Improving the Feasibility of 2G Ethanol Production from Lignocellulosic Hydrolysate Using Immobilized Recombinant Yeast: A Technical–Economic Analysis and Life Cycle Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview and Software
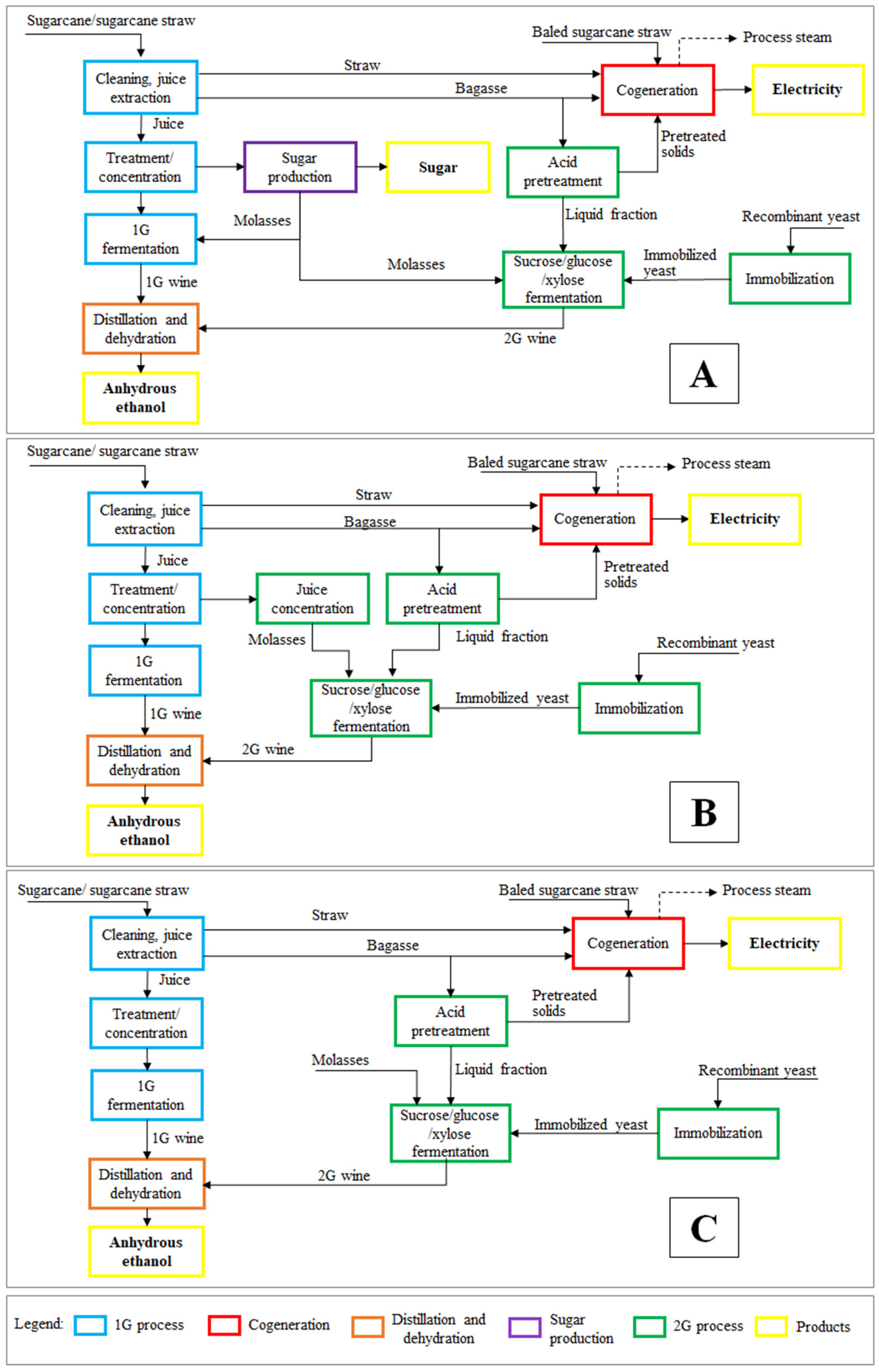
2.2. Process Description
2.2.1. The 1G Ethanol–Sugar Process (CsA Case Study)
2.2.2. The 1G Process (CsB Case Study)
2.2.3. The 1G2G Process of the Cs1
2.2.4. The 1G2G Process of the Cs2
2.2.5. The 1G2G Process of the Cs3
2.3. Economic Analysis
2.4. Life Cycle Assessment
3. Results and Discussion
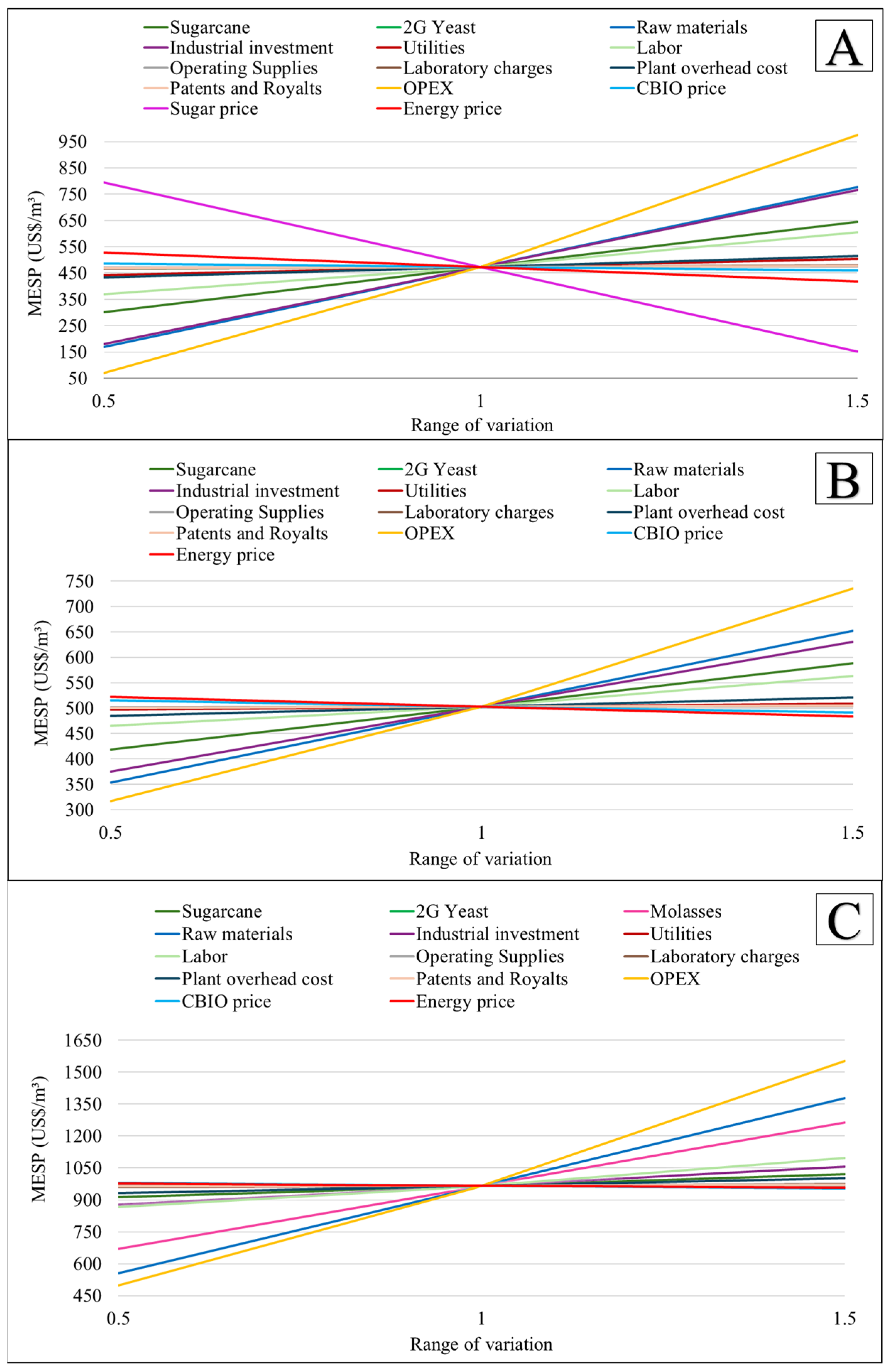
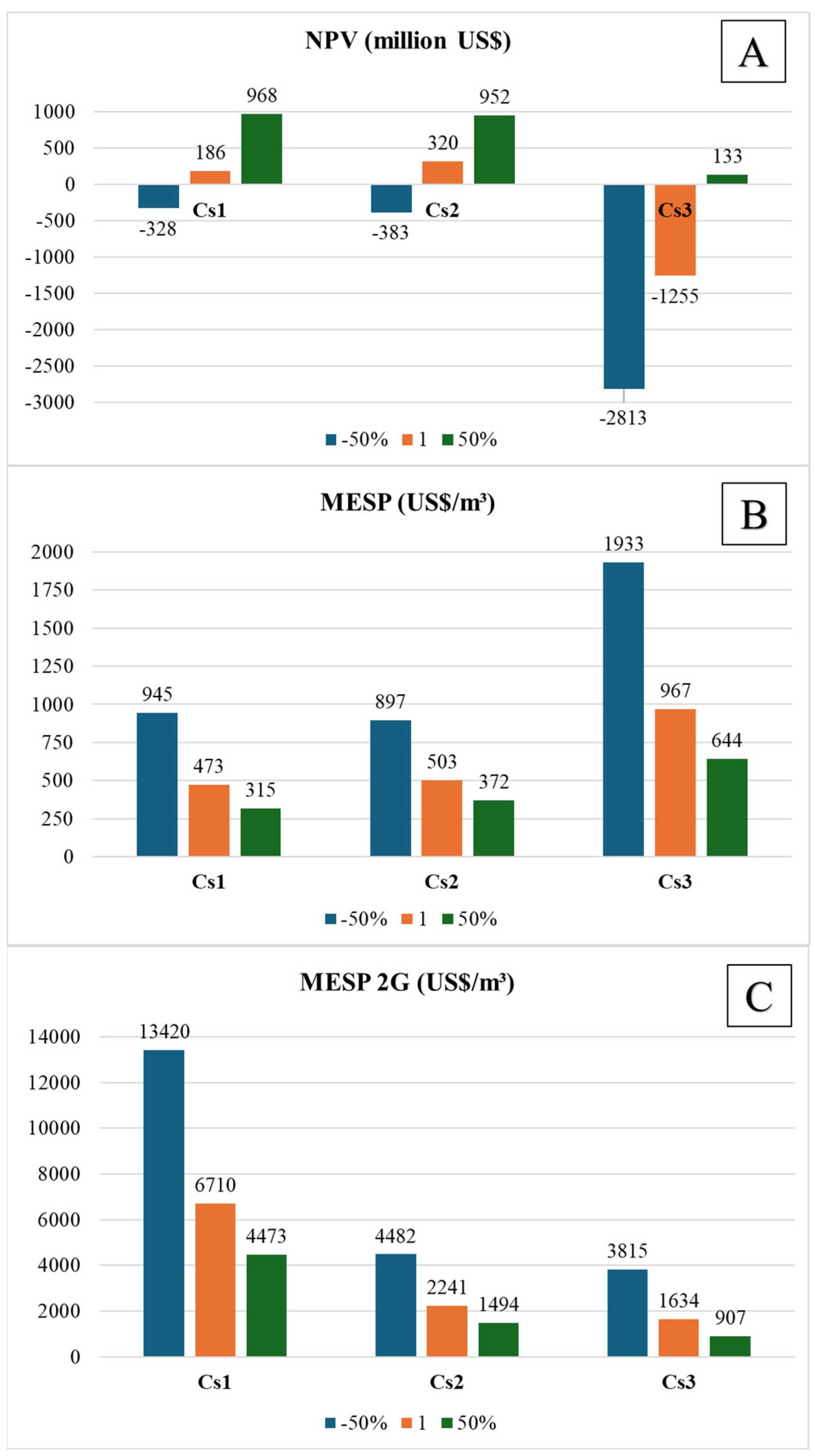
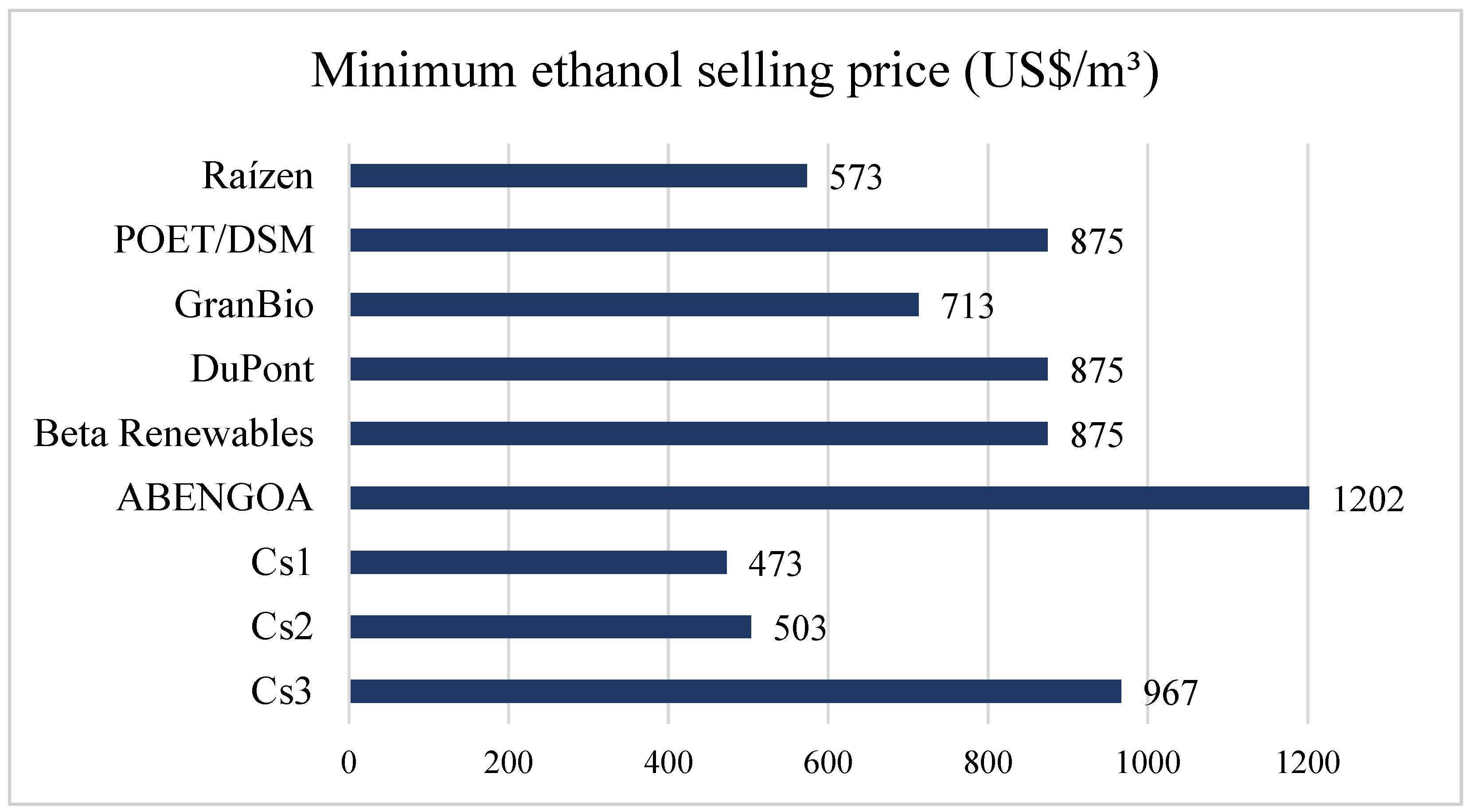
3.1. Economic Assessment
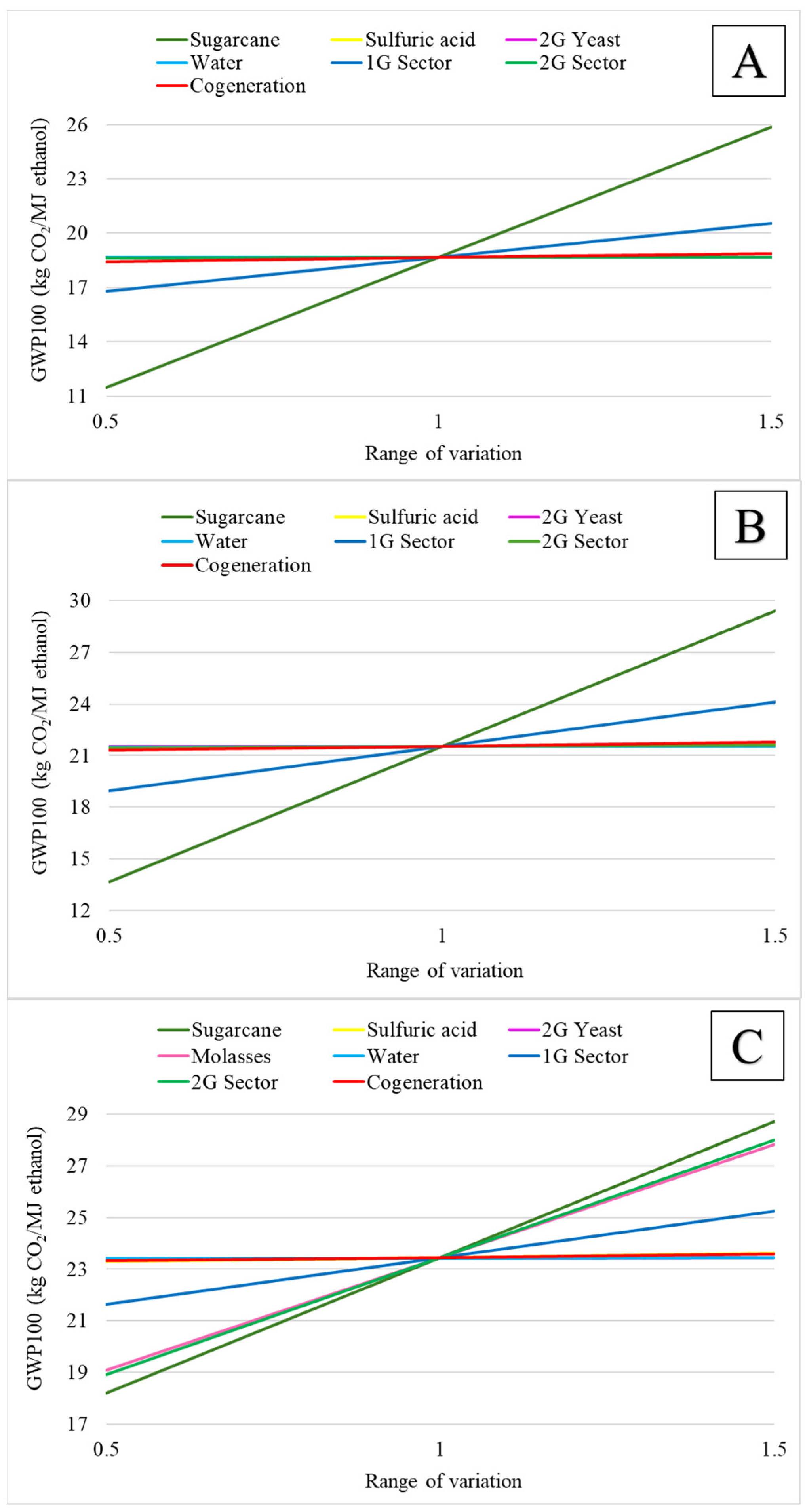
3.2. Environmental Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Summary for Policymakers. In Global Warming of 1.5 °C; Cambridge University Press: Cambridge, UK, 2022; pp. 1–24. [Google Scholar]
- Greenberg, M.R. Center for Geoeconomic Studies. Oil Price Volatility: Causes, Effects, and Policy Implications. 2016. Available online: https://www.cfr.org/report/oil-price-volatility-causes-effects-and-policy-implications (accessed on 1 February 2025).
- Min, H. Examining the Impact of Energy Price Volatility on Commodity Prices from Energy Supply Chain Perspectives. Energies 2022, 15, 7957. [Google Scholar] [CrossRef]
- Du, X.; Ouyang, T.; Shi, G.; Deng, L.; Gul, R. Role of Oil Price Volatility, Energy Efficiency, and Financial Stability on Sustainable Energy Production. Front. Environ. Sci. 2022, 10, 977733. [Google Scholar] [CrossRef]
- Lup, A.N.K.; Soni, V.; Keenan, B.; Son, J.; Taghartapeh, M.R.; Morato, M.M.; Poya, Y.; Montañés, R.M. Sustainable Energy Technologies for the Global South: Challenges and Solutions toward Achieving SDG 7. Environ. Sci. Adv. 2023, 2, 570–585. [Google Scholar] [CrossRef]
- Meramo, S.; Fantke, P.; Sukumara, S. Advances and Opportunities in Integrating Economic and Environmental Performance of Renewable Products. Biotechnol. Biofuels Bioprod. 2022, 15, 144. [Google Scholar] [CrossRef]
- Mignogna, D.; Szabó, M.; Ceci, P.; Avino, P. Biomass Energy and Biofuels: Perspective, Potentials, and Challenges in the Energy Transition. Sustainability 2024, 16, 7036. [Google Scholar] [CrossRef]
- Ou, L.; Dou, C.; Yu, J.H.; Kim, H.; Park, Y.C.; Park, S.; Kelley, S.; Lee, E.Y. Techno-Economic Analysis of Sugar Production from Lignocellulosic Biomass with Utilization of Hemicellulose and Lignin for High-Value Co-Products. Biofuels Bioprod. Biorefining 2020, 15, 404–415. [Google Scholar] [CrossRef]
- Souza, G.M.; Maciel Filho, R.; Nogueira, L.A.H.; Cantarella, H.; Rossetto, R.; Canabarro, I.; Silva-Ortiz, P.; Silva, J.F.L. Biofuels in Emerging Markets: Potential for Sustainable Production and Consumption; IEA Bioenergy: Rotorua, New Zealand, 2023. [Google Scholar]
- CONAB. Acompanhamento Da Safra Brasileira de Cana-de-Açúcar—Safra 2023/20224—4° Levantamento; CONAB: Brasília, Brazil, 2024.
- Vandenberghe, L.P.S.; Valladares-Diestra, K.K.; Bittencourt, G.A.; Zevallos Torres, L.A.; Vieira, S.; Karp, S.G.; Sydney, E.B.; de Carvalho, J.C.; Thomaz Soccol, V.; Soccol, C.R. Beyond Sugar and Ethanol: The Future of Sugarcane Biorefineries in Brazil. Renew. Sustain. Energy Rev. 2022, 167, 112721. [Google Scholar] [CrossRef]
- Guilherme, E.P.X.; Zanphorlin, L.M.; Sousa, A.S.; Miyamoto, R.Y.; Bruziquesi, C.G.O.; de Carvalho Mesquita, B.M.A.; Santos, S.H.S.; Aguiar-Oliveira, E.; Cota, J. Simultaneous Saccharification Isomerization and Co-Fermentation—SSICF: A New Process Concept for Second-Generation Ethanol Biorefineries Combining Immobilized Recombinant Enzymes and Non-GMO Saccharomyces. Renew. Energy 2022, 182, 274–284. [Google Scholar] [CrossRef]
- Iram, A.; Cekmecelioglu, D.; Demirci, A. Integrating 1G with 2G Bioethanol Production by Using Distillers’ Dried Grains with Solubles (DDGS) as the Feedstock for Lignocellulolytic Enzyme Production. Fermentation 2022, 8, 705. [Google Scholar] [CrossRef]
- Vasilakou, K.; Nimmegeers, P.; Thomassen, G.; Billen, P.; Van Passel, S. Assessing the Future of Second-Generation Bioethanol by 2030—A Techno-Economic Assessment Integrating Technology Learning Curves. Appl. Energy 2023, 344, 121263. [Google Scholar] [CrossRef]
- de Souza Queiroz, S.; Jofre, F.M.; dos Santos, H.A.; Hernández-Pérez, A.F.; Felipe, M.D.G.D.A. Xylitol and Ethanol Co-Production from Sugarcane Bagasse and Straw Hemicellulosic Hydrolysate Supplemented with Molasses. Biomass Convers. Biorefin. 2021, 13, 3143–3152. [Google Scholar] [CrossRef]
- Longati, A.A.; Lino, A.R.A.; Giordano, R.C.; Furlan, F.F.; Cruz, A.J.G. Biogas Production from Anaerobic Digestion of Vinasse in Sugarcane Biorefinery: A Techno-Economic and Environmental Analysis. Waste Biomass Valorization 2019, 11, 4573–4591. [Google Scholar] [CrossRef]
- Carpio, R.R.; de Carvalho Miyoshi, S.; Elias, A.M.; Furlan, F.F.; Giordano, R.C.; Secchi, A.R. Multi-Objective Optimization of a 1G-2G Biorefinery: A Tool towards Economic and Environmental Viability. J. Clean. Prod. 2021, 284, 125431. [Google Scholar] [CrossRef]
- da Silva Barreto, E.; da Fonseca, Y.A.; Adarme, O.F.H.; Silva, D.F.; Brandão, R.L.; Baêta, B.E.L.; Guimarães, V.M.; Gurgel, L.V.A. Optimization of 2G Ethanol Production from Sugarcane Bagasse: Upscaling of Soda Pretreatment with Redox Mediator Followed by Fed-Batch Enzymatic Hydrolysis and Co-Fermentation. Energy Convers. Manag. 2025, 323, 119225. [Google Scholar] [CrossRef]
- Fernandes Araújo, M.; Cardeal Volpi, M.P.; Mockaitis, G.; Morais Junior, M.A.; Carvalho da Costa, A.; Cândida Rabelo, S. Crude Glycerol Organosolv Pretreatment: Chain Integration for the Production of 2G Ethanol and Biogas. Fuel 2025, 379, 132984. [Google Scholar] [CrossRef]
- GranBio BioFlex. Available online: https://www.granbio.com.br/tecnologia/bioflex/ (accessed on 5 January 2024).
- Rosas-Vega, F.E.; Pozzan, R.; Martínez-Burgos, W.J.; Letti, L.A.J.; de Mattos, P.B.G.; Ramos-Neyra, L.C.; Spinillo Dudeque, G.; Amaro Bittencourt, G.; dos S. Costa, G.; Porto de Souza Vandenberghe, L.; et al. Enzymes Produced by the Genus Aspergillus Integrated into the Biofuels Industry Using Sustainable Raw Materials. Fermentation 2025, 11, 62. [Google Scholar] [CrossRef]
- GranBio. Individual and Consolidated Financial Statements; GranBio: São Paulo, Brazil, 2023. [Google Scholar]
- Raízen. The Journey. Available online: https://ri.raizen.com.br/e2g/ (accessed on 5 January 2024).
- Epbr. Começa a Operar Maior Usina de Etanol 2G Do Mundo. Available online: https://epbr.com.br/raizen-comeca-a-operar-maior-usina-de-etanol-2g-do-mundo/ (accessed on 5 January 2024).
- Raízen. Etanol de Segunda Geração: Potencial e Oportunidades. Available online: https://www.raizen.com.br/blog/etanol-de-segunda-geracao (accessed on 6 January 2024).
- Shibukawa, V.P.; Ramos, L.; Cruz-Santos, M.M.; Prado, C.A.; Jofre, F.M.; de Arruda, G.L.; da Silva, S.S.; Mussatto, S.I.; dos Santos, J.C. Impact of Product Diversification on the Economic Sustainability of Second-Generation Ethanol Biorefineries: A Critical Review. Energies 2023, 16, 6384. [Google Scholar] [CrossRef]
- Beig, B.; Riaz, M.; Raza Naqvi, S.; Hassan, M.; Zheng, Z.; Karimi, K.; Pugazhendhi, A.; Atabani, A.E.; Thuy Lan Chi, N. Current Challenges and Innovative Developments in Pretreatment of Lignocellulosic Residues for Biofuel Production: A Review. Fuel 2021, 287, 119670. [Google Scholar] [CrossRef]
- Hans, M.; Lugani, Y.; Chandel, A.K.; Rai, R.; Kumar, S. Production of First- and Second-Generation Ethanol for Use in Alcohol-Based Hand Sanitizers and Disinfectants in India. Biomass Convers. Biorefin. 2023, 13, 7423–7440. [Google Scholar] [CrossRef]
- Santana Júnior, D.B.; Kelbert, M.; De Araújo, P.H.H.; de Andrade, C.J. Application of Physical Pretreatment of Lignocellulosic Biomass to Produce Fermentable Sugars. 2025. Available online: https://sciety.org/articles/activity/10.20944/preprints202501.0170.v1 (accessed on 1 February 2025).
- Geng, B.; Jia, X.; Peng, X.; Han, Y. Biosynthesis of Value-Added Bioproducts from Hemicellulose of Biomass through Microbial Metabolic Engineering. Metab. Eng. Commun. 2022, 15, e00211. [Google Scholar] [CrossRef]
- Milessi, T.S.; Corradini, F.A.S.; Marçal, J.V.M.; Baldez, T.O.; Kopp, W.; Giordano, R.C.; Giordano, R.L.C. Xylooligosaccharides Production Chain in Sugarcane Biorefineries: From the Selection of Pretreatment Conditions to the Evaluation of Nutritional Properties. Ind. Crops Prod. 2021, 172, 114056. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Vergara, P.; Wojtusik, M.; Gutiérrez, S.; Santos, V.E.; Ladero, M.; Villar, J.C. Multi-Feedstock Lignocellulosic Biorefineries Based on Biological Processes: An Overview. Ind. Crops Prod. 2021, 172, 114062. [Google Scholar] [CrossRef]
- Perez, C.L.; Pereira, L.P.R.D.C.; Milessi, T.S.; Sandri, J.P.; Demeke, M.; Foulquié-Moreno, M.R.; Thevelein, J.M.; Zangirolami, T.C. Towards a Practical Industrial 2G Ethanol Production Process Based on Immobilized Recombinant S. Cerevisiae: Medium and Strain Selection for Robust Integrated Fixed-Bed Reactor Operation. Renew. Energy 2022, 185, 363–375. [Google Scholar] [CrossRef]
- Reis, V.R.; Bassi, A.P.G.; da Silva, J.C.G.; Ceccato-Antonini, S.R. Characteristics of Saccharomyces Cerevisiae Yeasts Exhibiting Rough Colonies and Pseudohyphal Morphology with Respect to Alcoholic Fermentation. Braz. J. Microbiol. 2013, 44, 1121–1131. [Google Scholar] [CrossRef]
- Rossi, L.M.; Gallo, J.M.R.; Mattoso, L.H.C.; Buckeridge, M.S.; Licence, P.; Allen, D.T. Ethanol from Sugarcane and the Brazilian Biomass-Based Energy and Chemicals Sector. ACS Sustain. Chem. Eng. 2021, 9, 4293–4295. [Google Scholar] [CrossRef]
- Milessi, T.S.; Perez, C.L.; Zangirolami, T.C.; Corradini, F.A.S.; Sandri, J.P.; Foulquié-Moreno, M.R.; Giordano, R.C.; Thevelein, J.M.; Giordano, R.L.C. Repeated Batches as a Strategy for High 2G Ethanol Production from Undetoxified Hemicellulose Hydrolysate Using Immobilized Cells of Recombinant Saccharomyces Cerevisiae in a Fixed-Bed Reactor. Biotechnol. Biofuels 2020, 13, 85. [Google Scholar] [CrossRef]
- Stojiljković, M.; Claes, A.; Deparis, Q.; Demeke, M.M.; Subotić, A.; Foulquié-Moreno, M.R.; Thevelein, J.M. Whole-Genome Transformation of Yeast Promotes Rare Host Mutations with a Single Causative SNP Enhancing Acetic Acid Tolerance. Mol. Cell Biol. 2022, 42, e00560-21. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.C.; Zangirolami, T.C.; Giordano, R.L.C.; Demeke, M.M.; Thevelein, J.M.; Milessi, T.S. Cell Immobilization Using Alginate-Based Beads as a Protective Technique against Stressful Conditions of Hydrolysates for 2G Ethanol Production. Polymers 2022, 14, 2400. [Google Scholar] [CrossRef]
- Demeke, M.M.; Echemendia, D.; Belo, E.; Foulquie-Moreno, M.R.; Thevelein, J.M. Enhancing Xylose-Fermentation Capacity of Engineered Saccharomyces Cerevisiae by Multistep Evolutionary Engineering in Inhibitor-Rich Lignocellulose Hydrolysate. FEMS Yeast Res. 2024, 24, foae013. [Google Scholar] [CrossRef]
- Cavalett, O.; Junqueira, T.L.; Dias, M.O.S.; Jesus, C.D.F.; Mantelatto, P.E.; Cunha, M.P.; Franco, H.C.J.; Cardoso, T.F.; Maciel Filho, R.; Rossell, C.E.V.; et al. Environmental and Economic Assessment of Sugarcane First Generation Biorefineries in Brazil. Clean. Technol. Environ. Policy 2012, 14, 399–410. [Google Scholar] [CrossRef]
- Demichelis, F.; Laghezza, M.; Chiappero, M.; Fiore, S. Technical, Economic and Environmental Assessement of Bioethanol Biorefinery from Waste Biomass. J. Clean. Prod. 2020, 277, 124111. [Google Scholar] [CrossRef]
- Macrelli, S.; Galbe, M.; Wallberg, O. Effects of Production and Market Factors on Ethanol Profitability for an Integrated First and Second Generation Ethanol Plant Using the Whole Sugarcane as Feedstock. Biotechnol. Biofuels 2014, 7, 26. [Google Scholar] [CrossRef]
- Pratto, B.; dos Santos-Rocha, M.S.R.; Longati, A.A.; de Sousa Júnior, R.; Cruz, A.J.G. Experimental Optimization and Techno-Economic Analysis of Bioethanol Production by Simultaneous Saccharification and Fermentation Process Using Sugarcane Straw. Bioresour. Technol. 2020, 297, 122494. [Google Scholar] [CrossRef]
- Junqueira, T.L.; Chagas, M.F.; Gouveia, V.L.R.; Rezende, M.C.A.F.; Watanabe, M.D.B.; Jesus, C.D.F.; Cavalett, O.; Milanez, A.Y.; Bonomi, A. Techno-Economic Analysis and Climate Change Impacts of Sugarcane Biorefineries Considering Different Time Horizons. Biotechnol. Biofuels 2017, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Losordo, Z.; McBride, J.; Van Rooyen, J.; Wenger, K.; Willies, D.; Froehlich, A.; Macedo, I.; Lynd, L. Cost Competitive Second-Generation Ethanol Production from Hemicellulose in a Brazilian Sugarcane Biorefinery. Biofuels Bioprod. Biorefining 2016, 10, 589–602. [Google Scholar] [CrossRef]
- Sampaio, I.L.M.; Cardoso, T.F.; Souza, N.R.D.; Watanabe, M.D.B.; Carvalho, D.J.; Bonomi, A.; Junqueira, T.L. Electricity Production from Sugarcane Straw Recovered Through Bale System: Assessment of Retrofit Projects. Bioenergy Res. 2019, 12, 865–877. [Google Scholar] [CrossRef]
- Gnansounou, E.; Vaskan, P.; Pachón, E.R. Comparative Techno-Economic Assessment and LCA of Selected Integrated Sugarcane-Based Biorefineries. Bioresour. Technol. 2015, 196, 364–375. [Google Scholar] [CrossRef]
- Moonsamy, T.A.; Mandegari, M.; Farzad, S.; Görgens, J.F. A New Insight into Integrated First and Second-Generation Bioethanol Production from Sugarcane. Ind. Crops Prod. 2022, 188, 115675. [Google Scholar] [CrossRef]
- Soares, R.D.P.; Secchi, A.R. European Symposium on Computer Aided Process Engineering-13, 36th European Symposium of the Working Party on Computer Aided Process Engineering. Comput. Aided Chem. Eng. 2003, 14, 947–952. [Google Scholar] [CrossRef]
- CONAB. Perfil Do Setor Do Açúcar e Do Etanol No Brasil; CONAB: Brasília, Brazil, 2019.
- Filho, G.Z.; Piccirilli, J.P. O Processo de Fabricação Do Açúcar e Álcool: Desde a Lavoura Da Cana Até o Produto Acabado, 1st ed.; Mira, G.A., Ed.; Editora Viena: Santa Cruz do Rio Prado, Brazil, 2012; ISBN 978-85-371-0265-7. [Google Scholar]
- Santos, F.; Borém, A.; Caldas, C. Cana-de-Açúcar: Bioenergia, Açúcar e Etanol: Tecnologias e Perpectivas, 2nd ed.; Almeida, A.M.D.G., Ed.; Editora Folha de Viçosa Ltda: Viçosa, Brazil, 2011; ISBN 978-85-60249-39-8. [Google Scholar]
- Elias, A.M.; Giordano, R.D.C.; Secchi, A.R.; Furlan, F.F. Integrating Pinch Analysis and Process Simulation within Equation-Oriented Simulators. Comput. Chem. Eng. 2019, 130, 106555. [Google Scholar] [CrossRef]
- Elias, A.M.; Longati, A.A.; Giordano, R.D.C.; Furlan, F.F. Retro-Techno-Economic-Environmental Analysis Improves the Operation Efficiency of 1G-2G Bioethanol and Bioelectricity Facilities. Appl. Energy 2021, 282, 116133. [Google Scholar] [CrossRef]
- Marcelino, P.R.F.; Peres, G.F.D.; Terán-hilares, R.; Pagnocca, F.C.; Rosa, C.A.; Lacerda, T.M.; Santos, J.C.; Silva, S.S. Biosurfactants Production by Yeasts Using Sugarcane Bagasse Hemicellulosic Hydrolysate as New Sustainable Alternative for Lignocellulosic Biore Fineries. Ind. Crops Prod. 2019, 129, 212–223. [Google Scholar] [CrossRef]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol; Technical Report; National Renewable Energy Lab (NREL): Golden, CO, USA, 2011; NREL/TP-51.
- Peters, M.S.; Timmerhaus, K.D.; West, R.E. Plant Design and Economics for Chemical Engineers, 5th ed.; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Davis, R.; Grundl, N.; Tao, L.; Biddy, M.J.; Tan, E.C.D.; Beckham, G.T.; Humbird, D.; Thompson, D.N.; Roni, M.S. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbon Fuels and Coproducts: 2018 Biochemical Design Case Update: Biochemical Deconstruction and Conversion of Biomass to Fuels and Products via Integrated Biorefinery Pathways; National Renewable Energy Lab (NREL): Golden, CO, USA, 2018.
- Aden, A.; Ruth, M.; Ibsen, K.; Jechura, J.; Neeves, K.; Sheehan, J.; Wallace, B.; Montague, L.; Slayton, A.; Lukas, J. Lignocellulosic Biomass to Ethanol Process Design and Economics Utilizing Co-Current Dilute Acid Prehydrolysis and Enzymatic Hydrolysis for Corn Stover; National Renewable Energy Lab (NREL): Golden, CO, USA, 2002.
- Davis, R.; Tao, L.; Scarlata, C.; Tan, E.C.D.; Ross, J.; Lukas, J.; Sexton, D. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbons: Dilute-Acid and Enzymatic Deconstruction of Biomass to Sugars and Catalytic Conversion of Sugars to Hydrocarbons; National Renewable Energy Lab (NREL): Golden, CO, USA, 2015.
- B3. Dados Por Ativo. Available online: http://estatisticas.cetip.com.br/astec/series_v05/paginas/lum_web_v05_series_introducao.asp?str_Modulo=Ativo&int_Idioma=1&int_Titulo=6&int_NivelBD=2/ (accessed on 31 January 2024).
- CCEE. Painel de Preços. Available online: https://www.ccee.org.br/web/guest/precos/painel-precos (accessed on 31 January 2024).
- CEPEA/ESALQ. Consultas ao Banco de Dados do Site. Available online: https://www.cepea.esalq.usp.br/br/consultas-ao-banco-de-dados-do-site.aspx (accessed on 31 January 2024).
- Macrelli, S.; Mogensen, J.; Zacchi, G. Techno-Economic Evaluation of 2 Nd Generation Bioethanol Production from Sugar Cane Bagasse and Leaves Integrated with the Sugar-Based Ethanol Process. Biotechnol. Biofuels 2012, 5, 22. [Google Scholar] [CrossRef]
- Julio, A.A.V.; Milessi, T.S.; Maya, D.M.Y.; Lora, E.S.; Palacio, J.C.E. Assessment of the Sustainability and Economic Potential of Hydrotreated Vegetable Oils to Complement Diesel and Biodiesel Blends in Brazil. Biofuels Bioprod. Biorefining 2023, 17, 312–323. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2023: Synthesis Report; Arias, P., Bustamante, M., Elgizouli, I., Flato, G., Howden, M., Méndez-Vallejo, C., Pereira, J.J., Pichs-Madruga, R., Rose, S.K., Saheb, Y., et al., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- EPE. RenovaBio: Biocombustíveis 2030—Nota Técnica: Papel. Dos. Biocombustíveis Na Matriz; EPE: Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Matsuura, M.I.D.S.F.; Seabra, J.E.A.; Ferreira Chagas, M.; Scachetti, M.T.; Morandi, M.A.B.; Moreira, M.M.R.; Novaes, R.M.L.; Ramos, N.P.; Cavalett, O.; Bonomi, A. Renovacalc: A calculadora do programa renovabio. In Proceedings of the VI Congresso Brasileiro Sobre Gestão do Ciclo de Vida; GCV: Brasília, Brazil, 2018; pp. 1–6. [Google Scholar]
- Furlan, F.F.; Filho, R.T.; Pinto, F.H.; Bb Costa, C.; Cruz, A.J.; Giordano, R.L.; Giordano, R.C. Bioelectricity versus Bioethanol from Sugarcane Bagasse: Is It Worth Being Flexible? Springer: Berlin/Heidelberg, Germany, 2013; Volume 6. [Google Scholar]
- Dias, M.O.S.; Junqueira, T.L.; Cavalett, O.; Cunha, M.P.; Jesus, C.D.F.; Mantelatto, P.E.; Rossell, C.E.V.; Maciel Filho, R.; Bonomi, A. Cogeneration in Integrated First and Second Generation Ethanol from Sugarcane. Chem. Eng. Res. Des. 2013, 91, 1411–1417. [Google Scholar] [CrossRef]
- Vand der Hoeven, D. Cellulosic Ethanol: Feedstock Costs Predominant, Sugar Cane Cheapest. Available online: https://www.biobasedpress.eu/2016/02/cellulosic-ethanol-feedstock-costs-predominant-sugar-cane-cheapest/ (accessed on 2 July 2024).
- Turboden Future Cogeneration: Energy Effiency and Sustainability. Available online: https://www.turboden.com/company/media/press/press-reviews/4581/future-of-cogeneration-energy-efficiency-and-sustainability#:~:text=Cogeneration%20plays%20a%20fundamental%20role,building%20heating%20or%20industrial%20processes (accessed on 1 February 2025).
- Brasil. Resolução N° 758 de 23 de Novembro de 2018; Ministério de Minas e Energia/Agência Nacional do Petróleo Gás Natural e Biocombustíveis: Brasília, Brazil, 2018.
- Hans, M.; Pellegrini, V.O.A.; Filgueiras, J.G.; de Azevedo, E.R.; Guimaraes, F.E.C.; Chandel, A.K.; Polikarpov, I.; Chadha, B.S.; Kumar, S. Optimization of Dilute Acid Pretreatment for Enhanced Release of Fermentable Sugars from Sugarcane Bagasse and Validation by Biophysical Characterization. Bioenergy Res. 2023, 16, 416–434. [Google Scholar] [CrossRef]
- Alves, L.A.; Felipe, M.G.A.; Silva, J.B.A.E.; Silva, S.S.; Prata, A.M.R. Pretreatment of Sugarcane Bagasse Hemicellulose Hydrolysate for Xylitol Production ByCandida Guilliermondii. Appl. Biochem. Biotechnol. 1998, 70–72, 89–98. [Google Scholar] [CrossRef]
- Carvalho, R.S.; Cruz, I.A.; Américo-Pinheiro, J.H.P.; Soriano, R.N.; de Souza, R.L.; Bilal, M.; Iqbal, H.M.N.; Bharagava, R.N.; Romanholo Ferreira, L.F. Interaction between Saccharomyces Cerevisiae and Lactobacillus Fermentum during Co-Culture Fermentation. Biocatal. Agric. Biotechnol. 2020, 29, 101756. [Google Scholar] [CrossRef]
- ComexStat. Exportação e Importação Geral. Available online: http://comexstat.mdic.gov.br/pt/geral (accessed on 9 October 2022).
- Claes, A.; Deparis, Q.; Foulquié-Moreno, M.R.; Thevelein, J.M. Simultaneous Secretion of Seven Lignocellulolytic Enzymes by an Industrial Second-Generation Yeast Strain Enables Efficient Ethanol Production from Multiple Polymeric Substrates. Metab. Eng. 2020, 59, 131–141. [Google Scholar] [CrossRef]
- Antunes, F.A.F.; Santos, J.C.; Chandel, A.K.; Milessi, T.S.S.; Peres, G.F.D.; da Silva, S.S. Hemicellulosic Ethanol Production by Immobilized Wild Brazilian Yeast Scheffersomyces shehatae UFMG-HM 52.2: Effects of Cell Concentration and Stirring Rate. Curr. Microbiol. 2015, 72, 133–138. [Google Scholar] [CrossRef]
- Bonomi, A.; Mariano, A.P.; de Jesus, C.D.F.; Cunha, M.P.; Mantelatto, P.E. The Virtual Sugarcane Biorefinery (VSB)—2011 Report; Issuu: Campinas, Brazil, 2012. [Google Scholar]
- Chandel, A.K.; Silveira, M.H.L. Advances in Sugarcane Biorefinery; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Chieppe Júnior, J.B. Tecnologia e Fabricação do Álcool; Instituto Federal de Educação, Ciência e Tecnologia: Inhumas, Brazil, 2012. [Google Scholar]
- Dantas, G.d.A. Alternativas de Investimento do Setor Sucroenergético Brasileiro para Aproveitamento de Bagaço e de Palha (Tese (Doutorado em Planejamento Energético)); Universidade Federal do Rio de Janeiro: Rio de Janeiro, Brazil, 2013. [Google Scholar]
- Elias Andrew, M.; Longati, A.A.; Ellamla, H.R.; Furlan, F.F.; Ribeiro, M.P.A.; Marcelino, P.R.F.; Dos Santos, J.C.; Da Silva, S.S.; Giordano, R.C. Techno-Economic-Environmental Analysis of Sophorolipid Biosurfactant Production from Sugarcane Bagasse. Ind. Eng. Chem. Res. 2021, 60, 9833–9850. [Google Scholar] [CrossRef]
- Gilpin, G.S. The Environmental Life Cycle Assessment of Selected 1 st Through 3 rd Generation Biofuels Within the Context of Sustainable Development (Tese (Doutorado em Filosofia)); Norwegian University of Life Sciences, Faculty of Environmental Science and Technology: Ås, Norway, 2016. [Google Scholar]
- Harding, K. A Generic Approach to Environmental Assessment of Microbial Bioprocesses through Life Cycle Assessment (LCA) (Tese (Doutorado em Filosofia)); University of Cape Town: Cape Town, South Africa, 2008. [Google Scholar]
- Oliveira, C.M.; Pavão, L.V.; Ravagnani, M.A.S.S.; Cruz, A.J.G.; Costa, C.B.B. Process integration of a multiperiod sugarcane biorefinery. Appl. Energy 2018, 213, 520–539. [Google Scholar] [CrossRef]
- Parra-Ramírez, D.; Martinez, A.; Cardona, C.A. Technical and economic potential evaluation of the strain Escherichia coli MS04 in the ethanol production from glucose and xylose. Biochem. Eng. J. 2018, 140, 123–129. [Google Scholar] [CrossRef]
- Pina, E.A.; Palacios-Bereche, R.; Chavez-Rodriguez, M.F.; Ensinas, A.V.; Modesto, M.; Nebra, S.A. Reduction of process steam demand and water-usage through heat integration in sugar and ethanol production from sugarcane–Evaluation of different plant configurations. Energy 2017, 138, 1263–1280. [Google Scholar] [CrossRef]
- Walter, A.; Dolzan, P.; U da Silva, C.R.; Piacente, F. A Sustainability Analysis of the Brazilian Ethanol Sustainable Development Potentials and Pathways for Biobased Economy Options: An Integrated Approach on Land Use, Energy System and Economy and Environment; View Project: Campinas, Brazil, 2008. [Google Scholar]


| Main Process Results | CsA | CsB | Cs1 | Cs2 | Cs3 |
|---|---|---|---|---|---|
| Specific anhydrous ethanol production (L/TC) | 47.46 | 89.81 | 49.01 | 99.11 | 161.26 |
| Anhydrous ethanol production (m3/h) | 39.55 | 74.84 | 40.85 | 82.59 | 134.38 |
| 1G ethanol (%) | 100 | 100 | 72 | 54 | 48.6 |
| 2G ethanol (%) | - | - | 28 | 46 | 51.4 |
| Electricity (MW) | 154.82 | 156.37 | 145.84 | 104.07 | 88.63 |
| Vinasse generated (m3/h) | 343.27 | 681.67 | 431.90 | 803.38 | 1421.54 |
| Specific vinasse production (m3vinasse/m3ethanol) | 8.68 | 9.11 | 10.58 | 9.73 | 10.58 |
| Sugar production (t/h) | 58.15 | - | 60.05 | - | - |
| Specific sugar production (kg/TC) | 69.78 | - | 72.06 | - | - |
| Molasses production (t/h) | 38.75 | - | 40.02 | 50.58 | - |
| Specific molasses production (kg/TC) | 46.50 | - | 48.02 | 60.70 | - |
| Steam consumption | |||||
| 6 bar (t/h) | 2.93 | 5.50 | 15.71 | 26.18 | 45.88 |
| 2.5 bar (t/h) | 263.95 | 247.91 | 300.25 | 701.48 | 601.22 |
| 0.1 bar (t/h) * | 453.71 | 466.46 | 397.16 | - | - |
| Total | 720.59 | 719.86 | 713.10 | 727.66 | 647.10 |
| Specific steam consumption | |||||
| 6 bar (kg/TC) | 3.51 | 6.59 | 18.85 | 31.41 | 55.06 |
| 2.5 bar (kg/TC) | 316.74 | 297.49 | 360.30 | 841.78 | 721.46 |
| 0.1 bar (kg/TC) | 544.88 | 559.75 | 476.59 | - | - |
| Boil fuel (t/h) | |||||
| Bagasse (50% humidity) | 240.90 | 240.90 | 215.67 | 170.26 | 38.90 |
| Straw (15% humidity) | 71.22 | 71.22 | 71.22 | 71.22 | 71.22 |
| 2G solids (50% humidity) | - | - | 17.05 | 47.90 | 136.96 |
| Main Process Results | CsA | CsB | Cs1 | Cs2 | Cs3 | |
|---|---|---|---|---|---|---|
| Industrial investment | ||||||
| Total (USD × 106) | 184.74 | 153.38 | 189.60 | 167.19 | 187.65 | |
| Extraction | 2.03 | 2.03 | 2.03 | 2.03 | 2.03 | |
| Treatment | 11.96 | 13.87 | 11.96 | 13.87 | 13.87 | |
| Concentration/fermentation | 23.86 | 39.35 | 22.70 | 26.72 | 39.35 | |
| Crystallization | 50.69 | - | 51.68 | - | - | |
| Cogeneration | 86.17 | 86.13 | 85.00 | 83.65 | 77.76 | |
| Distillation | 7.72 | 9.52 | 8.19 | 9.97 | 11.83 | |
| Pretreatment | - | - | 0.43 | 0.79 | 1.74 | |
| 2G fermentation | - | - | 4.55 | 26.43 | 36.43 | |
| Yeast immobilization | - | - | 0.44 | 0.67 | 0.70 | |
| Other costs | 2.31 | 2.50 | 2.62 | 3.06 | 3.94 | |
| Operational costs | ||||||
| Total (USD × 106) | 145.30 | 136.53 | 147.81 | 138.16 | 565.48 | |
| Raw materials | 96.97 | 92.27 | 99.09 | 98.73 | 442.25 | |
| Sugarcane | 59.60 | 59.60 | 59.60 | 59.60 | 59.60 | |
| 1G sector | 40.90 | 36.20 | 37.22 | 36.21 | 58.92 | |
| 2G sector | - | - | 0.51 | 1.44 | 4.08 | |
| Molasses | - | - | - | - | 318.09 | |
| 2G Yeast | - | - | 1.74 | 1.65 | 1.71 | |
| Utilities | 10.39 | 9.77 | 10.03 | 3.83 | 5.97 | |
| Labor | 20.79 | 19.05 | 21.19 | 19.57 | 68.12 | |
| Operating supplies | 0.59 | 0.49 | 0.60 | 0.53 | 0.60 | |
| Laboratory charges | 1.89 | 1.73 | 1.93 | 1.78 | 6.19 | |
| Plant overhead cost | 13.35 | 11.98 | 13.63 | 12.46 | 37.06 | |
| Patents and royalties | 1.32 | 1.25 | 1.34 | 1.26 | 5.28 | |
| Sales | ||||||
| Total (USD × 106) | 252.64 | 226.01 | 257.94 | 235.45 | 683.92 | |
| Ethanol | 95.01 | 192.78 | 97.35 | 209.44 | 654.61 | |
| Electricity | 23.96 | 24.23 | 22.60 | 16.13 | 13.74 | |
| Sugar | 128.62 | - | 132.81 | - | - | |
| CBIO | 4.99 | 9.00 | 5.17 | 9.88 | 15.58 | |
| Economic performance | ||||||
| Net present value (USD × 106) | 171.96 | 276.23 | 186.13 | 320.00 | −1254.91 | |
| Minimum ethanol-selling price (MESP) | 476.71 | 511.08 | 472.92 | 503.15 | 966.53 | |
| Minimum 2G ethanol-selling price (MESP 2G) | - | - | 6710.04 | 2240.76 | 1634.20 | |
| Energy Allocation Fraction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case Study | Ethanol | Electricity | Sugar | |||||||
| CsA | 0.37 | 0.23 | 0.40 | |||||||
| CsB | 0.74 | 0.26 | - | |||||||
| Cs1 | 0.38 | 0.21 | 0.41 | |||||||
| Cs2 | 0.83 | 0.17 | - | |||||||
| Cs3 | 0.90 | 0.10 | - | |||||||
| Environmental Indicators | ||||||||||
| Case Study | GWP a | AD b | ODP c | HT d | FWAET e | MAET f | TET g | PO h | AC i | EU j |
| CsA | 18.22 | 5.22 | 1.90 | 1.49 | 6.09 | 12.34 | 1.07 | 4.53 | 4.35 | 17.83 |
| CsB | 21.30 | 5.60 | 2.26 | 1.72 | 8.08 | 16.91 | 1.21 | 5.48 | 4.79 | 19.68 |
| Cs1 | 18.65 | 5.17 | 1.91 | 1.48 | 6.11 | 12.50 | 1.06 | 4.61 | 4.32 | 17.61 |
| Cs2 | 21.55 | 5.65 | 2.39 | 1.77 | 8.44 | 18.04 | 1.20 | 5.94 | 4.91 | 19.83 |
| Cs3 | 23.44 | 3.78 | 1.61 | 1.14 | 5.63 | 11.71 | 0.77 | 8.27 | 4.03 | 16.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, L.P.; Longati, A.A.; Elias, A.M.; Perez, C.L.; Pereira, L.P.R.d.C.; Zangirolami, T.C.; Furlan, F.F.; Giordano, R.d.C.; Milessi, T.S. Improving the Feasibility of 2G Ethanol Production from Lignocellulosic Hydrolysate Using Immobilized Recombinant Yeast: A Technical–Economic Analysis and Life Cycle Assessment. Fermentation 2025, 11, 116. https://doi.org/10.3390/fermentation11030116
Pinheiro LP, Longati AA, Elias AM, Perez CL, Pereira LPRdC, Zangirolami TC, Furlan FF, Giordano RdC, Milessi TS. Improving the Feasibility of 2G Ethanol Production from Lignocellulosic Hydrolysate Using Immobilized Recombinant Yeast: A Technical–Economic Analysis and Life Cycle Assessment. Fermentation. 2025; 11(3):116. https://doi.org/10.3390/fermentation11030116
Chicago/Turabian StylePinheiro, Luísa Pereira, Andreza Aparecida Longati, Andrew Milli Elias, Caroline Lopes Perez, Laís Portugal Rios da Costa Pereira, Teresa Cristina Zangirolami, Felipe Fernando Furlan, Roberto de Campos Giordano, and Thais Suzane Milessi. 2025. "Improving the Feasibility of 2G Ethanol Production from Lignocellulosic Hydrolysate Using Immobilized Recombinant Yeast: A Technical–Economic Analysis and Life Cycle Assessment" Fermentation 11, no. 3: 116. https://doi.org/10.3390/fermentation11030116
APA StylePinheiro, L. P., Longati, A. A., Elias, A. M., Perez, C. L., Pereira, L. P. R. d. C., Zangirolami, T. C., Furlan, F. F., Giordano, R. d. C., & Milessi, T. S. (2025). Improving the Feasibility of 2G Ethanol Production from Lignocellulosic Hydrolysate Using Immobilized Recombinant Yeast: A Technical–Economic Analysis and Life Cycle Assessment. Fermentation, 11(3), 116. https://doi.org/10.3390/fermentation11030116






