Abstract
Nitrogen source is a necessary nutrient factor in the process of mycelial growth and metabolite synthesis. To improve the yield of ergosterol, in the previous study, we used transcriptome technology to explore the difference in gene expression of Cordyceps cicadae by CO(NH2)2 (urea) in the process of synthesizing ergosterol. In the present study, CO(NH2)2 was used to examine its effects on cell membrane permeability and metabolic flow in the process of C. cicadae fermentation and ergosterol synthesis. Metabonomic results showed that CO(NH2)2 supplementation caused significant changes in five aspects: (1) CO(NH2)2 notably increased biomass growth and extracellular ergosterol, and decreased intracellular ergosterol concentration; (2) CO(NH2)2 boosted the level of inositol 1,4-bisphosphate, which implied the acceleration of cell membrane decomposition, the weakening of integrity, and the increase in permeability and change in metabolic regionalization; (3) CO(NH2)2 changed the metabolic fluxes and metabolic speed, including increasing the levels of amino acids, vitamins, hormones, and nucleotides, which exhibited an elevated biomass growth, promoting the synthesis of intracellular flavonoids, alkaloids, and terpenes, facilitating extracellular ergosterol synthesis and decreasing the degradation of ergosterol; (4) in the fermentation anaphase, CO(NH2)2 caused the inhibition of 6-phosphogluconate dehydrogenase and α-ketoglutarate dehydrogenase activities, decreased NADPH, NADH, and ATP synthesis, and finally inhibited biomass growth and ergosterol synthesis. Collectively, metabonomics was a valuable strategy to study the regulatory effects of medium composition and incubation conditions on ergosterol synthesis by C. cicadae.
1. Introduction
Cordyceps cicadae Shing (Chanhua) belongs to the genus Cordyceps (family Clavicipitaceae, Ascomycotina). C. cicadae, a rare traditional Chinese medicine, has been applied to the treatment of childhood palpitation, epilepsy, convulsions, and several types of eye diseases [1]. C. cicadae is rich in sphingolipids [2], polysaccharides [3], nucleosides [4], mannitol [5], ergosterol [6], and other active substances. Modern medical research has demonstrated that C. cicada possesses more potent immunoregulatory [7] and renal functions [8], as well as anti-diabetic [9], anti-bacterial [10], and anti-tumorigenic properties [11]. Ergosterol, one of the chemical components from mycelial cells, is the predominant sterol found in most fungi [12]. It can be divided into free ergosterol and esterified ergosterol [13]. Ergosterol plays an important role in ensuring cell viability, membrane fluidity, membrane-binding enzyme activity, membrane integrity, and cell material transport. Meanwhile, ergosterol is a precursor of fat-soluble vitamin D2 (VD2). When exposed to ultraviolet light, ergosterol is converted into VD2. VD2 is an important pharmaceutical and chemical ingredient used to prevent and treat rickets in children, osteomalacia in adults, and osteoporosis in the elderly [14]. In addition, ergosterol has anti-cancer [15], anti-tyrosinase [16], and anti-inflammatory [17] activities, together with applications in new drug formulations with antibiotics [18]. Yajaira et al. found that ergosterol exerts synergistic effects on cell proliferation [19]. Slominski et al. confirmed that ergosterol metabolites in vivo can inhibit the proliferation of skin cancer cells [20].
Due to the limitations of natural sources, liquid fermentation has become an alternative approach for obtaining active components of medical fungi. In the liquid fermentation process, the nitrogen source is not only one of main the nutrient components but also a key substance regulating the metabolism of mycelium. The nitrogen source plays an important role in controlling the growth process of the S. cerevisiae and T. delbrueckii yeasts. The optimum nitrogen sources have three traits: a relatively shorter lag phase time, a higher maximum growth rate, and a higher maximum dissolved oxygen [21] (Su et al., 2020). In a previous study, we proved that CO(NH2)2 (urea) is the most suitable nitrogen source for ergosterol synthesis by C. cicadae [22]. Metabonomics was applied to find that the expressions of many genes changed after the C. cicadae were cultured in the presence of CO(NH2)2, and the alteration of many genes played an important role in promoting the synthesis of ergosterol. However, metabonomics did not fully reveal the mechanism of ergosterol synthesis. Due to the lack of knowledge on the metabolic mechanism of ergosterol synthesized by C. cicadae, although we optimized the fermentation conditions of ergosterol synthesis, the yield of ergosterol was still very low.
Metabonomics allows for simultaneous qualitative analysis and quantitative measurement of all low-molecular-weight metabolites in an organism or cell in response to internal and external stimuli in an integrated biological system [23]. Metabonomics has shown broad application prospects in identifying biomarkers, diagnosing and predicting diseases, and elucidating the dynamic responses of organisms to disease or environmental changes [24,25,26,27,28,29,30]. In recent years, ultra-high-performance liquid chromatography–electrospray ionization–tandem mass spectrometry (UHPLC-ESI-MS/MS) technology has become a powerful tool for the separation and identification of active ingredients. UHPLC-ESI-MS/MS technology has a high chromatographic resolution, high sensitivity, high selectivity, and rapid separation, and can measure the accurate mass numbers of parent and daughter ions. Therefore, UHPLC-ESI-MS/MS has been widely used in metabonomics to investigate subtle metabolite alterations in complex mixtures [31,32,33,34]. Meanwhile, multivariate statistical methods such as principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) are widely used in different types of metabolite identification.
In order to further increase the ergosterol field in the fermentation process of C. cicadae, it is particularly important to reveal the metabolic regulation mechanism of ergosterol synthesized by C. cicadae. According to the general knowledge of microbial metabolic regulation, we speculated that the supplementation of CO(NH2)2 increased ergosterol synthesis from four levels, including cell membrane permeability, metabolic regionalization, metabolic fluxes direction, and metabolic rate. Thus, the aim the present study is to use UHPLC-ESI-MS/MS technology with PCA and OPLS-DA and an automated processing software platform to systematically screen the key biomarkers in the synthesis process of ergosterol to test our hypothesis and to lay foundations to further control fermentation conditions and increase ergosterol yield in the fermentation process of C. cicadae.
2. Materials and Methods
2.1. Reagents
Methanol and formic acid (FA) were reagent-grade, Sigma-Aldrich, and purchased from Sinopharm Chemical Reagent Co, Ltd. (Zhenjiang, China). Meal peptone, a biochemical reagent, was purchased from Sinopharm Chemical Reagent Co, Ltd. The water used for UHPLC-ESI-MS/MS analysis was re-distilled water. Other chemicals and reagents used were analysis-grade and purchased from Sinopharm Chemical Reagent Co, Ltd.
2.2. Microorganism and Culture Conditions
C. cicadae (NO. Bio-33088) were gifted by the Key Laboratory of Food Science of Jiangnan University and maintained on a potato dextrose agar (PDA) slant. The slants were incubated at 25 °C for 5 days and then stored at 4 °C. The seed medium was composed of (g/L) 20 wheat bran, 20 glucose, 10 corn flour, and 4 fish meal peptone. The control fermentation medium (CFM) was composed of (g/L) 36 wheat bran, 46 xylose, 28 fructose, 25 glycerol, 1.7 ZnSO4, and 1.8 KH2PO4. CFM+CO(NH2)2 was composed of CFM and 6.6 g/L CO(NH2)2.
2.3. The Preparation of C. cicadae Samples
The biomass of C. cicadae was prepared according to a method previously described, with minor modifications [22]. Briefly, the slant C. cicadae was aseptically inoculated into 250 mL Erlenmeyer flasks containing 100 mL seed culture solution. These flasks were incubated on a rotary shaker at 25 °C and 150 rpm for 72 h. The seed liquid was aseptically transferred into 250 mL Erlenmeyer flasks containing 100 mL CFM or CFM+CO(NH2)2 at an inoculation ratio of 10%. Subsequently, these flasks were incubated on a rotary shaker at 25 °C and 150 rpm for 94 h.
After incubation, the broth was centrifuged at 10,000× g for 10 min. One part of the biomass (sediment) was washed with sterile water five times and stored at −80 °C for metabonomic analysis. The other part of the biomass was used to determine the intracellular ergosterol content. The supernatant was used to determine the content of extracellular ergosterol.
The biomass (100 mg) individually ground in liquid nitrogen was mixed with 2 mL 80% methanol containing 0.1% formic acid by vortexing, and the mixture was allowed to stand in the ice bath for 5 min. The samples were centrifuged at 15,000× g, 4 °C for 20 min. Next, 0.2 mL supernatant was diluted by the water of LC-MS grade to a solution containing 53% methanol. The samples were subsequently transferred to fresh Eppendorf tubes and then centrifuged at 15,000× g, 4 °C for 20 min. Finally, the supernatant was injected into the LC-MS/MS system.
The biomass cultured in the CFM or CFM+CO(NH2)2 was used as the control blank (CB) or the sample of CO(NH2)2 response (NS), respectively.
2.4. The Qualitative and Quantitative Determination of Ergosterol
Briefly, 25 mL supernatant was dried to a constant weight at 70 °C. The dried material was extracted by 25 mL 60% ethanol in a water bath at 75 °C for 4 h, followed by centrifugation at 12,000× g, 4 °C for 10 min. The supernatant was collected to determine the content of intracellular ergosterol.
The contents of extracellular and intracellular ergosterol were determined according to the vanillin method. Briefly, 1 mL of sample solution was mixed with 0.2 mL of 5% vanillin–glacial acetic acid solution and 0.8 mL of perchloric acid. The mixture was heated at 60 °C for 15 min, cooled to room temperature, and then reacted with 3 mL of glacial acetic acid. The absorbance was determined at a wavelength of 570 nm. Ergosterol was used as the standard.
2.5. UHPLC-ESI-MS/MS Analysis
The analytical UHPLC-ESI-MS/MS consisted of a Vanquish UHPLC system (Thermo Fisher, Waltham, MA, USA) coupled with an Orbitrap Q Exactive HF-X mass spectrometer (Thermo Fisher, Bremen, Germany) as the detector and equipped with an electrospray source in both positive and negative ion modes. To acquire better signals of metabolites, the MS method was developed with a spray voltage of 3.2 kV, capillary temperature of 320 °C, sheath gas flow rate of 40 arb, and aux gas flow rate of 10 arb. Samples were separated by a Hypesil Gold column (100 × 2.1 mm, 1.9 μm) using a 17 min linear gradient at a flow rate of 0.2 mL/min. The developing agent for the positive polarity mode was composed of mobile phase A (0.1% FA in water) and mobile phase B (methanol). The developing agent for the negative polarity mode was composed of mobile phase A (5 mM ammonium acetate, pH 9.0) and mobile phase B (methanol). The developing agent gradient was set as follows: 2% B, 1.5 min; 2–100% B, 12.0 min; 100% B, 14.0 min; 100–2% B, 14.1 min; 2% B, 17 min.
2.6. Data Processing
The raw data files from UHPLC-ESI-MS/MS were processed by the Compound Discoverer 3.1 (CD3.1, Thermo Fisher) to perform peak alignment, peak picking, and quantitation for each metabolite. Some key parameters were set as follows: retention time tolerance, 0.2 min; actual mass tolerance, 5 ppm; signal intensity tolerance, 30%; signal/noise ratio, 3; and minimum intensity, 100,000. Subsequently, peak intensities were normalized to the total spectral intensity. The normalized data were used to predict the molecular formula based on additive ions, molecular ion peaks, and fragment ions. Then, peaks were matched with the mzclould (https://www.mzcloud.org/, access on 16 March 2021), mz Vaultand Masslistdatabase to obtain accurate qualitative and relative quantitative results. Statistical analyses were performed using the statistical software R (R version R-3.4.3), Python (Python 2.7.6 version), and CentOS (CentOS release 6.6). When data were not normally distributed, normal transformations were attempted using the area normalization method.
2.7. Differential Metabolites Identification
For statistical analysis, PCA and OPLS-DA were applied to analyze the significant metabolites between the CB and NS groups using the R package models. Together with Student’s t-test (threshold value 0.05), significant testing among metabolite data was conducted using the variable importance in projection (VIP) score of the OPLS-DA model. Those with a p value of the t-test < 0.05 and VIP ≥ 1 were considered differential metabolites between the two groups. Heatmap clustering was drawn using the heatmaps software package in the R language (version 1.0.12).
3. Results
3.1. Effect of CO(NH2)2 on the Contents of Intracellular and Extracellular Ergosterol
In the study, we firstly analyzed the effect of CO(NH2)2 on the extracellular and intracellular ergosterol concentrations and biomass products. Figure 1 shows that in the absence of CO(NH2)2, the concentrations of intracellular ergosterol, extracellular ergosterol, and biomass were 86.10 mg/L, 75.43 mg/L, and 10.3 g/L, respectively. After CO(NH2)2 was added, the concentrations of intracellular ergosterol, extracellular ergosterol, and biomass were 71.27 mg/L, 178.56 mg/L, and 26.5 g/L, respectively. Apparently, CO(NH2)2 could significantly increase the synthesis of extracellular ergosterol and biomass. In our previous study [22] (Su et al., 2021), we concluded that CO(NH2)2 increases the synthesis of the extracellular ergosterol by regulating the permeability of the cell membrane. According to the present results, the conclusion was further proved.
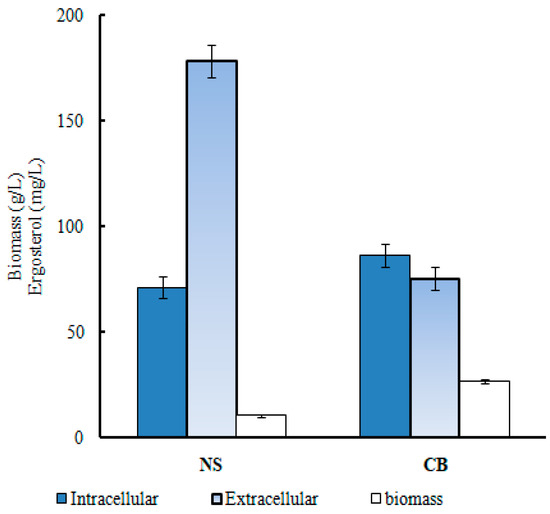
Figure 1.
Effects of various nitrogen sources on the yield of extracellular ergosterol. Blue: intracellular ergosterol; gray: extracellular ergosterol; white: biomass.
3.2. Analysis of Mycelial Metabolites
The PCA was established based on the ergosterol content in the biomass cultured in CB and NS. The PCA was applied in all samples using R package models (http://www.r-project.org/ access on 16 March 2021). All metabolites were divided into two groups. The metabolites with CO(NH2)2 added were grouped together and were indicated by a red dot. The metabolites without CO(NH2)2 were grouped together and were indicated by a small blue triangle. The separation effect of intracellular metabolites of C. cicadae was better, and the difference in metabolites of C. cicadae in the cation/anion model was significant. Figure 2A illustrates the PCA score of metabolites in the cation model. The two principal components in Figure 2A could explain 61.5% of the variables, PC1 = 51.9% and PC2 = 9.6%. In the anion model, the two principal components in Figure 2B could explain 62% of the variables, PC1 = 49.4%, and PC2 = 12.6%. The results showed that CO(NH2)2 caused a great difference in the metabolites of C. cicadae.
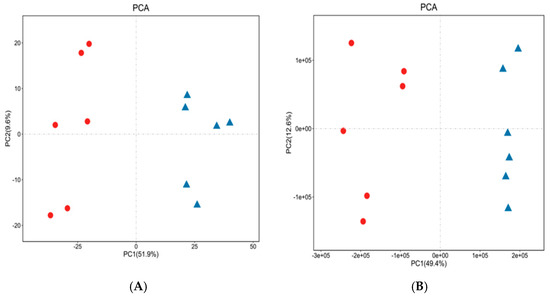
Figure 2.
The PCA of the biomass. The cation model (A); the anion model (B). ●: CB; ▲: NS.
The S-plot of OPLS-DA (in Figure 3A, the cation model, and in Figure 3B, THE ANION) showed that Rx2 was 0.757 and 0.568, Ry2 was 0.997 and 0.997, and Qy2 was 0.989 and 0.974, respectively. Figure 4A,B show that the intercept of R2y were all 0.69 in cation and anion models and the intercept of Q2 was −0.38 and −0.48 in the cation and anion models, respectively.
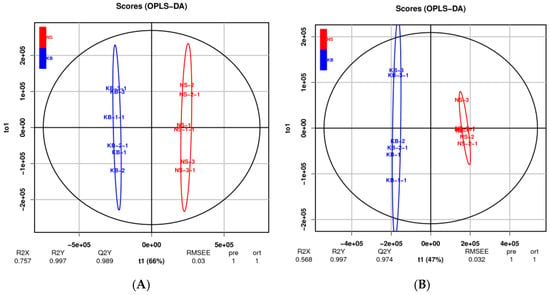
Figure 3.
Score plot with OPLS-DA. The cation model (A); the anion model (B).
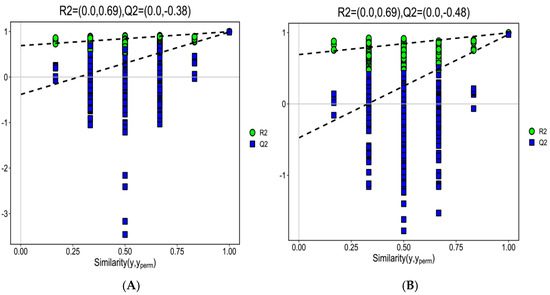
Figure 4.
The OPLS-DA model permutation test plots. The cation model (A); the anion model (B). ●: R2; ■: Q2.
Based on the results of OPLS-DA, the VIP of the OPLS-DA model was obtained, and the different metabolites caused by CO(NH2)2 were screened. Meanwhile, the different metabolites were further selected with fold change (FC). It is generally believed that the metabolites with an FC ≥ 2 or FC ≤ 0.5 and VIP≥ 1 are considered the differential metabolites. An FC ≥ 2 indicated that the metabolite was up-regulated. Conversely, an FC ≤ 0.5 indicated that the metabolite was down-regulated. The VIP value indicated the influence intensity of each corresponding metabolite to the model. The metabolite with a VIP ≥ 1 indicated a significantly different model. Based on the values of VIP and FC, the volcano map of differential metabolites was obtained (Figure 5A in the cation model; Figure 5B in the anion model). After comparing the volcano plots of two groups, 157 different metabolites caused by CO(NH2)2 were found in the cation model, of which 98 were down-regulated and 59 were up-regulated. Moreover, 105 different metabolites caused by CO(NH2)2 were found in the anion model, of which 61 were down-regulated and 44 were up-regulated. To gain insights into the metabolic mechanism in response to CO(NH2)2 and to provide more accurate information of ergosterol synthesis, these different metabolites obtained from the volcano map were made into a heatmap according to their VIP values (Figure 6). They could be divided into seven groups as follows: carbohydrate, amino acid, lipid, nucleotides, vitamins, hormones, and other secondary metabolisms.

Figure 5.
Volcano scatter plot analysis of differently expressed ion features detected by UHPLC-ESI-MS/MS. The cation model (A); the anion model (B). ●: up-regulation; ●: no sig. ●: down-regulation.
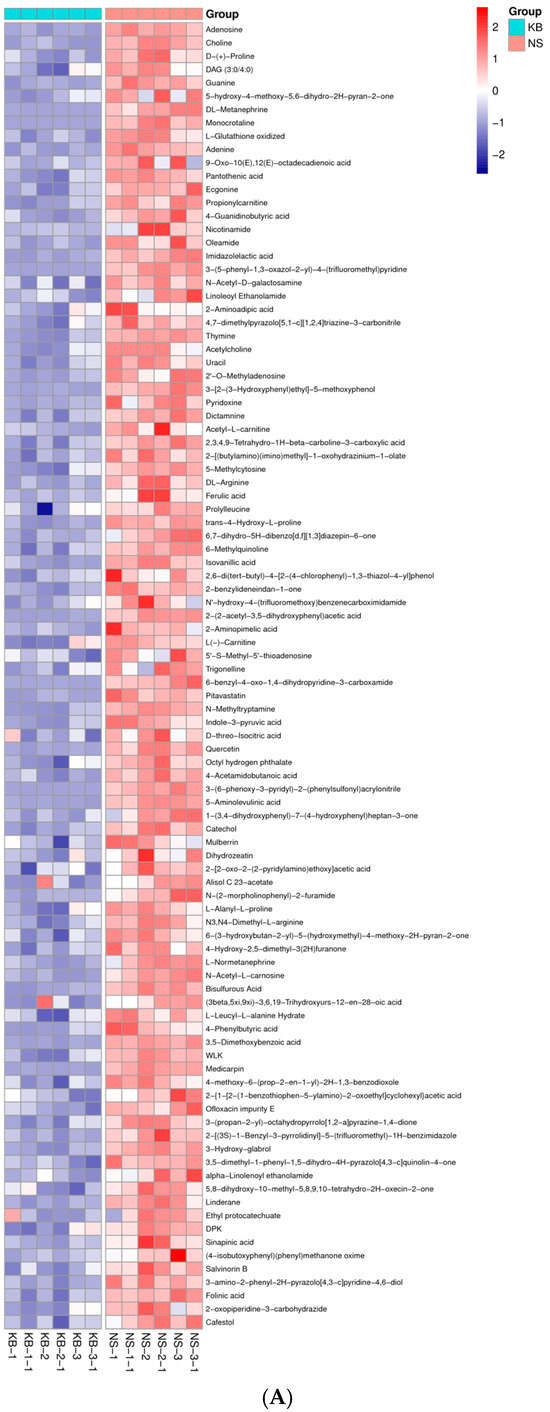
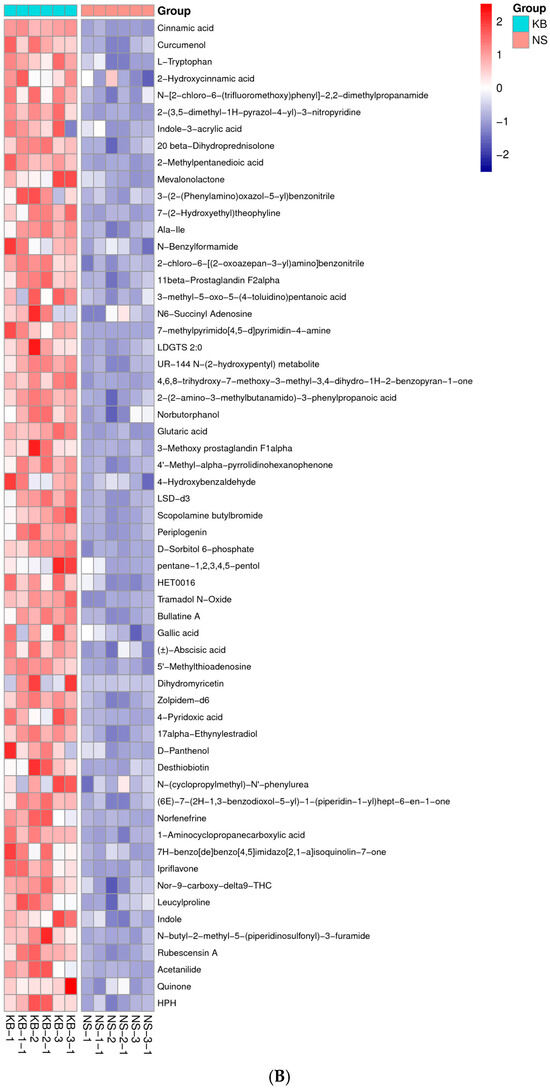
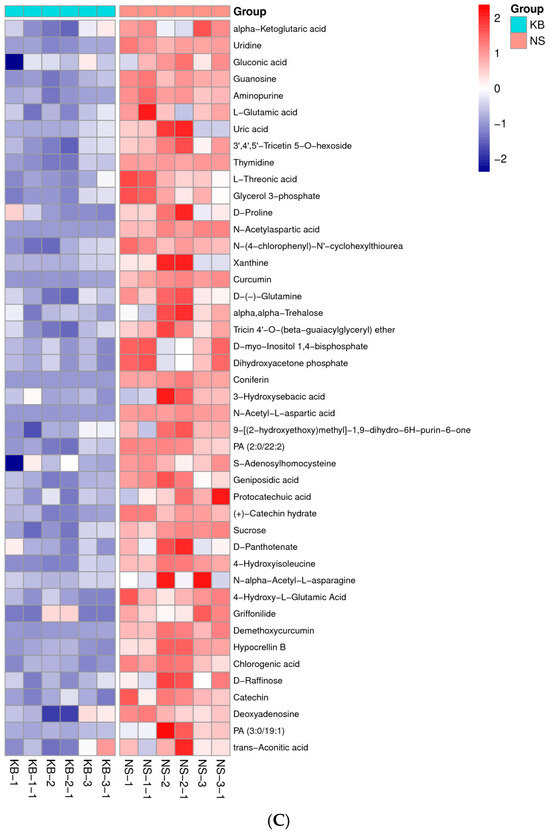
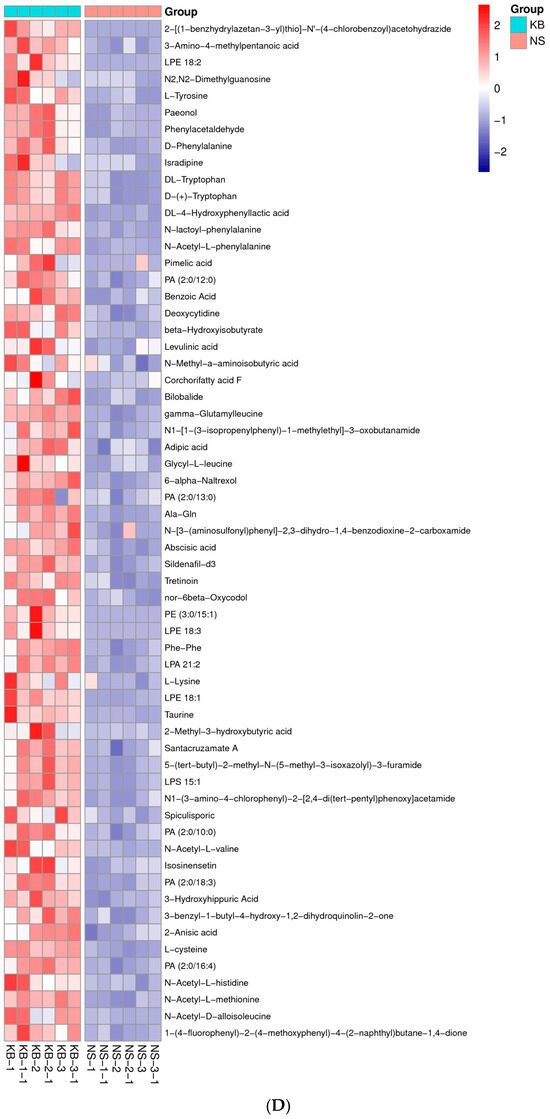
Figure 6.
The metabonomic heatmap. (A) Up-regulated metabolites in the cation model; (B) down-regulated metabolites in the cation model; (C) up-regulated metabolites in the anion model; (D) down-regulated metabolites in the anion model. Rows represent metabolites; columns represent samples; color key indicates metabolite expression value; red: highest; and blue: lowest.
4. Discussion
In the fermentation process, the nitrogen sources must meet the requirement of biomass growth and product synthesis. The effects of different nitrogen sources on biomass and product synthesis depend on the type of nitrogen sources [35]. Many nitrogen sources are involved in the synthesis of nitrogen-containing metabolites, such as amino acids, nucleotides, and vitamins. These amino acids and nucleotides are not only the basics of biomass growth but also the precursors of some secondary metabolites [36]. However, no information on the regulatory action of nitrogen source to the ergosterol synthesis of C. cicadae can be obtained. In the present study, after CO(NH2)2 was added, the concentration of intracellular ergosterol was significantly decreased, and the ergosterol content of per-unit biomass was further significantly decreased, implying that the content of intracellular ergosterol and its induced inhibitory action were lowered. To disclose the regulatory mechanism of CO(NH2)2 on ergosterol synthesis, the UHPLC-EIS-MS/MS-based metabonomic techniques were applied to analyze the C. cicadae metabolic profile changes to the CO(NH2)2 response.
Compounds with the same retention time Rt value and m/z value in different samples were considered to be the same compound. Multivariate statistical analysis was performed to find potential differential biomarkers caused by the CO(NH2)2 response. In the process of analysis, PCA was first performed on the overall response sample status to obtain an overview and classification, and to visualize the clustering trend of the CB and NS groups of samples. Then, OPLS-DA was conducted to obtain the variables with the largest inter-group differences. The S-plot of OPLS-DA visualizes the predictive variables. The similarity between samples can be determined based on the distance between samples; the farther the distance between samples, the larger the VIP value, and the stronger the difference. When the VIP value is greater than 1.0, the variable is generally considered a potential biomarker difference between the two samples.
Figure 2 shows that the intracellular metabolites without added CO(NH2)2 are mainly in the negative axis, and mainly in the positive axis with added CO(NH2)2, according to the first principal component; according to the second principal component, the differences were not very significant. According to the first principal component, the CB groups are mainly concentrated in the negative axis, and the NS groups are mainly concentrated in the positive axis. We deduced that CO(NH2)2 caused a great difference in the metabolites of C. cicadae.
To find out the difference in metabolites caused by CO(NH2)2, OPLS-DA was used to investigate the difference in metabolites between the CB and NS groups. OPLS-DA was applied in comparison groups using R package models (http://www.r-project.org/, access on 16 March 2021). Although PCA is a reliable grouping method, PCA cannot provide a clear grouping if the intra-group differences in samples are greater compared with the inter-group differences. The OPLS-DA screens differential metabolites and maximizes isolation by removing systemic variants that are not associated with the grouping. After the data are statistically analyzed according to the nature of the group, the key variables that affect the grouping are obtained accurately. Based on the relative content of total ergosterol obtained from NS and CB, the OPLS-DA model was established. Figure 3A (the cation model) and Figure 3B (the anion model) show that the OPLS-DA model clearly distinguished metabolites of the CB and NS groups, and the repeated samples are clustered together compactly, indicating the repeatability and reliability of the experiment. R2 indicated the total variation in the data matrix explained by the model. The results are shown in Figure 4. In the cation/anion model (Figure 3A or Figure 3B), Q2, Rx2, and Ry2 were all larger than 0.5, showing that the model had a good fit. Based on the intercept of Ry2 being larger than the intercept of Q2 (−0.38 and −0.48 in the cation and anion models, respectively) and the difference between R2y and Q2 being less than 0.3 in Figure 4, we inferred that the model had a better interpretation and prediction.
4.1. Carbohydrate
Proteins, nucleic acids, and fatty acids are the elementary components of the mycelium. The energy (ATP) is the hub in the syntheses of the three above-mentioned components. ATP is derived from the tricarboxylic acid cycle (TCA) pathway. Figure 6A shows that the contents of glycerol 3-phosphate and dihydroxyacetone phosphate were significantly increased after CO(NH2)2 was added. According to KEGG metabolic pathway, glycerol can directly access the Embden–Meyerhof pathway and TCA pathway and synthesize NADH and ATP only when they are converted to α-phosphoglycerol and dihydroxyacetone phosphate under the action of glycerol kinase and α-phosphoglycerol dehydrogenase. The increase in glycerol 3-phosphate and dihydroxyacetone phosphate caused by CO(NH2)2 showed that the pyruvate for the synthesis of NADH and ATP was significantly increased. The significant increase in the isocitric acid and α-ketoglutaric acid hinted that the activity of the α-ketoglutarate dehydrogenase complex was weakened in the metabolic flow through TCA cycle, and the amount of NADH and ATP decreased.
The glycerol 3-phosphate and dihydroxyacetone phosphate were synthesized to glucose 6-phosphate by the gluconeogenesis pathway, and then the glucose 6-phosphate was oxidized by glucose 6-phosphate dehydrogenase to produce gluconic acid 6-phosphate. Further, gluconic acid 6-phosphate was oxidized by 6-phosphogluconate dehydrogenase to form ribulose 5-phosphate, which entered the hexose monophosphate shunt (HMP) and simultaneously synthesized nicotinamide adenine dinucleotide phosphate (NADPH). When the activity of 6-phosphogluconate dehydrogenase was inhibited, the excess gluconic acid 6-phosphate was hydrolyzed into gluconic acid. Thus, the high level of gluconic acid indicated that 6-phosphogluconate dehydrogenase in the hexose monophosphate shunt (HMP) was inhibited [37]. 6-phosphogluconate dehydrogenase is the limiting enzyme, which catalyzed the oxidative dehydrogenation of 6-phospho-D-gluconic acid to NADPH. NADPH is the main hydrogen donor. According to the general theory, if biomass is synthesized in large quantities, HMP is activated, and the NADPH is synthesized in large quantities. In the present study, 6-phosphogluconate dehydrogenase was inhibited, the synthesis of NADPH was decreased, and ergosterol synthesis was decreased.
It has been reported that the main monosaccharides of C. cicadae polysaccharides are glucose, mannose, and galactose [38]. The insignificant increases in mannose and galactose in Figure 6 indicated that there was no increase in the content of the mycelial polysaccharides. Trehalose is a disaccharide compound and exists in many fungi. It is both an energy substance and a signal molecule to adjust metabolic pathways [39]. Meanwhile, trehalose can protect proteins and cellular membranes from inactivation or denaturation caused by CO(NH2)2. Figure 6 shows that the level of trehalose was significantly increased after supplementation of CO(NH2)2, which might show that CO(NH2)2 adjusted the metabolic fluxes of C. cicadae.
4.2. Protein and Nucleic Acid Metabolism
The premise of biomass growth is the excessive synthesis of proteins, nucleic acids, and fatty acids. Figure 6 shows that four amino acids, including Pro, Arg, Gln, and Glu, were up-regulated, and five amino acids, including Try, Tyr, Met, Lys, and Cys, were down-regulated. It can probably be attributed to the effect of CO(NH2)2 on nitrogen metabolism in vivo. Simultaneously, α-ketoglutaric acid is the precursor of Arg, Gln, and Glu synthesis. The increase in Arg, Gln, and Glu content hinted that the α-ketoglutaric acid dehydrogenase was inhibited as well. Asn is the main nitrogen storage and transport substance in the metabolic process of amide amino acids. Asn was up-regulated under the CO(NH2)2 stress in the process of the biomass incubation, which could improve the tolerance of biomass to high concentrations of CO(NH2)2. Meanwhile, the transamination of Asn provided nitrogen sources for the syntheses of nucleic acids and other amino acids. The up-regulation of Asp synthesis was closely related to the up-regulation of Asn synthesis. Asp was a synthetic precursor of amino acids, such as Lys, Thr, and Met, as well as purine and pyrimidine bases in the cells. The up-regulation of Asp was helpful for the syntheses of these amino acids, as well as purine and pyrimidine. The higher levels of various free amino acids in the biomass were accompanied by increases in intracellular amino acid levels. Glutathione is a reducing agent and can help the biomass to better grow under oxidative stress [40]. Up-regulated levels of Glu and Gln are related to glutathione metabolism, thereby improving the oxidative stress tolerance of the yeast. In the present study, however, because of the long incubation time, the levels of Lys and Met were down-regulated instead of up-regulated, and the level of L-glutathione oxidized was up-regulated instead of the glutathione. The up-regulated level of oxidized L-glutathione was associated with a decrease in NADH and ATP synthesis.
Nucleic acids are divided into RNA and DNA. Nucleic acids are composed of nitrogen-containing bases (purines and pyrimidines), pentose, and phosphoric acid. In the present study, some bases (adenine, guanine, thymine, uridine, aminopurine, xanthine, deoxyadenosine, and 5-methylcytosine) were up-regulated in response to CO(NH2)2 stress. Moreover, 5-methylcytosine is a type of epigenetic modification, and it can be found in a variety of locations. In addition, 5-methylcytosine can protect DNA from its methylation-sensitive restriction enzymes. Excessive synthesis of these bases indicates the enhanced synthesis of DNA and RNA. Deoxyadenosine is a characteristic compound of Cordyceps fungi. Deoxyadenosine can inhibit DNA replication, making it a potential anti-cancer and anti-viral drug. These results indicated a significant increase in metabolic fluxes related to biomass synthesis.
4.3. Lipid Metabolism
Phospholipid metabolism, a linoleic acid metabolism, is involved in the process of CO(NH2)2 response. Choline is the most abundant phospholipid in the cell membrane and an important metabolite of linoleic acid. The abnormal elevation in choline and linoleoyl ethanolamide (Figure 6) showed that supplementation of CO(NH2)2 enhanced the catabolism of linoleic acid. S-adenosylhomocysteine is a by-product of phosphatidylcholine. The increase in S-adenosylhomocysteine level indicated the increase in phosphatidylcholine synthesis. The synthesis level of glycerol 3-phosphate was increased due to CO(NH2)2 supplementation. The phosphatidylcholine and glycerol 3-phosphate were further converted into phospholipid. An increase in both phospholipid anabolism and catabolism suggested that CO(NH2)2 supplementation triggered a disturbance in lipid metabolism.
The unsaturated fatty acid is a major component of lipids. Oxygenation of unsaturated fatty acid leads to the production of a large number of structurally similar oxidized fatty acids, collectively known as oxygen lipids. Fungal oxygen lipids are mainly derived from oleic acid, linoleic acid, and linolenic acid. 9-Oxo-10(E),12(E)- octadecadienoic acid is an oxygen lipid, and its content was markedly increased with CO(NH2)2 supplementation.
After CO(NH2)2 was supplied, the concentrations of carnitine, propionyl carnitine, acetyl-L-carnitine, and isocitric acid were increased. Because carnitine is a carrier when acetyl-CoA crosses the mitochondrial membrane [41], we concluded that the amount of acetyl-CoA, a metabolite of the fatty acid β-oxidation, entering into the TCA cycle was increased. Then, acetyl-CoA reacted with amino acids and other compounds to produce large amounts of acetyl-like compounds, such as laevulinic acid and acetyl–aspartic acid. The excess acetyl-CoA also activates gluconeogenesis, and trehalose is produced from pyruvic acid and stored in the mycelium [42,43].
Inositol is both a precursor of many chemical compounds and a key membrane structural molecule. It may balance the intracellular environment and protect the cell from cell damage by changing the lipid composition of cells. Inositol 1,4-bisphosphate is the product of the enzymatic hydrolysis of phosphatidylinositol. The addition of CO(NH2)2 up-regulated the abundance of inositol 1,4-bisphosphate in C. cicadae; more inositol1,4-bisphosphate indicated that more lipids were hydrolyzed, and the membrane structure damage caused by CO(NH2)2 stresses was strengthened. The result was consistent with our previous findings. Metabolism in eukaryotes is compartmentalized within specific regions (organelles), and membrane structure damage is the basis for the regionalization of biochemical reactions. Membrane structures caused by CO(NH2)2 indicated that the regionalization of metabolism had been altered. In summary, CO(NH2)2 supplementation led to lipid metabolism disorder, increased cell membrane permeability, changes in metabolic region, and elevated ergosterol secretion and extracellular ergosterol concentration.
4.4. Hormones and Vitamins
Hormones are a class of trace organic molecules of living cells that regulate the metabolism or physiological function of target cells. After the supplementation of CO(NH2)2, the levels of these hormones were dramatically changed, including metanephrine, normetanephrine, indole, and abscisic acid. One of the most basic functions of abscisic acid is to inhibit the syntheses of DNA and protein. In the present study, the level of abscisic acid was down-regulated, which was consistent with the increased synthesis of nucleic acid and protein described above. We deduced that the significant increase in biomass with added CO(NH2)2 was related to the down-regulation of abscisic acid.
As a type of organic substance, vitamins are necessary for maintaining human life activity. They are also important active substances to keep humans healthy. Vitamins are scarce in the body but essential. In the present study, the levels of many vitamins, including pantothenic acid, panthenol, nicotinamide, pyridoxine, pyridoxic acid, folinic acid, and tretinoin, were increased. Pantothenic acid is VB5. Pantothenic acid aids in energy production in the biomass and can increase the rate at which fat and sugar are converted into energy. The increased pantothenic acid level proved that more fatty acids were oxidized and entered into the TCA cycle to produce energy. Nicotinamide is the precursor for coenzyme I (dihydrourocil dehydrogense, NAD+) and coenzyme II (nicotinamide–adenine dinucleotide phosphate, NADP+). NAD+ and NADP+ participate in the energy metabolism of cells and reducing power, respectively. The increased synthesis of NAD + and NADP+ was further proven through the decrease in NADH and NADPH synthesis. VB6 is pyridoxine, including pyridoxine, pyridoxal, and pyridoxamine. As a water-soluble vitamin, VB6 is closely related to the metabolism of amino acids. The increased concentration of pyridoxine showed the enhanced catabolism of amino acids. Folinic acid is the reduced form of folic acid. When the activity of folate reductase in the organism is reduced, folic acid cannot be converted into folinic acid. Folinic acid was the carrier of the one-carbon unit in vivo. The synthesis of many amino acids and nucleotides requires one-carbon units. Therefore, folinic acid was a key marker of nucleotides and amino acids. The increased concentration of folinic acid was directly related to the increased biomass. Tretinoin is an intermediate metabolite of vitamin A (VA). VA is an unsaturated monoalcohol containing an aliphatic ring. VA contributes to cell proliferation and growth. A decrease in tretinoin concentration exhibited an increase in vitamin A concentration, which was consistent with an increase in biomass.
4.5. Sterol
Although we found that the concentration of extracellular ergosterol was significantly increased by the supplementation of CO(NH2)2, a significant change in the ergosterol concentration in vivo was not found in this study. This may be explained by a reduction in ergosterol concentrations below significant levels. However, the periplogenin and cafestol concentrations were found to be significantly down-regulated and up-regulated, respectively. Periplogenin and cafestol are the other two sterols, whose molecular structures are similar to ergosteril (Figure 7C). Cafestol has been reported to inhibit the degradation of cholesterol by down-regulating cholesterol 7ɑ-hydroxylase and sterol 27-hydroxylase [44,45]. Ergosterol and cholesterol have the same matrix structure, but they have different substituent groups in their matrix structures (Figure 7A). Therefore, we hypothesized that cafestol could inhibit ergosterol degradation in the process of ergosterol synthesis and caused the synthetic ergosterol to be secreted extracellularly. When the concentration of cafestol was up-regulated, the inhibition action of cafestol became stronger, and more ergosterol was secreted extracellularly. The structure of periplogenin is similar to the structure of ergosterol and a change in its concentration can indicate the trend of intracellular ergosterol concentration. Here, the decrease in periplogenin showed the decrease in intracellular ergosterol concentration. In the previous study, we proved that the supplementation of CO(NH2)2 increased the synthesis, so we concluded that the supplementation of CO(NH2)2 promoted the secretion of more ergosterol extracellularly by reducing the degradation rate of ergosterol. In the fermentation anaphase, the synthesis of reducing power (NADPH) and energy materials (NADH and ATP) was significantly decreased due to CO(NH2)2. NADPH, NADH, and ATP were involved in many steps of ergosterol synthesis. Thus, we concluded that the inhibition of α-ketoglutarate dehydrogenase and 6-phosphogluconate dehydrogenase caused by CO(NH2)2 contributed to the decrease in ergosterol synthesis in the fermentation anaphase.
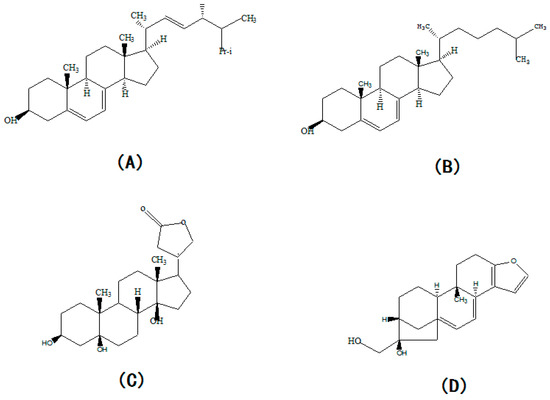
Figure 7.
The structural formula of ergosterol (A), cholesterol (B), periplogenin (C), and cafestol (D).
4.6. Other Secondary Metabolites
In the present study, the contents of some secondary metabolites with high biological activity in the fermentation broth of C. cicadae were significantly changed, including flavonoids, terpenes, saponins, and alkaloids. In the flavonoids, the concentrations of catechin, protocatechuic acid, 3-hydroxy-glabrol, mulberrin, medicarpin, and quercetin were up-regulated. The concentrations of dihydromyricetin and ipriflavone were down-regulated. In the alkaloids, monocrotaline, dictamnine, and trigonelline were up-regulated, while scopolamine butylbromide, bullatine A, and 7-(2-hydroxyethyl) theophylline were down-regulated. In the terpenes, the levels of coniferin, curcumin, lindane, and demethoxycurcumin were up-regulated, and the levels of curcumenol, rubescensin A, bilobalide (BB), and 6-α-naltrexol were down-regulated. BB belongs to a sesquiterpene lactone and is only found in ginkgo leaves [46,47]. BB possesses a remarkable curative effect on cardiovascular diseases [48], such as coronary heart disease, myocardial infarction, and arrhythmia. Notably, BB is frequently used in the prevention and treatment of Alzheimer disease [49] because of its excellent effect on mitigating age-related cognitive decline. To our knowledge, BB is the first ginkgolide to be found in the fungi biomass. Whether there are other ginkgolides in the mycelium of C. cicadae remains to be studied. Meanwhile, the biomass of C. cicadae contained so many bioactive materials, suggesting that the biomass of C. cicada is an excellent source of traditional Chinese medicine and worthy of further development.
5. Conclusions
In summary, biomass metabonomic analysis using UHPLC-ESI-MS/MS presented a useful tool for identifying physiological changes caused by a component in the medium. Based on biomarkers including isocitric acid and α-ketoglutaric acid, glycerol 3-phosphate and dihydroxyacetone phosphate, trehalose, choline, carnitine, propionyl carnitine, acetyl-L-carnitine, abscisic acid, folinic acid, and cafestol, we proved our speculation that CO(NH2)2 supplementation significantly promoted the growth and ergosterol synthesis of C. cicadae by increasing cell membrane permeability, changing the metabolic regionalization and metabolic flux direction, and regulating the metabolic rate. Therefore, the strategy of CO(NH2)2 supplementation was expected to be an effective way to improve ergosterol synthesis in the fermentation of C. cicadae.
Author Contributions
Z.Z.: writing—original draft, methodology, investigation, funding acquisition. M.W.: investigation. F.S.: investigation, data curation. W.W.: writing—review and editing, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 31301544).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Author Zhicai Zhang was employed by the company Zhenjiang Yemaikang Food Bio-Technology Co., Ltd. Author Weijie Wu was employed by the company Jiangsu Alphay Biotechnology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Nxumalo, W.; Elateeq, A.A.; Sun, Y. Can Cordyceps cicadae be used as an alternative to Cordyceps militaris and Cordyceps sinensis?—A review. J. Ethnopharmacol. 2020, 257, e112879. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Q.; Zhang, W.C.; Peng, F.; Lu, R.L.; Zhou, H.; Bao, G.H.; Wang, B.; Huang, B.; Li, Z.; He, L. Metabolomic variation in wild and cultured cordyceps and mycelia of Isaria cicadae. Biomed. Chromatogr. 2019, 33, e4487. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Chen, S.D.; Zeng, B.; Liang, H.L.; Wang, T.; Bai, L.Y.; Tao, J.; Zhang, X.; Wang, J. Protective effect and mechanism of polysaccharides from Cordyceps cicadae on acute liver injury induced by D-GlaN in mice. MedPlant 2018, 9, 102–106. [Google Scholar]
- Liu, N.H.; Zhou, S.; Olatunji, O.J.; Wu, Y. Nucleosides rich extract from Cordyceps cicadae alleviated cisplatin-induced neurotoxicity in rats: A behavioral, biochemical and histopathological study. Arab. J. Chem. 2022, 15, 103476. [Google Scholar] [CrossRef]
- Shi, C.E.; Song, W.L.; Gao, J.; Yan, S.B.; Guo, C.; Zhang, T.F. Enhanced production of cordycepic acid from Cordyceps cicadae isolated from a wild environment. Braz. J. Microbiol. 2022, 53, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Zheng, R.; Deng, Y.Y.; Chen, Y.P.; Zhang, S.W. Ergosterol peroxide from Cordyceps cicadae ameliorates TGF-β1-induced activation of kidney fibroblasts. Phytomedicine 2014, 21, 278–372. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.Y.; Li, C.; Shao, Y.; Chen, A.H. Polysaccharides from spores of Cordyceps cicadae protect against cyclophosphamide-induced immunosuppression and oxidative stress in mice. Foods 2022, 11, 515. [Google Scholar] [CrossRef]
- Zheng, R.; Wang, Y.H.; Zhong, Y.F.; Zhu, R. The mushroom, Cordyceps cicadae, ameliorates renal interstitial fibrosis via TLR2-mediated pathways. Trop. J. Pharm. Res. 2020, 19, 2153–2159. [Google Scholar] [CrossRef]
- Wang, Y.N.; Zeng, T.T.; Li, H.; Wang, Y.D.; Wang, J.H.; Yuan, H.B. Structural characterization and hypoglycemic function of polysaccharides from Cordyceps cicadae. Molecule 2023, 28, e526. [Google Scholar] [CrossRef]
- Li, I.C.; Lin, S.; Tsai, Y.T.; Hsu, J.H.; Chen, Y.L.; Lin, W.H.; Chen, C.C. Cordyceps cicadae mycelia and its active compound HEA exert beneficial effects on blood glucose in type 2 diabetic db/db mice. J. Sci. Food Agric. 2019, 99, 606–612. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Michael, W.; Wang, P.; Lu, H.; Zhao, H.; Liu, H.; Wang, S.; Sun, Y.; Liang, Z. Biological characteristics, bioactive components and antineoplastic properties of sporoderm-broken spores from wild Cordyceps cicadae. Phytomedicine 2017, 36, 217–228. [Google Scholar] [CrossRef]
- Feng, T.; Deng, W.Q.; Liu, J.K. Two highly conjugated ergosterols from the fungus Psathyrella rogueiana and their anti-inflammatory activity. J. Asian Nat. Prod. Res. 2023, 26, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.J.; Jiang, Y.L.; Peng, J.; Zhang, C.X.; Zhu, Q.; Wang, Q.Q.; Liao, Y.N.; Pi, W.L.; Dong, X.Y.; Yuan, J.P.; et al. Evaluation of ergosterol composition and esterification rate in fungi isolated from mangrove soil, long-term storage of broken spores, and two soils. Appl. Microbiol. Biotechnol. 2020, 104, 5461–5475. [Google Scholar] [CrossRef] [PubMed]
- Erica, M.; Sharon, R.; Gregory, J.S.; Gonzalez, T.; Abman, S.H.; Fleet, J.C. Maternal vitamin D deficiency induces transcriptomic changes in newborn rat lungs. J. Steroid. Biochem. Mol. Biol. 2020, 199, 105613. [Google Scholar]
- Nilkhet, S.; Vongthip, W.; Lertpatipanpong, P.; Prasansuklab, A.; Tencomnao, T.; Chuchawankul, S.; Baek, S.J. Ergosterol inhibits the proliferation of breast cancer cells by suppressing AKT/GSK-3beta/beta-catenin pathway. Sci. Rep. 2024, 14, e19664. [Google Scholar] [CrossRef] [PubMed]
- Taofiq, O.; Heleno, S.A.; Calhelha, R.C.; Fernandes, I.P.; Alves, M.J.; Barros, L.; Gonzalez-Paramas, A.M.; Ferreira, I.C.F.R.; Barreiro, M.F. Phenolic acids, cinnamic acid, and ergosterol as cosmeceutical ingredients: Stabilization by microencapsulation to ensure sustained bioactivity. Microchem. J. 2019, 147, 469–477. [Google Scholar] [CrossRef]
- Li, L.Y.; Zhu, Y.H.; Cheng, W.J.; Di, F.Q.; Zhang, J.C.; Wang, C.T. Efficacy of Ergosterol and Ergosterol peroxide in different anti-inflammatory models—A comparison study. Food Agric. Immunol. 2024, 35, 2423638. [Google Scholar] [CrossRef]
- Tintino, S.R.; Oliveira-Tintino, C.D.M.; Campina, F.F.; Costa, M.S.; Cruz, R.P.; Pereira, R.L.S.; Andrade, J.C.; Sousa, E.O.; Siqueira-Junior, J.P.; Coutinho, H.D.M.; et al. Cholesterol and ergosterol affect the activity of Staphylococcus aureus antibiotic efflux pumps. Microb. Pathogen. 2017, 104, 133–136. [Google Scholar] [CrossRef]
- Wei, M.S.; Li, X.X.; Liao, H.; Liu, L.L.; Li, Q.; Sun, W.G.; Chen, C.M.; Zhu, H.C.; Zhang, Y.H. Quadristerols A-G: Seven undescribed ergosterols from Aspergillus quadrilineata. Phytochemistry 2023, 213, 113785. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Semak, I.; Zjawiony, J.; Wortsman, J.; Gandy, M.N.; Li, J.; Zbytek, B.; Li, W.; Tuckey, R.C. Enzymatic metabolism of ergosterol by cytochrome P450scc to biologically active 17α,24-Dihydroxyergosterol. Chem. Biol. 2005, 12, 931–939. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Su, Y.; Seguinot, P.; Sanchez, I.; Ortiz-Julien, A.; Heras, J.M.; Querol, A.; Camarasa, C.; Guillamón, J.M. Nitrogen sources preferences of non-Saccharomyces yeasts to sustain growth and fermentation under winemaking conditions. Food Microbiol. 2020, 85, 103287. [Google Scholar] [CrossRef]
- Su, Q.; Zhang, Z.; Liu, X.; Wang, F. The transcriptome analysis on urea response mechanism in the process of ergosterol synthesis by Cordyceps cicadae. Sci. Rep. 2021, 11, 10927. [Google Scholar] [CrossRef]
- Nong, X.; Zhong, S.N.; Huang, L.Y.; Xiao, J.; Hu, Y.; Xie, Y. Nontargeted metabonomics analysis of Scorias spongiosa fruiting bodies at different growth stages. Front. Microbiol. 2024, 15, 1478887. [Google Scholar] [CrossRef]
- Xu, H.Y.; Zhou, Y.; Pan, Z.; Gao, Z.X. Osteoarthritis: Insights into potential causes and biomarkers from articular fluid metabonomics. Curr. Proteom. 2024, 21. in press. [Google Scholar] [CrossRef]
- Liu, J.Q.; Zhou, H.B.; Bai, W.F.; Wang, J.; Li, Q.; Fan, L.Y.; Chang, H.; Shi, S.L. Assessment of progression of pulmonary fibrosis based on metabonomics and analysis of intestinal microbiota. Artif. Cells Nanomed. Biotechnol. 2024, 52, 201–217. [Google Scholar] [CrossRef]
- Yu, Y.D.; Yao, Q.G.; Chen, D.Y.; Zhang, Z.H.; Pan, Q.L.; Yu, J.; Cao, H.C.; Li, L.; Li, L.J. Serum metabonomics reveal the effectiveness of human placental mesenchymal stem cell therapy for primary sclerosing cholangitis. Stem. Cell Res. Ther. 2024, 15, 346. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.S.; Wang, S.S.; Li, Y.Z. An untargeted UPLC-Q-TOF-MS-based plasma metabonomics revealed the effects of peperomin E in a prostate cancer nude mouse model. Pak. J. Pharm. Sci. 2024, 37, 1135–1150. [Google Scholar]
- Lv, X.; Liu, Y.Z.; Liu, S.J.; Liu, Y.H.; Qu, Y.; Cai, Q. Metabonomics and pharmacodynamics studies of Gentiana radix and wine-processed Gentiana radix in damp-heat jaundice syndrome rats. J. Ethnopharmacol. 2024, 332, e118291. [Google Scholar] [CrossRef] [PubMed]
- Han, P.P.; Chen, X.Y.; Liang, Z.W.; Liu, Y.W.; Yu, X.; Song, P.Y.; Zhao, Y.J.; Zhang, H.; Zhu, S.Y.; Shi, X.Y.; et al. Metabolic signatures and risk of sarcopenia in suburb-dwelling older individuals by LC-MS-based untargeted metabonomics. Front. Endocrinol. 2024, 15, 1308841. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.Y.; Liu, R.X.; Zhang, X.Y.; Li, Y.F.; Peng, F.; Tang, W.C. Analysis of cantharidin-induced kidney injury and the protective mechanism of resveratrol in mice determined by liquid chromatography/mass spectrometry-based metabonomics. J. Appl. Toxicol. 2024, 44, 990–1004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qian, L.; Wang, C.; Teng, M.M.; Duan, M.M.; Chen, X.G.; Li, X.F.; Wang, C.J. UPLC-TOF-MS/MS metabolomics analysis of zebrafish metabolism by spirotetramat. Environ. Pollut. 2020, 266, 115310. [Google Scholar] [CrossRef]
- Rosli, M.A.F.; Mediani, A.; Azizan, K.A.; Baharum, S.N.; Goh, H.H. UPLC-TOF-MS/MS-based metabolomics analysis reveals species-specific metabolite compositions in pitchers of Nepenthes ampullaria, Nepenthes rafflesiana, and their hybrid Nepenthes x hookeriana. Front. Plant Sci. 2021, 12, 655004. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Wang, Y.S.; Zhu, A.Q.; Zhang, L.Q.; Zhang, X.; Zhang, J.; Zhang, C.B. UPLC-TOF/MS-based metabolomics reveals the chemical changes and in vitro biological effects in fermentation of white ginseng by four probiotics. Front. Microbiol. 2022, 13, 1022200. [Google Scholar] [CrossRef]
- Li, J.; Yu, L.W.; Liang, Y.C.; Lan, B.H.; Chen, Y.T.; Wang, Q.Q.; Wu, Z.Q. Chemical analysis of different parts from agarwood columns by artificially agarwood-inducing method based on GC-MS and UPLC-TOF-MS. Fitoterapia 2024, 178, 106156. [Google Scholar] [CrossRef]
- Rojo, M.C.; Talia, P.M.; Lerena, M.C.; Ponsone, M.L.; Gonzalez, M.L.; Becerra, L.M.; Mercado, L.A.; Martín-Arranz, V.; Rodríuez-Gómez, F.; Arroyo-López, F.N.; et al. Evaluation of different nitrogen sources on growth and fermentation performance for enhancing ethanol production by wine yeasts. Heliyon 2023, 9, e22608. [Google Scholar] [CrossRef] [PubMed]
- Ventorim, R.Z.; Germano, V.K.D.; Fontes, P.P.; da Silveira, W.B. Effect of carbon and nitrogen concentrations on lipid accumulation and regulation of acetyl-CoA carboxylase in Papiliotrema laurentii. Antonie Leeuwenhoek 2023, 116, 1161–1170. [Google Scholar] [CrossRef]
- Wang, D.C.; Sun, C.H.; Liu, L.Y.; Sun, X.H.; Jin, X.W.; Song, W.L.; Liu, X.Q.; Wan, X.L. Serum fatty acid profiles using GC-MS and multivariate statistical analysis: Potential biomarkers of Alzheimer’s disease. Neurobiol. Aging 2012, 33, 1057–1066. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Feng, Y.; Olatunji, O.O.; Tang, J.; Wei, Y.; Ouyang, Z.; Su, Z. Polysaccharides purified from Cordyceps cicadae protects PC12 cells against glutamate-induced oxidative damage. Carbohyd. Polym. 2016, 153, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, G.D.; Paranhos, L.D.; Eleutherio, E.C.A. Trehalose promotes biological fitness of fungi. Fungal Biol. 2024, 128, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Cao, X.T.; Zhu, N.Q.; Jiang, L.H.; Zhang, X.G.; He, Q.M.; Wei, P.H. A stepwise control strategy for glutathione synthesis in Saccharomyces cerevisiae based on oxidative stress and energy metabolism. World J. Microb. Biotechnol. 2020, 36, 117. [Google Scholar] [CrossRef]
- Giangregorio, N.; Tonazzi, A.; Pierri, C.L.; Indiveri, C. Insights into transient dimerisation of carnitine/acylcarnitine carrier (SLC25A20) from sarkosyl/PAGE, cross-linking reagents, and comparative modelling analysis. Biomolecules 2024, 14, 1158. [Google Scholar] [CrossRef]
- Wang, Y.L.; Yang, H.; Geerts, C.; Furtos, A.; Waters, P.; Cyr, D.; Wang, S.P.; Mitchell, G.A. The multiple facets of acetyl-CoA metabolism: Energetics, biosynthesis, regulation, acylation and inborn errors. Mol. Gent. Metab. 2023, 138, 106966. [Google Scholar] [CrossRef]
- Thompson, S.N.; Borchardt, D.B. Glucogenic blood sugar formation in an insect Manduca sexta L.: Asymmetric synthesis of trehalose from 13C enriched pyruvate. Comp. Biochem. Phys. B 2003, 135, 461–471. [Google Scholar] [CrossRef]
- Hofman, M.K.; Weggemans, R.M.; Zock, P.L.; Schouten, E.G.; Katan, M.B.; Princen, H.M.G. CYP7A1 A-278C polymorphism affects the response of plasma lipids after dietary cholesterol or cafestol interventions in humans. J. Nutr. 2004, 9, 2200–2204. [Google Scholar] [CrossRef]
- Ricketts, M.L.; Boekschoten, M.V.; Kreeft, A.J.; Hooiveld, G.J.E.J.; Moen, C.J.A.; Müller, M.; Frants, R.R.; Kasanmoentalib, S.; Post, S.M.; Princen, H.M.G.; et al. The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors. Mol. Endocrinol. 2007, 21, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.F.; Hu, C.X.; Jin, J.L.; Chao, Y.Q.; Wang, D.Y.; Xia, F.L.; Ruan, C.X.; Jiang, C.; Guan, M.; Zou, C.C. Bilobalide ameliorates osteoporosis by influencing the SIRT3/NF-κB axis in osteoclasts and promoting M2 polarization in macrophages. Int. J. Biol. Macromol. 2024, 281, 136504. [Google Scholar] [CrossRef]
- Soetanto, D.A.; Li, F.N.; Boateng, I.D.; Yang, X.M. Thermal fixation technologies affect phenolic profile, ginkgolides, bilobalide, product quality, and ginkgolic acids in Ginkgo biloba leaf tea. J. Food Sci. 2024, 89, 4093–4108. [Google Scholar] [CrossRef] [PubMed]
- Song, W.F.; Chen, Z.; Zhang, M.; Fu, H.X.; Wang, X.Q.; Ma, J.F.; Zang, X.B.; Hu, J.; Ai, F.; Chen, K. Bilobalide prevents apoptosis and improves cardiac function in myocardial infarction. Mol. Biotechnol. 2023, 66, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kang, S.M.; Go, G.W. Exploring the multifaceted role of ginkgolides and bilobalide from Ginkgo biloba in mitigating metabolic disorders. Food Sci. Biotechnol. 2024, 33, 2903–2917. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).