Abstract
The excessive use of synthetic preservatives poses significant threats to food safety and human health. This study systematically investigated the genetic characteristics of the Limosilactobacillus fermentum (L. fermentum) z-6 strain, the antibacterial properties of the bacteriocin-like substance (FC) it produces, and its mechanism of action. The results demonstrated that this bacteriocin-like substance exhibited remarkable antimicrobial activity and exceptional stability, maintaining high activity across a broad pH range (4.0–8.0) and withstanding heat treatment at 100 °C and UV irradiation, indicating robust environmental adaptability. Its proteinaceous nature was confirmed by its detection below 1 kDa on Tricine-SDS-PAGE and its inactivation by trypsin and pepsin. The FC showed broad-spectrum inhibition against foodborne pathogens, including Salmonella enterica serovar Typhimurium, E. coli, A. baumannii, S. aureus, P. mirabilis and L. monocytogenes. Mechanistic investigation demonstrated that the FC exerts antibacterial effects primarily through membrane disruption, as evidenced by a live-dead staining assay confirming significantly enhanced permeability in Salmonella enterica serovar Typhimurium, and scanning electron microscopy revealing distinct pore formation on bacterial surfaces. It is speculated that the FC produced by z-6, due to its excellent properties and outstanding antibacterial performance, could potentially serve as a natural biopreservative.
1. Introduction
Salmonella enterica serovar Typhimurium, a Gram-negative bacterium belonging to the Enterobacteriaceae family, is a significant foodborne pathogen known to cause systemic illness and gastroenteritis [1]. It is widely disseminated through diverse contaminated food and water sources [2], with transmission routes known to be broader than many other foodborne pathogens. Human infections frequently occur through consumption of undercooked poultry, eggs, pork products, and raw dairy [3]. As the second most common non-typhoidal Salmonella serovar, it results in approximately 600 deaths annually, with infected individuals facing a 2.5-fold increase in mortality risk within one year post-infection [3,4]. Infections typically manifest as acute gastroenteritis characterized by diarrhea, fever, and abdominal cramping, which may progress to bacteremia in immunocompromised individuals [5]. The persistent challenge posed by this pathogen underscores the critical need for innovative control measures. Consequently, this study explores the potential of a bacteriocin-like substance from Limosilactobacillus fermentum z-6, a strain isolated and characterized in this work for the first time, as a natural alternative for controlling Salmonella enterica serovar Typhimurium in food systems, aligning with the growing interest in biological preservation strategies to enhance food safety.
Food safety faces multifaceted challenges in modern society. The escalating emergence of novel pathogenic microorganisms elevates risks of foodborne illnesses, while the complexity of modern supply chains exacerbates quality control challenges. Concurrently, consumer preferences are undergoing a shift towards foods with reduced chemical preservatives. Moreover, there is a growing awareness among consumers of food safety issues. Bacteriocins are extracellularly secreted proteins or peptides that exhibit antimicrobial activity against specific microorganisms [6]. Bacteriocin research has advanced significantly over the past decade. This classification system categorizes bacteriocins into two types: Class I lantibiotics, which are small peptides 3-methyllanthionine residues [7], and Class II bacteriocins (<10 kDa), which lack lanolthionine, typically exhibit thermal stability and they lack extensive post-translational modifications [8]. The development of bacteriocin as a natural food additive has become a subject of increasing interest in recent years [9]. Among the bacteriocin producers, lactic acid bacteria (LAB) are particularly esteemed for their “Generally Recognized as Safe” (GRAS) status and their prolific production of diverse bacteriocins. Furthermore, they have been demonstrated to exhibit targeted antimicrobial properties without compromising nutritional quality [10]. L. fermentum is a prevalent species within the LAB group, frequently isolated from traditional fermented foods and the human gut microbiome. Certain strains of L. fermentum have been demonstrated to produce bacteriocins or bacteriocin-like inhibitory substances (BLIS) with potent activity against various pathogens [11].
The selection of the L. fermentum z-6 strain was based on the broad-spectrum antimicrobial activity of the bacteriocin-like substances it produces, which demonstrated the strongest inhibitory effect against Salmonella enterica serovar Typhimurium. This study focused on evaluating the antibacterial activity of FC produced by L. fermentum z-6 against Salmonella enterica serovar Typhimurium and characterizing its biological properties. The antibacterial mechanism of this FC was systematically investigated through multiple approaches, including growth curve inhibition assays of indicator bacteria, live–dead staining assays, and scanning electron microscopy (SEM) observations. Furthermore, whole-genome sequencing was performed to conduct preliminary genetic characterization of the z-6 strain.
2. Materials and Methods
2.1. Materials, Preliminary Identification of Strains
In this study, the indicator strain was sourced from the microbial culture collection of the Key Laboratory of Preventive Veterinary Medicine at Jilin Agricultural Science and Technology University. Lactic acid bacteria (LAB) were isolated from pumpkins cultivated in Yanji City. Following aseptic sampling, the samples were inoculated into MRS broth and constant temperature culture at 37 °C for 24 h. The sample was inoculated onto MRS solid medium using the dilution coating method. After cultivation for 48 h, well-isolated colonies were obtained. The isolated strains were identified by 16S rRNA gene sequence analysis. PCR amplification was performed using universal primers 5′-AGAGTTTGATCCTGGCTCAG-3′ and 5′-GGTTACCTTGTTACGACTT-3′ [12]. The PCR reaction mixture (25.0 μL total volume) consisted of 12.5 μL of 2× Taq Master Mix, 1.0 μL of each primer, 1.0 μL of DNA template, and 9.5 μL of ddH2O. The PCR amplification protocol consisted of an initial denaturation step at 94 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 1 min, annealing at 50 °C for 1 min, and extension at 72 °C for 1 min, with a final extension at 72 °C for 10 min. The PCR products were sent to Sangon Biotech, Changchun, China, for sequencing and were compared with those deposited in the GenBank database using the online BLAST program (http://www.ncbi.nlm.nih.gov/BLAST/) [13], accessed on 27 October 2025.
All chemical reagents and analytical instruments utilized in this study were obtained from commercial sources. Mueller-Hinton (MH) agar, brain heart infusion (BHI) broth, de Man, Rogosa and Sharpe (MRS) broth, and MRS agar were purchased from Hope Bio-Technology Co., Ltd. (Qingdao, China). Dialysis tubing (MD44-1000) was acquired from Yibo Biotechnology Co., Ltd. (Hunan, China). Phosphate-buffered saline (PBS), papain, trypsin, proteinase K, pepsin, protein molecular weight markers (2.7–40 kDa), and Coomassie Brilliant Blue were procured from Sangon Biotech (Shanghai, China). Hydrochloric acid (HCl) was supplied by SINOPEC (Nanjing, China). Bacterial viability was assessed using a Bacterial Viability Kit from Servicebio (Wuhan, China). Scanning electron microscopy (SEM) observations were performed using a Hitachi SU8100 instrument (Tokyo, Japan), and fluorescence microscopy images were acquired with a Nikon microscope (Tokyo, Japan).
2.2. L. fermentum z-6 Whole-Genome Sequencing
Genomic DNA of L. fermentum z-6 was extracted and submitted for whole-genome sequencing. The strain was activated from a glycerol stock and cultivated in MRS broth at 37 °C with shaking at 180 rpm for 24 h. The high-quality genomic DNA extracted from the harvested cells was subjected to whole-genome sequencing, which was performed by BioMarker Technologies (Qingdao, China).
The complete genome of L. fermentum z-6 was sequenced using Oxford Nanopore technology. The filtered reads were assembled with Canu v1.5, and the initial assembly was subsequently polished using Racon v3.4.3 with the long reads to improve accuracy. Finally, the assembled genome was scanned with GenBlastA v1.0.4 to identify putative functional genes.
2.3. FC Production and Activity Identification
The experiment systematically evaluated the antibacterial activity of the strains through a standardized protocol: initially, Salmonella enterica serovar Typhimurium ATCC 14028 preserved in glycerol was streaked onto LB agar plates and cultured overnight at 37 °C for activation. Subsequently, a single colony was inoculated into 6 mL of LB broth and incubated at 37 °C with shaking for 4 h to prepare the indicator bacterial suspension. L. fermentum z-6 was inoculated at 0.5% (v/v) into 6 mL of MRS broth and cultured at 37 °C for 24 h. Subsequently, 1 mL of the bacterial suspension was centrifuged at 12,000× g for 5 min at 4 °C to obtain the cell-free (CFS) supernatant. The supernatant was collected by centrifugation at 12,000× g for 5 min at 4 °C. To eliminate interfering factors, the sample was treated as follows: the pH was adjusted to 7.0 with 1 mol/L NaOH to neutralize organic acids; hydrogen peroxide was removed by incubation with an equal volume of catalase (1 mg/mL) at 37 °C for 4 h; and a final sterile filtration was performed using a 0.22 μm filter. Antibacterial activity was determined using the Oxford cup method [14], 50 μL of the activated indicator bacterial suspension was evenly spread onto the (MH) agar plates, after adding 200 μL of the treated sterile supernatant to the Oxford cup, the flat panel was incubated at 37 °C for 18 h, the diameter of the inhibition zone was measured using a vernier caliper with an accuracy of 0.02 mm.
2.4. Antimicrobial Spectrum of z-6
The antibacterial activity of the FC substance z-6 against six foodborne pathogens was assessed by the agar diffusion method, with the test panel comprising four Gram-negative strains—Salmonella enterica serovar Typhimurium ATCC 14028, Escherichia coli ATCC 25922, Acinetobacter baumannii wyy-5, and Proteus mirabilis SH-8—and two Gram-positive strains: Staphylococcus aureus ATCC 25923 and Listeria monocytogenes ATCC 19115; these strains were, respectively, cultured in either LB broth (Salmonella enterica serovar Typhimurium ATCC 14028, E. coli ATCC 25922, S. aureus ATCC 25923, A. baumannii wyy−5, and P. mirabilis SH−8) or BHI broth (L. monocytogenes ATCC 19115 ) at 37 °C for 12 h, after which all indicator bacteria were adjusted to 1 × 106 CFU/mL and the inhibition zone diameters were measured using a Vernier caliper.
2.5. Growth Curve of z-6
L. fermentum z-6 was revived in MRS broth and cultured at 37 °C for 24 h until OD600 ≈ 0.5 to prepare a starter culture. This culture was inoculated at 2% (v/v) into 100 mL of MRS broth and incubated at 37 °C with shaking. Samples were taken every 2 h for 24 h. At each interval, 200 μL of culture was collected for OD600 and pH measurement, and 1 mL was taken to prepare supernatant for antibacterial activity assessment.
2.6. Estimation of Molecular Mass
L. fermentum z-6 was cultured in 1200 mL of MRS broth at 37 °C for 28 h with a 2% (v/v) inoculation rate, and its cell-free supernatant (CFS) was collected using the method described in Section 2.3. The collected CFS was then concentrated 10-fold using a rotary evaporator at 45 °C, followed by dialysis with a 1000 Da membrane at 4 °C for 24 h. Finally, the dialyzed liquid was again concentrated 10-fold and assayed for antibacterial activity.
We used Tricine-SDS-PAGE further to confirm the molecular weight of the target protein. We used a discontinuous gel system comprising 4% stacking gel, 10% spacer gel, and 18% separating gels [15]. Electrophoresis was performed in an ice-water bath. After loading 10 μL of samples and protein markers, the gel was run at constant voltages: 30 V for 10 min, 80 V until the dye front reached the separating gel interface, and 120 V until completion. Following electrophoresis, the gel was fixed for 30 min in a solution containing 50% methanol, 10% acetic acid, and 40% distilled water. After rinsing with distilled water, the gel was stained with Coomassie Brilliant Blue R-250 for 1 h. Destaining was then performed using a solution of 20% ethanol, 10% glacial acetic acid, and 70% distilled water with multiple changes until clear visualization of both sample bands and protein markers (comprising polypeptides ranging from 2.7 to 40 kDa) was achieved.
To further determine whether it is the target protein, the electrophoresis-completed and unstained gel is gently washed, placed onto (MH) agar plates, and overlaid with molten MH agar containing 0.4% (v/v) indicator bacteria. The plates were then incubated at constant temperature overnight for antimicrobial activity assessment [16].
2.7. Physicochemical Properties of FC
2.7.1. Temperature Stability
The FC was subjected to thermal treatment at varying temperatures (−40 °C to 4 °C, 37 °C, 60 °C, 80 °C, and 100 °C) for 1 h. After cooling to room temperature, the antimicrobial activity was evaluated using Salmonella enterica serovar Typhimurium ATCC 14028 as the indicator strain (with the 37 °C treatment serving as control).
2.7.2. Stability at Various pH
The FC was pH-adjusted to values ranging from 4.0 to 8.0 using 1 mol/L HCl or NaOH, followed by incubation in a 37 °C water bath for 2 h. Subsequently, all samples were readjusted to their initial pH (pH ≈ 4.0). Antimicrobial activity was then determined against Salmonella enterica serovar Typhimurium ATCC 14028 as the indicator strain, with the Original (pH ≈ 4.0) samples serving as control.
2.7.3. Protease Stability
The FC was treated with trypsin, papain, proteinase K, and pepsin, with the pH of each reaction system adjusted to the enzyme-specific optimal range using 1 mol/L HCl or NaOH. The enzymatic reactions were performed at a final protease concentration of 1 mg/mL, Water bath at 37 °C for 2 h, followed by enzyme inactivation at 100 °C for 10 min. A protease-free control group was included. Antimicrobial activity was then assessed against Salmonella enterica serovar Typhimurium ATCC 14028 as the indicator strain.
2.7.4. Ultraviolet Stability
The FC was subjected to UV irradiation (254 nm) for varying durations (0.5, 1, 1.5, 2, and 2.5 h). Following UV treatment, antimicrobial activity was evaluated using Salmonella enterica serovar Typhimurium ATCC 14028 as the indicator strain. The results were subsequently compared with the non-UV-exposed control group.
2.8. Live–Dead Staining Assay
The Live-Dead staining assay was performed to evaluate potential membrane damage in Salmonella enterica serovar Typhimurium ATCC 14028 caused by FC Briefly, the bacterial suspension was prepared as described for the antimicrobial assay and adjusted to approximately 6 × 107 CFU/mL using PBS. Following the protocol of the SYTO-9/PI Bacterial Viability Kit (Servicebio, Wuhan, China), the suspensions were incubated with FC at 37 °C for 1.5 h. A sample without FC served as a positive control. After incubation, the cells were washed three times with PBS and then stained in the dark for 15 min using SYTO 9 and propidium iodide (PI). Bacterial viability was finally observed under a fluorescence microscope (Nikon, Tokyo, Japan) using dark-field conditions.
2.9. Scanning Electron Microscope (SEM)
To investigate bacteriocin-induced cellular damage in Salmonella enterica serovar Typhimurium, mid-log phase bacterial suspensions (OD600 ≈ 0.6) were harvested by centrifugation (10,000× g, 4 °C, 10 min) and washed three times with phosphate-buffered saline (PBS). The FC was mixed with an equal volume of Salmonella enterica serovar Typhimurium suspension (1:1 ratio) and incubated at 37 °C for 2 h. A parallel control was prepared using PBS instead of bacteriocin. Following treatment, Salmonella enterica serovar Typhimurium cells were harvested and fixed overnight with 2.5% glutaraldehyde at 4 °C, followed by stepwise dehydration using an ethanol gradient series (30%, 50%, 70%, 80%, 90%, and 100%) at 4 °C. After natural drying, the immobilized bacteria were placed on a microscope slide and observed under a scanning electron microscope (SEM) to examine their morphological changes.
2.10. Statistical Analysis
To ensure the reliability and reproducibility of the experimental data, we conducted three independent parallel measurements, and the final results are expressed as the arithmetic mean of the three measurements. Data were subjected to mean and variance analysis using Microsoft Excel.
3. Results
3.1. Isolation and Identification of Lactic Acid Bacteria
After 24 h of incubation at 37 °C on agar plates, strain z-6 was identified as Gram-positive bacilli with smooth colony surfaces. Combined phenotypic and molecular characterization identified the isolate as Lactobacillus fermentum. designating it as z-6. 16SrRNA gene sequencing and BLAST alignment showed 99.93% sequence homology between strain z-6 (GenBank: PX456134.1) and L. fermentum z-9 (GenBank: PV088864.1). Phylogenetic analysis further confirmed the phylogenetic position of L. fermentum z-6, as shown in Figure 1.
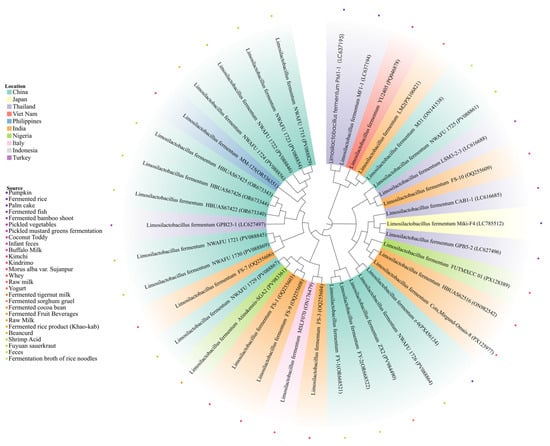
Figure 1.
The resulting phylogenetic tree is partitioned into 10 distinct clusters, with the color of each of the 34 nodes denoting its country of origin.
3.2. Identification of Strains and Their Whole Genome Sequencing
Whole-genome sequencing of strain z-6 revealed a high-quality assembly with characteristic bacterial genomic features, comprising 131,620 contigs (GC content: 51.13%). The sequencing data demonstrated reliable quality (mean Phred score = 11), with the chromosomal and plasmid sequences deposited under GenBank accession numbers CP171795 and CP171796, respectively.
3.3. Evaluation of Antimicrobial Activity
Following pH neutralization of organic acid-mediated antibacterial activity in the supernatant, catalase treatment produced only marginal attenuation of inhibitory effects (18.2% reduction in inhibition zone diameter). In contrast, enzymatic digestion with either pepsin or pancreatin substantially compromised antimicrobial activity, demonstrating 82.6% and 86.1% suppression rates, respectively. This activity gradient confirms that z-6’s antibacterial components include hydrogen peroxide, but its primary active substance is a protease-sensitive FC (Figure 2).

Figure 2.
The process of measuring the inhibition zone diameter following the determination of the antibacterial activity of z-6 FC against Salmonella enterica serovar Typhimurium ATCC 14028 utilizes the double-layer Oxford cup method. The diameter of the inhibition zone was measured using a vernier caliper.
3.4. The Analytics of Bacteriocin Molecular Weight Size
Tricine-SDS-PAGE analysis of the FC from z-6 showed a single protein band below 2.7 kDa (Figure 3). The corresponding in situ activity assay formed a clear inhibition zone against Salmonella enterica serovar Typhimurium ATCC 14028, confirming that the active substance is a low-molecular-mass antimicrobial peptide.
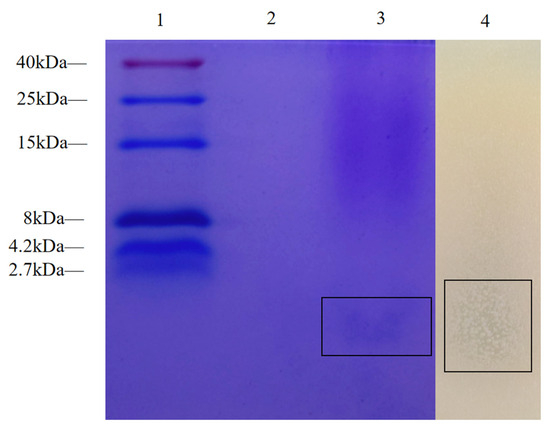
Figure 3.
The following study investigates the inhibition zones following in situ antimicrobial activity testing of Tricine-SDS-PAGE and cell-free culture supernates of L. fermentum z-6. Lane 1: This is a low-molecular-weight pre-stained marker. Lane 2: Control group Lane 3: FC (The black frame shows the target protein). Lane 4: After incubating overnight at 37 °C, overlay the same samples with MH agar containing Salmonella enterica serovar Typhimurium ATCC 14028.
3.5. Antibacterial Spectrum
As shown in Table 1, FC exhibits broad-spectrum antibacterial activity against both Gram-negative and Gram-positive bacteria. The largest inhibition zone was observed against Salmonella enterica serovar Typhimurium ATCC 14028, measuring 30.28 mm. Overall, FC demonstrated stronger antibacterial activity against Gram-negative bacteria than against Gram-positive ones. Notably, the substance was effective against several foodborne pathogens—including S. aureus, E. coli, L. monocytogenes, A. baumannii, and P. mirabilis—highlighting the potential health risks posed by these organisms. These findings indicate that z-6 holds promise for applications in food safety.

Table 1.
The antimicrobial spectrum of FC.
3.6. Effect of pH, Heat, Proteases, and UV Rays on the Antibacterial Activity
As shown in Figure 4a, the antibacterial activity of BLIS is relatively stable after heat treatment and reaches its peak at 37 °C. Although further temperature increases led to a decline in antibacterial activity, the FC produced by z-6 retained approximately 80% of its activity, demonstrating good thermal stability. As shown in Figure 4b, the antibacterial activity of the FC varied in a pH-dependent manner, with a maximum at pH ≈ 4.0. Subsequently, the activity gradually decreased as pH rose to 6.0, but showed recovery in the neutral to weakly alkaline range (pH 7.0–8.0). Figure 4c demonstrates that FC was highly sensitive to pepsin and trypsin, papain and proteinase K exhibited virtually no influence. The FC produced by z-6 showed a gradual decrease in antimicrobial activity under UV irradiation, though the effect was relatively minor compared to the control group (Figure 4d).
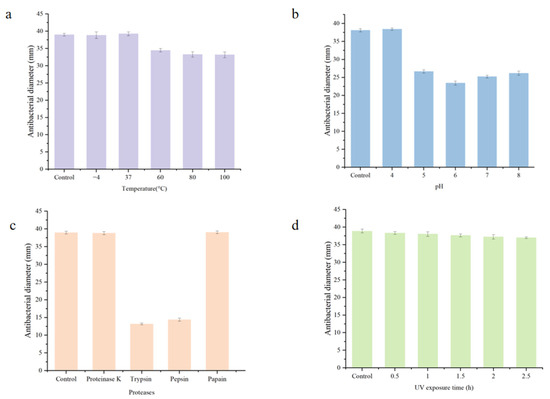
Figure 4.
(a–d) investigate the effects of temperature, pH, protease, and UV irradiation on the antibacterial activity of FC, respectively.
3.7. Production Kinetics of z-6
As shown in Figure 5, both synthesis and secretion activities showed a significant trend from 0 to 14 h, reaching peak levels at 14 h. Subsequently, the inhibitory activity of the culture medium decreased significantly from 14 to 24 h. A comprehensive analysis of the experimental results identified 14 h as the optimal incubation duration under these experimental conditions.
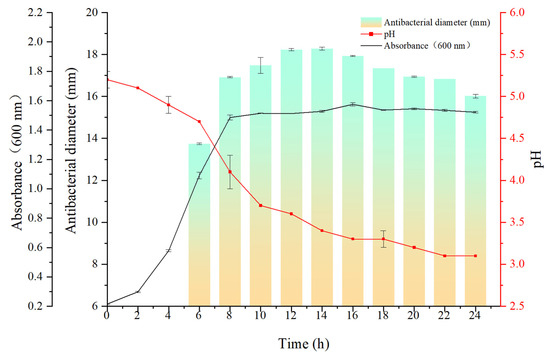
Figure 5.
Biosynthesis kinetics of FC produced by L. fermentum z-6, demonstrating the relationship between cultivation time and culture OD600 values, pH, and antimicrobial 3 activity.
3.8. Bactericidal Effect Observed Using Fluorescence Microscope
The bactericidal activity of FC against Salmonella enterica serovar Typhimurium ATCC 14028 was evaluated by fluorescence microscopy using the SYTO 9 and propidium iodide (PI) double staining method (Figure 6). SYTO 9, a membrane-permeant green fluorescent nucleic acid stain, labels all bacterial cells. In contrast, propidium iodide (PI), a red fluorescent dye, only enters cells with damaged membranes. In the control group (Figure 6a), the bacterial population displayed predominantly green fluorescence with minimal red signal, indicating a high proportion of cells with intact membranes and thus high viability. Conversely, in the FC-treated group (Figure 6b), a significant increase in red fluorescence intensity was observed, demonstrating that the treatment severely compromised the integrity of the bacterial inner membrane.
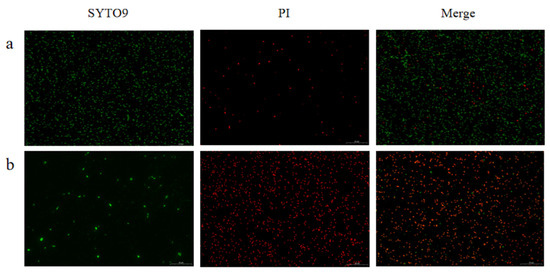
Figure 6.
Fluorescence microscopy was used to analyze Salmonella enterica serovar Typhimurium ATCC 14028 following treatment with FC. As shown in the micrographs, untreated control bacteria (a) were compared against those treated with FC for 1.5 h (b). Subsequent dual staining with SYTO 9 and propidium iodide (PI) revealed distinct fluorescence patterns: SYTO 9 (green, left panels) and PI (red, middle panels), with the corresponding merged images displayed on the right.
3.9. SEM Analysis
When Salmonella enterica serovar Typhimurium was exposed to FC, its cellular morphology and structure underwent significant alteration. Scanning electron microscopy (SEM) observations (Figure 7a) revealed that control cells maintained typical rod-shaped morphology with smooth, intact surfaces. In contrast, FC treated cells exhibited marked morphological aberrations after 2 h exposure, including cellular distortion and surface irregularities. Higher-magnification observation (Figure 7b) further revealed distinct membrane perforations in a subset of cells.
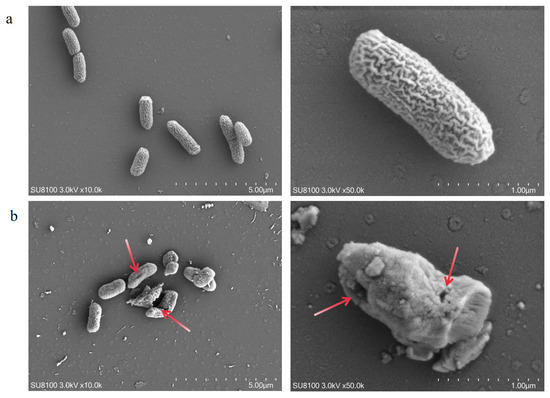
Figure 7.
Scanning electron microscopy (SEM) analysis confirmed the significant bactericidal effects of FC on Salmonella enterica serovar Typhimurium. (a) Negative control (PBS-treated for 2 h) showed intact cellular morphology; (b) FC treatment 2 h induced characteristic membrane damage, including pore formation, structural collapse, and morphological deformation (All images represent multi-angle observations of the same sample, The arrow points to the rupture site on the bacterium).
The results demonstrate that the FC produced by z-6 potently disrupt the cellular integrity of Salmonella enterica serovar Typhimurium. Membrane damage may lead to leakage of intracellular nutrients and genetic materials, while simultaneously impairing transmembrane transport functions. These structural disruptions likely impair essential metabolic processes and cell division machinery, leading to growth inhibition.
4. Discussion
The global food industry has faced growing challenges in mitigating Salmonella contamination in recent years. Research indicates that Salmonella displays extensive host adaptability, and its multidrug resistance further undermines the effectiveness of traditional intervention strategies [17]. This situation has prompted researchers to explore the development and application of natural antimicrobial agents. Bacteriocins derived from lactic acid bacteria offer unique benefits: their safety as natural antimicrobial peptides is well-established [18]; Notably, compared to chemical preservatives, bacteriocins exhibit environmentally friendly characteristics and a low propensity for inducing antimicrobial resistance [19]. These properties position bacteriocins as a promising research direction to overcome current bottlenecks in food preservation technologies. Particularly in industrial sectors such as ready-to-eat meats, dairy products, and fruit and vegetable preservation, bacteriocins hold broad application prospects as natural preservatives. By optimizing formulations and delivery systems, their antibacterial efficacy within complex food matrices can be further enhanced, providing technological support for developing safer and healthier food products.
This study defines a unique physicochemical identity for the bacterioin-like inhibitory substance (FC) produced by L. fermentum z-6. Its profile is characterized by an ultra-low molecular weight (<1 kDa), exceptional stability under acidic, thermal, and UV stress, and a distinct protease sensitivity profile. Comparative analysis revealed that this BLIS exhibits distinctive molecular characteristics: Tricine-SDS-PAGE coupled with dialysis experiments determined its molecular weight to be below 1 kDa, which is significantly lower than previously reported bacteriocin from L. fermentum strains (e.g., 2.056 kDa; 13.1 kDa; 42.5 kDa) [11,20,21]. This ultra-low molecular weight FC has not been reported in previous studies on L. fermentum. Its molecular weight of less than 1 kDa confers several advantages, including a simple structure, high biological activity, high stability, and ease of synthesis [22,23]. Protease assay results indicated that the bacteriocin was inactivated by pepsin and trypsin but remained stable following treatment with papain and proteinase K. This observed sensitivity profile can be rationalized by its small molecular size (<1 kDa). As a micropeptide, it possesses a limited number of potential cleavage sites. Its stability against the broad-specificity proteases (proteinase K and papain) suggests that its compact structure lacks the specific peptide bonds targeted by these enzymes. Conversely, its susceptibility to pepsin and trypsin confirms the presence of their highly specific cleavage sites (e.g., adjacent to aromatic or basic residues, respectively), which are likely essential for its bactericidal activity. Such a profile is consistent with several known small bacteriocins and provides critical insight into its structure-function relationship [24,25,26,27]. During pH stability testing, the antimicrobial substance demonstrated remarkable stability under acidic conditions (pH ≤ 5). This enhanced stability may result from protonation of acidic amino acid residues at lower pH values, which reduces intramolecular electrostatic repulsion and thereby improves structural integrity [28]. Under alkaline pH, the deprotonation of carboxyl groups (pKa ~4.5) on surface-exposed acidic residues creates anionic sites that can engage in electrostatic interactions (salt bridges) with proximal cationic amino acids, conferring structural stability and functional preservation [29,30,31]. Notably, the antimicrobial substance retained >90% antimicrobial activity after 1 h at 100 °C. This remarkable thermal stability may be attributed to its compact structure as a low-molecular-weight peptide, which resists thermal denaturation [32,33]. In addition, 4 h of UV irradiation had no significant effect on its activity, the same results were reported by Hassanet et al. [34]. This may be because protein molecules are structurally simple and lack long chains that are susceptible to UV damage, thus exhibiting higher stability [35]. Antimicrobial activity was first detected at 4 h of fermentation, peaked at 14 h (coinciding with pH decline to 3.4), and remained stable during stationary phase. This growth-phase-dependent production pattern suggests classification as a secondary metabolite [36]. To elucidate the antimicrobial mechanism, we employed an integrated multi-technique analytical approach. First, growth curve analysis confirmed its time-dependent bactericidal activity against Salmonella enterica serovar Typhimurium. To investigate whether membrane integrity was compromised, we conducted the following experiments. (1) live–dead staining assay revealed markedly increased fluorescence intensity after 2 h treatment, indicating substantial membrane integrity disruption; and (2) Scanning electron microscopy (SEM) directly visualized pore formation on bacterial surfaces following FC exposure. These results collectively support the mechanism hypothesis that “this antimicrobial substance exerts its antibacterial effect by disrupting cell membrane integrity” [37,38]. The FC likely exerts dual mechanisms: (1) indirect membrane depolarization via inhibition of respiratory chain complexes/transport proteins, leading to cellular content leakage [39,40]; and (2) direct binding to lipid II to block peptidoglycan biosynthesis [41]. The antimicrobial substance positively charged hydrophobic domain may initiate electrostatic interactions with negatively charged phosphate groups on bacterial membranes, ultimately leading to membrane permeabilization, cytoplasmic leakage and cell death [42]. Furthermore, the FC of L. fermentum z-6 found has a broad antibacterial spectrum, inhibiting Salmonella enterica serovar Typhimurium, E. coli, A. baumannii, S. aureus, P. mirabilis and L. monocytogenes. The whole-genome sequencing results revealed that L. fermentum z-6 possesses a GC content of 51.13%, which is significantly higher than that of most lactobacilli (typically ranging between 30 and 45%). A similarly high GC content has been reported in Levilactobacillus acidifarinae JCM 15949 (GC content 51.71%) based on whole-genome analysis, suggesting that elevated GC content may be associated with genomic evolution or environmental adaptation [43,44].
5. Conclusions
The antimicrobial substance produced by L. fermentum z-6 was preliminarily identified as a FC (<1 kDa), demonstrating exceptional environmental stability and potent antimicrobial activity. Its bactericidal mechanism primarily involves disruption of target cell membrane integrity. Our findings not only systematically elucidate the functional properties of this FC, but also provide both theoretical foundations and practical guidance for developing novel food-grade biopreservatives.
Author Contributions
Y.Z.: Writing—review and editing, Writing—original draft, Conceptualization, Investigation, Resources, Information analysis, Visualization, and Data curation. X.W.: Investigation, Data curation, validation, software, resources and Formal analysis. H.L.: Formal analysis, Investigation. N.X.: Information analysis, and Data curation. J.W.: Resources. B.Y.: Writing—review, Methodology, Supervision, and Data curation. W.D.: Writing—review, Methodology. W.S.: Writing—review, and Methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We sincerely thank our mentors for their careful guidance and selfless support throughout the project. Their professional insights and patient assistance provided direction for our research. We would also like to thank our colleagues in the laboratory, who not only shared their valuable experimental experience, but also provided key ideas for the project through in-depth discussions. Their spirit of collaboration greatly optimized the research process and significantly improved the reliability and innovation of the results.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hébrard, M.; Kröger, C.; Sivasankaran, S.K.; Händler, K.; Hinton, J.C. The challenge of relating gene expression to the virulence of Salmonella enterica serovar Typhimurium. Curr. Opin. Biotechnol. 2011, 22, 200–210. [Google Scholar] [CrossRef]
- Marchello, C.S.; Fiorino, F.; Pettini, E.; Crump, J.A.; Martin, L.B.; Breghi, G.; Canals, R.; A Gordon, M.; Hanumunthadu, B.; Jacobs, J.; et al. Incidence of non-typhoidal Salmonella invasive disease: A systematic review and meta-analysis. J. Infect. 2021, 83, 523–532. [Google Scholar] [CrossRef]
- Chen, J.; Huang, L.; An, H.; Wang, Z.; Kang, X.; Yin, R.; Jia, C.; Jin, X.; Yue, M. One Health approach probes zoonotic non-typhoidal Salmonella infections in China: A systematic review and meta-analysis. J. Glob. Health 2024, 14, 04256. [Google Scholar] [CrossRef]
- Helms, M.; Ethelberg, S.; Mølbak, K.; DT104 Study Group. International Salmonella typhimurium DT104 infections, 1992–2001. Emerg. Infect. Dis. 2005, 11, 859. [Google Scholar] [CrossRef]
- Lee, C.M.; Lee, M.S.; Yang, T.L.; Lee, K.L.; Yen, T.Y.; Lu, C.Y.; Hsueh, P.R.; Lee, P.I.; Chen, J.M.; Huang, L.M.; et al. Clinical features and risk factors associated with bacteremia of nontyphoidal salmonellosis in pediatric patients, 2010–2018. J. Formos. Med. Assoc. 2021, 120, 196–203. [Google Scholar] [CrossRef]
- Vesković-Moračanin, S.M.; Đukić, D.A.; Memiši, N.R. Bacteriocins produced by lactic acid bacteria: A review. Acta Period. Technol. 2014, 45, 271–283. [Google Scholar] [CrossRef]
- Riley, M.A.; Wertz, J.E. Bacteriocins: Evolution, ecology, and application. Annu. Rev. Microbiol. 2002, 56, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Novel bacteriocins from lactic acid bacteria (LAB): Various structures and applications. Microb. Cell Factories 2014, 13, S3. [Google Scholar] [CrossRef]
- Yang, S.C.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol 2014, 5, 241. [Google Scholar]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Halder, D.; Mandal, S. The in vitro studies combined with molecular docking and MM-GBSA binding free energy calculations reveal broad-spectrum antibacterial activity of bacteriocin from curd-derived Limosilactobacillus fermentum with probiotic attributes. Silico Res. Biomed. 2025, 1, 100009. [Google Scholar] [CrossRef]
- Uebanso, T.; Kano, S.; Yoshimoto, A.; Naito, C.; Shimohata, T.; Mawatari, K.; Takahashi, A. Effects of consuming xylitol on gut microbiota and lipid metabolism in mice. Nutrients 2017, 9, 756. [Google Scholar] [CrossRef]
- Leite, A.M.O.; Miguel, M.A.L.; Peixoto, R.S.; Ruas-Madiedo, P.; Paschoalin, V.M.F.; Mayo, B.; Delgado, S. Probiotic potential of selected lactic acid bacteria strains isolated from Brazilian kefir grains. Dairy Sci. 2015, 98, 3622–3632. [Google Scholar] [CrossRef]
- Zeng, Y.; He, W.; Li, K.; Zeng, X.; Wu, Z.; Guo, Y.; Bao, W.; Pan, D. The potential mechanism and key genes of bacteriocin production in Lactiplantibacillus plantarum ZY-1 induced by co-cultivation with Limosilactobacillus fermentum RC4. LWT 2025, 228, 118059. [Google Scholar] [CrossRef]
- Schägger, H. Tricine–sds-page. Nat. Protoc. 2006, 1, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Wannun, P.; Piwat, S.; Teanpaisan, R. Purification, characterization, and optimum conditions of fermencin SD11, a bacteriocin produced by human orally Lactobacillus fermentum SD11. Appl. Biochem. Biotechnol. 2016, 179, 572–582. [Google Scholar] [CrossRef]
- Mkangara, M. Prevention and control of human Salmonella enterica infections: An implication in food safety. Int. J. Food Sci. 2023, 2023, 8899596. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.B.; Worobo, R.W. Chemical and genetic characterization of bacteriocins: Antimicrobial peptides for food safety. J. Sci. Food Agric. 2014, 94, 28–44. [Google Scholar] [CrossRef]
- Johnson, E.M.; Jung, Y.-G.; Jin, Y.-Y.; Jayabalan, R.; Yang, S.H.; Suh, J.W. Bacteriocins as food preservatives: Challenges and emerging horizons. Crit. Rev. Food Sci. Nutr. 2018, 58, 2743–2767. [Google Scholar] [CrossRef]
- Heredia-Castro, P.Y.; Reyes-Díaz, R.; Rendón-Rosales, M.Á.; Beltrán-Barrientos, L.M.; Torres-Llanez, M.J.; Estrada-Montoya, M.C.; Hernández-Mendoza, A.; González-Córdova, A.F.; Vallejo-Cordoba, B. Novel bacteriocins produced by Lactobacillus fermentum strains with bacteriostatic effects in milk against selected indicator microorganisms. J. Dairy Sci. 2021, 104, 4033–4043. [Google Scholar] [CrossRef] [PubMed]
- Abramov, V.M.; Kosarev, I.V.; Machulin, A.V.; Priputnevich, T.V.; Deryusheva, E.I.; Nemashkalova, E.L.; Chikileva, I.O.; Abashina, T.N.; Panin, A.N.; Melnikov, V.G.; et al. Limosilactobacillus fermentum 3872 That Produces Class III Bacteriocin Forms Co-Aggregates with the Antibiotic-Resistant Staphylococcus aureus Strains and Induces Their Lethal Damage. Antibiotics 2023, 12, 471. [Google Scholar] [CrossRef]
- Zhang, Q.T.; Liu, Z.D.; Wang, Z.; Wang, T.; Wang, N.; Wang, N.; Zhang, B.; Zhao, Y.F. Recent advances in small peptides of marine origin in cancer therapy. Mar. Drugs 2021, 19, 115. [Google Scholar] [CrossRef] [PubMed]
- Rabbani-Chadegani, A.; Abdossamadi, S.; Bargahi, A.; Yousef-Masboogh, M. Identification of low-molecular-weight protein (SCP1) from shark cartilage with anti-angiogenesis activity and sequence similarity to parvalbumin. J. Pharm. Biomed. Anal. 2008, 46, 563–567. [Google Scholar] [CrossRef]
- Li, J.F.; Zhang, J.X.; Li, G.; Xu, Y.Y.; Lu, K.; Wang, Z.G.; Liu, J.P. Antimicrobial activity and mechanism of peptide CM4 against Pseudomonas aeruginosa. Food Funct. 2020, 11, 7245–7254. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Song, J.; Zeng, W.; Wang, H.; Zhang, Y.; Xin, J.; Suo, H. A broad-spectrum novel bacteriocin produced by Lactobacillus plantarum SHY 21–2 from yak yogurt: Purification, antimicrobial characteristics and antibacterial mechanism. LWT 2021, 142, 110955. [Google Scholar] [CrossRef]
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial peptides: An update on classifications and databases. Int. J. Mol. Sci. 2021, 22, 11691. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Z.; Guo, Y.; Liu, S.; Zhao, H.; Zhao, S.; Xiao, C.; Feng, S.; Yang, X.; Wang, F. Ultraviolet photodissociation reveals the molecular mechanism of crown ether microsolvation effect on the gas-phase native-like protein structure. J. Am. Chem. Soc. 2022, 145, 1285–1291. [Google Scholar] [CrossRef]
- Bosshard, H.R.; Marti, D.N.; Jelesarov, I. Protein stabilization by salt bridges: Concepts, experimental approaches and clarification of some misunderstandings. J. Mol. Recognit. 2004, 17, 1–16. [Google Scholar] [CrossRef]
- Wang, L.; Ding, B.; Hu, X.; Li, G.; Deng, Y. Rationally Engineering pH Adaptation of Acid-Induced Arginine Decarboxylase from Escherichia coli to Alkaline Environments to Efficiently Biosynthesize Putrescine. Adv. Sci. 2024, 11, 2307779. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, B.; Zeng, W.; Zhang, X.; Peng, Y. Proteomic and metabolomic profilings reveal crucial functions of γ-aminobutyric acid in regulating Ionic, water, and metabolic homeostasis in creeping bentgrass under salt stress. J. Proteome Res. 2020, 19, 769–780. [Google Scholar] [CrossRef]
- Liu, Z.; Lemmonds, S.; Huang, J.; Tyagi, M.; Hong, L.; Jain, N. Entropic contribution to enhanced thermal stability in the thermostable P450 CYP119. Proc. Natl. Acad. Sci. USA 2018, 115, E10049–E10058. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, J.; Ma, M.; You, T.; Ye, S.; Liu, S. Computational design towards a boiling-resistant single-chain sweet protein monellin. Food Chem. 2024, 440, 138279. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.U.; Nayab, H.; Rehman, T.U.; Williamson, M.P.; Haq, K.U.; Shafi, N.; Shafique, F. Characterisation of bacteriocins produced by Lactobacillus spp. isolated from the traditional Pakistani yoghurt and their antimicrobial activity against common foodborne pathogens. BioMed Res. Int. 2020, 2020, 8281623. [Google Scholar] [CrossRef] [PubMed]
- Jaenicke, R. Protein stability and molecular adaptation to extreme conditons. Eur. J. Biochem. 1991, 202, 715–728. [Google Scholar] [CrossRef]
- Bérdy, J. Thoughts and facts about antibiotics: Where we are now and where we are heading. J. Antibiot. 2012, 65, 385–395. [Google Scholar] [CrossRef]
- Li, Y.; Yu, S.; Weng, P.; Wu, Z.; Liu, Y. Purification and antimicrobial mechanism of a novel bacteriocin produced by Lactiplantibacillus plantarum FB-2. LWT 2023, 185, 115123. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J.; Zheng, W.; Zhao, S.; Yang, Q.; Yu, D.; Zhu, Y. Antibacterial activity and mechanism of a novel bacteriocin produced by Lactiplantibacillus plantarum against Escherichia coli and Staphylococcus aureus. Int. J. Food Sci. Technol. 2023, 58, 181–193. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, X.; Ding, X.; Tian, T.; Tseng, C.K.; Luo, X.; Chen, X.; Lo, C.J.; Leake, M.C.; Bai, F. Sensitive bacterial Vm sensors revealed the excitability of bacterial Vm and its role in antibiotic tolerance. Proc. Natl. Acad. Sci. USA 2023, 120, e2208348120. [Google Scholar] [CrossRef]
- Whittle, E.E.; Orababa, O.; Osgerby, A.; Siasat, P.; Element, S.J.; Blair, J.M.A.; Overton, T.W. Efflux pumps mediate changes to fundamental bacterial physiology via membrane potential. Mbio 2024, 15, e02370-24. [Google Scholar] [CrossRef]
- Bauer, R.; Dicks, L.M.T. Mode of action of lipid II-targeting lantibiotics. Int. J. Food Microbiol. 2005, 101, 201–216. [Google Scholar] [CrossRef]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, e0037425. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, M.; Nakamura, K.; Sk, R.; Tashiro, Y.; Shiwa, Y. Complete genome sequence of Levilactobacillus acidifarinae type strain JCM 15949 (DSM 19394). Microbiol. Resour. Announc. 2025, 14, e00374-25. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhang, G.; Bahadur, A.; Xu, Y.; Liu, Y.; Tian, M.; Ding, W.; Chen, T.; Zhang, W.; Liu, G. Genomic investigation of desert Streptomyces huasconensis D23 reveals its environmental adaptability and antimicrobial activity. Microorganisms 2022, 10, 2408. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).