Apple and Grape Waste Pomace Fermentation and Co-Ferment Product Chemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Materials
2.2. Fermentation
2.3. Chemical Properties of Cider Piquettes
2.4. Chromatic Properties of Cider Piquettes
2.5. Final Cider Piquette Characteristics
2.6. The Phenolics Concentrations and Antiradical Activities of Cider Piquettes
2.7. Flavonoids and Phenolic Acids in Cider Piquettes
2.8. Statistical Analysis
3. Results and Discussion
3.1. Pre-Fermentation Must Components
3.2. Pre-Fermentation Yeast Assimilable Nitrogen Content in the Must
3.3. Acids in Pre-Fermented Must
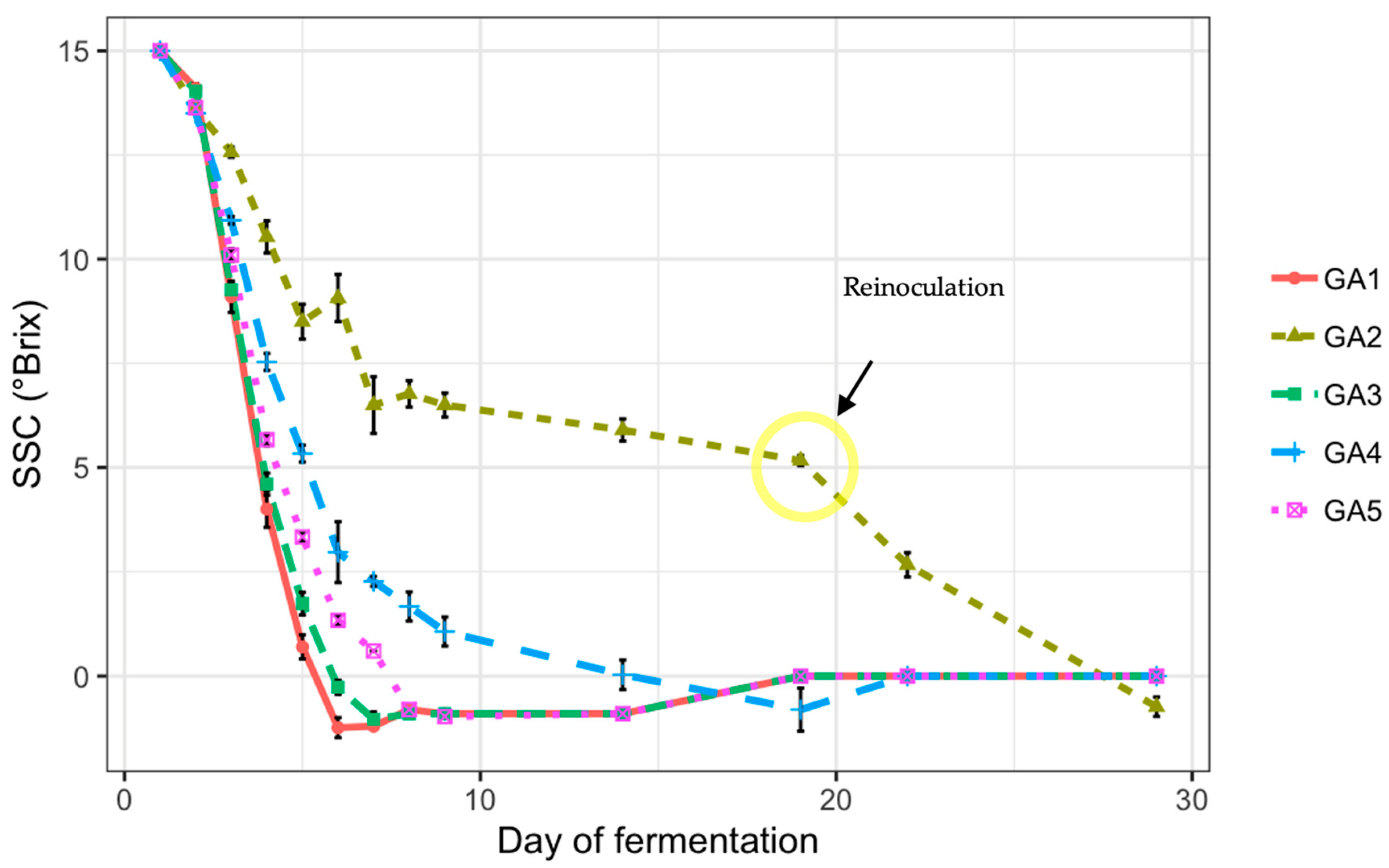
3.4. SSC Dynamic Changes During Fermentation
3.5. pHs Changes During Fermentation
3.6. Chromatic Dynamics During Fermentation
3.7. Final Wine Characteristics
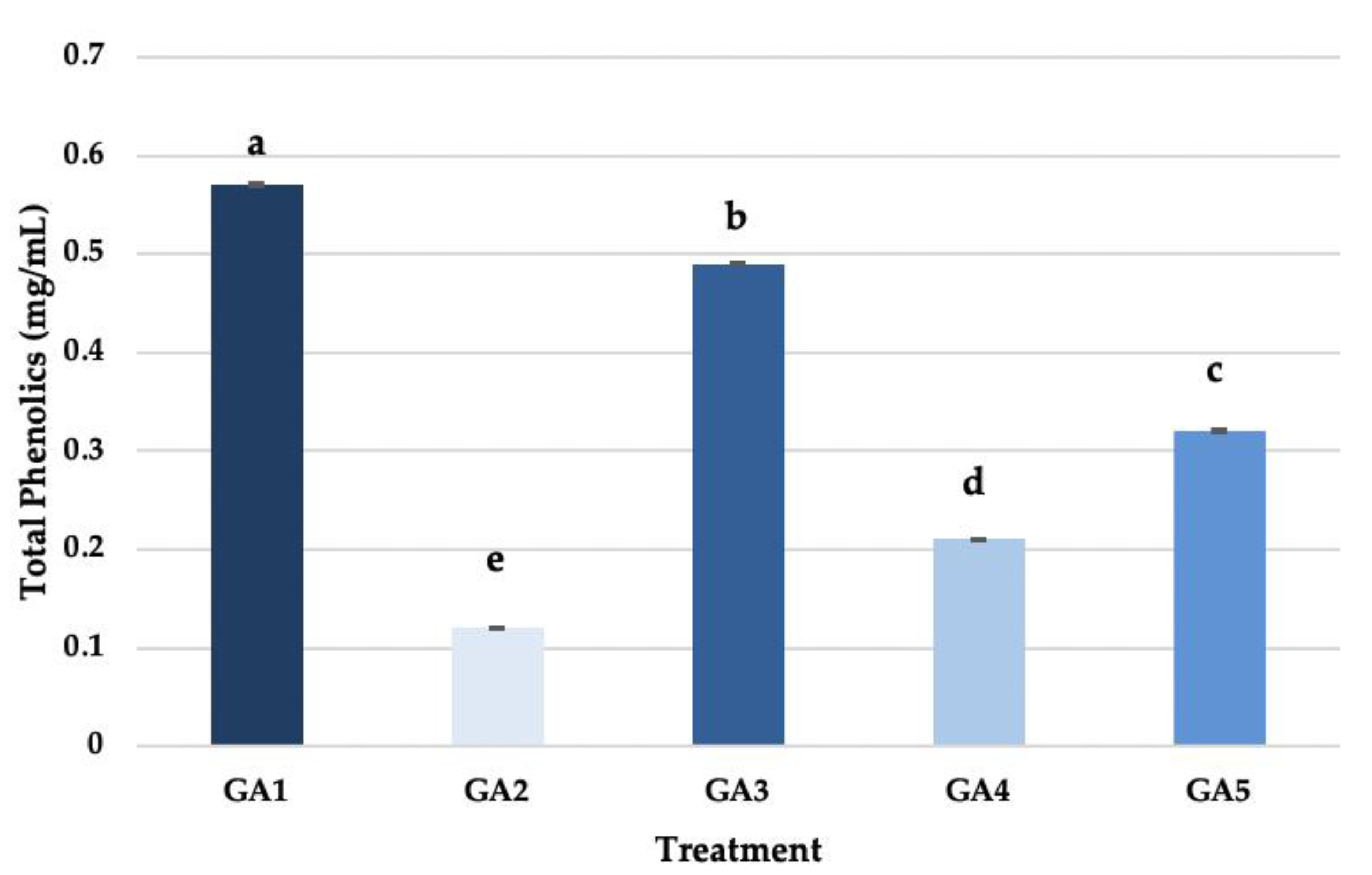
3.8. Final Wine Total Phenolics Content
3.9. Final Wine Antioxidant Capacities
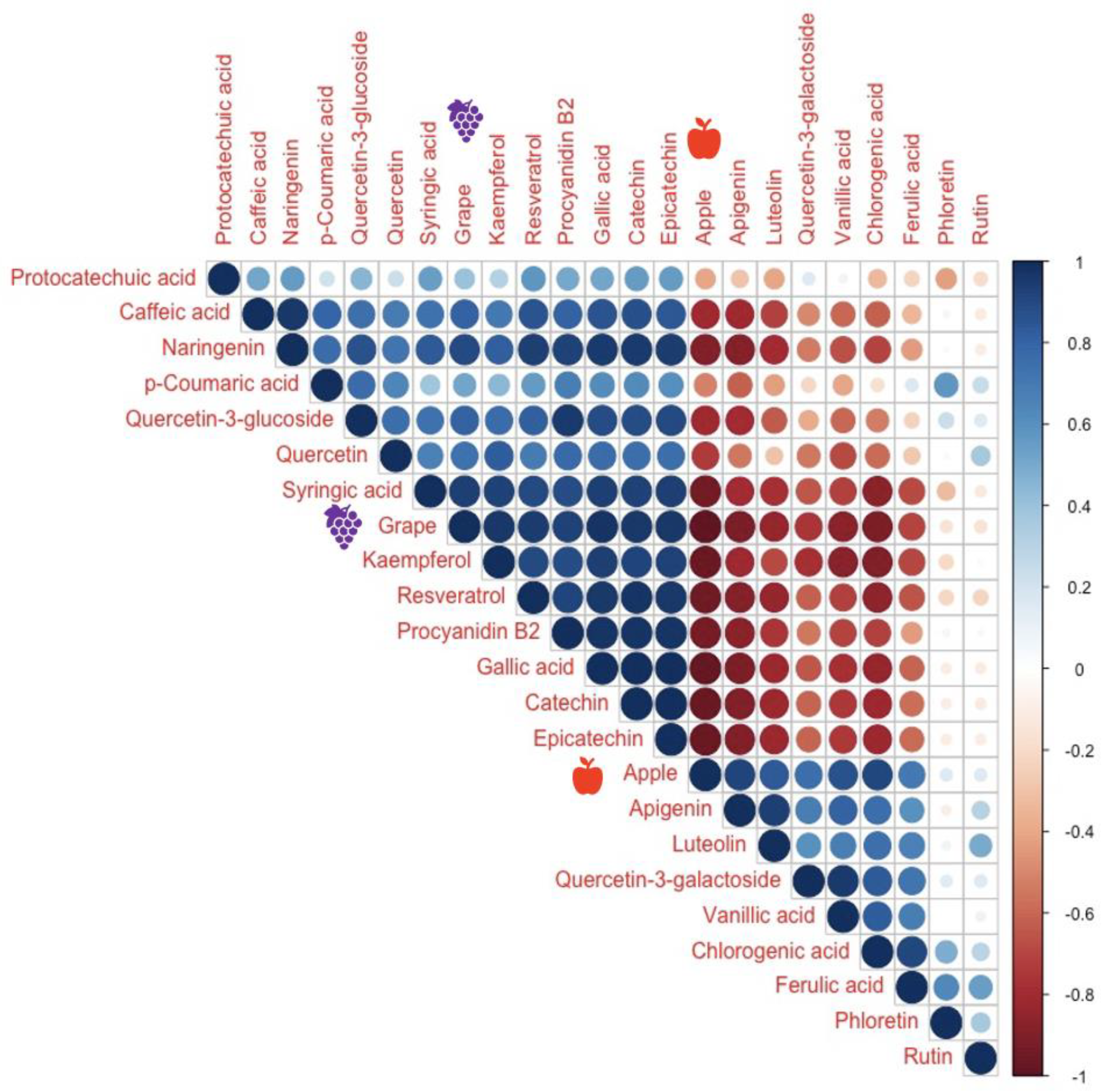
3.10. Final Wine Flavonoids and Phenolic Acids Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rakesh, B.; Mahendran, R. Upcycling of Food Waste and Food Loss—A Sustainable Approach in the Food Sector. Trends Food Sci. Technol. 2024, 143, 104274. [Google Scholar] [CrossRef]
- Jurgilevich, A.; Birge, T.; Kentala-Lehtonen, J.; Korhonen-Kurki, K.; Pietikäinen, J.; Saikku, L.; Schösler, H. Transition towards Circular Economy in the Food System. Sustainability 2016, 8, 69. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Teles, A.S.C.; Chávez, D.W.H.; Oliveira, R.A.; Bon, E.P.S.; Terzi, S.C.; Souza, E.F.; Gottschalk, L.M.F.; Tonon, R.V. Use of Grape Pomace for the Production of Hydrolytic Enzymes by Solid-State Fermentation and Recovery of Its Bioactive Compounds. Food Res. Int. 2019, 120, 441–448. [Google Scholar] [CrossRef]
- Shalini, R.; Gupta, D.K. Utilization of Pomace from Apple Processing Industries: A Review. J. Food Sci. Technol. 2010, 47, 365–371. [Google Scholar] [CrossRef]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.-M.; Tou, J.C. A Comprehensive Analysis of the Composition, Health Benefits, and Safety of Apple Pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef] [PubMed]
- Almanza-Oliveros, A.; Bautista-Hernández, I.; Castro-López, C.; Aguilar-Zárate, P.; Meza-Carranco, Z.; Rojas, R.; Michel, M.R.; Martínez-Ávila, G.C.G. Grape Pomace—Advances in Its Bioactivity, Health Benefits, and Food Applications. Foods 2024, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Harutyunyan, M.; Viana, R.; Granja-Soares, J.; Martins, M.; Ribeiro, H.; Malfeito-Ferreira, M. Adaptation of Ancient Techniques to Recreate ‘Wines’ and ‘Beverages’ Using Withered Grapes of Muscat of Alexandria. Fermentation 2022, 8, 85. [Google Scholar] [CrossRef]
- Campbell, C. The Botanist and the Vintner: How Wine Was Saved for the World; Algonquin Books: Chapel Hill, NC, USA, 2006; ISBN 978-1-56512-528-5. [Google Scholar]
- Piquette: The Perfect Summer Quencher. Available online: https://www.vinerra.com/in-depth-guides/piquette-the-perfect-summer-quencher (accessed on 10 February 2025).
- Shockey, C.; Shockey, K.K. The Big Book of Cidermaking: Expert Techniques for Fermenting and Flavoring Your Favorite Hard Cider; Storey Publishing: North Adams, MA, USA, 2020; ISBN 978-1-63586-113-6. [Google Scholar]
- Proulx, A. Making the Best Apple Cider; Storey Publishing: North Adams, MA, USA, 1980; ISBN 978-0-88266-222-0. [Google Scholar]
- Casassa, F.; Mawdsley, P.; Stoffel, E.; Williams, P.; Dodson, P.J.C. Fermenation: Chemical and Sensory Effects of Cofermentation and Post-Malolactic Fermentation Blending of Syrah with Rhone White Cultivars. Wine Vitic. J. 2021, 35, 22+24–28. [Google Scholar] [CrossRef]
- Calugar, P.C.; Coldea, T.E.; Salanță, L.C.; Pop, C.R.; Pasqualone, A.; Burja-Udrea, C.; Zhao, H.; Mudura, E. An Overview of the Factors Influencing Apple Cider Sensory and Microbial Quality from Raw Materials to Emerging Processing Technologies. Processes 2021, 9, 502. [Google Scholar] [CrossRef]
- Ogodo, A.C.; Ugbogu, O.C.; Ugbogu, A.E.; Ezeonu, C.S. Production of Mixed Fruit (Pawpaw, Banana and Watermelon) Wine Using Saccharomyces Cerevisiae Isolated from Palm Wine. SpringerPlus 2015, 4, 683. [Google Scholar] [CrossRef]
- Skorospelova, E.; Motovilov, O.; Nitsievskaya, K.; Sherbinin, V. Comparative Characteristics of Varietal and Blended Flavored Wines. IOP Conf. Ser. Earth Environ. Sci. 2019, 403, 012022. [Google Scholar] [CrossRef]
- Fracassetti, D.; Bottelli, P.; Corona, O.; Foschino, R.; Vigentini, I. Innovative Alcoholic Drinks Obtained by Co-Fermenting Grape Must and Fruit Juice. Metabolites 2019, 9, 86. [Google Scholar] [CrossRef]
- Tsegay, Z.T. Total Titratable Acidity and Organic Acids of Wines Produced from Cactus Pear (Opuntia-Ficus-Indica) Fruit and Lantana Camara (L. Camara) Fruit Blended Fermentation Process Employed Response Surface Optimization. Food Sci. Nutr. 2020, 8, 4449–4462. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, F.; Yang, L.; Li, J.; Zhu, X. Enhancement of the Aroma in Low-Alcohol Apple-Blended Pear Wine Mixed Fermented with Saccharomyces Cerevisiae and Non-Saccharomyces Yeasts. LWT 2022, 155, 112994. [Google Scholar] [CrossRef]
- Kovach, E. What Is Cider Piquette?|Ciderkin and Cider Piquette 101. Cider Cult. 2022. Available online: https://www.ciderculture.com/what-is-cider-piquette/ (accessed on 27 February 2024).
- Aipperspach, A.; Hammond, J.; Hatterman-Valenti, H. Utilizing Pruning and Leaf Removal to Optimize Ripening of Vitis Riparia-Based ‘Frontenac Gris’ and ‘Marquette’ Wine Grapes in the Northern Great Plains. Horticulturae 2020, 6, 18. [Google Scholar] [CrossRef]
- McIntosh Apple. Available online: https://www.thecanadianencyclopedia.ca/en/article/mcintosh-apple (accessed on 27 February 2024).
- Jones, D.S.; McManus, P.S. Distinctive Symptoms and Signs of Downy Mildew on Cold-Climate Wine Grape Cultivars. Plant Health Prog. 2017, 18, 192–195. [Google Scholar] [CrossRef]
- Wang, Z.; Svyantek, A.; Miller, Z.; Watrelot, A.A. Fermentation Process Effects on Fermented McIntosh Apple Ciders. Fermentation 2024, 10, 115. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Li, P.; Lv, Y.; Nan, H.; Wen, L.; Wang, Z. Research Progress of Wine Aroma Components: A Critical Review. Food Chem. 2023, 402, 134491. [Google Scholar] [CrossRef]
- ENGİN, S.; MERT, C. The Effects of Harvesting Time on the Physicochemical Components of Aronia Berry. Turk. J. Agric. For. 2020, 44, 361–370. [Google Scholar] [CrossRef]
- Panasiuk, O.; Talley, F.B.; Sapers, G.M. Correlation Between Aroma and Volatile Composition of Mcintosh Apples. J. Food Sci. 1980, 45, 989–991. [Google Scholar] [CrossRef]
- 2023 USDA Plant Hardiness Zone Map|USDA Plant Hardiness Zone Map. Available online: https://planthardiness.ars.usda.gov/ (accessed on 31 January 2025).
- Web Soil Survey|Natural Resources Conservation Service. Available online: https://www.nrcs.usda.gov/resources/data-and-reports/web-soil-survey (accessed on 31 January 2025).
- Murray, M. Intermountain Commercial Tree Fruit Production Guide 2024-25; Colorado, United States. Available online: https://intermountainfruit.org/files/IntermountainTFG-2024.pdf (accessed on 31 January 2025).
- Farr, J.E.; Sigurdson, G.T.; Giusti, M.M. Influence of Cyanidin Glycosylation Patterns on Carboxypyranoanthocyanin Formation. Food Chem. 2018, 259, 261–269. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing|BibSonomy. Available online: https://www.bibsonomy.org/bibtex/7469ffee3b07f9167cf47e7555041ee7 (accessed on 20 March 2024).
- Horwitz, W.; Latimer, G.W. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005; ISBN 978-0-935584-75-2. [Google Scholar]
- Lamuela-Raventós, R.M. Folin–Ciocalteu Method for the Measurement of Total Phenolic Content and Antioxidant Capacity. In Measurement of Antioxidant Activity & Capacity; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 107–115. ISBN 978-1-119-13538-8. [Google Scholar]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Devaki, M. The Ferric Reducing/Antioxidant Power (FRAP) Assay for Non-Enzymatic Antioxidant Capacity: Concepts, Procedures, Limitations and Applications. In Measurement of Antioxidant Activity & Capacity; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 77–106. ISBN 978-1-119-13538-8. [Google Scholar]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 16 November 2023).
- Wickham, H. Programming with Ggplot2. In ggplot2: Elegant Graphics for Data Analysis; Wickham, H., Ed.; Use R! Springer International Publishing: Cham, Switzerland, 2016; pp. 241–253. ISBN 978-3-319-24277-4. [Google Scholar]
- Berthels, N.J.; Cordero Otero, R.R.; Bauer, F.F.; Thevelein, J.M.; Pretorius, I.S. Discrepancy in Glucose and Fructose Utilisation during Fermentation by Saccharomyces Cerevisiae Wine Yeast Strains. FEMS Yeast Res. 2004, 4, 683–689. [Google Scholar] [CrossRef]
- Su, Y.; Heras, J.M.; Gamero, A.; Querol, A.; Guillamón, J.M. Impact of Nitrogen Addition on Wine Fermentation by S. Cerevisiae Strains with Different Nitrogen Requirements. J. Agric. Food Chem. 2021, 69, 6022–6031. [Google Scholar] [CrossRef]
- The Australian Wine Research Institute. Yeast Assimilable Nitrogen (YAN); The Australian Wine Research Institute: Urrbrae, Australia, 2025. [Google Scholar]
- Boudreau, T.F.; Peck, G.M.; O’Keefe, S.F.; Stewart, A.C. Free Amino Nitrogen Concentration Correlates to Total Yeast Assimilable Nitrogen Concentration in Apple Juice. Food Sci. Nutr. 2017, 6, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Gibney, P.; Cheng, L.; Liu, S.; Peck, G. Yeast Assimilable Nitrogen Concentrations Influence Yeast Gene Expression and Hydrogen Sulfide Production During Cider Fermentation. Front. Microbiol. 2020, 11, 1264. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Su, J.; Yang, H.; Feng, L.; Wang, M.; Xu, G.; Shao, J.; Ma, C. Grape Tartaric Acid: Chemistry, Function, Metabolism, and Regulation. Horticulturae 2023, 9, 1173. [Google Scholar] [CrossRef]
- Lima, M.M.M.; Choy, Y.Y.; Tran, J.; Lydon, M.; Runnebaum, R.C. Organic Acids Characterization: Wines of Pinot Noir and Juices of ‘Bordeaux Grape Varieties’. J. Food Compos. Anal. 2022, 114, 104745. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Li, J.; Qin, S.; Yang, W.; Ma, X.; Qiao, X.; Yang, B. Effects of Genetic Background and Altitude on Sugars, Malic Acid and Ascorbic Acid in Fruits of Wild and Cultivated Apples (Malus Sp.). Foods 2021, 10, 2950. [Google Scholar] [CrossRef]
- Mocke, L. Kinetic Modelling of Wine Fermentations: Why Does Yeast Prefer Glucose to Fructose; Stellenbosch University: Stellenbosch, South Africa, 2013. [Google Scholar]
- Wang, D.; Xu, Y.; Hu, J.; Zhao, G. Fermentation Kinetics of Different Sugars by Apple Wine Yeast Saccharomyces Cerevisiae. J. Inst. Brew. 2004, 110, 340–346. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; de Freitas, V. Influence of Phenolics on Wine Organoleptic Properties. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 529–570. ISBN 978-0-387-74118-5. [Google Scholar]
- Brady, J. Descriptive Analysis of Frontenac Gris and Brianna Wine Grape and Wine Varieties; University of Minnesota: Minneapolis, MN, USA, 2017. [Google Scholar]
- Techakanon, C.; Sirimuangmoon, C. The Effect of Pasteurization and Shelf Life on the Physicochemical, Microbiological, Antioxidant, and Sensory Properties of Rose Apple Cider during Cold Storage. Beverages 2020, 6, 43. [Google Scholar] [CrossRef]
- Hensel, M.; Scheiermann, M.; Fahrer, J.; Durner, D. New Insights into Wine Color Analysis: A Comparison of Analytical Methods to Sensory Perception for Red and White Varietal Wines. J. Agric. Food Chem. 2024, 72, 2008–2017. [Google Scholar] [CrossRef]
- The Elemental Profile of Ciders Made from Different Varieties of Apples. Available online: https://colab.ws/articles/10.21603%2F2308-4057-2024-2-604 (accessed on 6 March 2024).
- Frontenac Gris Grape Cultivar|Minnesota Hardy. Available online: https://mnhardy.umn.edu/frontenac-gris (accessed on 6 March 2024).
- Sparrow, A.M.; Dambergs, R.G.; Close, D.C. Grape Skins as Supplements for Color Development in Pinot Noir Wine. Food Res. Int. 2020, 133, 108707. [Google Scholar] [CrossRef]
- Possner, E.; Kliewer, W.M. The Localisation of Acids, Sugars, Potassium and Calcium in Developing Grape Berries. Available online: https://ojs.openagrar.de/index.php/VITIS/article/view/5976 (accessed on 6 March 2024).
- Ginni, G.; Adish Kumar, S.; Mohamed Usman, T.M.; Pakonyi, P.; Rajesh Banu, J. Chapter 13—Integrated Biorefineries of Food Waste. In Food Waste to Valuable Resources; Banu, J.R., Kumar, G., Gunasekaran, M., Kavitha, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 275–298. ISBN 978-0-12-818353-3. [Google Scholar]
- Remize, F.; Roustan, J.L.; Sablayrolles, J.M.; Barre, P.; Dequin, S. Glycerol Overproduction by Engineered Saccharomyces Cerevisiae Wine Yeast Strains Leads to Substantial Changes in By-Product Formation and to a Stimulation of Fermentation Rate in Stationary Phase. Appl. Environ. Microbiol. 1999, 65, 143–149. [Google Scholar] [CrossRef]
- Mutton, M.J.R.; Ferrari, F.C.S.; de Freita, L.A.; de Freita, C.M.; Andrietta, M.d.G.S.; Andrietta, S.R. Interaction between the Production of Ethanol and Glycerol in Fed-Batch Bioreactors. Braz. J. Microbiol. 2019, 50, 389. [Google Scholar] [CrossRef] [PubMed]
- The Australian Wine Research Institute. Acidity and pH; The Australian Wine Research Institute: Urrbrae, Australia, 2025. [Google Scholar]
- Waterhouse, A.L. Wine Phenolics. Ann. N. Y. Acad. Sci. 2002, 957, 21–36. [Google Scholar] [CrossRef]
- Rocchetti, G.; Gregorio, R.P.; Lorenzo, J.M.; Barba, F.J.; Oliveira, P.G.; Prieto, M.A.; Simal-Gandara, J.; Mosele, J.I.; Motilva, M.-J.; Tomas, M.; et al. Functional Implications of Bound Phenolic Compounds and Phenolics–Food Interaction: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 811–842. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of Antiradical Properties of Antioxidants Using DPPH Assay: A Critical Review and Results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Reis, S.F.; Rai, D.K.; Abu-Ghannam, N. Apple Pomace as a Potential Ingredient for the Development of New Functional Foods. Int. J. Food Sci. Technol. 2014, 49, 1743–1750. [Google Scholar] [CrossRef]
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.-L. Wine By-Products: Phenolic Characterization and Antioxidant Activity Evaluation of Grapes and Grape Pomaces from Six Different French Grape Varieties. Molecules 2014, 19, 482–506. [Google Scholar] [CrossRef] [PubMed]
- Živković, N.M.; Čakar, U.D.; Petrović, A.V. Effects of Spontaneous and Inoculated Fermentation on the Total Phenolic Content and Antioxidant Activity of Cabernet Sauvignon Wines and Fermented Pomace. Food Feed Res. 2024, 51, 119–129. [Google Scholar] [CrossRef]
- Grigoras, C.G.; Destandau, E.; Fougère, L.; Elfakir, C. Evaluation of Apple Pomace Extracts as a Source of Bioactive Compounds. Ind. Crops Prod. 2013, 49, 794–804. [Google Scholar] [CrossRef]
- Zanwar, A.A.; Badole, S.L.; Shende, P.S.; Hegde, M.V.; Bodhankar, S.L. Chapter 21—Antioxidant Role of Catechin in Health and Disease. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 267–271. ISBN 978-0-12-398456-2. [Google Scholar]
- German, I.J.S.; Pomini, K.T.; Andreo, J.C.; Shindo, J.V.T.C.; Castro, M.V.M.d.; Detregiachi, C.R.P.; Araújo, A.C.; Guiguer, E.L.; Fornari Laurindo, L.; Bueno, P.C.d.S.; et al. New Trends to Treat Muscular Atrophy: A Systematic Review of Epicatechin. Nutrients 2024, 16, 326. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.H.A.; Salgado, H.R.N. Gallic Acid: Review of the Methods of Determination and Quantification. Crit. Rev. Anal. Chem. 2016, 46, 257–265. [Google Scholar] [CrossRef]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef]
- Guo, S.; Guan, L.; Cao, Y.; Li, C.; Chen, J.; Li, J.; Liu, G.; Li, S.; Wu, B. Diversity of Polyphenols in the Peel of Apple (Malus Sp.) Germplasm from Different Countries of Origin. Int. J. Food Sci. Technol. 2016, 51, 222–230. [Google Scholar] [CrossRef]
- Zhang, M.; Swarts, S.G.; Yin, L.; Liu, C.; Tian, Y.; Cao, Y.; Swarts, M.; Yang, S.; Zhang, S.B.; Zhang, K.; et al. Antioxidant Properties of Quercetin. In Proceedings of the Oxygen Transport to Tissue XXXII; LaManna, J.C., Puchowicz, M.A., Xu, K., Harrison, D.K., Bruley, D.F., Eds.; Springer US: Boston, MA, USA, 2011; pp. 283–289. [Google Scholar]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, K.; Jing, Y.; Liu, S.; Qin, S.; Peng, F.; Li, D.; Peng, C. Procyanidin B2: A Promising Multi-Functional Food-Derived Pigment for Human Diseases. Food Chem. 2023, 420, 136101. [Google Scholar] [CrossRef]
- Kiliç, I.; Yeşiloğlu, Y. Spectroscopic Studies on the Antioxidant Activity of p-Coumaric Acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed]
- Frémont, L. Biological Effects of Resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Kim, M.-C.; Um, J.-Y.; Hong, S.-H. The Beneficial Effect of Vanillic Acid on Ulcerative Colitis. Molecules 2010, 15, 7208–7217. [Google Scholar] [CrossRef] [PubMed]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic Acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The Potential Effects of Chlorogenic Acid, the Main Phenolic Components in Coffee, on Health: A Comprehensive Review of the Literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Harutyunyan, M.; Viana, R.; Granja-Soares, J.; Asryan, A.; Marques, J.C.; Malfeito-Ferreira, M. Consumer Acceptance of Sweet Wines and Piquettes Obtained by the Adaptation of Ancient Wine-Making Techniques. J. Sens. Stud. 2023, 38, e12823. [Google Scholar] [CrossRef]



| Treatment | Grape Pomace:Apple Pomace Ratios (w/w) | Must Total Volume (L) | SSC °Brix Before Adjustment | SSC °Brix After Adjustment |
|---|---|---|---|---|
| GA1 | 1:0 | 3 | 8.40 ± 0.10 | 15.00 ± 0.01 |
| GA2 | 0:1 | 3 | 4.37 ± 0.05 | 15.00 ± 0.01 |
| GA3 | 3:1 | 3 | 7.33 ± 0.15 | 15.00 ± 0.11 |
| GA4 | 1:3 | 3 | 5.40 ± 0.10 | 15.00 ± 0.01 |
| GA5 | 1:1 | 3 | 6.43 ± 0.11 | 15.00 ± 0.01 |
| Sugar Contents and Ratios in Treatments | |||
|---|---|---|---|
| Treatment | Glucose (g/L) | Fructose (g/L) | G:F ratio |
| GA1 | 119.24 ± 0.31 a | 41.35 ± 1.97 a | 2.89 ± 0.13 b |
| GA2 | 113.89 ± 0.80 ab | 25.54 ± 2.67 b | 4.49 ± 0.45 a |
| GA3 | 108.52 ± 6.44 b | 31.31 ± 5.71 ab | 3.52 ± 0.43 ab |
| GA4 | 107.71 ± 6.44 b | 28.05 ± 2.45 b | 3.86 ± 0.31 a |
| GA5 | 116.49 ± 2.18 ab | 36.81 ± 1.31 a | 3.17 ± 0.07 b |
| Treatment | Tartaric Acid (g/L) | Malic Acid (g/L) | Citric Acid (g/L) |
|---|---|---|---|
| GA1 | 1.59 ± 0.18 a | 0.99 ± 0.06 a | 0.04 ± 0.02 a |
| GA2 | 0.00 ± 0.00 c | 1.14 ± 0.13 a | 0.00 ± 0.05 a |
| GA3 | 1.43 ± 0.20 a | 1.17 ± 0.13 a | 0.09 ± 0.06 a |
| GA4 | 0.78 ± 0.33 b | 1.19 ± 0.12 a | 0.01 ± 0.05 a |
| GA5 | 0.89 ± 0.30 b | 1.01 ± 0.06 a | 0.04 ± 0.02 a |
| Treatment | pH | Ethanol (%vol) | Titratable Acidity (pH = 8.2) (g/L) | Volatile Acids (g/L) | Glycerol (g/L) | Glucose (g/L) | Fructose (g/L) |
|---|---|---|---|---|---|---|---|
| GA1 | 3.26 ± 0.17 a | 8.13 ± 0.06 a | 5.45 ± 0.12 b | 0.05 ± 0.01 b | 6.00 ± 0.00 a | 1.06 ± 0.12 c | 0.40 ± 0.10 a |
| GA2 | 3.21 ± 0.0 b | 7.87 ± 0.05 b | 4.79 ± 0.08 c | 0.15 ± 0.02 a | 5.43 ± 0.15 b | 2.47 ± 0.06 a | 0.17 ± 0.06 a |
| GA3 | 3.24 ± 0.01 ab | 8.16 ± 0.07 a | 5.56 ± 0.05 b | 0.08 ± 0.03 b | 5.83 ± 0.23 ab | 1.43 ± 0.15 bc | 0.30 ± 0.00 a |
| GA4 | 3.24 ± 0.03 ab | 7.88 ± 0.09 b | 5.51 ± 0.04 b | 0.21 ± 0.06 a | 5.53 ± 0.25 ab | 2.20 ± 0.15 a | 0.53 ± 0.61 a |
| GA5 | 3.26 ± 0.01 a | 7.99 ± 0.06 b | 5.73 ± 0.05 a | 0.13 ± 0.02 ab | 5.53 ± 0.12 ab | 1.70 ± 0.10 b | 0.03 ± 0.06 a |
| Treatment | FRAP Assay | DPPH Assay |
|---|---|---|
| (mM Ferrous Equivalent/µL) | TEAC (µm/µL) | |
| GA1 | 25.97 ± 0.11 a | 705.79 ± 49.82 a |
| GA2 | 1.02 ± 0.02 e | 530.84 ± 2.17 b |
| GA3 | 6.19 ± 0.25 b | 668.69 ± 9.21 a |
| GA4 | 2.57 ± 0.12 d | 595.38 ± 52.31 b |
| GA5 | 4.21 ± 0.22 c | 601.91 ± 7.99 b |
| Treatment | Dominant Phenolic Compounds (mg/L) | |||||
|---|---|---|---|---|---|---|
| Catechin | Epicatechin | Gallic Acid | Quercetin-3-Glucoside | Quercetin-3-Galactoside | Quercetin | |
| GA1 | 64.77 ± 4.25 a | 48.54 ± 2.12 a | 29.90 ± 0.98 a | 53.13 ± 11.77 a | 7.76 ± 1.30 b | 20.47 ± 1.50 a |
| GA2 | 0.22 ± 0.02 c | 0.79 ± 0.06 c | 0.05 ± 0.00 c | 14.54 ± 3.14 b | 15.15 ± 2.27 a | 9.27 ± 0.75 c |
| GA3 | 51.11 ± 11.38 a | 37.61 ± 7.87 a | 23.96 ± 3.50 ab | 52.66 ± 9.93 a | 9.88 ± 2.07 a | 18.97 ± 3.25 a |
| GA4 | 15.23 ± 2.46 bc | 11.97 ± 1.73 bc | 7.74 ± 1.03 b | 34.88 ± 6.18 a | 11.85 ± 2.89 a | 18.78 ± 1.81 a |
| GA5 | 30.94 ± 11.33 b | 23.33 ± 8.65 ab | 14.57 ± 4.41 b | 44.12 ± 15.91 a | 10.72 ± 3.16 a | 14.89 ± 0.80 b |
| p-Value | <0.001 | <0.001 | <0.001 | <0.01 | <0.05 | <0.001 |
| Treatment | Moderate Quantity Phenolic Compounds | ||||||
|---|---|---|---|---|---|---|---|
| Caffeic Acid (mg/L) | p-Coumaric Acid (mg/L) | Ferulic Acid (mg/L) | Kaempferol (mg/L) | Procyanidin B2 (mg/L) | Phloretin (mg/L) | Resveratrol (mg/mL) | |
| GA1 | 7.68 ± 3.14 b | 1.72 ± 0.46 bc | 0.91 ± 0.11 b | 4.97 ± 0.49 a | 8.88 ± 0.83 a | 0.12 ± 0.04 c | 1.43 ± 0.21 a |
| GA2 | 2.10 ± 0.05 a | 0.70 ± 0.05 c | 1.64 ± 0.14 a | 0.04 ± 0.02 c | 1.47 ± 0.09 c | 0.68 ± 0.12 b | ND |
| GA3 | 8.05 ± 2.28 b | 2.51 ± 0.87 abc | 1.38 ± 0.18 a | 3.47 ± 0.25 a | 7.74 ± 1.19 a | 2.17 ± 0.30 a | 1.16 ± 0.30 a |
| GA4 | 3.50 ± 0.67 a | 1.77 ± 0.40 ab | 1.93 ± 0.24 a | 1.98 ± 0.15 b | 4.30 ± 0.54 b | 2.51 ± 0.07 a | 0.15 ± 0.02 b |
| GA5 | 5.52 ± 1.32 a | 2.22 ± 0.64 ab | 1.65 ± 0.45 a | 2.15 ± 0.46 b | 5.86 ± 2.20 ab | 3.60 ± 1.25 a | 0.53 ± 0.15 a |
| p-Value | <0.05 | <0.05 | <0.05 | <0.01 | <0.01 | <0.001 | <0.001 |
| Treatment | Small Quantity Phenolic Compounds | ||||||
|---|---|---|---|---|---|---|---|
| Apigenin (ng/mL) | Chlorogenic Acid (ng/mL) | Luteolin (ng/mL) | Naringenin (ng/mL) | Rutin (ng/mL) | Syringic Acid (ng/mL) | Vanillic Acid (ng/mL) | |
| GA1 | 1.20 ± 0.20 a | 27.20 ± 7.08 b | 6.16 ± 0.73 b | 81.67 ± 13.59 a | 77.07 ± 32.14 a | 398.60 ± 48.58 a | 457.73 ± 33.29 b |
| GA2 | 6.33 ± 0.30 b | 804.43 ± 74.99 a | 15.00 ± 0.91 a | 28.97 ± 1.01 c | 17.47 ± 22.2 a | 119.33 ± 23.01 b | 1074.83 ± 123.02 a |
| GA3 | 1.40 ± 0.36 a | 335.17 ± 86.40 b | 7.63 ± 1.24 b | 82.23 ± 11.89 a | 116.77 ± 53.76 a | 281.80 ± 7.78 a | 602.80 ± 83.77 a |
| GA4 | 5.80 ± 0.26 b | 743.60 ± 138.60 a | 18.33 ± 1.70 a | 42.43 ± 3.86 b | 422.13 ± 213.9 a | 165.20 ± 24.56 b | 769.00 ± 123.88 a |
| GA5 | 2.00 ± 0.34 a | 648.77 ± 210.90 a | 8.73 ± 1.75 b | 58.83 ± 15.13 ab | 98.80 ± 87.9 a | 197.03 ± 36.34 ab | 624.77 ± 174.25 a |
| p-Value | <0.001 | <0.001 | <0.001 | <0.001 | <0.01 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Svyantek, A.; Miller, Z. Apple and Grape Waste Pomace Fermentation and Co-Ferment Product Chemistry. Fermentation 2025, 11, 126. https://doi.org/10.3390/fermentation11030126
Wang Z, Svyantek A, Miller Z. Apple and Grape Waste Pomace Fermentation and Co-Ferment Product Chemistry. Fermentation. 2025; 11(3):126. https://doi.org/10.3390/fermentation11030126
Chicago/Turabian StyleWang, Zhuoyu, Andrej Svyantek, and Zachariah Miller. 2025. "Apple and Grape Waste Pomace Fermentation and Co-Ferment Product Chemistry" Fermentation 11, no. 3: 126. https://doi.org/10.3390/fermentation11030126
APA StyleWang, Z., Svyantek, A., & Miller, Z. (2025). Apple and Grape Waste Pomace Fermentation and Co-Ferment Product Chemistry. Fermentation, 11(3), 126. https://doi.org/10.3390/fermentation11030126







