Effects of Rumen-Degradable Starch Levels on In Vitro Rumen Fermentation and Microbial Protein Synthesis in Alfalfa Silage
Abstract
1. Introduction
2. Materials and Methods
2.1. Composition and Nutritional Level of Experimental Diet
2.2. In Vitro Experiment
2.3. Data Processing and Analysis
3. Results
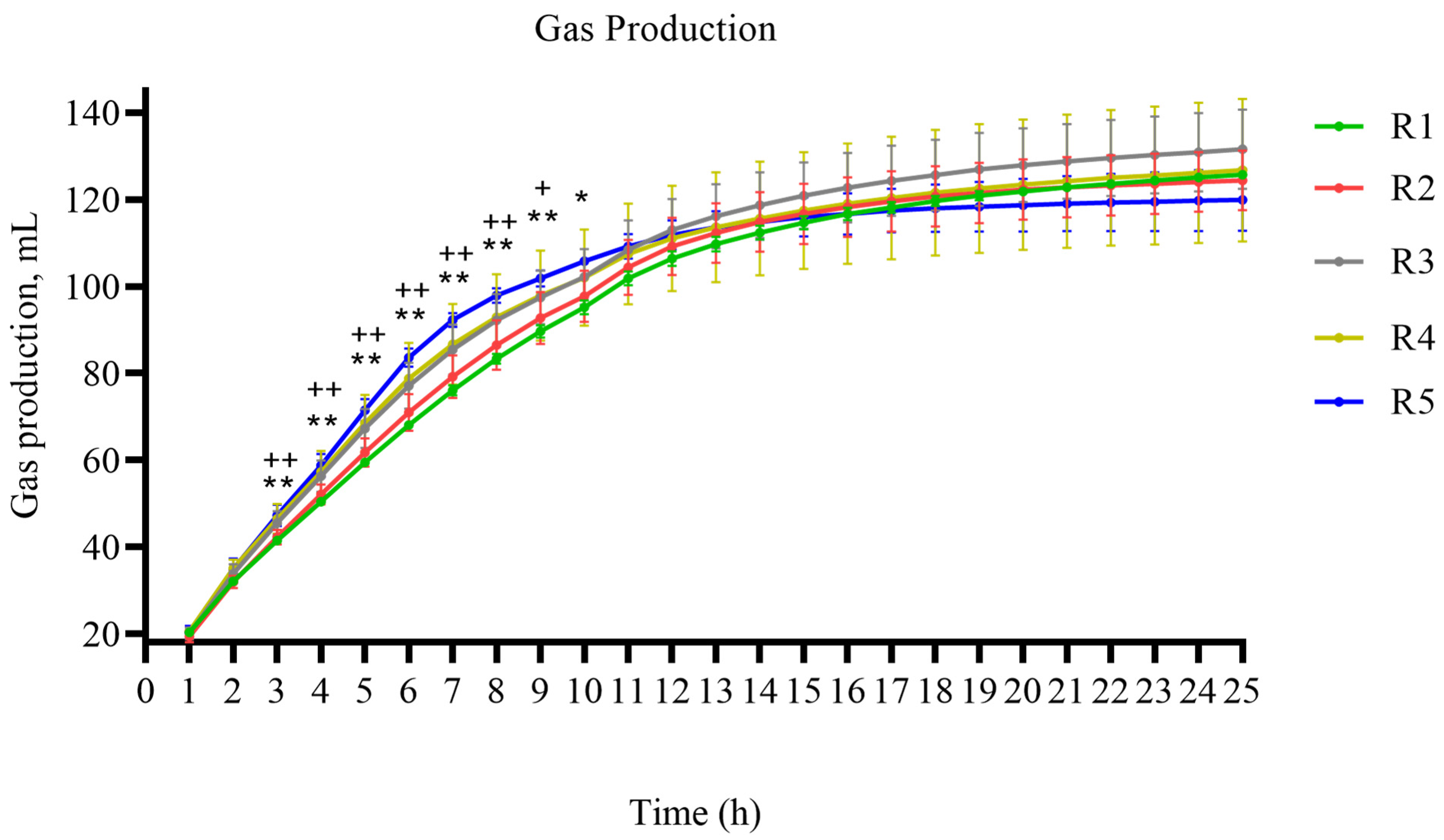
3.1. Effects of RDS Levels on Total Gas Production During In Vitro Rumen Fermentation of Alfalfa Silage
3.2. Effects of RDS Levels In Vitro Rumen Fermentation Parameters of Alfalfa Silage
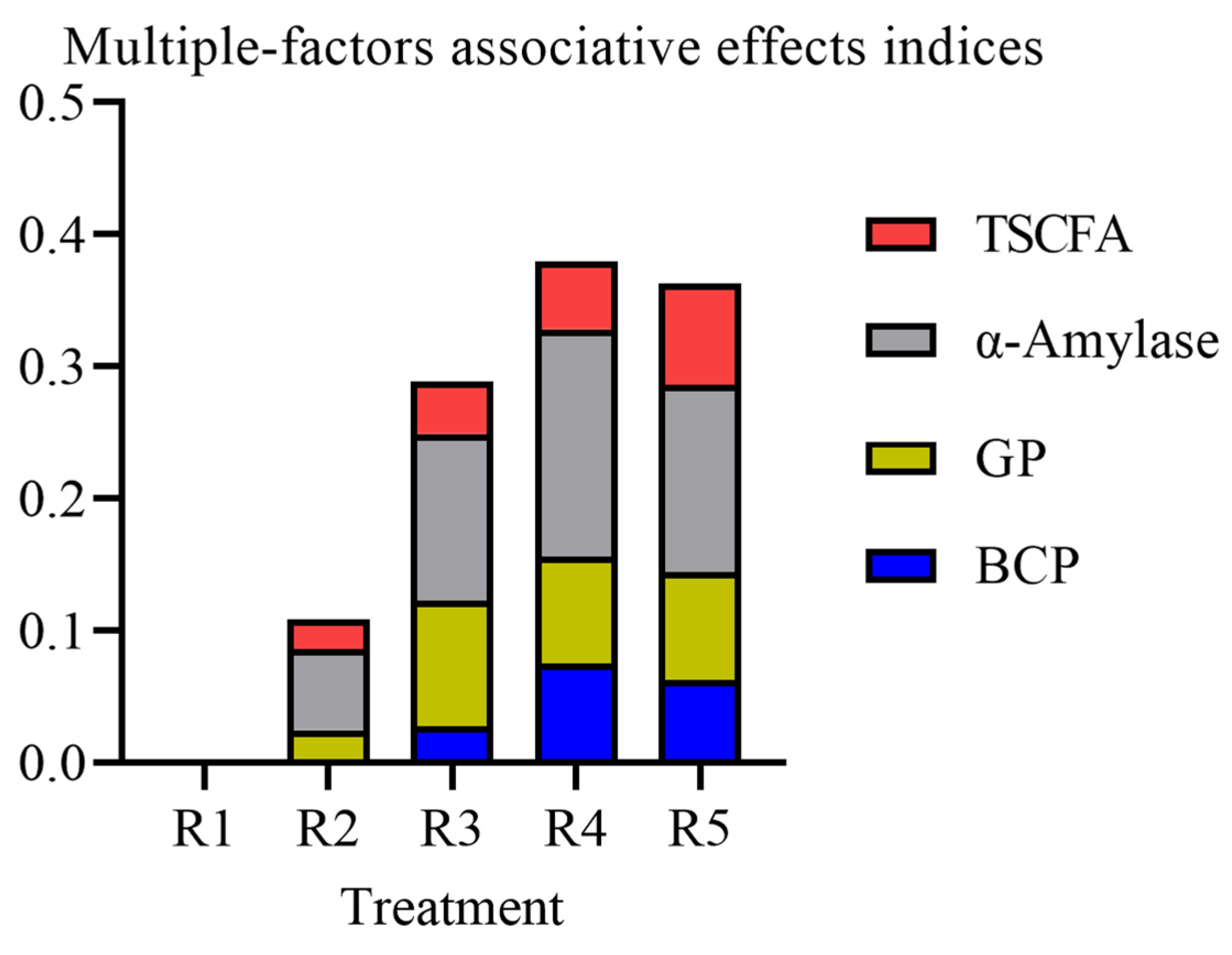
3.3. The Effects of RDS Levels on In Vitro Rumen Fermentation SFAEI and MFAEI of Alfalfa Silage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RDS | rumen-degradable starch |
| NH3-N | ammonia nitrogen |
| BCP | bacterial protein |
| SCFA | short-chain fatty acid |
| RDP | rumen-degradable protein |
| DM | dry matter |
| CP | crude protein |
| EE | ether extract |
| ADF | acid detergent fiber |
| NDF | neutral detergent fiber |
References
- Wayne, K.C.; Matthew, S.A.; Burney, A.K. Storage characteristics and nutritive value of moist large-round bales of alfalfa or alfalfa–grass hay treated with a propionic acid–based preservative. Appl. Anim. Sci. 2020, 36, 455–470. [Google Scholar]
- Radovic, J.; Sokolovic, D.; Markovic, J. Alfalfa-most important perennial forage legume in animal husbandry. Biotechnol. Anim. Husb. 2009, 25, 465–475. [Google Scholar] [CrossRef]
- Sun, J.; Wang, J.; Bai, C.; Zhao, J.; Yun, Y.; Yu, Z.; Xue, Y.; Zhang, T.; Bao, W. Natural fermentation quality, bacteria, and functional profiles of three cuttings of alfalfa silage in a year in Inner Mongolia, China. Front. Microbiol. 2023, 14, 1083620. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Rode, L.M.; Eliason, M.V. Chewing activities and milk production of dairy cows fed alfalfa as hay, silage, or dried cubes of hay or silage. J. Dairy Sci. 1997, 80, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Hristov, A.N.; Huhtanen, P.; Rode, L.M.; Acharya, S.N.; McAllister, T.A. Comparison of the ruminal metabolism of nitrogen from 15N-labeled alfalfa preserved as hay or as silage. J. Dairy Sci. 2001, 84, 2738–2750. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Delaby, L.; Moloney, A.; Boland, T.; Lewis, E. Nutritive value of forage legumes used for grazing and silage. Ir. J. Agric. Food Res. 2009, 48, 167–187. [Google Scholar]
- Lee, M.R.F.; Connelly, P.L.; Tweed, J.K.S.; Dewhrrst, R.J.; Scollan, N.D. Effects of high-sugar ryegrass silage and mixtures with red clover silage on ruminant digestion. 2. Lipids. J. Anim. Sci. 2006, 84, 3061–3070. [Google Scholar] [CrossRef] [PubMed]
- Agca, C.; Broderick, G.A. Effect of grinding of high moisture corn on yield of lactating cows fed alfalfa silage. J. Anim. Sci. 1995, 78 (Suppl. S1), 220. [Google Scholar]
- Vagnoni, D.B.; Broderick, G.A. Effects of supplementation of energy or ruminally undegraded protein to lactating cows fed alfalfa hay or silage. J. Dairy Sci. 1997, 80, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.S.; Longuski, R.A.; Ying, Y. Effects of corn grain endosperm type and fineness of grind on site of digestion, ruminal digestion kinetics, and flow of nitrogen fractions to the duodenum in lactating dairy cows. J. Dairy Sci. 2021, 104, 7641–7652. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lei, Y.; Zhang, Y.; Liu, J.; Shi, X.; Jia, H.; Wang, C.; Chen, F.; Chu, Q. Rational trade-offs between yield increase and fertilizer inputs are essential for sustainable intensification: A case study in wheat-maize cropping systems in China. Sci. Total Environ. 2019, 20, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, Z.; Zhang, Y. Effects of the Partial Substitution of Corn with Wheat or Barley on the Growth Performance, Blood Antioxidant Capacity, Intestinal Health and Fecal Microbial Composition of Growing Pigs. Antioxidants 2022, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Moate, P.J.; Williams, S.R.; Jacobs, J.L.; Hannah, M.C.; Beauchemin, K.A.; Eckard, R.J.; Wales, W.J. Wheat is more potent than corn or barley for dietary mitigation of enteric methane emissions from dairy cows. J. Dairy Sci. 2017, 100, 7139–7153. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zheng, L.; Chen, X.; Han, X.; Cao, Y.; Yao, J. Metagenomic Analyses of Microbial and Carbohydrate-Active Enzymes in the Rumen of Dairy Goats Fed Different Rumen Degradable Starch. Front. Microbiol. 2020, 11, 1003. [Google Scholar] [CrossRef]
- Zhang, Z.A.; Wang, L.; Li, Q.W.; Li, F.; Ma, Z.Y.; Li, F.D.; Wang, Z.L.; Chen, L.; Yang, X.; Wang, X.J.; et al. Effects of dietary forage neutral detergent fiber and rumen degradable starch ratios on chewing activity, ruminal fermentation, ruminal microbes and nutrient digestibility of Hu sheep fed a pelleted total mixed ration. J. Anim. Sci. 2024, 102, p.skae100. [Google Scholar] [CrossRef]
- Ferraretto, L.F.; Crump, P.M.; Shaver, R.D. Effect of cereal grain type and corn grain harvesting and processing methods on intake, digestion, and milk production by dairy cows through a meta-analysis. J. Dairy Sci. 2013, 96, 533–550. [Google Scholar] [CrossRef]
- Gao, Z.H.; Raza, S.H.A.; Ma, B.Y.; Zhang, F.S.; Wang, Z.Y.; Hou, S.Z.; Almohaimeed, H.M.; Alhelaify, S.S.; Alzahrani, S.S.; Alharthy, O.M.; et al. Effects of dietary wheat supplementation levels on growth performance, rumen bacterial community and fermentation parameters in Chinese Tibetan Sheep. J. Anim. Physiol. Anim. Nutr. 2024, 108, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Na, M.; Liu, S.; Li, K.; Du, H.; Zhang, J.; Na, R. Rumen-Degradable Starch Improves Rumen Fermentation, Function, and Growth Performance by Altering Bacteria and Its Metabolome in Sheep Fed Alfalfa Hay or Silage. Animals 2025, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Savin, K.W.; Moate, P.J.; Williams, S.R.O.; Bath, C.; Hemsworth, J.; Wang, J.H.; Ram, D.; Zawadzki, J.; Rochfort, S.; Cocks, B.G. Dietary wheat and reduced methane yield are linked to rumen microbiome changes in dairy cows. PLoS ONE 2022, 17, e0268157. [Google Scholar] [CrossRef]
- Plascencia, A.; González, V.; Víctor, M.; Zinn, R.A. Comparative effects of grain source on digestion characteristics of finishing diets for feedlot cattle: Steam_flaked corn, barley, wheat, and oats. Can. J. Anim. Sci. 2018, 98, 794–800. [Google Scholar] [CrossRef]
- Xu, N.N.; Wang, D.M.; Wang, B.; Wang, J.K.; Liu, J.X. Different endosperm structures in wheat and corn affected in vitro rumen fermentation and nitrogen utilization of rice straw-based diet. Animal 2019, 13, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, Y.; Shen, Y.; Cao, Y.; Li, Q.; Wang, M.; Liu, M.; Wang, Z.; Huo, Z.; Ren, S.; et al. Effect of Dietary Rumen-Degradable Starch to Rumen-Degradable Protein Ratio on In Vitro Rumen Fermentation Characteristics and Microbial Protein Synthesis. Animals 2022, 12, 2633. [Google Scholar] [CrossRef]
- Sauvant, D.; Perez, J.M.; Tran, G. Tables of Composition and Nutritional Value of Feed Materials: Pig, Poultry, Sheep, Goats, Rabbits, Horses, Fish; Agricultural and Food Sciences; MTT Agrifood Research Finland: Jokioinen, The Finland, 2004; p. 10. [Google Scholar]
- Menke, K.H.; Steingass, H. Estimation of the Energetic Feed Value Obtained by Chemical Analysis and In Vitro Gas Production Using Rumen Fluid; Wageningen Academic Publisher: Wageningen, The Netherlands, 1988. [Google Scholar]
- Winichayakul, S.; Beechey-Gradwell, Z.; Muetzel, S.; Molano, G.; Crowther, T.; Lewis, S.; Xue, H.; Burke, J.; Bryan, G.; Roberts, N.J. In vitro gas production and rumen fermentation profile of fresh and ensiled genetically modified high-metabolizable energy ryegrass. J. Dairy Sci. 2020, 103, 2405–2418. [Google Scholar] [CrossRef]
- Seo, J.; Jung, J.K.; Seo, S. Evaluation of nutritional and economic feed values of spent coffee grounds and Artemisia princeps residues as a ruminant feed using in vitro ruminal fermentation. PeerJ 2015, 3, e1343. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.; Lee, S.S.; Mamuad, L.; Choi, Y.J.; Jeong, C.D.; Son, A.; Cho, K.K.; Kim, E.T.; Kim, S.B.; Lee, S.S. Enhancing Butyrate Production, Ruminal Fermentation and Microbial Population through Supplementation with Clostridium saccharobutylicum. J. Microbiol. Biotechnol. 2019, 29, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Chanjula, P.; Cherdthong, A. Effects of spent mushroom Cordyceps militaris supplementation on apparent digestibility, rumen fermentation, and blood metabolite parameters of goats. J. Anim. Sci. 2018, 96, 1150–1158. [Google Scholar] [CrossRef]
- Brewster, A.N.; Pless, L.A.; McLean, D.J.; Armstrong, S.A. Time of rumen fluid collection relative to feeding alters in vitro fermentation gas parameters. Transl. Anim. Sci. 2018, 2 (Suppl. S1), S97. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yang, X.J.; Cao, Y.C.; Li, S.X.; Yao, J.H.; Li, Z.J.; Sun, F.F. Effects of dietary effective fiber to rumen degradable starch ratios on the risk of sub-acute ruminal acidosis and rumen content fatty acids composition in dairy goat. Anim. Feed Sci. Technol. 2014, 189, 54–62. [Google Scholar] [CrossRef]
- Xue, Z.; Liu, N.; Wang, Y.; Yang, H.; Wei, Y.; Moriel, P.; Palmer, E.; Zhang, Y. Combining Orchardgrass and Alfalfa: Effects of Forage Ratios on In Vitro Rumen Degradation and Fermentation Characteristics of Silage Compared with Hay. Animals 2019, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Hristov, A.N.; Broderick, G.A. Synthesis of microbial protein in ruminally cannulated cows fed alfalfa silage, alfalfa hay, or corn silage. J. Dairy Sci. 1996, 79, 1627–1637. [Google Scholar] [CrossRef]
- Belanche, A.; Doreau, M.; Edwards, J.E.; Moorby, J.M.; Pinloche, E.; Newbold, C.J. Shifts in the rumen microbiota due to the type of carbohydrate and level of protein ingested by dairy cattle are associated with changes in rumen fermentation. J. Nutr. 2012, 142, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.B.; Huntington, G.B. Nutrient synchrony: Sound in theory, elusive in practice. J. Anim. Sci. 2007, 86, E287–E292. [Google Scholar] [CrossRef]
- Gallo, A.; Giuberti, G.; Atzori, A.S.; Masoero, F. Short communication: In vitro rumen gas production and starch degradation of starch-based feeds depend on mean particle size. J. Dairy Sci. 2018, 101, 6142–6149. [Google Scholar] [CrossRef]
- Calabrò, S.; Cutrignelli, M.I.; Bovera, F.; Piccolo, G.; Infascelli, F. In vitro fermentation kinetics of carbohydrate fractions of fresh forage, silage and hay of Avena sativa. J. Sci. Food Agric. 2005, 85, 1838–1844. [Google Scholar] [CrossRef]
- Bashir, K.; Aggarwal, M. Physicochemical, structural and functional properties of native and irradiated starch: A review. J. Food Sci. Technol. 2019, 56, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Lettat, A.; Nozière, P.; Silberberg, M.; Morgavi, D.P.; Berger, C.; Martin, C. Rumen microbial and fermentation characteristics are affected differently by bacterial probiotic supplementation during induced lactic and subacute acidosis in sheep. BMC Microbiol. 2012, 12, 142. [Google Scholar] [CrossRef]
- Russo, V.M.; Leury, B.J.; Kennedy, E.; Hannah, M.C.; Auldist, M.J.; Wales, W.J. Forage type influences milk yield and ruminal responses to wheat adaptation in late-lactation dairy cows. J. Dairy Sci. 2018, 101, 9901–9914. [Google Scholar] [CrossRef] [PubMed]
- Schwandt, E.F.; Hubbert, M.E.; Thomson, D.U.; Vahl, C.I.; Bartle, S.J.; Reinhardt, C.J. A survey of starch availability of steam-flaked corn in commercial feedlots evaluating roll size and flake density. Prof. Anim. Sci. 2016, 32, 550–560. [Google Scholar] [CrossRef]
- Shen, J.S.; Song, L.J.; Sun, H.Z.; Wang, B.; Chai, Z.; Chacher, B.; Liu, J.X. Effects of corn and soybean meal types on rumen fermentation, nitrogen metabolism and productivity in dairy cows. Asian-Australas. J. Anim. Sci. 2015, 28, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Hess, P.S.; Moate, P.J.; Williams, S.R.O.; Jacobs, J.L.; Beauchemin, K.A.; Durmic, Z.; Hannah, M.C.; Eckard, R.J. The effect of diet of the donor cows on in vitro measurements of methane production from wheat and corn incubated in various forage-to-grain ratios. J. Sci. Food Agric. 2019, 99, 3451–3458. [Google Scholar] [CrossRef]
- Moate, P.J.; Williams, S.R.O.; Deighton, M.H.; Hannah, M.C.; Ribaux, B.E.; Morris, G.L.; Jacobs, J.L.; Hill, J.; Wales, W.J. Effects of feeding wheat or corn and of rumen fistulation on milk production and methane emissions of dairy cows. Anim. Prod. Sci. 2019, 59, 891–905. [Google Scholar] [CrossRef]
- Liu, Y.F.; Zhao, H.B.; Liu, X.M.; You, W.; Cheng, H.J.; Wan, F.C.; Liu, G.F.; Tan, X.W.; Song, E.L.; Zhang, X.L. Substitution of Wheat for Corn in Beef Cattle Diets: Digestibility, Digestive Enzyme Activities, Serum Metabolite Contents and Ruminal Fermentation. Asian Australas. J. Anim. Sci. 2015, 29, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.Q.; Costa, S.F.; Lopes, F.; Guerreiro, M.C.; Armentano, L.E.; Pereira, M.N. Rumen morphometrics and the effect of digesta pH and volume on volatile fatty acid absorption. J. Anim. Sci. 2013, 91, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.; Jensen, B.B.; Engberg, R.M. The effect of pectin, corn and wheat starch, inulin and pH on in vitro production of methane, short chain fatty acids and on the microbial community composition in rumen fluid. Anaerobe 2012, 18, 83–90. [Google Scholar] [CrossRef]
- Shen, Y.Z.; Zhao, F.F.; Yu, L.H.; Yang, W.Z.; Wang, M.Z.; Wang, H.R. Starch sources and concentration in diet of dairy goats affected ruminal pH and fermentation, and inflammatory response. Anim. Prod. Sci. 2019, 59, 1640–1647. [Google Scholar] [CrossRef]
- Clark, J.M.A.; Sun, D. Guidelines for the ethical review of laboratory animal welfare People’s Republic of China National Standard GB/T 35892-2018. Anim. Models Exp. Med. 2020, 3, 107–117. [Google Scholar]
| Nutrient 1 | Corn Stalk | Alfalfa Silage | Corn | Wheat | Soybean Meal | Wheat Bran |
|---|---|---|---|---|---|---|
| DM | 94.44 | 37.40 | 86.90 | 85.70 | 91.20 | 90.2 |
| DE, MJ/kg | 9.58 | 10.77 | 14.86 | 14.91 | 16.41 | 12.81 |
| CP | 4.52 | 19.10 | 8.50 | 13.50 | 47.60 | 17.4 |
| starch | 3.5 | 4.80 | 70.40 | 63.10 | - | - |
| ADF | 42.92 | 26.58 | 3.60 | 4.20 | 10.10 | 13.80 |
| NDF | 71.32 | 36.67 | 9.80 | 12.50 | 19.60 | 40.12 |
| EE | 3.35 | 3.50 | 3.84 | 1.98 | 7.12 | 4.39 |
| RDS | - | - | 38.08 | 49.71 | - | - |
| RDP | - | 58.00 | - | - | - | - |
| Ingredients | Treatment 1 | Nutrient 3 | Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | R1 | R2 | R3 | R4 | R5 | ||
| Corn stalk | 10 | 10 | 10 | 10 | 10 | DM | 66.74 | 66.13 | 65.43 | 65.27 | 65.12 |
| Alfalfa silage | 40 | 40 | 40 | 40 | 40 | DE, MJ/kg | 12.03 | 12.07 | 12.08 | 12.07 | 11.98 |
| Wheat | 0 | 10 | 23 | 33 | 43.5 | CP | 14.64 | 14.67 | 14.45 | 14.56 | 14.73 |
| Corn | 39 | 30 | 19 | 10 | 0 | starch | 28.55 | 28.47 | 28.85 | 28.80 | 28.37 |
| Soybean meal | 5 | 4 | 2 | 1 | 0 | ADF | 21.27 | 21.20 | 21.08 | 21.07 | 21.12 |
| Wheat bran | 4 | 3.5 | 3 | 3 | 3.5 | NDF | 30.22 | 30.19 | 30.14 | 30.32 | 30.65 |
| Soybean oil | 0 | 0.5 | 1 | 1 | 1 | EE | 4.05 | 4.25 | 4.30 | 4.04 | 3.78 |
| Calcium Hydrogen Phosohate | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | RDS | 14.85 | 16.40 | 18.67 | 20.21 | 21.62 |
| Limestone | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | RDP | 6.32 | 6.32 | 6.32 | 6.32 | 6.32 |
| Salt | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | RDS/RDP | 2.35 | 2.60 | 2.95 | 3.20 | 3.47 |
| Premix 2 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | ||||||
| Items | Groups 1 | SEM | p-Values 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | RDS | L | Q | ||
| 3 h | |||||||||
| pH | 6.67 | 6.65 | 6.65 | 6.63 | 6.64 | 0.01 | 0.278 | 0.035 | 0.101 |
| NH3-N, mg/100 mL | 21.83 | 21.94 | 20.64 | 20.38 | 21.44 | 0.37 | 0.285 | 0.060 | 0.082 |
| BCP, mg/100 mL | 36.34 c | 35.12 c | 38.44 bc | 42.98 a | 40.33 ab | 0.69 | 0.004 | 0.003 | 0.017 |
| α-amylase, U/dL | 272.39 c | 302.15 b | 319.89 ab | 317.60 ab | 343.35 a | 26.6 | 0.002 | 0.001 | 0.001 |
| 6 h | |||||||||
| pH | 6.46 | 6.40 | 6.41 | 6.37 | 6.34 | 0.03 | 0.462 | 0.063 | 0.191 |
| NH3-N, mg/100 mL | 23.21 | 23.00 | 22.36 | 20.92 | 21.78 | 0.44 | 0.177 | 0.030 | 0.097 |
| BCP, mg/100 mL | 40.08 | 40.05 | 41.76 | 43.82 | 41.45 | 0.64 | 0.860 | 0.358 | 0.662 |
| α-amylase, U/dL | 256.16 c | 275.42 bc | 295.26 ab | 299.93 a | 286.51 ab | 37.08 | 0.005 | 0.005 | 0.001 |
| 12 h | |||||||||
| pH | 6.18 | 6.14 | 6.13 | 6.09 | 6.09 | 0.03 | 0.632 | 0.099 | 0.263 |
| NH3-N, mg/100 mL | 26.19 | 26.55 | 25.10 | 24.25 | 23.77 | 0.67 | 0.412 | 0.047 | 0.141 |
| BCP, mg/100 mL | 47.85 | 47.64 | 48.60 | 50.50 | 51.97 | 0.37 | 0.162 | 0.013 | 0.296 |
| α-amylase, U/dL | 203.72 | 235.77 | 262.66 | 274.68 | 244.35 | 15.87 | 0.171 | 0.068 | 0.038 |
| 24 h | |||||||||
| pH | 6.04 | 6.00 | 5.98 | 5.94 | 6.01 | 0.04 | 0.813 | 0.496 | 0.055 |
| NH3-N, mg/100 mL | 31.80 | 31.76 | 30.90 | 31.40 | 31.67 | 0.45 | 0.773 | 0.670 | 0.576 |
| BCP, mg/100 mL | 55.57 | 57.13 | 55.76 | 54.18 | 54.89 | 0.37 | 0.757 | 0.376 | 0.635 |
| α-amylase, U/dL | 187.89 | 179.14 | 195.48 | 240.41 | 211.82 | 30.38 | 0.075 | 0.043 | 0.141 |
| Items | Groups 1 | SEM | p-Values 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | RDS | L | Q | ||
| 3 h | |||||||||
| Acetate | 12.47 | 12.98 | 12.99 | 13.36 | 12.88 | 0.83 | 0.976 | 0.655 | 0.815 |
| Propionate | 4.74 | 5.07 | 5.17 | 5.16 | 5.24 | 0.31 | 0.844 | 0.264 | 0.488 |
| Isobutyrate | 2.29 | 2.20 | 2.10 | 2.15 | 2.00 | 0.25 | 0.964 | 0.445 | 0.756 |
| Butyrate | 2.53 | 2.47 | 2.36 | 2.38 | 2.17 | 0.23 | 0.880 | 0.278 | 0.556 |
| Isovaleric | 4.27 | 3.75 | 4.02 | 4.19 | 3.81 | 0.43 | 0.924 | 0.753 | 0.947 |
| Valerianic | 2.05 | 2.04 | 1.91 | 1.94 | 1.77 | 0.19 | 0.877 | 0.279 | 0.557 |
| TSCFA | 28.35 | 28.51 | 28.54 | 29.18 | 27.87 | 1.96 | 0.996 | 0.975 | 0.960 |
| A/P | 2.66 | 2.56 | 2.51 | 2.59 | 2.44 | 0.10 | 0.687 | 0.206 | 0.464 |
| 6 h | |||||||||
| Acetate | 14.63 c | 15.21 c | 15.55 bc | 16.69 ab | 17.21 a | 0.68 | 0.011 | 0.001 | 0.001 |
| Propionate | 5.62 c | 5.80 c | 6.05 bc | 6.48 b | 7.00 a | 0.33 | 0.001 | 0.001 | 0.001 |
| Isobutyrate | 2.71 | 2.73 | 2.69 | 2.57 | 2.55 | 0.11 | 0.757 | 0.197 | 0.406 |
| Butyrate | 2.96 | 3.01 | 3.02 | 3.07 | 3.07 | 0.14 | 0.986 | 0.547 | 0.837 |
| Isovaleric | 4.47 | 4.53 | 4.46 | 4.52 | 4.60 | 0.18 | 0.989 | 0.678 | 0.897 |
| Valerianic | 3.35 | 3.22 | 3.25 | 3.26 | 3.04 | 0.11 | 0.456 | 0.121 | 0.270 |
| TSCFA | 33.73 | 34.50 | 35.02 | 36.59 | 37.47 | 1.14 | 0.091 | 0.003 | 0.014 |
| A/P | 2.60 | 2.62 | 2.57 | 2.58 | 2.46 | 0.08 | 0.659 | 0.188 | 0.311 |
| 12 h | |||||||||
| Acetate | 23.26 | 24.87 | 24.62 | 24.24 | 25.88 | 1.03 | 0.544 | 0.170 | 0.405 |
| Propionate | 9.05 | 9.79 | 10.26 | 9.97 | 10.24 | 0.39 | 0.162 | 0.029 | 0.448 |
| Isobutyrate | 3.33 | 3.23 | 3.29 | 3.43 | 2.96 | 0.27 | 0.831 | 0.562 | 0.697 |
| Butyrate | 4.69 | 4.47 | 5.19 | 5.00 | 5.05 | 0.27 | 0.353 | 0.135 | 0.328 |
| Isovaleric | 6.10 | 6.00 | 6.25 | 5.64 | 5.64 | 0.25 | 0.296 | 0.118 | 0.219 |
| Valerianic | 6.54 | 6.42 | 7.38 | 6.87 | 6.44 | 0.33 | 0.187 | 0.723 | 0.245 |
| TSCFA | 52.98 | 54.77 | 56.98 | 55.14 | 56.20 | 1.34 | 0.287 | 0.096 | 0.139 |
| A/P | 2.57 | 2.54 | 2.40 | 2.43 | 2.53 | 0.07 | 0.481 | 0.392 | 0.250 |
| 24 h | |||||||||
| Acetate | 27.02 | 27.60 | 27.46 | 28.09 | 29.16 | 1.31 | 0.863 | 0.276 | 0.526 |
| Propionate | 9.65 | 10.22 | 10.32 | 10.75 | 11.09 | 0.56 | 0.483 | 0.053 | 0.165 |
| Isobutyrate | 3.82 | 4.23 | 4.30 | 4.62 | 4.94 | 0.53 | 0.696 | 0.121 | 0.314 |
| Butyrate | 5.80 | 5.63 | 5.83 | 6.66 | 6.79 | 0.59 | 0.565 | 0.246 | 0.116 |
| Isovaleric | 7.53 | 7.39 | 7.15 | 7.08 | 8.50 | 0.84 | 0.811 | 0.591 | 0.518 |
| Valerianic | 7.34 | 7.44 | 7.94 | 7.64 | 8.97 | 0.82 | 0.699 | 0.201 | 0.387 |
| TSCFA | 61.16 | 62.51 | 63.00 | 64.83 | 69.45 | 4.02 | 0.696 | 0.151 | 0.314 |
| A/P | 2.80 | 2.69 | 2.66 | 2.61 | 2.66 | 0.10 | 0.824 | 0.296 | 0.461 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Liu, Y.; Na, M.; Zhang, Y.; Na, R. Effects of Rumen-Degradable Starch Levels on In Vitro Rumen Fermentation and Microbial Protein Synthesis in Alfalfa Silage. Fermentation 2025, 11, 106. https://doi.org/10.3390/fermentation11020106
Guo W, Liu Y, Na M, Zhang Y, Na R. Effects of Rumen-Degradable Starch Levels on In Vitro Rumen Fermentation and Microbial Protein Synthesis in Alfalfa Silage. Fermentation. 2025; 11(2):106. https://doi.org/10.3390/fermentation11020106
Chicago/Turabian StyleGuo, Wenliang, Yulan Liu, Meila Na, Yu Zhang, and Renhua Na. 2025. "Effects of Rumen-Degradable Starch Levels on In Vitro Rumen Fermentation and Microbial Protein Synthesis in Alfalfa Silage" Fermentation 11, no. 2: 106. https://doi.org/10.3390/fermentation11020106
APA StyleGuo, W., Liu, Y., Na, M., Zhang, Y., & Na, R. (2025). Effects of Rumen-Degradable Starch Levels on In Vitro Rumen Fermentation and Microbial Protein Synthesis in Alfalfa Silage. Fermentation, 11(2), 106. https://doi.org/10.3390/fermentation11020106






