Abstract
Small black soybeans (Seomoktae, SBS), traditionally regarded as medicinal beans in East Asia, contain abundant bioactive compounds with antioxidant and anti-inflammatory potential. This study aimed to develop a functional plant-based milk substitute by fermenting soymilk (yellow soybean, YS) supplemented with SBS (25% or 50%) using Streptococcus thermophilus JAMI_LB_02 and Lactiplantibacillus plantarum JAMI_LB_05 (patented LABs) to enhance probiotic functionality and nutritional value. Fermentation characteristics, microbial viability, antioxidant activity, anthocyanin content, and free amino acid profiles were evaluated. After 72 h, total acidity in all samples exceeded 0.81%, and viable LAB counts reached 10.07–10.21 log CFU/mL, surpassing the Korean Food Code. The 50% SBS formulation exhibited significantly higher radical scavenging activity, total phenol and flavonoid contents, and anthocyanin levels (p < 0.05). Digestive enzyme treatment increased total free amino acid in SBS 50%, particularly functional amino acids such as arginine, alanine, and asparagine. Heatmap analysis classified products with high SBS content as Group A and analyzed the correlation between redness, antioxidant activity, and water-soluble amino acid content. Overall, SBS-fermented soymilk is an improved protein digestibility, probiotic-rich, and alternative to dairy-based fermented products, aligning with the growing consumer demand for plant-based functional foods.
1. Introduction
Fermentation is an ancient food preservation method that encourages the growth of specific microorganisms, thereby reducing microbial contamination. A fermentation starter introduces specific microorganisms into the food, allowing them to become the dominant bacteria, ultimately leading to successful fermentation [1]. Lactic acid bacteria (LAB) are widely distributed in nature and are found in various fermentation products such as cheese, kimchi, kefir, and natto. Lactobacillus used in fermented foods exhibit acid and bile resistance, enabling them to survive in the body and function as probiotics only when intestinal adhesion is high [2]. The initial proliferation of these lactobacilli is crucial in the production of fermented foods, and the predominant bacteria may vary depending on the fermentation conditions. In fermented dairy products, species such as Streptococcus, Lactobacillus and Lactococcus serve as dominant organisms [3]. The careful selection of strains as starter cultures or co-cultures in fermentation processes can enhance the in situ expression of desired properties [1]. Therefore, controlling the dominant species in the production of fermented foods is vital in the food industry.
Plant-based milk alternatives, made from grains, legumes, nuts, and fruits, have rapidly gained popularity in response to vegetarian and health-conscious consumer trends. In the food industry, milk alternatives are derived from various plant-based raw materials, including grains, legumes, nuts, and fruits. Specifically, these alternatives primarily consist of beverages made from oats, rice, soybeans, almonds, and coconut [4,5]. Plant-based milk alternatives are rich in plant-based proteins and fibers and possess antioxidant properties from anthocyanins and polyphenols found in plants, offering distinct nutritional benefits [6,7]. Additionally, with the recent rise in veganism and consumer preferences for health-oriented products, the demand for vegan options is growing, leading to an increase in companies producing products aimed at the vegan market, such as milk alternatives [8]. A key advantage of plant-based milk alternatives is their lack of lactose and casein. Lactose intolerance, caused by a deficiency of the enzyme lactase, can manifest as symptoms such as flatulence, abdominal pain, bloating, and diarrhea [9]. Therefore, people with conditions such as lactose intolerance are recommended to choose alternatives, such as plant-based drinks.
Among various plant-based sources, black soybeans have attracted attention due to their superior nutritional properties. Black soybeans and yellow soybeans (YS) are both classified as Glycine max. Black soybeans, in particular, are classified into Seoritae, Seomoktae, and Heuktae based on the color of the testa and cotyledon, and they are used differently depending on their intended purpose. Among legumes, Seomoktae is smaller and glossier than Seoritae, and both are known as medicinal beans in East Asia, having been used for lowering blood sugar and lowering inflammation since ancient times [10,11]. Small black soybeans (SBS) are rich in antioxidants, such as anthocyanins and isoflavones, which can prevent aging and improve blood circulation. Due to their high protein content, they are effective in strengthening bodily components and promoting hair and scalp health. Recent studies have focused on the management of age-related diseases and their antidiabetic effects [12,13]. In addition, it is known that functional components increase through fermentation of black bean soymilk using LAB, as isoflavones in the form of glucosidase are converted to aglycones, increasing antioxidant activity and exhibiting cytotoxicity to human carcinoma cells, Hep 3B, indication an anticancer effect [14,15].
In this study, we developed a plant-based fermented food product using Streptococcus thermophilus JAMI_LB_02 and Lactiplantibacillus plantarum JAMI_LB_05, which have been identified and patented for their probiotic and functional potential. These strains were assessed for microbial identity, gastrointestinal viability, antibiotic resistance, and notably, strong adhesion to intestinal epithelial cells [16]. We evaluated plant-based fermented products in terms of fermentation efficiency, antioxidant capacity, anthocyanin content, and free amino acid profile. This study aims to develop functional plant-based milk alternatives using patented LAB strains and to determine whether their probiotic functionalities can be effectively expressed in soybean-based fermentation systems.
2. Materials and Methods
2.1. Sample Preparation
YS and SBS were prepared by washing them several times with water and then immersing them in ten times their volume of water for 24 h. For each sample group (YS 100%, SBS 25%, and SBS 50%), a total of 100 g of soybeans, 500 mL of water, and 10 g of sugar were added and ground at 90 °C for 25 min using a soymilk maker (IOM-801A, Soylove, Ronic, Seoul, Republic of Korea). Soymilk was prepared by filtering the mixture to remove the bean pulp. The resulting soymilk was sterilized at 121 °C for 15 min and stored at 4 °C before use in the experiment. The milk used as the control was prepared by adding 100 mL of milk and 10 g of sugar and fermenting it under the same conditions as the soymilk samples.
The patented strains (Korea: 10-2024-0167216) obtained in previous studies were used as lactobacilli for fermentation. Streptococcus thermophilus JAMI_LB_02 (KACC 92623P) and Lactiplantibacillus plantarum JAMI_LB_05 (KACC 92624P) that increases intestinal adhesion when used as a mixed culture were used in the experiments. Each strain was inoculated into MRS broth with one colony and cultured at 37 °C for 24 h. The culture medium for each strain was mixed to adjust the bacterial count to 109 CFU/mL. The mixed culture was then inoculated into the soymilk at a concentration of 5% and cultured in an incubator at 37 °C for 72 h to prepare the sample.
2.2. Physicochemical Properties
The physicochemical properties were determined using the supernatant obtained from centrifugation of the sample (4 °C, 10,000× g, 10 min). The pH was measured with a pH meter (Professional Meter PP-15, Sartorius, Goettingen, Germany), and water-soluble solids were analyzed using a digital refractometer (PAL-1, Atago, Tokyo, Japan). Total acidity was titrated with a 0.1 N NaOH solution until a scarlet color was maintained for 30 s or more after adding 40 µL of 1% phenolphthalein solution following a 10-fold dilution of the sample. This value was calculated using the following equation: Total acidity (%) = ((V × F × A × D)/S) × 100. Where V is the volume (mL) of NaOH used for titration, F is the normality of NaOH, A is the lactic acid equivalent factor, D is the dilution factor, and S is the weight (g) of the sample. The chromaticity of the samples was measured using a colorimeter (CM-5, Konica Minolta, Tokyo, Japan), and the brightness (L*), redness (a*), and yellowness (b*) values were measured three times and expressed as average values.
2.3. Viable Bacterial Count Measurement
The sample was continuously diluted to 109 in MRS broth and spread on MRS agar. After incubating for 24 h at 37 °C, the number of probiotics per 1 mL of culture was calculated by multiplying the average colony count by the dilution factor.
2.4. Antioxidant Activity Measurement
Freeze-dried samples were extracted with ethanol at a concentration of 10 mg/mL. For the hydrogen donation capacity to 2,2-Diphenyl-1-picrylhydrazyl (DPPH), 20 μL of the sample solution and 80 μL of the DPPH solution were mixed in a 96-well plate, left at 37 °C for 30 min in the dark, and the absorbance was measured with a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA) at 517 nm. The radical scavenging effect was expressed as a percentage compared to the control group, which contained no added sample. L-ascorbic acid served as a positive control. The total phenolic compounds (TPC) of the sample were measured by mixing 500 µL of 10% Folin & Ciocalteu’s phenol reagent with 100 µL of the sample extract and allowing the reaction to proceed at 37 °C for 5 min. After adding 600 µL of 2% sodium carbonate solution, the reaction was continued at room temperature for 30 min. Absorbance was measured using a microplate reader at 765 nm. Gallic acid was used for the standard curve, and the content was expressed as mg gallic acid equivalent (GAE)/g. The total flavonoid content (TFC) was measured by modifying the method of Sembiring et al. [17]. The sample extract and 10% aluminum chloride (w/v) were mixed in a 1:1 ratio and reacted at 37 °C for 5 min, after which absorbance was measured with a microplate reader at a wavelength of 415 nm. Quercetin was used to establish the standard curve, and its content was expressed as mg quercetin equivalent (QE)/g.
2.5. Total Anthocyanin Content (TAC) Analysis
The TAC of the samples was measured using an anthocyanin assay kit (KB03015, Bioquochem, Oviedo, Asturias, Spain). The sample was freeze-dried and ground, followed by ultrasonic extraction with the addition of 10 mL of 70% ethanol to the sample (0.5 g). A portion of the extract was centrifuged (4 °C, 10,000× g, 10 min) to obtain a supernatant, which was then analyzed by filtration using a 0.45 µm membrane filter. Twenty microliters of the sample were mixed with 200 µL of reagent A and allowed to react at room temperature for 10 min, after which absorbance was measured with a microplate reader at wavelengths of 500 and 765 nm. The blank value of the sample was determined by mixing 20 µL of the sample with 200 µL of reagent B in the same manner. The absorbance was calculated using the following equation: Anthocyanins (mg/L) = (A × DF × MW × 1000)/ε × l. Where A is the absorbance previously calculated, DF is the dilution factor, MW is the molecular weight for cyanidin-3-glucoside (449.2 g/mol), 1000 is a factor for conversion from g to mg, ε is the molar extinction coefficient for cyanidin-3-glucoside (26,900 L mol−1 cm−1, and l is the pathlength in cm (0.6 cm for a 96-well microplate).
2.6. Digestive Model Based on Enzyme Treatment
The digestive model of the sample was created by referring to INFOGEST (static in vitro simulation of gastric food digestion) [18]. All enzymes were prepared prior to the experiment. Salivary amylase (Sigma Chemical Co., St. Louis, MO, USA) was prepared at a concentration of 1600 U/mg, rabbit lipase (Sigma Chemical Co., St. Louis, MO, USA) at 740 U/mg, pepsin (Sigma Chemical Co., St. Louis, MO, USA) at 2500 U/mg, and pancreatin (Sigma Chemical Co., St. Louis, MO, USA) at 200 U/mg. One milliliter of each sample, fermented for 72 h, was taken, mixed with preheated amylase (1.5 mL), and allowed to react for 2 min. Subsequently, 3 mL of pepsin was added, the pH was adjusted to 3.0 with 0.1 M HCl, and the mixture was reacted for 2 h at 37 °C with shaking. After adding 3 mL of lipase and pancreatin, the pH was adjusted to 7.0 with 0.1 M NaOH, and the reaction continued for 2 h with shaking. Once the reaction was complete, the supernatant was obtained by centrifugation (10,000× g, 5 min), and the reaction was terminated by cooling on ice. The blank was prepared by adding the enzyme to the phosphate-buffered saline (137 mM NaCl, 10 mM phosphate, and 2.7 mM KCl; pH 7.4). The samples were then analyzed for free amino acids.
2.7. Free Amino Acid Analysis
Free amino acids in the samples were measured by modifying the method described by Lee et al. [19]. A specific amount of the sample was homogenized with 70% ethanol, and the proteins were precipitated and removed by allowing the sample to sit at room temperature. Subsequently, the supernatant was centrifuged at 10,000× g for 10 min, collected, and dried using hot water. The sample was then diluted 10 times in 0.12 N sample dilution buffer (pH 2.20) and filtered through a 0.22 μm membrane filter (Millipore, Burlington, MA, USA) before being analyzed using an amino acid analyzer (S433, SYKAM, Eresing, Germany).
2.8. Heatmap Analysis
The relationships among physicochemical properties, amino acid composition, and antioxidant activity of samples were visualized using a heatmap. Data were normalized using Z-score standardization to eliminate differences in scale among variables. The heatmap was generated using the pheatmap package in R software (version 4.2.2; R Foundation for Statistical Computing, Vienna, Austra). The color gradient was set from red (high value) to blue (low value), indicating relative abundance or activity levels. Cluster groups of metabolites and physicochemical parameters were manually divided into three groups (A, B, and C) according to the dendrogram results. The sample classes were labeled as milk, YS, SBS 25%, and SBS 50%, representing different substitution ratios of soybean sprout extract.
2.9. Statistical Analysis
The results obtained from each experiment were analyzed using SPSS Statistics (version 12.0; SPSS, Inc., Chicago, IL, USA). The significance among each sample was assessed using one-way ANOVA and Duncan’s multiple range test at p < 0.05, while an independent sample t-test was conducted simultaneously for comparison between specific samples.
3. Results
3.1. Fermentation Characteristics
Samples prepared from milk and soybean raw materials were collected at 12 h intervals of fermentation to measure pH, soluble solid content, total acidity, and chromaticity (Figure 1). The pH of the milk decreased significantly from 6.25 ± 0.01 to 3.55 ± 0.01 after 72 h of fermentation. All soymilk samples (YS, SBS 25%, and SBS 50%) exhibited similar fermentation patterns, with pH values significantly decreasing from an initial range of 6.72–6.82 to 3.50–3.61 after 60 h of fermentation. In a comparative study [20], the pH values of yogurt prepared from milk and soymilk were 4.1 and 5.4 after 12 h of fermentation, confirming that fermentation progressed more rapidly in milk under the experimental conditions. The soluble solids in the soymilk samples decreased with fermentation time from the level of 5.05°Brix before fermentation, reaching 3.35 to 3.55°Brix at the end of fermentation. Meanwhile, milk was relatively high at 13.70°Brix before fermentation and significantly decreased to 8.80°Brix after 72 h of fermentation. LAB is known to produce lactic acid by consuming sucrose and glucose according to fermentation [4], and the decrease in sugar content was also confirmed in the samples prepared in this study. The total acidity of all samples increased in parallel with the decrease in pH. In milk, total acidity rose significantly from an initial value of 0.18% to 1.40 ± 0.06% after 72 h of fermentation. The total acidity of the soymilk samples increased significantly over the fermentation period, reaching 0.81 to 0.92% by the end of fermentation. Previous studies have reported that the optimal quality of conventional milk yogurt is associated with total acidity ranging from 1.0% to 1.1% [21]. This study confirmed that the total acidity of the milk samples was slightly higher than the optimal acidity at the end of fermentation, whereas that of the soymilk samples was significantly lower. As shown in Figure 1D–F, the chromaticity of the samples was determined from the quantitative values of brightness and color coordinates (L*, a*, b*). As the amount of SBS added increased, the L* (lightness) value decreased, while the a* (redness) value tended to increase. As fermentation progressed, the L* values tended to increase in all samples, although there was no significant change.
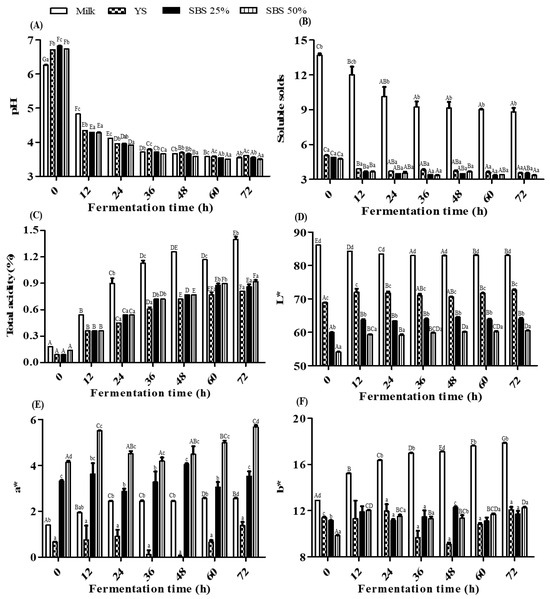
Figure 1.
Changes in physicochemical properties of the sample according to fermentation period. (A) pH; (B) Soluble solids; (C) Total acidity; (D) L* (lightness); (E) a* (green–red coordinate); (F) b* (blue–yellow coordinate). YS refers to yellow soybean, while SBS refers to small black soybean. Mean values ± SD (n = 3) are presented. Different uppercase letters (A, B, C, etc.) indicate time-dependent differences in the same sample, and lowercase letters (a, b, c, etc.) indicate differences in the sample compared to the same fermentation time according to Duncan’s multiple rage test (p < 0.05).
3.2. Viable Bacterial Count
The results for viable LAB in the samples are presented in Table 1. All samples exhibited the highest viable LAB counts after 24 h of fermentation, followed by a decreasing trend after 60 h. Milk reached the highest viable bacteria count of 12.56 log CFU/mL after 24 h, while the soymilk samples showed similar maximum values ranging from 10.04 to 10.21 log CFU/mL, although the time to reach the stationary phase varied for each sample. Viable LAB counts are critical quality indicators in fermented dairy products, and maintaining sufficient counts is essential for the development of probiotic products [22]. According to the Korea Food Code, beverages processed by fermenting dairy or plant-based raw materials must contain at least 106 viable LAB per mL [23]. In this study, all soymilk samples exceeded this standard after 12 h of fermentation, and the viable counts either increased or remained stable thereafter, highlighting their potential as probiotic-rich, plant-based products.

Table 1.
Viable bacterial count according to the sample’s fermentation period.
3.3. Sample Extract Antioxidant Activity
The antioxidant activities of the extracts were evaluated using a DPPH radical scavenging assay, and the results are shown in Figure 2. All the samples exhibited increased antioxidant activity after 24 h of fermentation. The antioxidant activity of YS rapidly rose from 4.42 ± 0.96% to 25.93 ± 1.60% after 24 h, reaching 24.23 ± 1.56% by the end of fermentation. Antioxidant activity was significantly enhanced by the addition of SBS, with the SBS 50% group demonstrating the highest antioxidant activity, with a DPPH radical scavenging activity of 33.40 ± 1.34%.
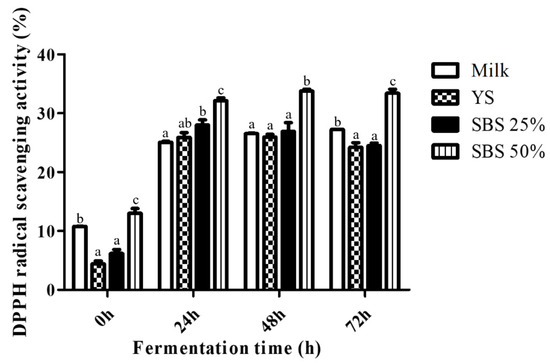
Figure 2.
DPPH radical scavenging activity of the samples over different fermentation times. YS refers to yellow soybean, while SBS refers to small black soybean. Mean values ± SD (n = 3) are presented. Lowercase letters (a, b, c, etc.) at the same fermentation time indicate significant differences according to Duncan’s multiple range test (p < 0.05).
Among the plant-based fermented beverages, soybeans are the most widely used due to their high nutrient content, comparable to that of milk [24]. During fermentation, bioactive compounds such as polyphenols, tocopherols, and peptides are generated through microbial metabolism and contribute to antioxidant activity [25,26]. In this study, the fermented product containing YS and SBS exhibited antioxidant activity comparable to that of milk after 72 h, while SBS 50% demonstrated the greatest improvement.
The TPC and TFC of the sample extracts are presented in Table 2. The TPC significantly increased with the addition of SBS, whereas milk consistently showed lower values, ranging from 39.06 to 46.02 µg GAE/g, compared to soymilk samples at all time points. SBS 50% recorded the highest TPC of 108.59 ± 6.15 µg GAE/g before fermentation, which gradually decreased to 94.52 ± 1.78 µg GAE/g at 72 h. Consistent with our findings, Hong et al. [27] reported higher polyphenol content in SBS extracts, measuring 22.89 ± 0.25 mg/g and 26.22 ± 1.02 mg/g in the ethanol extract, compared with YS, supporting the positive association between SBS content and TPC levels. The TFC value did not significantly change with fermentation time in all samples but varied according to the addition of SBS. Milk measured 2.17 ± 1.42 µg QE/g before fermentation, which was significantly lower than that of the plant-based samples (5.32–7.14 µg QE/g). At the end of fermentation, milk measured 2.29 ± 0.27 µg QE/g, while YS, SBS 25%, and SBS 50% were measured at 7.06 ± 0.01 µg QE/g, 7.83 ± 0.93 µg QE/g, and 9.83 µg QE/g, respectively, showing significant differences.

Table 2.
TPC and TFC according to the sample’s fermentation time.
3.4. Total Anthocyanin Content (TAC)
The TAC of the extracts is presented in Figure 3. The TAC of milk fluctuated from 45.69 ± 21.07 µg/L during the fermentation process to 21.57 ± 12.75, 67.03 ± 40.36, and 40.12 ± 29.76 µg/L at 24 h intervals, respectively, but no consistent change was observed during fermentation. In contrast, the TAC of soymilk samples was higher than that of milk (323.65 ± 43.15, 398.80 ± 60.49, and 381.17 ± 98.80 µg/L) and showed a tendency to gradually increase during fermentation. Notably, the SBS 50% group exhibited a significant rise to 508.26 ± 44.18 µg/L at 48 h, which further increased to 536.09 ± 44.18 µg/L at 72 h, demonstrating a significantly higher TAC than the YS group.
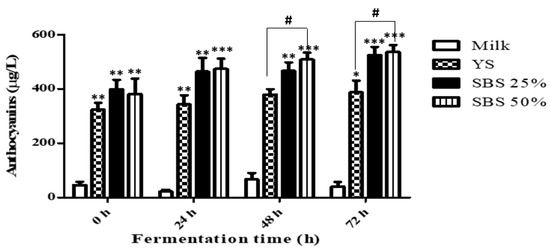
Figure 3.
Total anthocyanin content of the samples based on fermentation time. YS represents yellow soybean, while SBS denotes small black soybean. Values are expressed as mean ± SD from three independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. milk. # p < 0.05 vs. YS.
The soymilk samples showed significantly higher contents when fermented alongside milk. Specifically, SBS 50% was measured at 508.26 ± 44.18 µg/L and 536.09 ± 44.18 µg/L after 48 h of fermentation, indicating a significantly higher content than YS, a plant-based fermented product.
3.5. Free Amino Acid Conversion by the INFOGEST Experiment
The free amino acid contents and conversion rates before and after digestive enzyme treatment are shown in Table 3. Most free amino acids increased following digestive enzyme treatment. Branched-chain amino acids (BCAAs: leucine, isoleucine, and valine) were higher in milk prior to enzyme treatment but increased by more than 97% in the soymilk samples after treatment. Similarly, while the levels of most essential amino acids, except lysine, were higher in fermented milk before enzyme treatment, soymilk samples exhibited comparable or greater levels afterward. Histidine and arginine, which are essential for children’s growth, were found in higher concentrations in fermented soymilk, with arginine in SBS 50% exceeding that of milk by more than two-fold after enzyme treatment.

Table 3.
Free amino acid content and digestibility according to digestive enzyme treatment.
Except for proline, most non-essential amino acids were higher in the soymilk samples after enzyme treatment, with values increasing in proportion to the SBS content. Glycine and serine, which impact sleep quality and skin health, reached their highest levels at 1093.2 nmol/mL and 336.4 nmol/mL in SBS 50%, respectively. Alanine and asparagine, which affect liver metabolism, peaked at 1065.2 nmol/mL and 434.5 nmol/mL in SBS 50% samples, respectively. Glutamine, which supports muscle growth and recovery after exercise, measured 1215.7 nmol/mL in SBS 25%, more than twice that observed in fermented milk. The total free amino acid content of milk before digestive enzyme treatment was 2773.8 nmol/mL, which was higher than that of all soymilk samples, with greater SBS supplementation yielding higher contents and confirming improved conversion rates.
3.6. Heatmap Analysis
Figure 4 presents a heatmap of the physicochemical properties and component changes in the fermented products prepared using different raw materials. The blue color in the heatmap indicates relatively low values, while the red color indicates relatively high values. Based on 30 parameters, including physicochemical properties, color, viable cell count, antioxidant activity, anthocyanin content, and post-digestion amino acid content, the samples were grouped into four classes and three clusters. Cluster A was characterized by high a*, TPC, TFC, anthocyanins, and polar amino acids. As the SBS content increased, the anthocyanin content also increased, which was interpreted as an indication of enhanced antioxidant activity. Furthermore, a higher SBS content was associated with increased polar amino acid content, as water-soluble amino acids are known to contribute to microbial metabolism. Cluster B was primarily associated with characteristics of fermented milk products, such as L*, b*, soluble solids, total acidity, and viable bacterial count. Cluster C was distinguished by pH and nonpolar amino acids. These nonpolar, hydrophobic amino acids have low water solubility and are precursors of flavor-related compounds during fermentation.
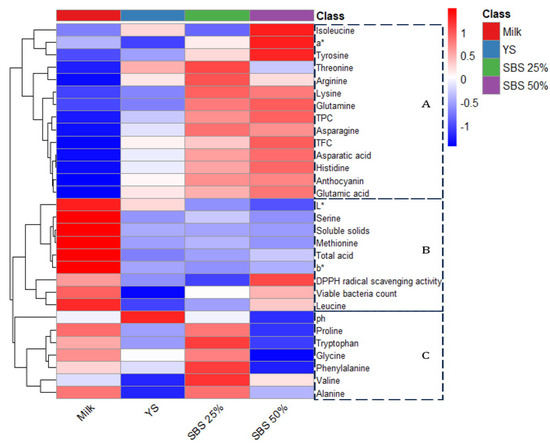
Figure 4.
Heatmap illustrating clusters of sample fermentation characteristics. Blue indicates values below average, while red indicates values above average.
4. Discussion
LAB typically utilize lactose and casein as their primary substrates to produce lactic acid, which increases acidity during dairy fermentation. In contrast, soymilk lacks lactose, and fermentation relies on the addition of sugars or plant-derived proteins such as glycinin. Although the fermentation products prepared in this study were manufactured similarly, milk had a higher initial sugar content than the plant-based fermentation products, and the number of viable cells increased dramatically 24 h after fermentation. However, using sucrose as a carbon source also induced similar levels of fermentation in milk.
Soymilk has been reported to exhibit strong radical scavenging activity due to its microbial protease and β-glucosidase activities, which convert glycosylated polyphenols into aglycones [28,29]. In this study, SBS-added soymilk demonstrated significantly higher antioxidant activity than milk under identical fermentation conditions. Antioxidants in plant components regulate reactive radical formation and can compensate for the decline in antioxidant defenses without the adverse effects of synthetic antioxidants, such as liver damage and carcinogenesis [30].
Anthocyanins, which are abundant in SBS seed coats, further contribute to the functional properties of fermented products. These pigments exhibit pH-dependent stability and are more stable in acidic environments due to the formation of flavylium cations, which enhance their water solubility [31,32]. Anthocyanins are recognized for their antioxidant and anticancer activities, with darker pigmentation typically associated with stronger bioactivity [33]. Previous studies have reported that compounds such as delphinidin-3-glucoside, cyanidin-3-glucoside, and cyanidin-3-arabinoside are enriched in colored crops, including black rice and black soybeans [34]. Consistent with these findings, the 50% SBS formulation in this study demonstrated lower redness and stable anthocyanin retention under acidic conditions, supporting its enhanced antioxidant potential. This increased antioxidant activity may be attributed not only to the higher anthocyanin content but also to synergistic interactions between phenolic compounds and peptides generated during fermentation, which can enhance free radical scavenging capacity and reduce oxidative stress [35].
In addition to antioxidant pigments, fermentation also affects protein hydrolysis and amino acid conversion, further enhancing nutritional quality. Amino acid conversion through digestive enzyme treatment showed that SBS-added samples produced markedly higher levels of free amino acids than milk, particularly functional amino acids such as arginine, alanine, asparagine, and glutamine. These results show that soybean-based fermentation products increase the free amino acid content due to microbial protein degradation [36,37]. In addition, soybean fermentation increases nutritional digestibility by treatment with digestive enzymes in the intestinal tract [38]. This effect may be due to microbial hydrolysis and structural modifications during fermentation, which increase the accessibility of protein substrates. Similarly, TPC and TFC were higher to those reported in other fermented black soybeans, further supporting the antioxidant and nutritional potential of SBS [39]. This can be linked to the accelerated physical digestion of SBS, which was slower than that of YS [40].
A heatmap analysis of the overall fermentation characteristics of the lactic acid bacteria fermentation product revealed that the parameters in Cluster A (Figure 3) exhibited high values, particularly in the 50% SBS sample, and correlated with antioxidant activity and water-soluble amino acids. This suggests that raw materials influence microbial substrate utilization and overall product functionality.
In this study, we developed fermented soymilk products using YS and SBS and investigated their fermentation characteristics and digestibility to assess their potential as alternatives to conventional fermented milk. The SBS-enriched formulation demonstrated significantly enhanced antioxidant activity, higher anthocyanin content, and increased free amino acid content after digestion. Additionally, LAB viable cell counts exceeded the standards outlined in the Korea Food Code. Thus, SBS-based fermented soymilk is a nutritionally enhanced, probiotic-rich, and sustainable alternative to dairy-based fermented products.
5. Conclusions
This study demonstrates that SBS-added soymilk fermented with a patented lactic acid bacteria starter culture is a promising alternative to conventionally fermented milk. The SBS formulation exhibited improved functional properties, including enhanced antioxidant capacity, anthocyanin stability, and free amino acid content. Furthermore, viable cell counts exceeded the minimum recommended levels for probiotic products, supporting microbiological stability and potential health benefits. Although a formal sensory evaluation was not conducted in this study, fermented soybean–based foods have a long history of consumption in East Asian cultures, suggesting that such products may be generally acceptable to consumers familiar with soy-based flavors. These results suggest that SBS-based fermented soymilk could serve as a sustainable and nutritionally superior alternative to meet the growing consumer demand for plant-based and functional foods.
6. Patents
The strain used in this study was registered under the Republic of Korea patent (Patent No. 10-2024-0167216).
Author Contributions
Conceptualization: E.A.S. and E.-G.M.; Normal Analysis: E.A.S. and H.K. Methodology, Investigation, Data Curation, Writing—Original Draft Preparation: E.A.S.; Writing—Review and Editing: E.-G.M. and S.-Y.K.; Visualization: E.A.S.; Supervision: S.-Y.K.; Project Administration: E.-G.M. and S.-Y.K.; Funding Acquisition: S.-Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The bacterial strains analyzed in this study were deposited at the Korean Agricultural Culture Collection (KACC), Republic of Korea, under accession numbers KACC 92623P and KACC 92624P.
Acknowledgments
The authors express their gratitude to Jeonju-si for their support in completing this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Leroy, F.; De Vuyst, L. Lactic Acid Bacteria as Functional Starter Cultures for the Food Fermentation Industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Seo, J.-H.; Lee, H. Characteristics and Immunomodulating Activity of Lactic Acid Bacteria for the Potential Probiotics. Korean J. Food Sci. Technol. 2007, 39, 681–687. [Google Scholar]
- Zapaśnik, A.; Sokołowska, B.; Bryła, M. Role of Lactic Acid Bacteria in Food Preservation and Safety. Foods 2022, 11, 1283. [Google Scholar] [CrossRef]
- Grasso, N.; Alonso-Miravalles, L.; O’Mahony, J.A. Composition, Physicochemical and Sensorial Properties of Commercial Plant-Based Yogurts. Foods 2020, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Domínguez, R.; Budaraju, S.; Roselló-Soto, E.; Barba, F.J.; Mallikarjunan, K.; Roohinejad, S.; Lorenzo, J.M. Effect of Innovative Food Processing Technologies on the Physicochemical and Nutritional Properties and Quality of Non-dairy Plant-Based Beverages. Foods 2020, 9, 288. [Google Scholar] [CrossRef]
- Shin, J.-S.; Kim, B.-H.; Kim, H.-S.; Baik, M.-Y. Optimization of Pea Protein and Citrus Fiber Contents for Plant Based Stirred Soymilk Yogurt Using Response Surface Methodology. Food Sci. Biotechnol. 2022, 31, 1691–1701. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Nazzaro, F.; Cruz, A.G.; Faria, J.A.F. Functional Foods and Nondairy Probiotic Food Development: Trends, Concepts, and Products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 292–302. [Google Scholar] [CrossRef]
- Oh, J.; Park, C.; Ahn, D.; Byun, J.; Jung, S.P. Veganomics: Current Status and Challenges. J. Korean Soc. Environ. Eng. 2023, 45, 296–310. [Google Scholar] [CrossRef]
- Fassio, F.; Facioni, M.S.; Guagnini, F. Lactose maldigestion, malabsorption, and intolerance: A comprehensive review with a focus on current management and future perspectives. Nutrients 2018, 10, 1599. [Google Scholar] [CrossRef]
- Kim, M.J.; Ha, B.J. Antihyperglycemic and Antihyperlipidemic Effects of Fermented Rhynchosia nulubilis in Alloxan-Induced Diabetic Rats. Toxicol. Res. 2013, 29, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.-J. The Inhibitory Effects of Roasted Black Bean (Rhynchosia nulubilis) Extracts on RANKL-Mediated RAW264. 7 Cells Differentiation. Food Sci. Biotechnol. 2016, 25, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.-Y.; Kim, K.H. Hair Growth Promotion with Black Soybean Extracts: Case Series. J. Pharmacopuncture 2022, 25, 63–67. [Google Scholar] [CrossRef]
- Park, H.; Yu, S.; Kim, W. Amelioration of Aging-Induced Muscular Decline by Black Soybean (Rhynchosia nulubilis) and Black Rice (Oryza sativa L.) Extracts. Front. Immunol. 2025, 16, 1554941. [Google Scholar] [CrossRef]
- Leksono, B.Y.; Cahyanto, M.N.; Rahayu, E.S.; Yanti, R.; Utami, T. Enhancement of antioxidant activities in black soy milk through isoflavone aglycone production during indigenous lactic acid bacteria fermentation. Fermentation 2022, 8, 326. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Lin, J.-T.; Liu, W.-H. Extracts from fermented black soybean milk exhibit antioxidant and cytotoxic activities. Food Technol. Biotechnol. 2011, 49, 111–117. [Google Scholar]
- Sim, E.A.; Kim, S.-Y.; Kim, S.; Mun, E.-G. Probiotic Potential and Enhanced Adhesion of Fermented Foods-Isolated Lactic Acid Bacteria to Intestinal Epithelial Caco-2 and HT-29 Cells. Microorganisms 2024, 13, 32. [Google Scholar] [CrossRef]
- Sembiring, E.N.; Elya, B.; Sauriasari, R. Phytochemical Screening, Total Flavonoid and Total Phenolic Content and Antioxidant Activity of Different Parts of Caesalpinia bonduc (L.) Roxb. Pharmacogn. J. 2017, 10, 123–127. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static In Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kwon, J.H.; Yoon, S.R.; Woo, S.M.; Jang, S.Y.; Yeo, S.H.; Choi, J.H.; Jeong, Y.J. Quality Characteristics of Brown Rice Vinegar by Different Yeasts and Fermentation Condition. J. Korean Soc. Food Sci. Nutr. 2010, 39, 1366–1372. [Google Scholar] [CrossRef]
- Farinde, E.O.; Adesetan, T.O.; Obatolu, V.A.; Oladapo, M.O. Chemical and Microbial Properties of Yogurt Processed from Cow’s Milk and Soymilk. J. Food Process. Preserv. 2009, 33, 245–254. [Google Scholar] [CrossRef]
- Shin, Y.S.; Sung, H.J.; Kim, D.H.; Lee, K.S. Preparation of Yogurt Added with Potato and Its Characteristics. Korean J. Food Sci. Technol. 1994, 26, 266–271. [Google Scholar]
- Lim, Y.; Hong, S.; Shin, Y.K.; Kang, S.H. Changes in the Viability of Lactic Acid Bacteria During Storage of Freeze-Dried Yogurt Snacks. J. Dairy Sci. Biotechnol. 2015, 33, 203–207. [Google Scholar]
- KFDA. Food Code; Ministry of Food and Drug Safety: Cheongju, Republic of Korea, 2021; Available online: https://various.foodsafetykorea.go.kr/fsd/#/ext/Document/FC (accessed on 26 August 2025).
- Vanga, S.K.; Raghavan, V. How Well Do Plant Based Alternatives Fare Nutritionally Compared to Cow’s Milk? J. Food Sci. Technol. 2018, 55, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Marazza, J.A.; Nazareno, M.A.; de Giori, G.S.; Garro, M.S. Enhancement of the Antioxidant Capacity of Soymilk by Fermentation with Lactobacillus rhamnosus. J. Funct. Foods. 2012, 4, 594–601. [Google Scholar] [CrossRef]
- Tonolo, F.; Moretto, L.; Folda, A.; Scalcon, V.; Bindoli, A.; Bellamio, M.; Feller, E.; Rigobello, M.P. Antioxidant Properties of Fermented Soy During Shelf Life. Plant Foods Hum. Nutr. 2019, 74, 287–292. [Google Scholar] [CrossRef]
- Hong, J.Y.; Shin, S.R.; Kong, H.J.; Choi, E.M.; Woo, S.C.; Lee, M.H.; Yang, K.M. Antioxidant Activity of Extracts from Soybean and Small Black Bean. Food Sci. Preserv. 2014, 21, 404–411. [Google Scholar]
- Lodha, D.; Das, S.; Hati, S. Antioxidant Activity, Total Phenolic Content and Biotransformation of Isoflavones During Soy Lactic-Fermentations. J. Food Process. Preserv. 2021, 45, e15583. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, J.; Zhao, H.; Li, Y.; Zhang, H.; Yang, B.; Guo, S. Antioxidant Properties of Fermented Soymilk and Its Anti-Inflammatory Effect on DSS-Induced Colitis in Mice. Front. Nutr. 2022, 9, 1088949. [Google Scholar] [CrossRef]
- Meenakshi, S.; Gnanambigai, D.M.; Mozhi, S.T.; Arumugam, M.; Balasubramanian, T. Total Flavanoid and In Vitro Antioxidant Activity of Two Seaweeds of Rameshwaram Coast. Glob. J. Pharmacol. 2009, 3, 59–62. [Google Scholar]
- Turturică, M.; Oancea, A.M.; Râpeanu, G.; Bahrim, G. Anthocyanins: Naturally Occurring Fruit Pigments with Functional Properties. Ann. Univ. Dunarea Jos Galati Fascicle VI Food Technol. 2015, 39, 9–24. [Google Scholar]
- Bąkowska-Barczak, A. Acylated Anthocyanins as Stable, Natural Food Colorants-A Review. Pol. J. Food Nutr. Sci. 2005, 14, 107–116. [Google Scholar]
- Sa, J.H.; Shin, I.C.; Jeong, K.J.; Shim, T.H.; Oh, H.S.; Kim, Y.J.; Cheung, E.H.; Kim, G.G.; Choi, D.S. Antioxidative Activity and Chemical Characteristics from Different Organs of Small Black Soybean (Yak-Kong) Grown in the Area of Jungsun. Korean J. Food Sci. Technol. 2003, 35, 309–315. [Google Scholar]
- Jeong, I.-H.; Oh, M.-S.; Jeon, J.-S.; Kim, H.-T.; Hong, S.-R.; Park, K.-H.; Yoon, M.-H. A Comparative Study on Anthocyanin and Polyphenol Contents in Colored Agricultural Products. J. Food Hyg. Saf. 2017, 32, 371–380. [Google Scholar] [CrossRef]
- Liang, Z.; Huang, Y.; Zhang, P.; Fang, Z. Impact of fermentation on the structure and antioxidant activity of selective phenolic compounds. Food Biosci. 2023, 56, 103147. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, Y.; Yu, J.; Wang, F.; Li, X.; Liu, Y.; Ma, X. Changes of proteins and amino acids in soymilk during lactic acid fermentation and subsequent storage. J. Food Meas. Charact. 2022, 16, 4728–4737. [Google Scholar] [CrossRef]
- Ren, Y.; Li, L. The influence of protease hydrolysis of lactic acid bacteria on the fermentation induced soybean protein gel: Protein molecule, peptides and amino acids. Food Res. Int. 2022, 156, 111284. [Google Scholar] [CrossRef]
- Yan, H.; Jin, J.Q.; Yang, P.; Yu, B.; He, J.; Mao, X.B.; Yu, J.; Chen, D.W. Fermented soybean meal increases nutrient digestibility via the improvement of intestinal function, anti-oxidative capacity and immune function of weaned pigs. Animal 2022, 16, 100557. [Google Scholar] [CrossRef]
- Juan, M.-Y.; Chou, C.C. Enhancement of antioxidant activity, total phenolic and flavonoid content of black soybeans by solid state fermentation with Bacillus subtilis BCRC 14715. Food Microbiol. 2010, 27, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Sim, E.A.; Mun, E.-G.; Oh, B.-J.; Jeong, S.-I. Fermentation Characteristics of Cheonggukjang Prepared from Non-germinated Pungsannamul-Soybeans. JKFN 2024, 53, 70–78. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).