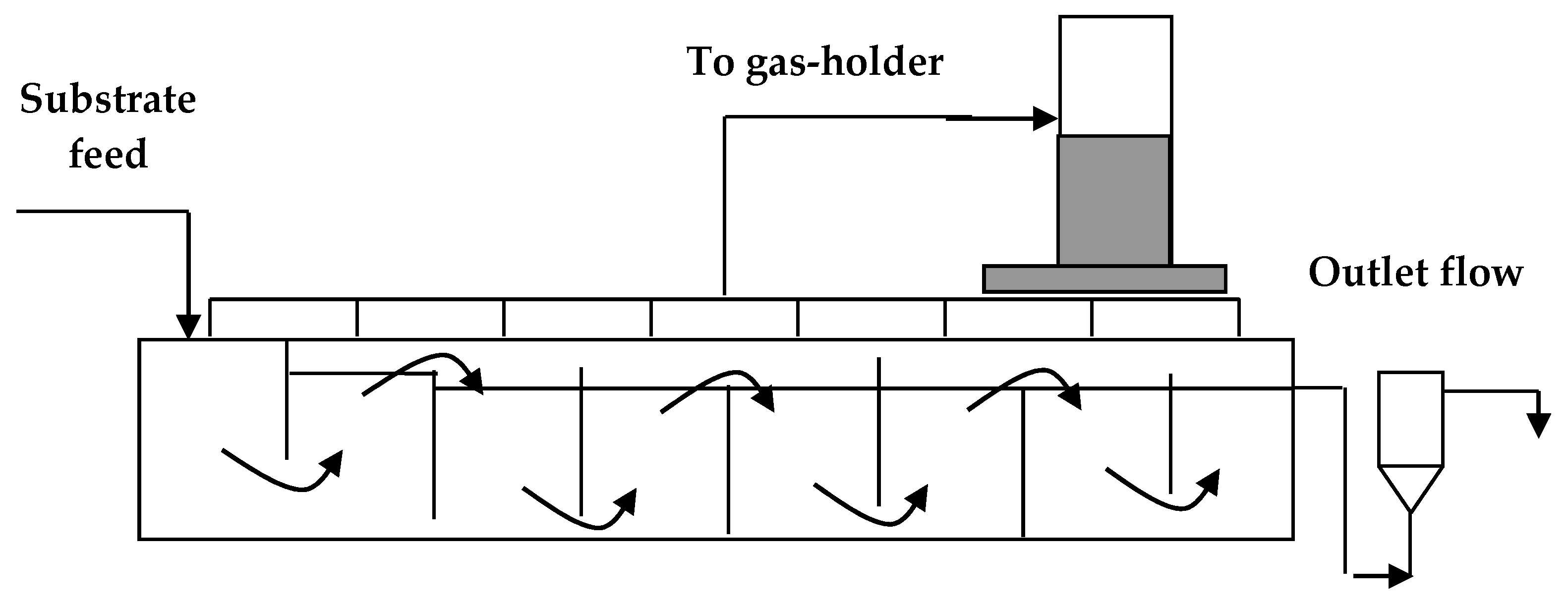
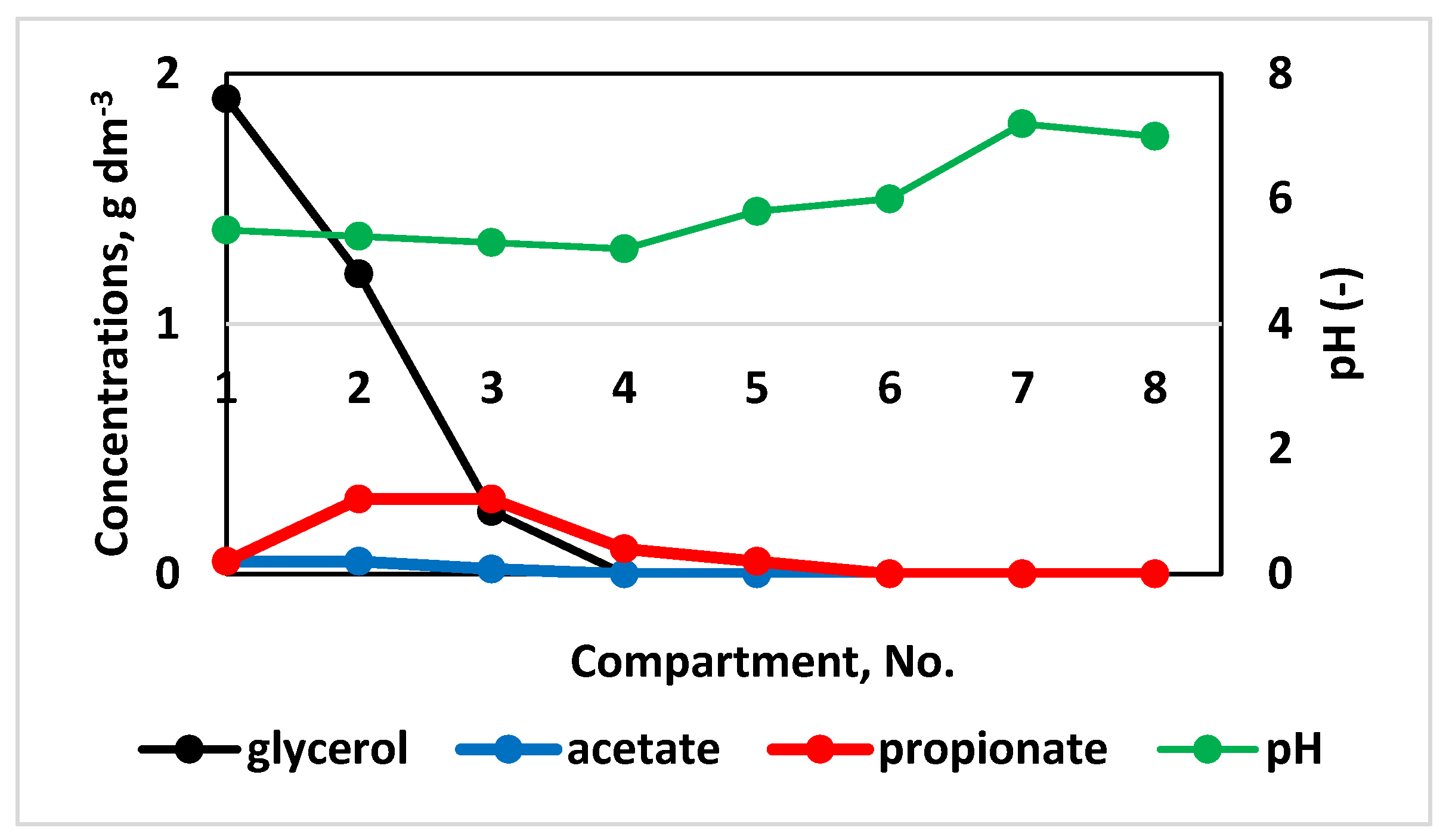
2.3. Case Study: Glycerol as a Substrate in a Multi-Step Bioreactor
2.4. Formation of Hydrogen vs. VFAs
2.5. VFA Removal from Fermentation Broth During Anaerobic Digestion
3. Conclusions
- Volatile fatty acids are inevitable intermediate products of anaerobic digestion with biogas formation. Their excessive accumulation may lead to strong inhibition of methanogenic microbes and a lack of methane in the biogas.
- However, volatile fatty acids (formic, acetic, propionic, and butyric ones) are useful commodities with various applications. That is why their targeted production with a desired production rate may shift the aims of the anaerobic digestion from biogas to volatile fatty acid production.
- Combining VFA production with biogas release can be an integrated and self-consistent process with biogas production sufficient enough to supply the target processes with energy. The biogas can also be utilized as a feedstock for value-added chemicals or fuels (light hydrocarbons) and hydrogen.
- The maintenance of optimum VFA concentrations can be accomplished by simultaneous VFA removal from the fermentation broth, thus integrating the product recovery with the maintenance of optimum operation conditions in the digester.
- The substrate preparation and the operating conditions (organic loading rate and hydraulic retention time) are of crucial importance for a successful fermentation process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yentekakis, I.V.; Goula, G. Biogas management: Advanced utilization for production of renewable energy and added-value chemicals. Front. Environ. Sci. 2017, 5, 7. [Google Scholar] [CrossRef]
- de Jong, E.; Jungmeier, G. Biorefinery concepts in comparison to petrochemical refineries, Chapter 1. In Industrial Biorefineries and White Biotechnology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–33. [Google Scholar] [CrossRef]
- Alemán-Nava, G.S.; Meneses-Jácome, A.; Cárdenas-Chávez, D.L.; Díaz-Chavez, R.; Scarlat, N.; Dallemand, J.F.; Ornelas-Soto, N.; Garcia-Arrazola, R.; Parra, R. Bioenergy in Mexico: Status and perspective. Biofuels Bioprod. Bioref. 2015, 9, 8–20. [Google Scholar] [CrossRef]
- Virmond, E.; Rocha, J.D.; Moreira, R.F.P.M.; José, H.J. Valorization of agroindustrial solid residues and residues from biofuel production chains by thermochemical conversion: A review, citing Brazil as a case study. Braz. J. Chem. Eng. 2013, 30, 197–229. [Google Scholar]
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popielm, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef]
- Garcia-Heras, J.L. Reactor sizing, process kinetics, and modelling of anaerobic digestion of complex wastes. In Biomethanization of the Organic Fractions in Municipal Solid Wastes; Mata-Alvarez, J., Ed.; IWA-Publishing: London, UK, 2003. [Google Scholar]
- Manimegalai, R.; Gopinath, L.R.; Merlin Christy, P.; Divya, D. Isolation and identification of acetogenic and methanogenic bacteria from anoxic black sediments and their role in biogas production. Int. J. Plant Anim. Environ. Sci. 2014, 4, 156–164. [Google Scholar]
- Tampio, E.A.; Blasco, L.; Vainio, M.M.; Kahala, M.M.; Rasi, S.E. Volatile fatty acids (VFAs) and methane from food waste and cow slurry: Comparison of biogas and VFA fermentation processes. GCB Bioenergy 2019, 11, 72–84. [Google Scholar] [CrossRef]
- Ferry, J.G. Enzymology of the fermentation of acetate to methane by Methanosarcina thermophila. Biofactors 1997, 6, 25–35. [Google Scholar]
- Conrad, R. Quantification of methanogenic pathways using stable carbon isotopic signatures: A review and a proposal. Org. Geochem. 2005, 36, 739–752. [Google Scholar]
- Games, L.M.; Hayes, J.M.; Gunsalus, R.P. Methane-producing bacteria: Natural fractionations of the stable carbon isotopes. Geochim.Cosmochim. Acta 1978, 42, 1295–1297. [Google Scholar]
- Fuchs, G.; Thauer, R.; Ziegler, H.; Stickler, N. Carbon isotope fractionation in Methanobacterium thermoautotrophicum. Arch. Microbiol. 1979, 120, 135–137. [Google Scholar]
- Wainaina, S.; Lukitawesa; Awasthi, M.K.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.; Yaswanth Lingam, Y.; Venkata Mohan, S. Understanding acidogenesis towards green hydrogen and volatile fatty acid production—Critical analysis and circular economy perspective. Chem. Eng. J. 2023, 464, 141550. [Google Scholar] [CrossRef]
- Ramos-Suarez, M.; Zhang, Y.; Outram, V. Current perspectives on acidogenic fermentation to produce volatile fatty acids from waste. Rev. Environ. Sci. Biotechnol. 2021, 20, 439–478. [Google Scholar] [CrossRef]
- Agnihotri, S.; Yin, D.-M.; Mahboubi, A.; Sapmaz, T.; Varjani, S.; Qiao, W.; Koseoglu-Imer, D.Y.; Taherzadeh, M.J. A Glimpse of the World of Volatile Fatty Acids Production and Application: A review. Bioengineered 2022, 13, 1249–1275. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, P.; Zhang, R.; Jianning Chang, J.; Chen, L.; Zhang, G.; Wang, A. Bioconversion of volatile fatty acids from organic wastes to produce high-value products by photosynthetic bacteria: A review. Environ. Res. 2024, 242, 117796. [Google Scholar]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar]
- Ehsanipour, M.; Suko, A.V.; Bura, R. Fermentation of lignocellulosic sugars to acetic acid by Moorella thermoacetica. J. Ind. Microbiol. Biotechnol. 2016, 43, 807–816. [Google Scholar] [CrossRef]
- Raspor, P.; Goranovič, D. Biotechnological applications of acetic acid bacteria. Crit. Rev. Biotechnol. 2008, 28, 101–124. [Google Scholar] [CrossRef]
- Perstorp, ProSid™ MI 700. No Year and 2012, Online Brochure and Product Data Sheet. Available online: https://www.perstorp.com (accessed on 10 February 2025).
- Ranaei, R.; Pilevar, Z.; Khaneghah, A.M.; Hosseini, H. Propionic acid: Method of production, current state and perspectives. Food Technol. Biotechnol. 2020, 58, 115–127. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, S.-T. Propionic acid production from glycerol by metabolically engineered Propionibacterium acidipropionici. Process Biochem. 2009, 44, 1346–1351. [Google Scholar]
- Himmi, E.H.; Bories, A.; Boussaid, A.; Hassani, L. Propionic acid fermentation of glycerol and glucose by Propionibacterium acidipropionici and Propionibacterium freudenreichii ssp. Shermanii. Appl. Microbiol. Biotechnol. 2000, 53, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Li, J.; Yuan, Y.; Barai, N.R.; Ai, B. A review on bio-butyric acid production and its optimization. Int. J. Agric. Biol. 2014, 16, 1019–1024. [Google Scholar]
- den Boer, E.; den Boer, J. Production of volatile fatty acids in biorefineries. Chapter 6. In Waste Biorefinery; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 159–178. [Google Scholar] [CrossRef]
- Garcia Alvaro, A.; Ruiz Palomar, C.; Hermosilla Redondo, D.; Mucoz Torre, R.; de Godos Crespo, I. Simultaneous production of biogas and volatile fatty acids through anaerobic digestion using cereal straw as substrate. Environ. Technol. Innov. 2023, 31, 103215. [Google Scholar]
- Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Production of volatile fatty acids through co-digestion of sewage sludge and external organic waste: Effect of substrate proportions and longterm operation. Waste Manag. 2020, 112, 30–39. [Google Scholar]
- Battista, F.; Bolzonella, D. Beyond anaerobic digestion: New perspectives for the development of a biorefinery platform for the simultaneous production of medium-chain fatty acids by chain elongation and biogas from food wastes. ACS Sustain. Chem. Eng. 2024, 12, 15294–15306. [Google Scholar] [CrossRef]
- Wu, K.K.; Zhao, L.; Wang, Z.H.; Sun, Z.F.; Wu, J.T.; Chen, C.; Xing, D.F.; Yang, S.S.; Wang, A.J.; Zhang, Y.F.; et al. Simultaneous biogas upgrading and medium-chain fatty acids production using a dual membrane biofilm reactor. Water Res. 2024, 249, 120915. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Loh, K.C. Review and perspectives of enhanced volatile fatty acids production from acidogenic fermentation of lignocellulosic biomass wastes. Bioresour. Bioprocess. 2021, 8, 68. [Google Scholar] [CrossRef]
- Varghese, V.K.; Poddara, B.J.; Shah, M.P.; Purohit, H.J.; Khardenavis, A.A. A comprehensive review on current status and future perspectives of microbial volatile fatty acids production as platform chemicals. Sci. Total Environ. 2024, 815, 152500. [Google Scholar] [CrossRef]
- Karuppiah, T.; Azariah, V.E. Biomass pretreatment for enhancement of biogas production. In Anaerobic Digestion; Rajesh Banu, J., Ed.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar] [CrossRef]
- Dahiya, S.; Sarka, R.O.; Swamy, Y.V.; Venkata Mohan, S. Acidogenic fermentation of food waste for volatile fatty acid production with co-generation of biohydrogen. Bioresour. Technol. 2015, 182, 103–113. [Google Scholar]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar]
- Zhang, B.; Zhang, L.L.; Zhang, S.C.; Shi, H.Z.; Cai, W.M. The influence of pH on hydrolysis and acidogenesis of kitchen wastes in two-phase anaerobic digestion. Environ. Technol. 2005, 26, 329–340. [Google Scholar] [PubMed]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour Technol. 2014, 161, 395–401. [Google Scholar] [PubMed]
- Al-Sulaimi, I.N.; Nayak, J.K.; Alhimali, H.; Sana, A.; Al-Mamun, A. Effect of volatile fatty acids accumulation on biogas production by sludge-feeding thermophilic anaerobic digester and predicting process parameters. Fermentation 2022, 8, 184. [Google Scholar] [CrossRef]
- Magdalena, J.A.; Greses, S.; González-Fernández, C. Impact of organic loading rate in volatile fatty acids production and population dynamics using microalgae biomass as substrate. Sci. Rep. 2019, 9, 18374. [Google Scholar]
- Lim, S.J.; Kim, B.J.; Jeong, C.M.; Choi, J.D.R.; Ahn, Y.H.; Chang, H.N. Anaerobic organic acid production of food waste in once-a-day feeding and drawing-off bioreactor. Bioresour. Technol. 2008, 99, 7866–7874. [Google Scholar]
- Dennehy, C.; Lawlor, P.G.; Gardiner, G.E.; Jiang, Y.; Cormican, P.; McCabe, M.S.; Zhan, X. Process stability and microbial community composition in pig manure and food waste anaerobic co-digesters operated at low HRTs. Front. Environ. Sci. Eng. 2017, 11, 4. [Google Scholar] [CrossRef]
- Zhang, W.; Lang, Q.; Pan, Z.; Jiang, Y.; Liebetrau, J.; Nelles, M.; Dong, H.; Dong, R. Performance evaluation of a novel anaerobic digestion operation process for treating highsolids content chicken manure: Effect of reduction of the hydraulic retention time at a constant organic loading rate. Waste Manag. 2017, 64, 340–347. [Google Scholar] [CrossRef]
- Li, J.; Rong, L.; Zhao, Y.; Li, S.; Zhang, C.; Xiao, D.; Foo, J.L.; Yu, A. Next-generation metabolic engineering of non-conventional microbial cell factories for carboxylic acid platform chemicals. Biotechnol. Adv. 2020, 43, 107605. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, M.; Miao, H.; Huang, Z.; Gao, S.; Ruan, W. In situ volatile fatty acids influence biogas generation from kitchen wastes by anaerobic digestion. Bioresour. Technol. 2014, 163, 186–192. [Google Scholar] [CrossRef]
- Kuo, T.-C.; Chen, H.-Y.; Chong, B.; Lin, M. Cost benefit analysis and carbon footprint of biogas energy through life cycle assessment. Clean. Environ. Syst. 2024, 15, 10024. [Google Scholar]
- Damyanova, S.; Beschkov, V. Biogas as a source of energy and chemicals. In Biorefinery Concepts, Energy and Products; Beschkov, V., Ed.; Intech Open: London, UK, 2020; pp. 17–30. [Google Scholar]
- Grobicki, A.; Stuckey, D.C. Hydrodynamic characteristics of the anaerobic baffled reactor. Water Res. 1992, 26, 371–378. [Google Scholar]
- Wilkens, E.; Ringel, A.K.; Hortig, D.; Willke, T.; Vorlop, K.D. High-level production of 1,3-propanediol from crude glycerol by Clostridium butyricum AKR102a. Appl. Microbiol. Biotechnol. 2012, 93, 1057–1063. [Google Scholar] [PubMed]
- Oh, B.R.; Seo, J.W.; Heo, S.Y.; Hong, W.K.; Luo, L.H.; Son, J.H.; Park, D.H.; Kim, C.H. Fermentation strategies for 1,3-propanediol production from glycerol using a genetically engineered Klebsiella pneumoniae strain to eliminate by-product formation. Bioprocess Biosyst. Eng. 2012, 35, 159–165. [Google Scholar] [CrossRef]
- Saxena, R.; Anand, P.; Saran, S.; Isar, J. Microbial production of 1,3-propanediol: Recent developments and emerging opportunities. Biotechnol. Adv. 2009, 27, 895. [Google Scholar]
- Wang, Z.; Yang, S.T. Propionic acid production in glycerol/glucose co-fermentation by Propionibacterium freudenreichii subsp. Shermanii. Bioresour. Technol. 2013, 137, 116–123. [Google Scholar] [PubMed]
- Boyaval, P.; Corre, C. Production of propionic acid. Lait 1995, 75, 453–461. [Google Scholar]
- Maerkl, H. Fortschritte der Verfahrenstechnik; VDI-Verlag: Düsseldorf, Germany, 1980; p. 509. [Google Scholar]
- da Silva, G.P.; Mack, M.; Contiero, J. Glycerol: A promising and abundant carbon source for industrial microbiology. Biotechnol. Adv. 2009, 27, 30–39. [Google Scholar]
- Zhang, Y.; Huang, Z.; Du, C.; Li, Y.; Cao, Z. Introduction of an NADH regeneration system into Klebsiella oxytoca leads to an enhanced oxidative and reductive metabolism of glycerol. Metab. Eng. 2009, 11, 101–106. [Google Scholar]
- González-Pajuelo, M.; Meynial-Salles, I.; Mendes, F.; Soucaille, P.; Vasconcelos, I. Microbial Conversion of glycerol to 1,3-propanediol: Physiological comparison of a natural producer, Clostridium butyricum VPI 3266, and an engineered strain, Clostridium acetobutylicum DG1(pSPD5). Appl. Environ. Microbiol. 2006, 72, 96–101. [Google Scholar]
- Reungsang, A.; Sittijunda, S.; Angelidaki, I. Simultaneous production of hydrogen and ethanol from waste glycerol by Enterobacter aerogenes KKU-S1. Int. J. Hydrogen Energy 2013, 38, 1813–1825. [Google Scholar]
- Nwachukwu, R.E.S.; Shahbazi, A.; Wang, L.; Worku, M.; Ibrahim, S.; Schimmel, K. Optimization of cultural conditions for conversion of glycerol to ethanol by Enterobacter aerogenes S012. AMB Express 2013, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Jitrwung, R.; Yargeau, V. Biohydrogen and bioethanol production from biodiesel-based glycerol by Enterobacter aerogenes in a continuous stir tank reactor. Int. J. Mol. Sci. 2015, 16, 10650–10664. [Google Scholar] [CrossRef] [PubMed]
- Beschkov, V.; Angelov, I.; Petrova, P. Biogas production from glycerol in a multistage anaerobic digestor. Curr. Top. Biotechnol. 2012, 7, 61–69. [Google Scholar]
- Angelidaki, I.; Sanders, W. Assessment of the anaerobic biodegradability of macropollutants. Rev. Environ. Sci. Biotechnol. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- Daugulis, A.J. Integrated reaction and product recovery in bioreactor systems. Biotechnol. Prog. 1988, 4, 113–122. [Google Scholar] [CrossRef]
- Kertes, A.S.; King, C. Extraction chemistry of fermentation product carboxylic acids. Biotechnol. Bioeng. 1986, 28, 269–282. [Google Scholar] [CrossRef]
- Yabannavar, V.M.; Wang, D.I.C. Extractive fermentation for lactic acid production. Biotechnol. Bioeng. 1991, 37, 1095–1100. [Google Scholar] [CrossRef]
- Timmer, J.K.M.; Kromkamp, J.; Robbertsen, T. Lactic acid separation from fermentation broth by reverse osmosis and nanofiltration. J. Membr. Sci. 1994, 2, 185–197. [Google Scholar] [CrossRef]
- Zyakun, A.M.; Bondar, V.A.; Laurinavichus, K.S.; Shipin, S.S.; Belyaev, S.S.; Ivanov, M.V. Fractionation of carbon isotopes under the growth of methane-producing bacteria on various substrates. Mikrobiol. Zhurnal 1988, 50, 16–22. [Google Scholar]
- Gelwicks, J.T.; Risatti, J.B.; Hayes, J.M. Carbon isotope effects associated with aceticlastic methanogenesis. Appl. Environ. Microbiol. 1994, 60, 467–472. [Google Scholar] [CrossRef]
- Wainaina, S.; Parchami, M.l.; Mahboubi, A.; Mahboubi, A.; Sarvari-Horvath, I.; Taherzadeh, M. Food waste-derived volatile fatty acids platform using an immersed membrane bioreactor. Bioresour Technol. 2019, 274, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Akinbomi, J.; Taherzadeh, M.J. Evaluation of fermentative hydrogen production from single and mixed fruit wastes. Energies 2015, 8, 4253–4272. [Google Scholar] [CrossRef]
- Kim, S.H.; Han, S.K.; Shin, H.S. Feasibility of biohydrogen production by anaerobic co-digestion of food waste and sewage sludge. Int. J. Hydrogen Energy 2004, 29, 1607–1616. [Google Scholar] [CrossRef]
- Van Ginkel, S.; Logan, B.E. Inhibition of biohydrogen production by undissociated acetic and butyric acids. Environ. Sci. Technol. 2005, 39, 9351–9356. [Google Scholar] [CrossRef]
- Cata Saady, N.M. Homoacetogenesis during hydrogen production by mixed cultures dark fermentation: Unresolved challenge. Int. J. Hydrogen Energy 2013, 38, 13172–13191. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrere, H.; Steyer, J.P. Hydrogen production from agricultural waste by dark fermentation: A review. Int. J. Hydrogen Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- Sydney, E.B.; Larroche, C.; Novak, A.C.; Nouaille, R.; Sarma, S.J.; Brar, S.K.; Letti, K.A., Jr.; Soccol, V.T.; Soccol, C.R. Economic process to produce biohydrogen and volatile fatty acids by a mixed culture using vinasse from sugarcane ethanol industry as nutrient source. Bioresour. Technol. 2014, 159, 380–386. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Y.; Dai, K.; Shen, N.; Zeng, R.J. The glucose metabolic distribution in thermophilic (55 °C) mixed culture fermentation: A chemostat study. Int. J. Hydrogen Energy 2015, 40, 919–926. [Google Scholar] [CrossRef]
- Cardena, R.; Valencia-Ojeda, C.; Chazaro-Ruiz, L.F.; Razo-Flores, E. Regulation of the dark fermentation products by electro-fermentation in reactors without membrane. Int. J. Hydrogen Energy 2024, 49, 107–116. [Google Scholar] [CrossRef]
- James, G.; Gȍrgens, J.F.; Pott, R.W.M. Co-production of volatile fatty acids and biogas from an anaerobic digestion system using in situ extraction. Separ. Purif. Technol. 2021, 257, 117891. [Google Scholar] [CrossRef]
- Morison, S.D.; van Rensburg, E.; Pott, R.W.M. Extraction of Volatile Fatty Acids from Wastewater Anaerobic Digestion Using Different Extractant-Diluent Mixtures. Biomass Conv. Bioref. 2024, 14, 16515–16533. [Google Scholar] [CrossRef]
- Beschkov, V.N. Ion exchange in downstream processing in biotechnology. Phys. Sci. Rev. 2020, 5, 63–78. [Google Scholar] [CrossRef]
- Zacharof, M.P.; Lovitt, R.W. Recovery of volatile fatty acids (VFA) from complex waste effluents using membranes. Water Sci. Technol. 2014, 63, 495–503. [Google Scholar]
- Dasa, K.T.; Westman, S.Y.; Millati, R.; Cahyanto, M.N.; Taherzadeh, M.J.; Niklasson, C. Inhibitory effect of long-chain fatty acids on biogas production and the protective effect of membrane bioreactor. BioMed Res. Int. 2016, 2016, 7263974. [Google Scholar] [CrossRef]
- Jones, R.J.; Massanet-Nicolau, J.; Fernandez–Feito, R.; Dinsdale, R.M.; Guwy, A.J. Fermentative volatile fatty acid production and recovery from grass using a novel combination of solids separation, pervaporation, and electrodialysis technologies. Bioresour. Technol. 2021, 342, 12592. [Google Scholar]
- Aktij, S.A.; Zirehpour, A.; Mollahosseini, A.; Taherzadeh, M.J.; Tiraferri, A.; Rahimpour, A. Feasibility of membrane processes for the recovery and purification of bio-based volatile fatty acids: A comprehensive review. J. Ind. Eng. Chem. 2020, 81, 24–40. [Google Scholar]
- Yesil, H.; Taner, H.; Ugur Nigiz, F.; Hilmioglu, N.; Tugtas, A.E. Pervaporative separation of mixed volatile fatty acids: A study towards integrated VFA production and separation. Waste Biomass Valor. 2020, 11, 1737–1753. [Google Scholar] [CrossRef]


| Fatty Acid | Areas of Applications | Market Price, EUR/ton | Annual Production ktons/Year |
|---|---|---|---|
| Acetic | Food additives, plasticizers, and dyes | 400–800 | 14,000–17,000 |
| Propionic | Resins, pharmaceuticals, and paints | 1250–1700 | 350–470 |
| Butyric | Perfumes, textiles, varnishes, and plastics | 1500–1650 | 90–105 |
| Valeric * | Perfumes, plasticizers, and lubricants | 1500–1650 | 720 * |
| Caproic * | Rubber, grease, and tobacco flavor | 1500–1650 | 150 * |
| Compartment | Aerobes, Facultative Aerobes, Count of Bacteria in 1 mL | Anaerobes, Number of Bacteria in 1 mL | Methanogens, Genera | Methane Production |
|---|---|---|---|---|
| 1 | Fungi, Bacillus ~1 × 102 | ~1 × 101 | - | |
| 2 | Klebsiella ~1 × 103 | ~1 × 105 | Methanosarcina | Acetate, CO2 + H2 [67,68] |
| 3 | Klebsiella ~1 × 103 | ~1 × 105 | Methanobacterium | CO2 + H2 [68] |
| 4 | Klebsiella ~2 × 103 | ~1 × 106 | Methanobacterium | CO2 + H2 [12] |
| 5 | Klebsiella 6–8 × 102 | ~4-5 × 106 | Methanobacterium | CO2 + H2 [12] |
| 6 | Klebsiella ~4 × 102 | ~2 × 106 | Methanobrevibacter | CO2 + H2 [68] |
| 7 | Klebsiella 1–2 × 103 | ~1 × 106 | Methanobrevibacter | CO2 + H2 [12] |
| 8 | Bacillus, Klebsiella ~1 × 102 | ~1 × 105 | Methanobrevibacter | CO2 + H2 [12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beschkov, V.N.; Angelov, I.K. Volatile Fatty Acid Production vs. Methane and Hydrogen in Anaerobic Digestion. Fermentation 2025, 11, 172. https://doi.org/10.3390/fermentation11040172
Beschkov VN, Angelov IK. Volatile Fatty Acid Production vs. Methane and Hydrogen in Anaerobic Digestion. Fermentation. 2025; 11(4):172. https://doi.org/10.3390/fermentation11040172
Chicago/Turabian StyleBeschkov, Venko N., and Ivan K. Angelov. 2025. "Volatile Fatty Acid Production vs. Methane and Hydrogen in Anaerobic Digestion" Fermentation 11, no. 4: 172. https://doi.org/10.3390/fermentation11040172
APA StyleBeschkov, V. N., & Angelov, I. K. (2025). Volatile Fatty Acid Production vs. Methane and Hydrogen in Anaerobic Digestion. Fermentation, 11(4), 172. https://doi.org/10.3390/fermentation11040172






