Retention of Original Flavor Characteristics in Defluorinated Instant Qingzhuan Brick Tea Prepared Using Membrane Separation Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Feasibility Analysis of Highly Selective Fluoride Removal
2.3. Factors Influencing Defluorination Using Nanofiltration
2.4. Analysis of Defluorinated Instant Brick Tea Products Prepared Through Nanofiltration
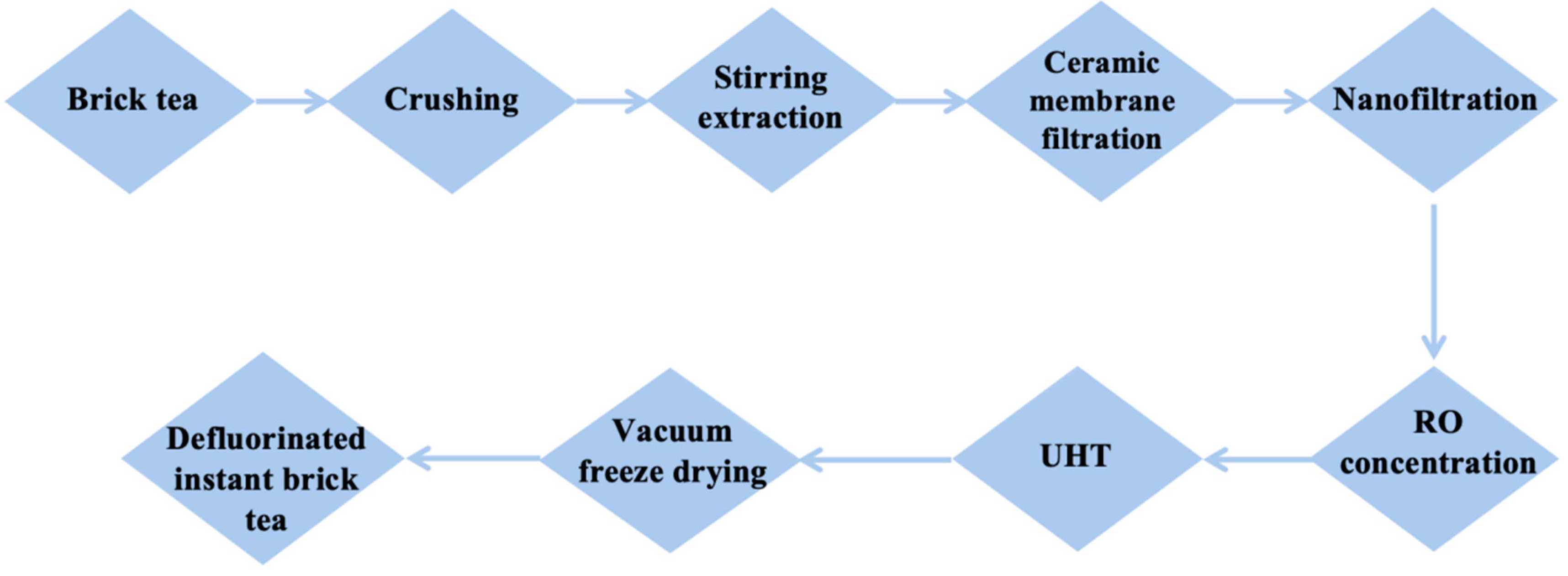
2.4.1. Preparation of Defluorinated Brick Tea
2.4.2. Sensory Evaluation
2.4.3. Quality Analytical Methods
2.5. Calculations
2.6. Statistical Analysis
3. Results and Discussion
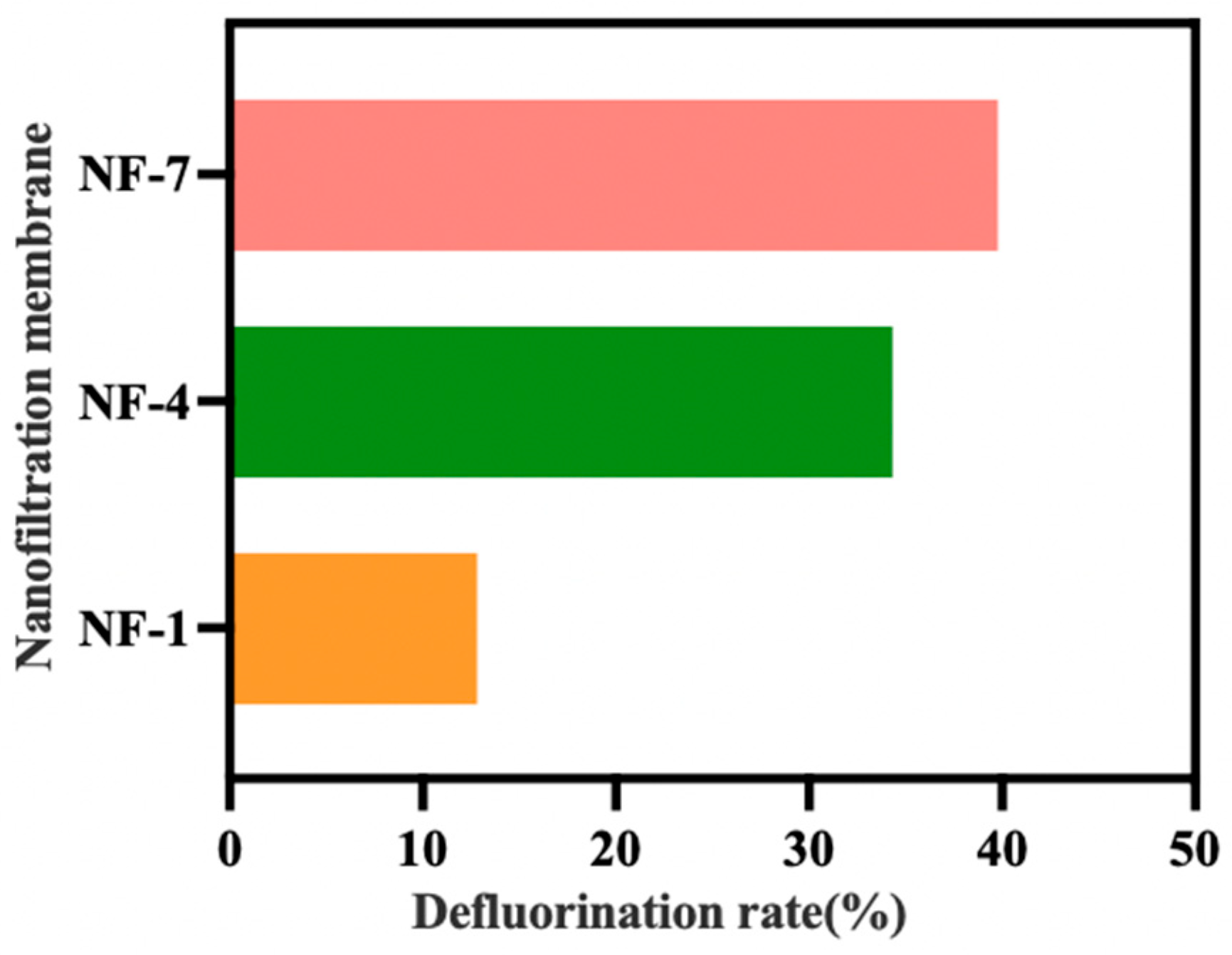
3.1. Feasibility Analysis of Highly Selective Fluoride Removal Using Nanofiltration Technology
3.2. Factors Influencing Defluorination Using Nanofiltration
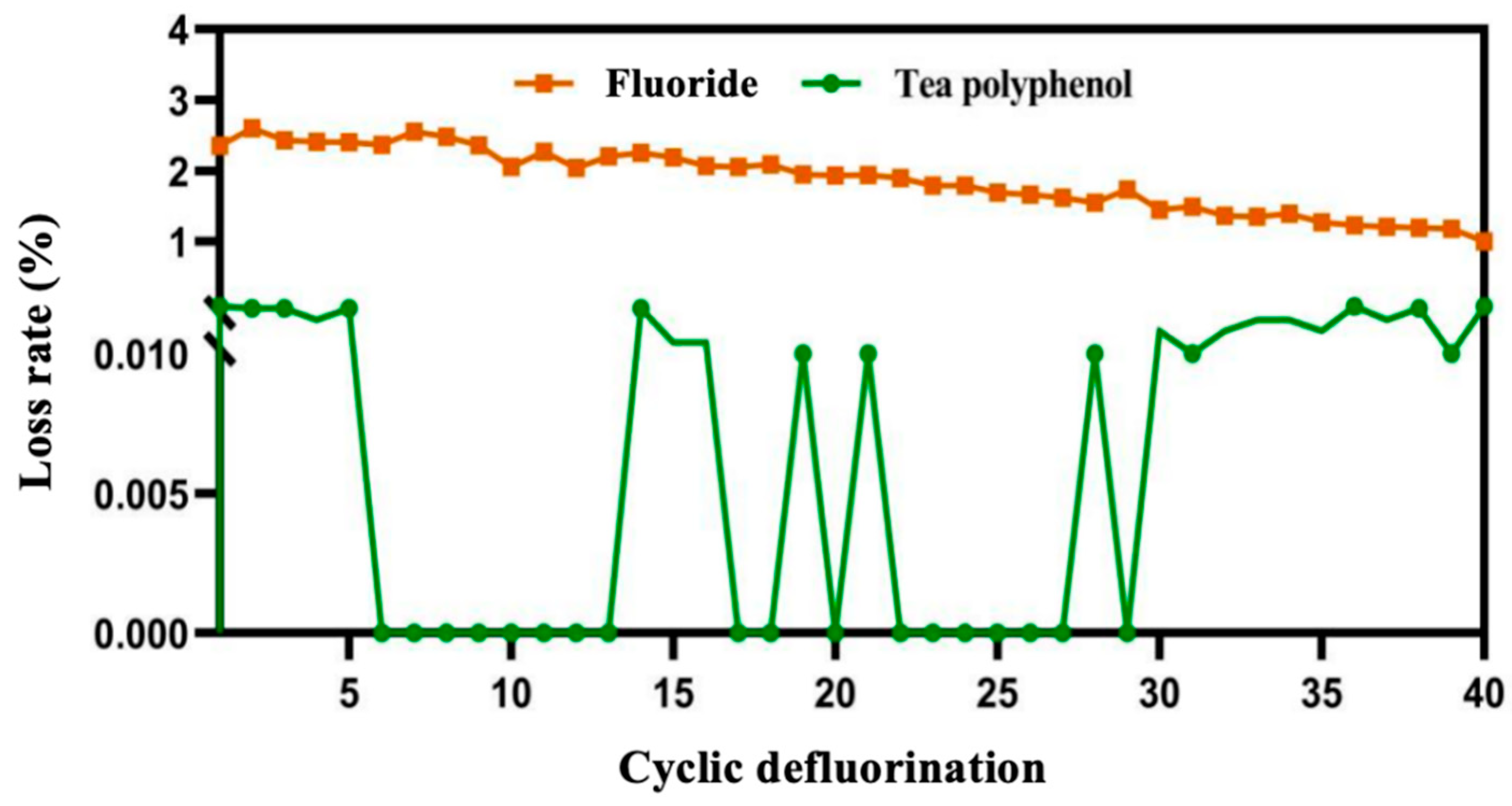
3.2.1. Characteristics of Cyclic Defluorination
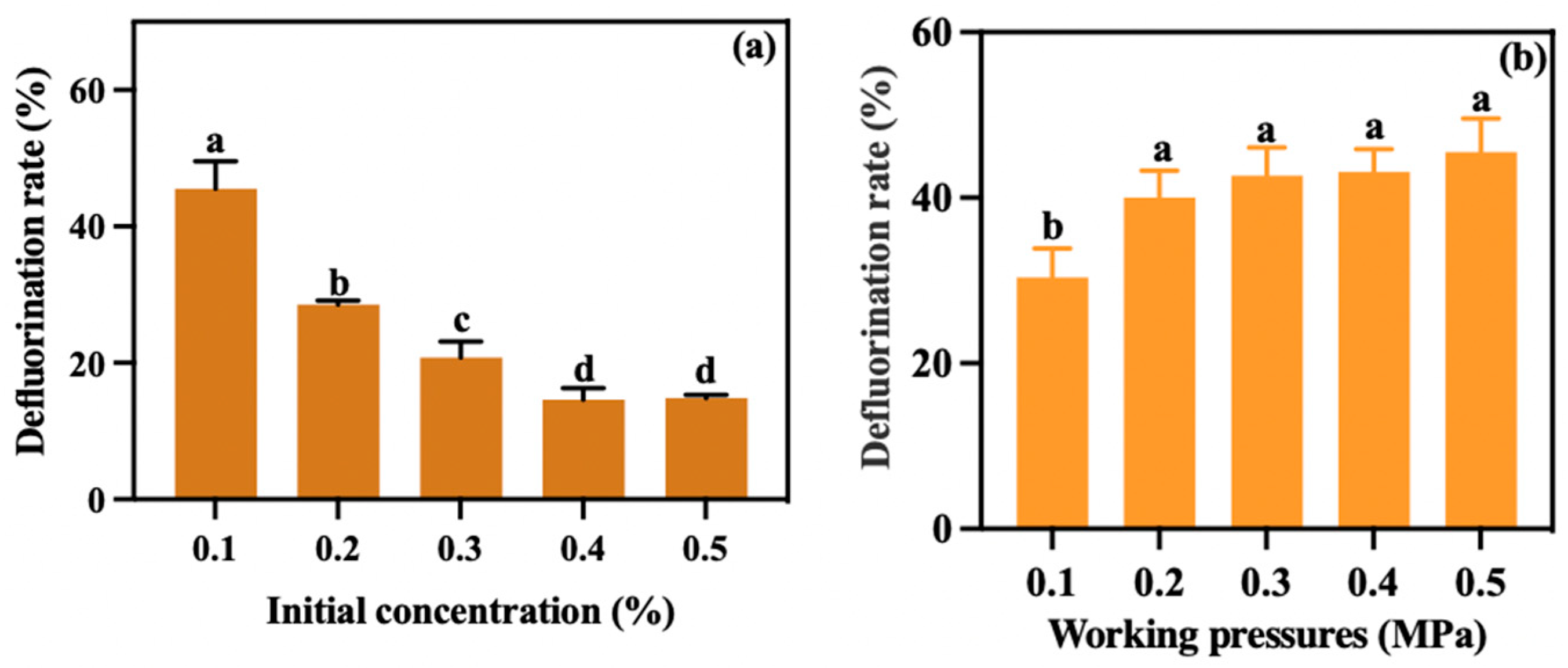
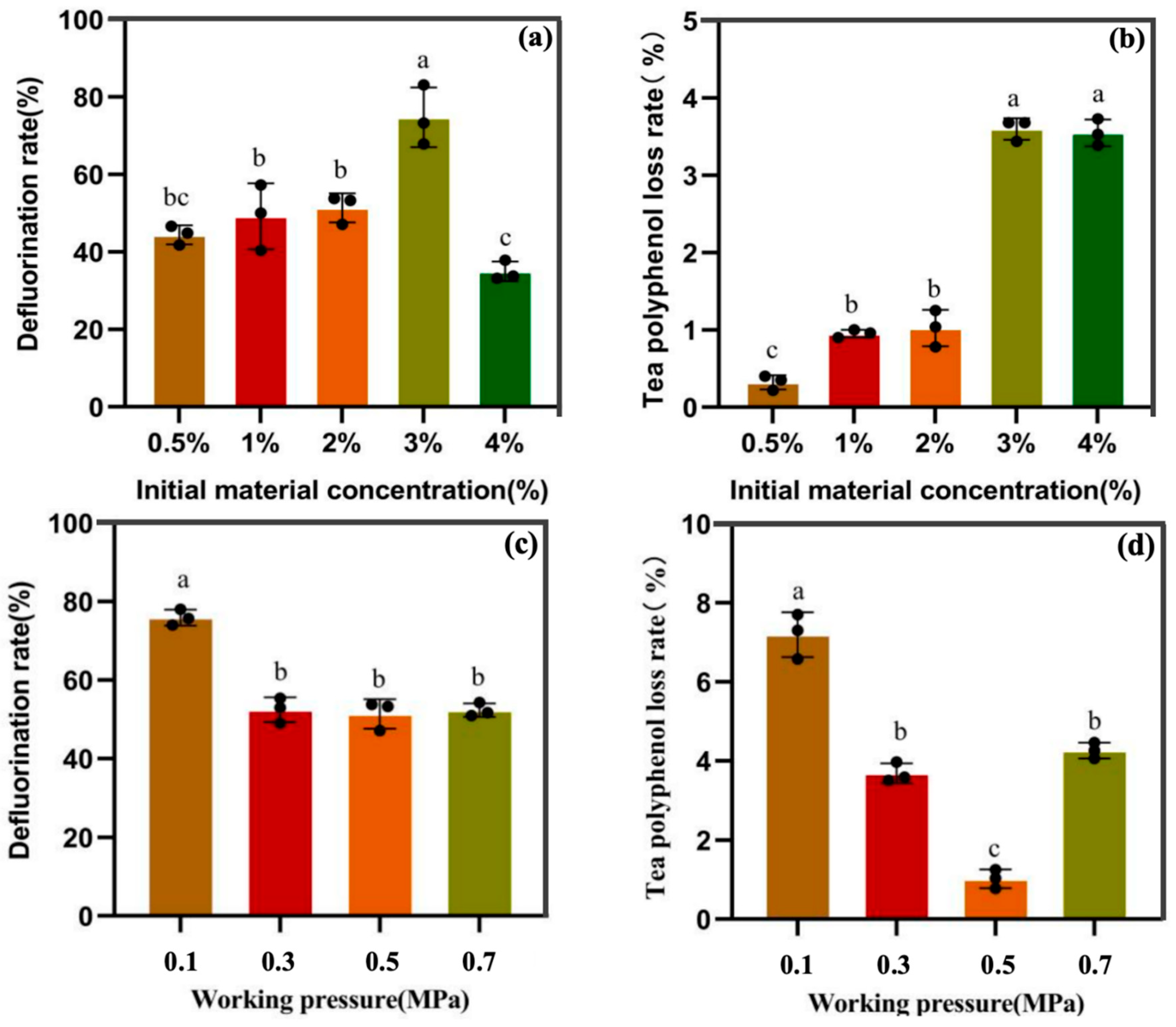
3.2.2. Regulation of Defluorination Under Different Initial Concentrations and Working Pressures
3.3. Analysis of Defluorinated Instant Brick Tea Products Prepared Through Nanofiltration
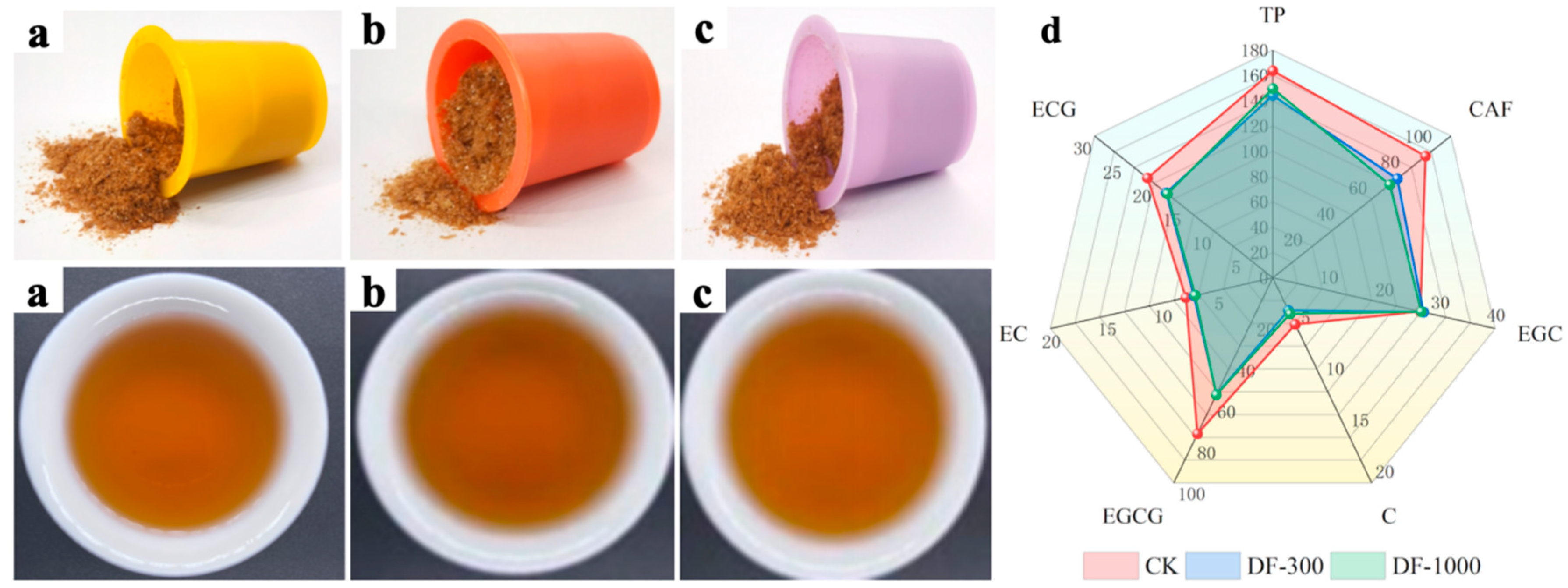
3.3.1. Preparation of Defluorinated Instant Brick Tea Products
3.3.2. Analysis of Defluorinated Instant Brick Tea Products
3.3.3. Analysis of Key Volatiles in Defluorinated Instant Brick Tea Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, C.Y.; Xu, X.F.; Ren, Y.F.; Niu, H.L.; Yang, Y.Q.; Hou, R.Y.; Wan, X.C.; Cai, H.M. Fluoride absorption, transportation and tolerance mechanism in Camellia sinensis, and its bioavailability and health risk assessment: A systematic review. J. Sci. Food Agric. 2021, 101, 379–387. [Google Scholar] [CrossRef]
- Chinese Nutrition Society. Chinese Dietary Reference Intakes for Residents (2013 Edition); Science Press: Beijing, China, 2014. [Google Scholar]
- Lin, F.J.; Wei, X.L.; Liu, H.Y.; Li, H.; Xia, Y.; Wu, D.T.; Zhang, P.Z.; Gandhi, G.R.; Li, H.B.; Gan, R.Y. State-of-the-art review of dark tea: From chemistry to health benefits. Trends Food Sci. Technol. 2021, 109, 126–138. [Google Scholar] [CrossRef]
- Liu, P.P.; Feng, L.; Chen, J.; Wang, S.P.; Wang, X.P.; Han, Y.N.; Ma, M.J.; Liu, Z.H.; Zheng, P.C. Unlocking the secrets of Qingzhuan tea: A comprehensive overview of processing, flavor characteristics, and health benefits. Trends Food Sci. Technol. 2024, 147, 104450. [Google Scholar] [CrossRef]
- Whyte, M.P.; Essmyer, K.; Gannon, F.H.; Reinus, W.R. Skeletal fluorosis and instant tea. Am. J. Med. 2005, 118, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Wang, Y.; Yao, Y.; Ma, W.W.; Li, C.H.; Zhang, T.; Liu, F. Processing technology and quality of low-fluoride brick tea. Agric. Sci. Technol. 2017, 18, 1680–1685. [Google Scholar]
- Joshi, A.N. A review of processes for separation and utilization of fluorine from phosphoric acid and phosphate fertilizers. Chem. Pap. 2022, 76, 6033–6045. [Google Scholar] [CrossRef]
- Yang, X.D.; Ni, K.; Shi, Y.Z.; Yi, X.Y.; Zhang, Q.F.; Fang, L.; Ma, L.F.; Ruan, J. Effects of long-term nitrogen application on soil acidification and solution chemistry of a tea plantation in China. Agric. Ecosyst. Environ. 2018, 252, 74–82. [Google Scholar] [CrossRef]
- Xi, J.J.; Zhang, L.; Peng, C.Y.; Zhou, J.; Peng, Y.; Xu, L.Y.; Chen, B.; Meng, Q.; Hou, R.Y.; Li, D.X.; et al. Flavor augmentations affect fluoride bioavailability from brewed dark tea. LWT 2019, 109, 270–275. [Google Scholar] [CrossRef]
- Xue, C.; Chen, X.; Yang, K. Effects of selenium and zinc on the absorption, excretion, and accumulation of fluoride in rats. J. Environ. Health 2002, 19, 170–172. [Google Scholar]
- Yadav, D.; Karki, S.; Ingole, P.G. Current advances and opportunities in the development of nanofiltration (NF) membranes in the area of wastewater treatment, water desalination, biotechnological and pharmaceutical applications. J. Environ. Chem. Eng. 2022, 10, 108109. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Fíla, V. Membrane-based technologies as an emerging tool for separating high-added-value compounds from natural products. Trends Food Sci. Technol. 2018, 82, 8–20. [Google Scholar] [CrossRef]
- Kotsanopoulos, K.V.; Arvanitoyannis, I.S. Membrane processing technology in the food industry: Food processing, wastewater treatment, and effects on physical, microbiological, organoleptic, and nutritional properties of foods. Crit. Rev. Food Sci. Nutr. 2015, 55, 1147–1175. [Google Scholar] [CrossRef]
- Firman, L.R.; Ochoa, N.A.; Marchese, J.; Pagliero, C.L. Deacidification and solvent recovery of soybean oil by nanofiltration membranes. J. Membr. Sci. 2013, 431, 187–196. [Google Scholar] [CrossRef]
- Salehi, F. Current and future applications for nanofiltration technology in the food processing. Food Bioprod. Process. 2014, 92, 161–177. [Google Scholar] [CrossRef]
- Nath, K.; Dave, H.K.; Patel, T.M. Revisiting the recent applications of nanofiltration in food processing industries: Progress and prognosis. Trends Food Sci. Technol. 2018, 73, 12–24. [Google Scholar] [CrossRef]
- Chen, G.Q.; Leong, T.S.H.; Kentish, S.E.; Ashokkumar, M.; Martin, G.J.O. Chapter 8—Membrane separations in the dairy industry. In Separation of Functional Molecules in Food by Membrane Technology; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 267–304. [Google Scholar] [CrossRef]
- Figueira, M.; Rodríguez-Jiménez, D.; López, J.; Reig, M.; Cortina, J.L.; Valderrama, C. Evaluation of the nanofiltration of brines from seawater desalination plants as pre-treatment in a multimineral brine extraction process. Sep. Purif. Technol. 2023, 322, 124232. [Google Scholar] [CrossRef]
- Uyttebroek, M.; Vandezande, P.; Van Dael, M.; Vloemans, S.; Noten, B.; Bongers, B.; Porto-Carrero, W.; Muñiz Unamunzaga, M.; Bulut, M.; Lemmens, B. Concentration of phenolic compounds from apple pomace extracts by nanofiltration at lab and pilot scale with a techno-economic assessment. J. Food Process Eng. 2018, 41, e12629. [Google Scholar] [CrossRef]
- Morgante, C.; Lopez, J.; Cortina, J.L.; Tamburini, A. New generation of commercial nanofiltration membranes for seawater/brine mining: Experimental evaluation and modelling of membrane selectivity for major and trace elements. Sep. Purif. Technol. 2024, 340, 126758. [Google Scholar] [CrossRef]
- Liu, Z.Q. Studies on the Technology to Optimize the Quality of Instant Black Tea by In-Vitro Oxidation with Polyphenol Oxidase. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2012. [Google Scholar]
- GB/T 21728; Determination of Fluoride Content in Brick Tea. Standardization Administration of the People’s Republic of China (SAPRC): Beijing, China, 2008.
- GB/T 8313; Determination of Total Polyphenols and Catechins Content in Tea. Standardization Administration of the People’s Republic of China (SAPRC): Beijing, China, 2008.
- Deng, G.J.; Huang, L.F.; Wang, W.Y.; Yu, T.Z.; Ning, J.M.; Wang, Y.J. Mechanism of key volatiles in osmanthus scented green tea on the improvement of its aroma and taste quality. Food Chem. 2024, 460, 140560. [Google Scholar] [CrossRef]
- Ramdani, A.; Deratani, A.; Taleb, S.; Drouiche, N.; Lounici, H. Performance of NF90 and NF270 commercial nanofiltration membranes in the defluoridation of Algerian brackish water. Desalin. Water Treat. 2021, 212, 286–296. [Google Scholar] [CrossRef]
- Ben Nasr, A.B.; Charcosset, C.; Amar, R.B.; Walha, K. Defluoridation of water by nanofiltration. J. Fluorine Chem. 2013, 150, 92–97. [Google Scholar] [CrossRef]
- Conidi, C.; Castro-Muñoz, R.; Cassano, A. Membrane-based operations in the fruit juice processing industry: A review. Beverages 2020, 6, 18. [Google Scholar] [CrossRef]
- Hu, K.; Dickson, J.M. Nanofiltration membrane performance on fluoride removal from water. J. Membr. Sci. 2006, 279, 529–538. [Google Scholar] [CrossRef]
- Salgado, C.M.; Fernández-Fernández, E.; Palacio, L.; Hernández, A.; Prádanos, P. Alcohol reduction in red and white wines by nanofiltration of musts before fermentation. Food Bioprod. Process. 2015, 96, 285–295. [Google Scholar] [CrossRef]
- Salgado, C.M.; Palacio, L.; Prádanos, P.; Hernández, A.; González-Huerta, C.; Pérez-Magariño, S. Comparative study of red grape must nanofiltration: Laboratory and pilot plant scales. Food Bioprod. Process. 2015, 94, 610–620. [Google Scholar] [CrossRef]
- Talebi, S.; Suarez, F.; Chen, G.Q.; Chen, X.; Bathurst, K.; Kentish, S.E. Pilot study on the removal of lactic acid and minerals from acid whey using membrane technology. ACS Sustain. Chem. Eng. 2020, 8, 2855–2864. [Google Scholar] [CrossRef]
- Liu, P.P.; Zheng, P.C.; Feng, L.; Gong, Z.M.; Zheng, L.; Gao, S.W.; Wang, X.P.; Ye, F.; Huang, J.N.; Liu, Z.H. Dynamic changes in the aroma profile of Qingzhuan tea during its manufacture. Food Chem. 2022, 375, 131847. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.D.; Liu, Z.; Zhao, Y.; Cheng, Y.; Li, J.; Ning, J.; Yang, Y.; Huang, J.A. Comparative study of vegetative and reproductive growth of different tea varieties response to different fluoride concentrations stress. Plant Physiol. Biochem. 2020, 154, 419–428. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Ma, L.F.; Ruan, J.Y. Characterization of fluoride uptake by roots of tea plants (Camellia sinensis (L.) O. Kuntze). Plant Soil. 2012, 366, 659–669. [Google Scholar] [CrossRef]
| Initial Concentration (%) | Fluoride (mg)/Tea Polyphenols (mg) | Working Pressure (MPa) | Fluoride (mg)/Tea Polyphenols (mg) | ||||
|---|---|---|---|---|---|---|---|
| Original Tea Infusion | Permeate | Permeate/Original Tea Infusion | Original Tea Infusion | Permeate | Permeate/Original Tea Infusion | ||
| 0.10 | 0.33 | 3.67 | 11.03 | 0.1 | 0.24 | 10.48 | 44.41 |
| 0.20 | 0.33 | 6.38 | 19.19 | 0.2 | 0.28 | 3.17 | 11.33 |
| 0.30 | 0.32 | 11.82 | 37.04 | 0.3 | 0.30 | 19.18 | 63.89 |
| 0.40 | 0.33 | 4.02 | 12.10 | 0.4 | 0.30 | 36.50 | 123.68 |
| 0.50 | 0.30 | 7.95 | 26.71 | 0.5 | 0.33 | 3.67 | 11.04 |
| Products | Color of Instant Brick Tea (10%) | Color of Tea Liquid (25%) | Aroma (25%) | Taste (30%) | Solubility (10%) | Total Score |
|---|---|---|---|---|---|---|
| CK | 82.50 | 84.60 | 84.60 | 78.90 | 84.10 | 82.63 |
| DF-300 | 76.90 | 87.70 | 85.60 | 75.80 | 88.00 | 82.56 |
| DF-1000 | 78.70 | 86.80 | 85.60 | 78.80 | 89.80 | 83.59 |
| Key Components (μg/mL) | CK | DF-1000 | DF-300 | Odorthresholds | Odor Quality |
|---|---|---|---|---|---|
| Linalool | 0.0884 ± 0.0101 b | 0.1385 ± 0.1051 a | 0.0993 ± 0.0782 ab | 0.0006 | Citrus-like, flowery |
| (E)-2-nonanal | 0.0239 ± 0.0031 a | ND b | 0.014 ± 0.0078 a | 0.0028 | Citrus-like, soapy |
| Decanal | 0.0080 ± 0.0028 a | ND a | 0.0032 ± 0.0021 a | / | / |
| Safranal | 0.0470 ± 0.0057 a | 0.0432 ± 0.0327 a | 0.0298 ± 0.0235 a | / | Saffron-like |
| Hexanal | 0.2284 ± 0.0300 a | 0.0331 ± 0.0221 b | 0.0154 ± 0.0114 b | 0.0024 | Green, grassy |
| (E,E)-2,4-heptadienal | 0.0041 ± 0.0027 a | ND b | ND b | 0.000032 | Fatty, flowery |
| 1-octen-3-one | 0.0025 ± 0.0002 a | ND b | 0.0002 ± 0.0001 a | 0.000016 | Mushroom-like |
| α-ionone | 0.0364 ± 0.0041 a | 0.0319 ± 0.0219 a | 0.0209 ± 0.0151 a | 0.0004 | Flowery, violet-like |
| (E)-β-ionone | 0.0284 ± 0.0079 a | 0.0205 ± 0.0116 a | 0.0111 ± 0.0083 a | 0.000021 | Flowery, violet-like |
| 6-methyl-3,5-heptadien-2-one | 0.0914 ± 0.0102 a | 0.0198 ± 0.0215 b | NDc | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; Xie, Y.-Y.; Liu, X.-Y.; Yi, H.-H.; Xu, H.-J.; Zhang, L.; Cai, H.-M.; Liu, Z.-Q.; Li, D.-X.; Yang, Y.-Q.; et al. Retention of Original Flavor Characteristics in Defluorinated Instant Qingzhuan Brick Tea Prepared Using Membrane Separation Technology. Fermentation 2025, 11, 609. https://doi.org/10.3390/fermentation11110609
Huang R, Xie Y-Y, Liu X-Y, Yi H-H, Xu H-J, Zhang L, Cai H-M, Liu Z-Q, Li D-X, Yang Y-Q, et al. Retention of Original Flavor Characteristics in Defluorinated Instant Qingzhuan Brick Tea Prepared Using Membrane Separation Technology. Fermentation. 2025; 11(11):609. https://doi.org/10.3390/fermentation11110609
Chicago/Turabian StyleHuang, Run, Ying-Ying Xie, Xin-Yu Liu, Huai-Hui Yi, Hao-Jie Xu, Liang Zhang, Hui-Mei Cai, Zheng-Quan Liu, Da-Xiang Li, Yun-Qiu Yang, and et al. 2025. "Retention of Original Flavor Characteristics in Defluorinated Instant Qingzhuan Brick Tea Prepared Using Membrane Separation Technology" Fermentation 11, no. 11: 609. https://doi.org/10.3390/fermentation11110609
APA StyleHuang, R., Xie, Y.-Y., Liu, X.-Y., Yi, H.-H., Xu, H.-J., Zhang, L., Cai, H.-M., Liu, Z.-Q., Li, D.-X., Yang, Y.-Q., Wan, X.-C., & Peng, C.-Y. (2025). Retention of Original Flavor Characteristics in Defluorinated Instant Qingzhuan Brick Tea Prepared Using Membrane Separation Technology. Fermentation, 11(11), 609. https://doi.org/10.3390/fermentation11110609










